Abstract
Invertebrates rely on Dicer to cleave viral dsRNA, and Drosophila Dicer-2 distinguishes dsRNA substrates by their termini. Blunt termini promote processive cleavage, while 3’ overhanging termini are cleaved distributively. To understand this discrimination, we used cryo-electron microscopy to solve structures of Drosophila Dicer-2 alone and in complex with blunt dsRNA. While the Platform-PAZ domains have been considered the only Dicer domains that bind dsRNA termini, unexpectedly, we found that the helicase domain is required for binding blunt, but not 3’ overhanging, termini. We further showed that blunt dsRNA is locally unwound and threaded through the helicase domain in an ATP-dependent manner. Our studies reveal a previously unrecognized mechanism for optimizing antiviral defense and set the stage for discovery of helicase-dependent functions in other Dicers.
One Sentence Summary:
The helicase domain of Dicer-2 recognizes dsRNA termini to initiate processive cleavage by a threading mechanism.
Dicer ribonucleases cleave double-stranded RNA (dsRNA) precursors to generate microRNAs (miRNAs) and small interfering RNAs (siRNAs) (1, 2). In concert with Argonautes, these small RNAs bind complementary mRNAs to downregulate their expression. miRNAs are processed by Dicer from small hairpins, while siRNAs are typically processed from longer dsRNA, from endogenous sources (3), or exogenous sources such as viral replication intermediates (4–6). Some organisms, such as Homo sapiens and Caenorhabditis elegans, encode one Dicer that generates miRNAs and siRNAs, but other organisms have multiple Dicers with specialized functions.
Dicers exist throughout eukaryotes, and a subset have an N-terminal helicase domain of the RIGI-like receptor (RLR) subgroup (7) (Fig. 1A, Fig. S1A). RLRs often function in innate immunity (8), and Dicer helicase domains sometimes show differences in activity that correlate with roles in immunity. For example, Drosophila melanogaster expresses two Dicers, one specialized for processing miRNAs (dmDcr-1), and a second for siRNAs (dmDcr-2) (9). dmDcr-1 has a degenerate helicase domain and is an ATP-independent enzyme (10), while dmDcr-2, with dedicated antiviral roles (11–13), has a conserved helicase domain that hydrolyzes ATP (14–17). Under certain conditions Homo sapiens Dicer-1 (hsDcr-1) also generates viral siRNAs (18, 19). However, despite conservation of its helicase domain, hsDcr-1 does not hydrolyze ATP in vitro (20), and its helicase domain is not implicated in viral siRNA biogenesis in vivo (19). Differences in activities of the helicase domain of vertebrate and invertebrate Dicers may reflect distinct roles in antiviral defense.
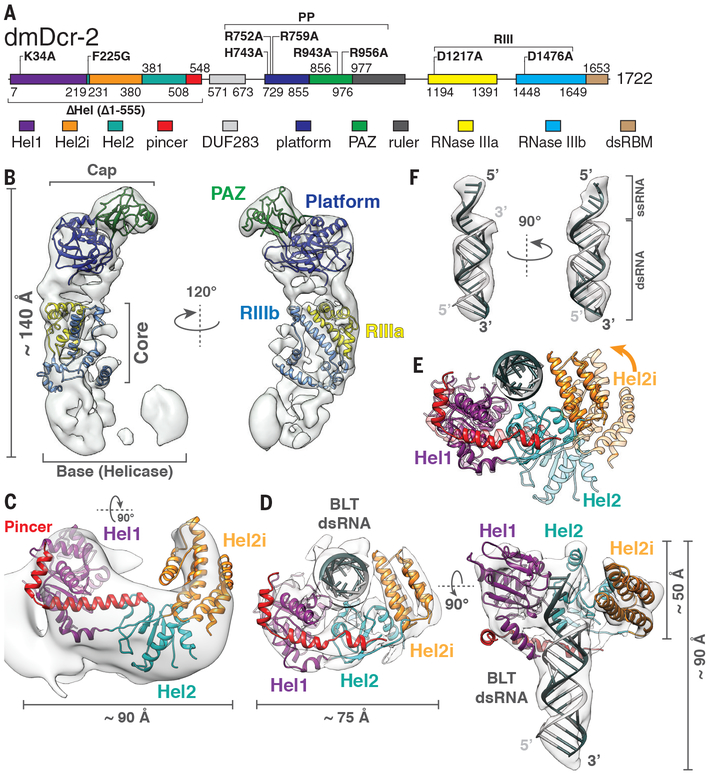
Fig. 1. Cryo-EM reconstructions of apo-dmDcr-2 and dmDcr-2•BLT dsRNA•ATP-γS.
