Abstract
Background:
Anticholinergic medication exposure has been associated with increased risk for dementia. No study has examined the association between anticholinergic medication use and neuropathologic lesions in a community-based sample.
Objective:
To examine the relationship between anticholinergic exposure and dementia-related neuropathologic changes.
Methods:
Within a community-based autopsy cohort (N=420), we ascertained use of anticholinergic medications over a 10-year period from automated pharmacy data and calculated total standardized daily doses (TSDDs). We used modified Poisson regression to calculate adjusted relative risks (RRs) and 95% confidence intervals (CIs) for the association between anticholinergic exposure and dementia-associated neuropathology. Inverse probability weighting was used to account for selection into the autopsy cohort.
Results:
Heavy anticholinergic exposure (≥1096 TSDDs) was not associated with greater neuropathologic changes of Alzheimer’s disease; the adjusted RRs for heavy use of anticholinergics (≥1096 TSDDs) compared to no use were 1.22 (95% CI 0.81–1.88) for neuritic plaque scores and 0.89 (0.47–1.66) for extent of neurofibrillary degeneration. Moderate (91–1095 TSDD) and heavy use of anticholinergics was associated with a significantly lower cerebral microinfarct burden compared with no use with adjusted RRs of 0.44 (0.21–0.89) and 0.24 (0.09–0.62), respectively. Anticholinergic exposure was not associated with macroscopic infarcts or atherosclerosis.
Conclusions:
Use of anticholinergic medications is not associated with Alzheimer’s disease-related neuropathologic changes but is associated with lower cerebral microinfarct burden. Further research into biological mechanisms underlying the anticholinergic-dementia link is warranted.
Keywords: Alzheimer’s disease, neuropathology, neurofibrillary tangles, neuritic plaques, cholinergic antagonists
Introduction
Medications with anticholinergic activity (i.e., or anticholinergics) are used to treat a variety of health conditions, such as overactive bladder, seasonal allergies, and depression. Guidelines recommend minimizing medications with anticholinergic activity in older adults,[1, 2] because of the risk for falls, poor cognitive outcomes and other adverse drug events. Nonetheless, anticholinergic medication use remains high with a prevalence of use between 10–27% in community-dwelling older adults.[3–6]
Cholinergic neurotransmission plays an important role in memory and learning. Although acute cognitive impairment is a well-known risk associated with anticholinergic medications, mounting evidence suggests that anticholinergic medications may increase risk for dementia.[7–9] The biological processes by which anticholinergics may lead to neurodegeneration are unknown, but limited evidence in human studies suggests that anticholinergics may be associated with a greater burden of Alzheimer Disease (AD)-related brain pathology (e.g., amyloid plaques and neurofibrillary tangles) in patients with Parkinson’s disease,[10] or increased brain atrophy and dysfunction.[11, 12] Yet, other theories gleaned from animal models suggest that anticholinergic medications may increase risk by other mechanisms not related to traditional neuropathologic findings visible macroscopically such as alterations in synaptic membrane, synaptic numbers or inflammation.[13, 14]
In previous work, we reported that older adults with heavy anticholinergic exposure had higher risk of dementia than those with no use.[8] To explore possible biological mechanisms, we sought to examine the association between anticholinergic use and dementia-related neuropathology, drawing on data from a representative community-based autopsy cohort. We hypothesized that, compared to older adults with no anticholinergic exposure, those who have heavier use would have higher burden of neuropathologic outcomes.
Materials and Methods
Overview
Adult Changes in Thought (ACT) is a prospective cohort study set within Kaiser Permanente Washington (KPW, previously Group Health), an integrated healthcare delivery system in the Pacific Northwest. Study methods have been described in detail elsewhere.[15] The sample for this study included participants who died and underwent autopsy. To account for differences that may exist between the analytic sample and the broader ACT cohort, we used statistical methods to account for potential selection bias. Both the autopsy sample and the broader ACT cohort are described below. Study procedures are approved by the Human Subjects Review Committee, and participants provide written informed consent.