(A) dmDcr-2 domains numbered at boundaries. Mutations/deletions in bold, and designated in text as superscripts. All mutations were in the context of the full-length protein, unless specified by Δ. Two full-length variants had multiple mutations: PP (H743A, R752A, R759A, R943A, R956A); RIII (D1217A, D1476A) (B) Cryo-EM density map of apo-dmDcr-2 (7.1 Å) fitted with homology models of subdomains. RIIIa, RNase IIIa; RIIIb, RNase IIIb. (C) Homology model of apo-dmDcr-2 in open conformation, based on apo-RIG-I, and fitted as rigid body into 8.7 Å cryo-EM density map (also Fig. S4A and Fig. S5, B to D). (D) Cryo-EM reconstruction of dmDcr-2RIII•BLT dsRNA•ATP-γS showing helicase in closed, ligand-bound conformation. (E) Superimposition of open (light) and closed (dark) helicase conformations showing clamping of Hel2 and Hel2i on BLT dsRNA. Arrow, direction of clamping. (F) EM density and modeling of BLT single and double-stranded RNA.
dmDcr-2 activity depends on termini of its dsRNA substrates (15, 16). Blunt (BLT) termini promote a processive reaction whereby multiple siRNAs are produced before dmDcr-2 dissociates, and this reaction requires a functional helicase domain and ATP (15, 17). In contrast, dsRNAs with 3’ overhanging (3’ovr) termini promote an ATP-independent, distributive cleavage, whereby dmDcr-2 dissociates after each cleavage. hsDcr-1 does not require ATP for processing BLT or 3’ovr termini (fig. S1B, C and D, lanes 5, 7, 10, 12), suggesting that, at least in vitro, cleavage of BLT dsRNA is not dependent on its helicase domain.
To understand the mechanism of termini discrimination by dmDcr-2, we used cryo-electron microscopy (cryo-EM) to determine structures of dmDcr-2 alone and in complex with a BLT 52 base pair (bp) dsRNA (52 dsRNA) and ATP-γS (Fig. 1, figs. S2 to S10, Table S1). We used full-length dmDcr-2 with a point mutation in each RNase III domain to preclude dsRNA cleavage (dmDcr-2RIII; Fig. 1A). ATP hydrolysis is required for processive cleavage of BLT dsRNA, and dmDcr-2 cannot hydrolyze ATP-γS, which stabilizes a helicase-dependent conformation of dmDcr-2 (16).
The structure of apo-dmDcr-2RIII (Fig. 1, B and C, and figs. S2 to S5) reiterated the “L shape” of lower resolution (~15–30 Å) EM reconstructions of hsDcr-1 (21–23). Our 7.1 Å EM density map (Fig. 1B, fig. S2C) enabled fitting (fig. S4, B to F) and homology modeling (Fig. 1B) of Platform–PAZ domains at the Cap and tandem RNase III domains in the Core. An additional round of 3D classification (fig. S3) revealed an 8.7 Å map (fig. S2C) allowing assignment of the helicase domain at the base (Fig. 1C, and fig. S4A). Fitting of related apo-helicases into the EM density is consistent with the helicase domain adopting an open conformation (fig. S5, A to D).
The 2D class averages of the dmDcr-2 complex revealed protein with well-resolved secondary structure features bound to the BLT dsRNA terminus (fig. S6, B and C). Some protein density was missing, and since control experiments indicated protein on the grid was intact (fig. S7A), this was likely due to inherent flexibility (fig. S7B). Measurements of the dsRNA, guided by major grooves, showed visible protein footprinted ~8–9 bps (fig. S6B). The crystal structure of RIG-I’s helicase domain bound to dsRNA has a similar footprint (24, 25), suggesting dmDcr-2’s helicase domain bound to BLT dsRNA termini. Indeed, our 6.8 Å reconstruction of the complex (fig. S6E and fig. S8) resembled RIG-I in a closed conformation (Fig.1D and fig. S9). The Hel1 and Hel2 subdomains of RIG-I’s helicase, along with the pincer helices (Fig. S1A) could be fit as a single rigid body (fig. S9A). The reconstruction also revealed a helical bundle characteristic of the Hel2i subdomain that could be fitted separately as a rigid body (fig. S9B). These fittings enabled a homology model of dmDcr-2’s helicase domain bound to BLT dsRNA (Fig. 1D and fig. S9, D and E).
Our models of dmDcr-2’s helicase in open (apo) and closed (substrate-bound) conformations implied clamping of the helicase on BLT dsRNA termini (Fig. 1E, Movie S1 and S2). In the open conformation, Hel2 and Hel2i extend away from Hel1 creating a C-shaped opening for substrate engagement (Fig. 1C). In the BLT dsRNA-bound state, Hel2 and Hel2i swivel towards Hel1 to clamp on the terminus (Fig. 1 D and E, Movie S1 and S2).
Within the helicase domain, density was only observed for one RNA strand, indicative of local unwinding (Fig. 1F and fig. S9C). Unwinding would likely require ATP hydrolysis, and possibly was enabled by contaminating ATP in commercial preparations of ATP-γS (16). Using dsRNAs with a nick in sense or antisense strands (fig. S11A), we performed in vitro unwinding assays (fig. S11B). With ATP, dmDcr-2RIII, but not the ATPase-defective Walker A mutant dmDcr-2RIII,K34A (16), unwound BLT dsRNA termini (Fig. S11B, compare lanes 3, 7, top and bottom panels).
The unwound single strand maintained an A-form conformation (Fig. 1F), likely to minimize entropic costs of reannealing before cleavage in RNase III sites. Whether the RIG-I helicase unwinds dsRNA is controversial (26, 27), but related helicases exhibit unwinding activity (28). Local unwinding may facilitate dmDcr-2’s helicase domain in binding and translocating along dsRNA.
The Platform-PAZ domains have been considered the only Dicer domains that bind dsRNA termini (29–32), but our structures suggested the helicase domain also binds termini. To test this idea, we purified dmDcr-2ΔHel,RIII (Fig. 2A), which lacked the helicase domain (Fig. 1A). Consistent with previous studies (16), in gel-shift assays full-length dmDcr-2RIII bound both BLT and 3’ovr dsRNA (Fig. 2B, dsRNA design; Fig. 2C, top); ATP increased affinity for BLT dsRNA and decreased affinity for 3’ovr dsRNA (Fig. 2, C and D and Table S2). However, while dmDcr-2ΔHel,RIII bound 3’ovr dsRNA with an affinity similar to dmDcr-2RIII, its binding to BLT dsRNA was not detected (Fig. 2C, bottom panel and Fig. 2D and Table S2). The inability of dmDcr-2ΔHel,RIII to bind BLT dsRNA was not due to the absence of ATP hydrolysis because dmDcr-2RIII,K34A bound BLT dsRNA efficiently (fig. S12, A and B and Table S2). Thus, the helicase domain is required for binding BLT, but not 3’ovr, dsRNA.
Fig. 2. dmDcr-2’s helicase domain is required to recognize and cleave BLT dsRNA.
(A) Gelfiltration and SDS-PAGE analyses of dmDcr-2ΔHel,RIII. (B) Cartoon of dsRNA used in (C-F), showing modifications that block binding at one end. (C) Gel mobility shift assays of dmDcr-2RIII (top) and dmDcr-2ΔHel,RIII (bottom) with 52 BLT or 3’ovr dsRNA, −/+ 5 mM ATP (n≥3). (D) Binding curves using data as in (C). Data points, mean ± SD (n=3). (E) Single-turnover cleavage assays of 52 BLT or 3’ovr dsRNA (1 nM) with dmDcr-2WT or dmDcr-2ΔHel (30 nM), −/+ 5 mM ATP (n=3). Only initial cleavage is monitored, since this removes 5’ 32P. Arrow, 22 nucleotide (nt) siRNA product. AH, alkaline hydrolysis. Left, nt lengths. (F) Quantification of cleavage assays as in (E). Percent dsRNA cleaved (all dsRNA except uncleaved) and percent siRNAs (21–23 nt products) resulting from 1st cleavage were quantified. Data points, mean ± SD (n=3). *, p< 0.05; **, p< 0.01; ***, p< 0.001.