Study Population
ACT recruits KPW members living in or near Seattle, Washington who are at least 65 years old, community dwelling, and dementia-free. Participants were enrolled during three waves: the original cohort between 1994 and 1996 (n=2581), the expansion cohort between 2000 and 2003 (n=811), and continuous enrollment of 10–15 participants per month beginning in 2004. Participants were assessed at study entry and at two-year intervals to evaluate cognitive function and collect information about demographic characteristics, medical history, health behaviors, and functional measures. [15]
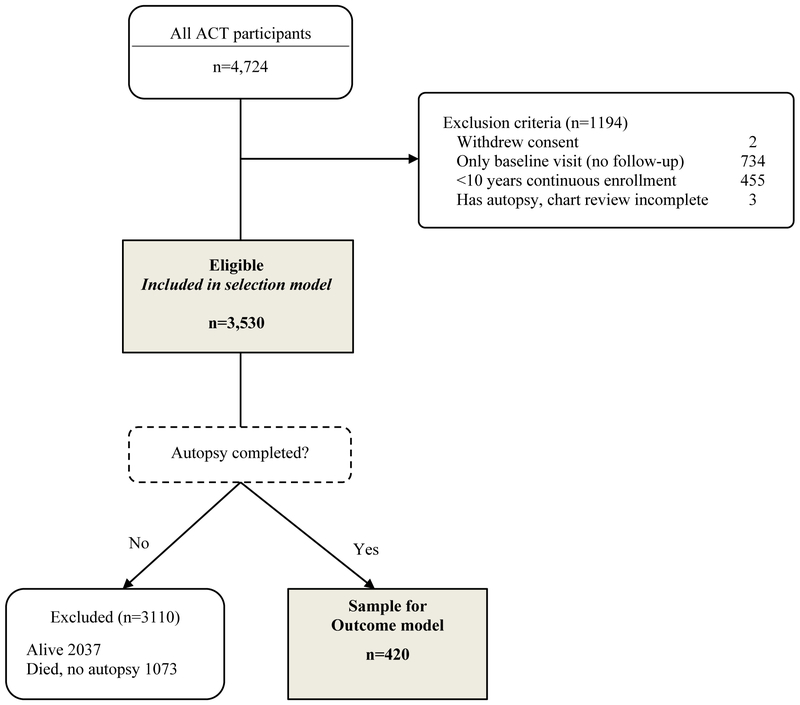
As of September 30, 2012, ACT had enrolled 4,724 people. Participants were excluded from the current analyses if they had no follow-up visits (n=734), had less than 10 years of continuous enrollment in the health plan prior to the index date (defined below) (n=455), or withdrew study participation or had not completed chart review (n=5). Of the 3530 participants meeting eligibility criteria, 2,037 were still alive, 1,073 died without autopsy, and 420 died with autopsy and comprise the analytic sample (Figure 1). Information on the distribution of demographic and clinical characteristics between those included and excluded in this analysis can be found in Supplementary Table 1.
Figure 1.
Study sample for neuropathology analyses. Abbreviations: ACT, Adult Changes in Thought.
Defining an Index Date
We defined the time window in which to measure anticholinergic exposure based on our hypothesis that anticholinergic exposure before dementia onset is the most etiologically relevant. The presence of dementia might influence medication exposure, either through increased use of certain medications to treat dementia symptoms or through avoidance of contraindicated medications, thus including exposures accrued in the period after dementia diagnosis could introduce bias.[16] Therefore, we measured exposure in relation to an index date defined as the estimated date of dementia onset for participants who had developed dementia. To define a comparable exposure window for participants who had not developed dementia, we matched each one to a pool of similar participants who had developed dementia and randomly selected an index date from the pool. The methods used to define the index date are described in Supplemental Table 1. The average (SD) time between index date and death was 4.8 (2.6) years for people with dementia and 4.4 (2.4) years for those without dementia. Anticholinergic exposure and covariates were defined based on a 10-year period prior to the index date.
Medication Exposure
Given the challenge in estimating the effect of individual medications with anticholinergic activity on the brain, researchers have relied on various scales to estimate overall anticholinergic activity from a person’s drug regimen. These scales vary considerably in the included medications and there is no gold standard.[17] For this study, we focused on medications with strong anticholinergic activity as outlined in the Beers Criteria (Supplemental Table 3).[1] We identified these medications from automated pharmacy dispensing data.[8] We converted prescription fills to standardized daily doses (SDDs) as in prior studies.[8, 16] To compute SDDs, we multiplied the number of pills by the pill strength and then divided by the minimum effective dose per day recommended for use in older adults according to a well-respected geriatric pharmacy reference.[18] We then defined each participant’s cumulative anticholinergic exposure as the total standardized daily doses (TSDDs), that is, the sum of SDDs across all prescriptions dispensed in the 10-year exposure window. We selected 10 years based on study hypotheses, methodologic and practical considerations and to align with the exposure-window used for our prior analyses assessing the association between anticholinergic use and incident dementia.[8] We excluded medication use that occurred during the 1 year prior to the index date because prodromal symptoms of dementia could affect medication usage, therefore exposure was assessed during the 10 year period from 11 years to one year prior to the index date.
We categorized cumulative anticholinergic use as 0, 1–90, 91–1095 or ≥1096 TSDDs.[8] To translate into clinical terms a person would reach the highest level of exposure if they took any of the following medications daily for more than 3 years: oxybutynin 5 mg, chlorpheniramine 4 mg, olanzapine 2.5 mg, meclizine 25 mg or doxepin 10 mg.