Single-turnover cleavage assays showed that neither dmDcr-2WT nor dmDcr-2ΔHel (fig. S12C) cleaved BLT dsRNA without ATP (Fig. 2E and F, lanes 5, 6). With ATP, cleavage of BLT dsRNA by dmDcr-2WT gave heterogeneous cleavage products characteristic of Dicer enzymes with ATPase activity (16, 33), but strikingly, dmDcr-2ΔHel was incapable of cleaving BLT dsRNA (Fig. 2E and F, compare lanes 9, 10). As expected (15, 16), cleavage of 3’ovr dsRNA was independent of ATP, and with both dmDcr-2WT and dmDcr-2ΔHel produced a single siRNA-sized 22 nt band (Fig. 2E, compare lanes 7, 8, 11, 12). dmDcr-2ΔHel cleaved 3’ovr dsRNA more efficiently than dmDcr-2WT (Fig. 2, E and F, compare lanes 7, 8, 11, 12), suggesting the helicase domain hinders cleavage of 3’ovr dsRNA. This observation is reminiscent of autoinhibition by hsDcr-1’s helicase domain in processing 3’ovr dsRNAs (34).
Our biochemical and structural studies indicated that dmDcr-2 has two modes of substrate recognition and cleavage: one mediated by Platform-PAZ domains and resulting in precise cleavage of 3’ovr dsRNAs into 22mer siRNAs, and a second mediated by the helicase domain and resulting in heterogeneous cleavage of BLT dsRNAs. We searched for amino acids that might separately affect cleavage of a 3’ovr or BLT dsRNA. We created one variant of dmDcr-2 (Fig. 1A, PP) containing five point mutations in the Platform and PAZ domains (35). Multiple crystal structures show a phenylalanine in the C-terminal domain (CTD) of RIG-I recognizes BLT dsRNA by stacking on the terminal base pair (24). Dicer enzymes do not have a CTD, but for the second variant we searched for regions in dmDcr-2 with sequence similarity to the CTD. Within the region identified (fig. S13, A to C) we mutated a single phenylalanine to a glycine (dmDcr-2F225G).
We compared activities of purified dmDcr-2PP and dmDcr-2F225G (fig. S13D) to dmDcr-2WT using single-turnover cleavage assays (Fig. 3, A and B). As expected, cleavage of BLT dsRNA was not observed without ATP (Fig. 3, A and B, lanes 5–7). However, with ATP, cleavage of BLT dsRNA by dmDcr-2WT or dmDcr-2PP appeared nearly identical (Fig. 3 A and B, lanes 11 and 13), while cleavage was completely disrupted by the helicase point mutation in dmDcr-2F225G (Fig. 3, A and B, lane 12). (At least part of this effect is due to weakened BLT dsRNA binding, fig. S13, E and F and Table S2). By contrast, cleavage of 3’ovr dsRNA was independent of ATP and minimally affected by the F225G helicase mutation (Fig. 3, A and B, lanes 8, 9, 14, 15). However, cleavage was eliminated by mutations in the Platform-PAZ domains (Fig. 3, A and B, lanes 10, 16). These data reiterate that cleavage of 3’ovr dsRNA is mediated by Platform-PAZ domains, while the helicase domain coordinates recognition and cleavage of BLT dsRNA.
Fig. 3. Helicase and Platform-PAZ domains differentially contribute to cleavage of BLT and 3’ovr dsRNA.