Neuropathologic Outcomes
We examined several neuropathology outcomes associated with dementia. AD-related neuropathologic changes included neuritic plaques assessed by Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) staging,[19, 20] neurofibrillary degeneration assessed by Braak staging,[21, 22] and cerebral amyloid angiopathy.[23] Vascular brain injury assessment included cerebral microinfarcts, macroscopic infarcts, and atherosclerosis. [24, 25] A board-certified neuropathologist performed these evaluations according to standard procedures, blinded to the participants’ medication exposures and clinical dementia status.[26] The majority of brains had a postmortem interval of less than 48 hours, with 40% having an interval of less than 8 hours. Once removed, brains were fixed in 10% neutral buffered formalin for at least 14 days.
We dichotomized each neuropathologic outcome to define a level of lesion burden that has been associated with clinical dementia. [23, 26] We have used these definitions in several prior studies.[16, 23, 26, 27] The outcomes are defined as follows: neuritic plaques (moderate or frequent versus sparse or none); neurofibrillary degeneration (Braak stages V-VI versus 0-IV), cerebral amyloid angiopathy (any versus none); cerebral microinfarcts (≥3 versus <3), macroscopic infarcts (≥1 versus none) and atherosclerosis (moderate or severe versus none or mild).
Covariates
Information about potential confounders came from ACT study visits, electronic health records and manual chart review. Demographic characteristics included age, sex, and years of education. Body mass index (BMI) was calculated from measured weight in kilograms divided by height in meters squared. Participants were asked about smoking, self-rated health and exercise. Regular exercise was defined as ≥ 15 minutes of activity at least three times a week of the following activities: hiking, bicycling, aerobics or calisthenics, swimming, water aerobics, weight training or stretching, or other exercise.[28] Depressive symptoms were assessed using a modified version of the Center for Epidemiologic Studies Depression scale.[29] Medical records were reviewed to collect information about medical conditions including diabetes, stroke, and coronary artery disease (CAD). CAD was defined as the presence of myocardial infarction, history of coronary artery bypass grafting, coronary angioplasty, or angina. Hypertension was defined as receiving two or more fills for an antihypertensive medication in a 1-year period.
Participants were screened for dementia at study entry and every two years using the Cognitive Abilities Screening Instrument (CASI).[30] Those screening positive on the CASI (score ≤85) underwent a standardized diagnostic evaluation which included neuropsychological testing.[15] A multidisciplinary committee assigned diagnoses of dementia and AD using research criteria.[31, 32] The date of dementia onset is imputed as the date halfway between the visit triggering the evaluation and the prior study visit.[15]
Statistical Analysis
We estimated relative risks using modified Poisson regression to assess the associations between cumulative anticholinergic exposure and binary neuropathology outcomes. This approach uses a generalized linear model with Poisson error distribution and log link, and uses generalized estimating equations to estimate regression parameters and account for misspecification of the mean-variance relationship.[33] A separate model was estimated for each neuropathologic outcome, adjusting for ACT study cohort (wave of enrollment), age at death, sex, education, hypertension, diabetes, stroke and CAD (models for macroscopic infarcts did not adjust for stroke, because of the collinearity expected between these variables). For variables that can change over time, we used values from the 10-year exposure window. Diabetes, stroke, CAD and hypertension were defined as present if they were present any time during this window.
In sensitivity analyses, outcome models additionally adjusted for self-rated health, depression, physical activity and BMI. We used the value closest to the index date for self-rated health, depression and physical activity. For BMI, we used the average over the exposure window. Due to sparse data, models for the outcomes of cerebral amyloid angiopathy and micro-infarcts did not converge for the sensitivity analyses with the additional adjustment variables. APOE ε4 allele was not available on all autopsy subjects, which precluded our ability to adjust for this potential confounder in our primary analysis. However, we conducted post-hoc complete case analyses additionally adjusting for APOE ε4 allele status (n=383).
Accounting for selection bias:
Inverse probability weighting was used to account for potential selection bias. Selection bias may occur if demographic and clinical factors are associated with mechanisms related to inclusion in the autopsy cohort (including consent to autopsy, study withdrawal, and death), leading to an analytic sample that is not representative of the overall cohort.[34] Logistic regression models were used to estimate the probability of inclusion in the autopsy cohort. Selection models were estimated using all ACT study participants meeting eligibility criteria (Figure 1), and predictors included age at study entry, ACT study cohort, sex, education, dementia, anticholinergic exposure, and history of stroke and CAD. All covariates were as defined as described previously, except for stroke and CAD. We used a combination of ACT interview data and electronic health record data because the selection models included all ACT participants and medical record reviews have not been completed for those without autopsy. The inverse of the estimated probabilities of selection were used as weights in the primary outcome models. We accounted for the uncertainty in the estimated selection weights by using bias corrected accelerated bootstrap confidence intervals for model parameters[35] as used in prior work.[16, 27, 36] Analyses were performed using STATA/MP 13.1 for Windows (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP) and R, version 2.15.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Overall, 185 participants had dementia, with the most common types including AD (n=110; 60%), vascular dementia (n= 25; 14%) and mixed dementia (n= 33; 18%). Of the 420 participants included in this analysis, 85 (20%) had no anticholinergic exposure, 113 (27%) had low (1–90 TSDDs), 137 (33%) had moderate (91–1095 TSDDs) and 85 (20%) had heavy (≥1096 TSDDs) exposure during the 10-year window. The most common anticholinergic medication classes used included tricyclic antidepressants (64% of all exposure), antihistamines (18%), gastrointestinal antispasmodics (7%) and bladder antimuscarinics (7%) (Supplementary Table 3). Among participants with heavy exposure, 71.8% were using three or more anticholinergic medication classes and the median TSDD was 2933 (interquartile range of 1828 to 5671). Compared to people with no anticholinergic exposure, higher proportions of people with heavy exposure were female, obese, and had hypertension, diabetes, CAD, and a history of stroke (Table 1). Higher proportions of people with heavy use also reported fair or poor health and had more depressive symptoms.
Table 1:
Characteristics of the Autopsy Sample, Overall and by Level of Cumulative Anticholinergic Exposure*,†
| Anticholinergic exposure (TSDD) | |||||
|---|---|---|---|---|---|
| All Participants | No use | 1 – 90 | 91 – 1095 | ≥1096 | |
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Total | 420 | 85 | 113 | 137 | 85 |
| Age at death, median (IQR) | 89 (84, 93) | 88 (84, 93) | 89 (84, 93) | 89 (84, 94) | 89 (85, 92) |
| Male | 179 (42.6) | 43 (50.6) | 52 (46.0) | 57 (41.6) | 27 (31.8) |
| At least some college | 275 (65.5) | 56 (65.9) | 71 (62.8) | 93 (67.9) | 55 (64.7) |
| Treated hypertension†† | 287 (68.3) | 50 (58.8) | 77 (68.1) | 93 (67.9) | 67 (78.8) |
| Diabetes** | 64 (15.2) | 7 (8.2) | 15 (13.3) | 23 (16.8) | 19 (22.4) |
| Stroke** | 107 (25.5) | 11 (12.9) | 27 (23.9) | 39 (28.5) | 30 (35.3) |
| Coronary artery disease*** | 148 (35.2) | 21 (24.7) | 41 (36.3) | 45 (32.9) | 41 (48.2) |
| Fair or poor self-rated health‡ | 141 (33.7) | 20 (23.5) | 32 (28.6) | 50 (36.5) | 39 (45.9) |
| High depressive symptoms (CESD score ≥ 10) ‡ | 89 (21.3) | 16 (18.8) | 15 (13.4) | 32 (23.5) | 26 (30.6) |
| Regular exercise‡,§ | 232 (55.4) | 46 (54.1) | 70 (62.5) | 75 (54.7) | 41(48.2) |
| Obese (BMI >30 kg/m2) ‡ | 50 (11.9) | 5 (5.9) | 10 (8.9) | 20 (14.6) | 15 (17.7) |
TSDD, Total Standardized Daily Dose; IQR, interquartile range; CESD, Center for Epidemiologic Studies Depression scale questionnaire.
Covariates assessed during the 10-year period prior to the index date during which anticholinergic medication exposure was assessed
Column percentages based on non-missing information. Only 3 autopsied participants were missing information on covariates in the outcome model.
From automated pharmacy data; having at least 2 fills of an antihypertensive medication within a 1 year window any time during the 10 year window prior to the index date
From medical record review.
From medical record review. Coronary artery disease was defined as history of myocardial infarction, angina, angioplasty, or coronary artery bypass grafting.
ACT study data at the last study visit prior to index date.
Defined as exercising for 15 or more minutes at least 3 times per week.
Neuropathologic outcomes related to AD did not differ significantly according to level of anticholinergic exposure (Table 2). Relative to participants with no anticholinergic exposure, those with heavy anticholinergic exposure had similar rates of high burden of neuritic plaques (49% vs. 47%; adjusted RR 1.22; 95% CI, 0.81–1.88) and neurofibrillary degeneration (25% vs. 33%; adjusted RR 0.89; 95% CI 0.47–1.66). All levels of anticholinergic exposure were associated with a lower risk for cerebral microinfarct burden relative to no use, with medium and heavy exposure reaching statistical significance. A 56% and 76% lower risk of cerebral microinfarct burden was found with medium and high anticholinergic exposure, respectively. Anticholinergic exposure was not associated with other vascular brain injury outcomes.