(A) Single-turnover cleavage assays of 52 BLT or 3’ovr dsRNA (1 nM) with dmDcr-2WT, dmDcr-2F225G and dmDcr-2PP (30 nM), −/+ 5 mM ATP (n=3). Substrates were as described in Fig. 2, B and E. AH, arrow, as in 2E. (B) Quantification of cleavage assays as in (A). Data points, mean ± SD (n=3). *, p< 0.05; **, p< 0.01; ***, p< 0.001; ****, p< 0.0001; p>0.05, n.s. (non-significant). (C) Substrates used in (D-E), with additional details in Fig. 2, B and E. In chimeras, nts 21–23 from 5’ end of sense strand, and nts 32–34 from 5’ end of antisense strand were deoxyribonucleotides (dashed circles). (D) Single-turnover cleavage assays of regular or chimeric 52 BLT or 3’ovr dsRNA (1 nM) with dmDcr-2WT (30 nM), −/+ 5 mM ATP (n=4). (E) Quantification of cleavage assays as in (D). Data points, mean ± SD (n=4).
While dmDcr-2PP cleaved BLT dsRNA to yield a pattern nearly identical to dmDcr-2WT, levels of 22 nt siRNA decreased (Fig. 3, A and B, lanes 11, 13). Since 22 nt siRNA was not observed with dmDcr-2F225G (Fig. 3A, lane 12), we hypothesized this species derived from dsRNA that was threaded through the helicase domain until the BLT terminus encountered the Platform-PAZ domains. To confirm that smaller products (<22 nt) did not result from degradation of 22 nt siRNA, we monitored cleavage of chimeric dsRNAs containing deoxynucleotides at positions 21–23 from the 5’ terminus (Fig. 3C). Cleavage of 3’ovr dsRNA was eliminated with chimeric molecules (Fig. 3, D and E, compare lanes 8, 9, 15, 16), as expected for Platform-PAZ-mediated cleavage. However, for BLT dsRNA, while 22 nt siRNA was absent, all other fragments were visible (Fig. 3, D and E, compare lanes 6, 13), consistent with a helicase-mediated threading mechanism.
Studies of Dicer from other organisms indicate Platform-PAZ domains bind termini of 3’ovr dsRNA to allow measuring to RNase III active sites and production of an siRNA length (29, 30). Our cryo-EM structure of the dmDcr-2 complex and subsequent biochemical studies suggested that BLT dsRNA is cleaved differently, and in an ATP-dependent manner, threaded through the helicase domain to encounter the RNase III active sites. We tested the threading model by designing dsRNA with blocks at specific positions (Fig. 4A). Measurements using our EM density maps predicted that BLT dsRNAs are threaded through the helicase domain ~20 bp before encountering RNase III domains. To trap threading intermediates, we put biotin-dT analogs on both strands of 52 BLT dsRNA, at positions 28 or 37, counting from the 5’ end of the sense strand (Fig. 4A); there was no significant difference in cleavage of these modified dsRNAs (figs. S14, A and B). However, we hypothesized that addition of streptavidin to biotin-dT-substituted dsRNAs (Block dsRNAs) would arrest threading of dsRNAs through the helicase. When dsRNA was incubated with streptavidin before initiating cleavage with dmDcr-2WT and ATP, we trapped early (<11 nt, Block-28) and intermediate (11–20 nt, Block-37) threading products, without observing 22 nt siRNAs (Fig 4B and4C; fig. S14C for schematic). By contrast, cleavage by hsDcr-1 was unaffected by blocks, indicating that, at least under these conditions, hsDcr-1 cannot thread dsRNA through its helicase domain (Fig. 4D, compare lanes 12–14 to lanes 15–17).
Fig. 4. BLT dsRNA threads through helicase domain.
(A) Substrates for (B-D); some features described in Fig. 2, B and E. Blocked dsRNAs contained biotin-dT (red B) on both strands, with 28 and 37 indicating position from 5’ end of sense strand. (B) Single-turnover cleavage assays of blocked or unblocked 52 BLT dsRNA (1 nM) with dmDcr-2WT (30 nM), 5 mM ATP and 80 nM streptavidin (n=3). dsRNA was pre-incubated with streptavidin before adding dmDcr-2WT. Arrow, AH, as in 2E. (C) Quantification of cleavage at 75 min as in (B). dsRNA cleaved (%) is plotted based on all products (total), those >23 nt, siRNAs (21–23 nt products), those 11–20 nts, and those <11 nts. Data points, mean ± SD (n=3). *, p< 0.05; **, p< 0.01; ***, p< 0.001; **** p< 0.0001; p>0.05, n.s. (non-significant). (D) Single-turnover cleavage assays as in (B) with dmDcr-2WT or hsDcr-1WT, −/+ 80 nM streptavidin (n=3). Black arrow, siRNA product (22 nt) with dmDcr-2WT; green arrow, siRNA product (26 nt) with hsDcr-1WT. (E) Model for recognition and cleavage of BLT and 3’ovr dsRNA by dmDcr-2. Dotted yellow arrow, clamping of helicase on BLT dsRNA; dotted white arrow, unwinding; dotted gray box, dmDcr-2RIII•BLT dsRNA•ATP-γS complex shown in Fig. 1D (also see fig. S10); red arrow, cleavage. Model for 3’ovr recognition from data reported here and elsewhere (23, 34).