Table 2:
Association Between Anticholinergic Exposure and Neuropathologic Outcomes
| Cumulative Anticholinergic Exposure | |||||
|---|---|---|---|---|---|
| No use N=85 |
1–90 TSDD N=113 |
91–1095 TSDD N=137 |
≥1096 TSDD N=85 |
||
| n (%) | n (%) | n (%) | n (%) | p-value | |
| AD-related neuropathology | |||||
| Neuritic plaque scores* | |||||
| None/sparse | 45 (53) | 55 (49) | 63 (46) | 43 (51) | |
| Intermediate/frequent | 40 (47) | 58 (51) | 74 (54) | 42 (49) | |
| RR (95% CI)† | Ref. | 1.15 (0.80, 1.74) | 1.29 (0.90, 1.91) | 1.22 (0.81, 1.88) | 0.59 |
| Neurofibrillary degeneration | |||||
| Braak stage 0-IV | 57 (67) | 80 (71) | 89 (65) | 64 (75) | |
| Braak stage V-VI | 28 (33) | 33 (29) | 48 (35) | 21 (25) | |
| RR (95% CI)† | Ref. | 0.88 (0.52, 1.51) | 1.21 (0.75, 2.00) | 0.89 (0.47, 1.66) | 0.49 |
| Amyloid angiopathy | |||||
| None | 52 (61) | 90 (80) | 89 (65) | 58 (70) | |
| Any | 33 (39) | 22 (20) | 48 (35) | 25 (30) | |
| RR (95% CI)† | Ref. | 0.53 (0.29, 0.99) | 0.84 (0.53, 1.38) | 0.97 (0.56, 1.71) | 0.18 |
| Vascular brain injury-related neuropathology | |||||
| Cerebral microinfarcts | |||||
| 0–2 | 67 (81) | 95 (84) | 116 (85) | 75 (89) | |
| 3 or more | 16 (19) | 18 (16) | 21 (15) | 9 (11) | |
| RR (95% CI)† | Ref. | 0.51 (0.24, 1.14) | 0.44 (0.21, 0.89) | 0.24 (0.09, 0.62) | 0.02 |
| Macroscopic infarcts†† | |||||
| 0 | 54 (64) | 76 (68) | 85 (63) | 49 (60) | |
| ≥ 1 | 30 (36) | 35 (32) | 49 (37) | 33 (40) | |
| RR (95% CI) ** | Ref. | 0.80 (0.48, 1.37) | 0.91 (0.58, 1.44) | 0.90 (0.55, 1.53) | 0.87 |
| Atherosclerosis | |||||
| None/mild | 33 (40) | 48 (44) | 56 (42) | 25 (31) | |
| Moderate/severe | 49 (60) | 61 (56) | 77 (58) | 56 (69) | |
| RR (95% CI)† | Ref. | 0.92 (0.68, 1.27) | 0.99 (0.72, 1.36) | 1.11 (0.80, 1.53) | 0.64 |
Abbreviations: ACT, Adult Changes in Thought; AD, Alzheimer’s Disease; CI, confidence interval; HR, hazard ratio; TSDD, Total Standardized Daily Doses
Consortium to Establish a Registry for Alzheimer’s Disease scoring system.
Adjusted for ACT study cohort, age at death, sex, education, treated hypertension, diabetes, stroke and coronary artery disease
Cystic infarcts and acute or subacute infarcts.
Adjusted for ACT study cohort, age at death, sex, education, treated hypertension, diabetes and coronary artery disease
In sensitivity analyses that adjusted for self-rated health, depression, physical activity and BMI, results were essentially unchanged. Furthermore, results did not change materially with adjustment for APOE genotype (data not shown).
Discussion
This is the first study to our knowledge to examine anticholinergic exposure and neuropathologic changes related to dementia in a community-based autopsy cohort of older adults. The aim of this study was to understand the potential biological mechanisms underlying the results of our previous work, where heavier anticholinergic exposure was associated with an increased risk for AD and dementia.[8] In contrast to our hypothesis, heavy use of anticholinergic medications was not associated with a higher burden of neuropathologic changes associated with AD (plaques or tangles) compared with no use. Unexpectedly, we found that older adults with anticholinergic exposure equivalent to 3 months or greater of typical doses had a lower risk for cerebral microinfarct burden. No association was found between anticholinergic exposure and other vascular brain injury neuropathologic changes.
Despite these findings, anticholinergics may still contribute to neurodegeneration through mechanisms that are not reflected in conventional neuropathologic evaluation as examined in this study. Evidence from animal models suggest that anticholinergics may cause neurotoxicity by decreasing synaptic numbers through two mechanisms: increasing the formation of potentially toxic amyloid-β or lowering levels of phosphatidylcholine.[13] Furthermore, blockade of muscarinic M1 receptors may induce cell death in basal forebrain cholinergic neurons.[37]
Prior studies attempting to understand the underlying biology of the cognition impairing effects of anticholinergics have focused on changes in brain structure and function, which are not directly comparable to our findings. In a cross-sectional study by Risacher et al., participants with at least 1 month of anticholinergic medication use had reduced brain glucose metabolism and increased brain atrophy compared with participants without anticholinergic medication use.[11] Given that this was a cross-sectional analysis, a temporal relationship cannot be established and reverse causation bias cannot be ruled out. Exposure to anticholinergic medications may be a consequence of treatment of prodromal symptoms of dementia (e.g. depression, anxiety, insomnia) rather than a cause of changes in brain structure and/or function. A second study examined midlife anticholinergic exposure and reported greater rates of atrophy in total cortical gray matter volume and in specific areas of the brain (e.g. right posterior cingulate, right middle frontal and left superior temporal gyri) with use of possible anticholinergic medications relative to non-use, but not with use of definite anticholinergics. [12] This finding is counterintuitive and one would expect that risk would be increased with use of definite anticholinergics as well. The investigators noted that potential reasons for not finding changes in brain outcomes with use of definite anticholinergics may be related to lower frequency and duration of use compared with possible anticholinergic use. Overall, the study was limited in that frequency and duration of use was not available for approximately half of participants.
Ideally, to examine the association between medication exposures and neuropathologic lesions, the assessment of these outcomes would occur at the time of dementia diagnosis, but this is not possible for obvious reasons. We realize that neuropathology assessed at death may not reflect what would have been present at the index date, which is a limitation that is inherent with studies of this nature. . Nonetheless, given that pathologic changes accumulate slowly over decades, our group and others have demonstrated that despite the delay in assessment of neuropathologic changes, these analyses are valid and relevant.[26, 38] In order to establish a temporal relationship, it was necessary to include only exposure prior to the diagnosis of dementia (or comparable index date). Thus, for some individuals the time from the index date to death may have been several years and anticholinergic use was not accounted for during this period. One question that remains unanswered is the etiologically relevant exposure window for anticholinergic-related damage. The choice of 10 years prior to dementia was selected to mirror our prior work but is somewhat arbitrary, and the etiologically relevant time-period could be earlier in life.
The explanation for finding less cerebral microinfarct burden among participants with anticholinergic use is unclear. Microinfarcts are most closely associated with insulin resistance and hypertension and are nearly as strongly associated with dementia in the ACT study as AD neuropathologic change.[26] Muscarinic receptors play a role in insulin release from the pancreas and a small study demonstrated that insulin sensitivity was higher during a short-term infusion of a cholinergic antagonist (i.e. atropine) compared to saline in 12 subjects without diabetes.[39] Our findings should be interpreted cautiously as they are based on few participants with heavy microinfarct burden, and the association may be due to residual confounding or bias. Further research to explore this association is needed.
This study has several strengths. Data come from a large community-based autopsy cohort. We have access to computerized pharmacy data about medication exposures going back many years. The ACT study’s rich data allow adjustment for many potential confounders. We used statistical methods to account for potential selection bias.
We also recognize that this study has limitations. Although the cohort is relatively large for an autopsy study, the confidence intervals were wide, and we were not able to rule out associations of a magnitude that would be clinically important, e.g., a relative risk of 1.9 for the association between heavy anticholinergic use and high neuritic plaque scores and 1.6 for high neurofibrillary degeneration. Some anticholinergics are available over the counter, and while our data sources capture over-the-counter medications purchased at KPW pharmacies, participants may also have purchased anticholinergics from pharmacies outside of KPW. Because of the sample size, we were unable to examine individual medication classes separately. The relationship between anticholinergic exposure and neuropathologic outcomes could differ by medication sub-class, which would be obscured in analyses of cumulative burden. Lastly, although the neuropathologic outcomes are measured using state-of-the-art methods, these methods are limited as they are semi-quantitative, restricted to a few pre-selected brain regions and generate only a single score for the entire brain. Newer methodologies to quantify Aβ1–42 and pathological phospho-tau burden might offer greater accuracy for measuring AD-related pathologic outcomes.[40–42]
In conclusion, in this large community-based autopsy study, we did not observe higher risk of dementia-associated neuropathology in people with heavy exposure to anticholinergics. Our prior work demonstrated an association between anticholinergic exposure and increased risk of dementia, thus future studies are needed to more fully understand the biological mechanism underlying the effect of anticholinergic medications on the brain. Other techniques that better quantify brain pathologic changes may provide more power to explore these associations.
Supplementary Material
ACKNOWLEDGMENTS
Funding: This study was supported by the National Institute on Aging (U01AG006781, R03AG042930, and P50AG05136), the National Institute of Neurological Disorders and Stroke (P50NS062684), the Barton Family Foundation, the Nancy and Buster Alvord Endowment, and the Branta Foundation.
Footnotes
Disclaimer: This work does not necessarily reflect the views of the National Institutes of Health.
Disclosures: Rod Walker has received funding as a biostatistician from an unrelated research grant awarded to KPW Health Research Institute from Pfizer. Dr. Dublin received a Merck/American Geriatrics Society New Investigator Award. Drs. Larson and Keene receive royalties from UpToDate. Ms. Yu has received funding from unrelated research grants awarded to KPW Health Research Institute from Bayer.
References
- [1].By the American Geriatrics Society Beers Criteria Update Expert Panel (2015) American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 63, 2227–2246. [DOI] [PubMed] [Google Scholar]
- [2].O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P (2015) STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 44, 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham BG, Harris TB, Hanlon JT, Rubin SM, Shorr RI, Bauer DC, Abernethy DR (2007) A drug burden index to define the functional burden of medications in older people. Arch Intern Med 167, 781–787. [DOI] [PubMed] [Google Scholar]
- [4].Kachru N, Carnahan RM, Johnson ML, Aparasu RR (2015) Potentially inappropriate anticholinergic medication use in community-dwelling older adults: a national cross-sectional study. Drugs Aging 32, 379–389. [DOI] [PubMed] [Google Scholar]
- [5].Marcum ZA, Wirtz HS, Pettinger M, LaCroix AZ, Carnahan R, Cauley JA, Bea JW, Gray SL (2016) Anticholinergic medication use and falls in postmenopausal women: findings from the women’s health initiative cohort study. BMC Geriatr 16, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ness J, Hoth A, Barnett MJ (2006) Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother 4, 42–51. [DOI] [PubMed] [Google Scholar]
- [7].Carriere I, Fourrier-Reglat A, Dartigues JF, Rouaud O, Pasquier F, Ritchie K, Ancelin ML (2009) Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med 169, 1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, Yu O, Crane PK, Larson EB (2015) Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 175, 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jessen F, Kaduszkiewicz H, Daerr M, Bickel H, Pentzek M, Riedel-Heller S, Wagner M, Weyerer S, Wiese B, van den Bussche H, Broich K, Maier W (2010) Anticholinergic drug use and risk for dementia: target for dementia prevention. Eur Arch Psychiatry Clin Neurosci 260 Suppl 2, S111–115. [DOI] [PubMed] [Google Scholar]
- [10].Perry EK, Kilford L, Lees AJ, Burn DJ, Perry RH (2003) Increased Alzheimer pathology in Parkinson’s disease related to antimuscarinic drugs. Ann Neurol 54, 235–238. [DOI] [PubMed] [Google Scholar]
- [11].Risacher SL, McDonald BC, Tallman EF, West JD, Farlow MR, Unverzagt FW, Gao S, Boustani M, Crane PK, Petersen RC, Jack CR Jr., Jagust WJ, Aisen PS, Weiner MW, Saykin AJ, Alzheimer’s Disease Neuroimaging Initiative (2016) Association Between Anticholinergic Medication Use and Cognition, Brain Metabolism, and Brain Atrophy in Cognitively Normal Older Adults. JAMA Neurol 73, 721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chuang Y-F, Elango P, Gonzalez CE, Thambisetty M (2017) Midlife anticholinergic drug use, risk of Alzheimer’s disease, and brain atrophy in community-dwelling older adults. Alzheimers Dement (N Y) 3, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wurtman RJ (2015) How Anticholinergic Drugs Might Promote Alzheimer’s Disease: More Amyloid-beta and Less Phosphatidylcholine. J Alzheimers Dis 46, 983–987. [DOI] [PubMed] [Google Scholar]
- [14].Yoshiyama Y, Kojima A, Itoh K, Uchiyama T, Arai K (2012) Anticholinergics boost the pathological process of neurodegeneration with increased inflammation in a tauopathy mouse model. Neurobiol Dis 45, 329–336. [DOI] [PubMed] [Google Scholar]
- [15].Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L, Larson EB (2002) Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol 59, 1737–1746. [DOI] [PubMed] [Google Scholar]
- [16].Dublin S, Walker RL, Gray SL, Hubbard RA, Anderson ML, Yu O, Montine TJ, Crane PK, Sonnen JA, Larson EB (2017) Use of Analgesics (Opioids and Nonsteroidal Anti-Inflammatory Drugs) and Dementia-Related Neuropathology in a Community-Based Autopsy Cohort. J Alzheimers Dis 58, 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Naples JG, Marcum ZA, Perera S, Gray SL, Newman AB, Simonsick EM, Yaffe K, Shorr RI, Hanlon JT, Health A, Body Composition S (2015) Concordance Between Anticholinergic Burden Scales. J Am Geriatr Soc 63, 2120–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Semla TP, Beizer JL, Higbee MD (2010) Geriatric Dosage Handbook. 15th ed Lexicomp, Hudson OH. [Google Scholar]
- [19].Mirra SS (1997) The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiol Aging 18, S91–94. [DOI] [PubMed] [Google Scholar]
- [20].Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41, 479–486. [DOI] [PubMed] [Google Scholar]
- [21].Braak E, Griffing K, Arai K, Bohl J, Bratzke H, Braak H (1999) Neuropathology of Alzheimer’s disease: what is new since A. Alzheimer? Eur Arch Psychiatry Clin Neurosci 249 Suppl 3, 14–22. [DOI] [PubMed] [Google Scholar]
- [22].Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259. [DOI] [PubMed] [Google Scholar]
- [23].Greenberg SM, Vonsattel JP (1997) Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke 28, 1418–1422. [DOI] [PubMed] [Google Scholar]
- [24].Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT, National Institute on Aging; Alzheimer’s Association (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ (2007) Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol 62, 406–413. [DOI] [PubMed] [Google Scholar]
- [27].Sonnen JA, Larson EB, Walker RL, Haneuse S, Crane PK, Gray SL, Breitner JC, Montine TJ (2010) Nonsteroidal anti-inflammatory drugs are associated with increased neuritic plaques. Neurology 75, 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W (2006) Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 144, 73–81. [DOI] [PubMed] [Google Scholar]
- [29].Andresen EM, Malmgren JA, Carter WB, Patrick DL (1994) Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med 10, 77–84. [PubMed] [Google Scholar]
- [30].Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, Sugimoto K, Yamaguchi T, Sasaki H, Chiu D, White LR (1994) The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr 6, 45–58; discussion 62. [DOI] [PubMed] [Google Scholar]
- [31].American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, American Psychiatric Association, Washington DC. [Google Scholar]
- [32].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944. [DOI] [PubMed] [Google Scholar]
- [33].Zou G (2004) A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159, 702–706. [DOI] [PubMed] [Google Scholar]
- [34].Haneuse S, Schildcrout J, Crane P, Sonnen J, Breitner J, Larson E (2009) Adjustment for selection bias in observational studies with application to the analysis of autopsy data. Neuroepidemiology 32, 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Efron B, Tibshirani RJ (1993) An Introduction to the Bootstrap, Chapman & Hall, New York. [Google Scholar]
- [36].Dublin S, Anderson ML, Heckbert SR, Hubbard RA, Sonnen JA, Crane PK, Montine TJ, Larson EB (2014) Neuropathologic changes associated with atrial fibrillation in a population-based autopsy cohort. J Gerontol A Biol Sci Med Sci 69, 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Del Pino J, Zeballos G, Anadon MJ, Moyano P, Diaz MJ, Garcia JM, Frejo MT (2016) Cadmium-induced cell death of basal forebrain cholinergic neurons mediated by muscarinic M1 receptor blockade, increase in GSK-3beta enzyme, beta-amyloid and tau protein levels. Arch Toxicol 90, 1081–1092. [DOI] [PubMed] [Google Scholar]
- [38].White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, Masaki K, Launer L, Markesbery WR (2002) Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci 977, 9–23. [DOI] [PubMed] [Google Scholar]
- [39].Svensson MK, Jansson PA, Persson AL, Sjostrand M, Eriksson JW (2011) Atropine improves insulin sensitivity in both lean and abdominally obese subjects. J Clin Endocrinol Metab 96, E1843–1847. [DOI] [PubMed] [Google Scholar]
- [40].Flanagan ME, Larson EB, Walker RL, Keene CD, Postupna N, Cholerton B, Sonnen JA, Dublin S, Crane PK, Montine TJ (2017) Associations between Use of Specific Analgesics and Concentrations of Amyloid-beta 42 or Phospho-Tau in Regions of Human Cerebral Cortex. J Alzheimers Dis. doi: 10.3233/JAD-170414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Postupna N, Keene CD, Crane PK, Gonzalez-Cuyar LF, Sonnen JA, Hewitt J, Rice S, Howard K, Montine KS, Larson EB, Montine TJ (2015) Cerebral cortical Abeta42 and PHF-tau in 325 consecutive brain autopsies stratified by diagnosis, location, and APOE. J Neuropathol Exp Neurol 74, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Postupna N, Rose SE, Bird TD, Gonzalez-Cuyar LF, Sonnen JA, Larson EB, Keene CD, Montine TJ (2012) Novel antibody capture assay for paraffin-embedded tissue detects wide-ranging amyloid beta and paired helical filament-tau accumulation in cognitively normal older adults. Brain Pathol 22, 472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.