We anticipated that short threading intermediates (<22 nts) might be unique to the initial cleavage event. However, threading intermediates were observed with dmDcr-2WT under multiple-turnover conditions using internally 32P-labeled dsRNAs, increasing proportionally with 22mers through the reaction time course (fig. S14, D and E). Thus, at least in vitro, threading intermediates are recurring by-products of processive cleavage and not specific to the initial cleavage. dmDcr-2’s highly efficient, helicase-dependent, processive cleavage is likely advantageous in antiviral defense. The generation of heterogeneous cleavage products during processive cleavage is predicted to dampen the phasing signal of viral siRNAs and is consistent with the overlapping and discontinuous viral siRNAs observed in invertebrate cells (6, 13, 36).
The dsRNA binding protein (dsRBP) Loquacious-PD (Loqs-PD) allows dmDcr-2 to cleave independent of termini (16, 37) and is required for processing endogenous siRNAs (38), but not for an antiviral response (13). This suggests dmDcr-2’s intrinsic termini preferences function in viral defense, while Loqs-PD allows processing of endogenous dsRNA with diverse termini. By monitoring cleavage of dsRNAs with 5’ovr termini, or overhangs on both strands (fig. S15, B to E), we determined that dsRNA with an accessible 3’ terminus is preferentially recognized by the Platform-PAZ domain, and without this feature, is processed by threading through the helicase domain.
RIG-I distinguishes capped termini of cellular transcripts from tri- and di-phosphorylated termini of viral transcripts, and this is inferred to allow self versus non-self discrimination (8). We found that, like RIG-I, dmDcr-2 cannot efficiently process dsRNAs capped at the 5’-terminus, although the phosphorylation state does not affect cleavage (Fig. S16, A to B; Table S3). These results may reflect dmDcr-2’s ability to process precursors of both endogenous and viral siRNAs.
We show that dmDcr-2 has two modes of cleavage (Fig. 4E, Movie S3 and S4). dmDcr-2 is capable of using its Platform-PAZ domain to recognize 3’ovr dsRNAs in vitro, but it is unknown if dmDcr-2 processes such dsRNAs in vivo. dmDcr-2’s cognate dsRBP, R2D2, may inhibit recognition and processing of substrates with 3’ovr termini (17). As such, the Platform-PAZ domains of dmDcr-2 may function solely on dsRNAs that are threaded through the helicase domain. At least in vitro, hsDcr-1 does not distinguish termini and does not exhibit helicase-dependent threading. Unlike dmDcr-2, hsDcr-1 may rely on the Platform-PAZ domain for generating viral siRNAs. Indeed, mutations to the Platform-PAZ domains of hsDcr-1 disrupt viral siRNA biogenesis (19). However, given the conservation of hsDcr-1’s helicase domain, it is intriguing to consider that, under certain conditions, perhaps with additional factors, hsDcr-1 might mediate processive cleavage by threading of dsRNA through the helicase domain.
Supplementary Material
Acknowledgments
We thank D. Cazalla and E. Cao for critique of the manuscript, and P. J. Aruscavage for technical assistance. EM was performed at University of Utah EM Core Laboratory with computational support from Utah Center for High Performance Computing. RNA was synthesized by the DNA/Peptide facility (Health Sciences Center Cores at University of Utah). This work was supported by funding from the National Institute of General Medical Sciences (R01GM121706) and the H.A. and Edna Benning Presidential Endowed Chair (to B.L.B.). The authors declare no competing financial interests. The models and cryo-EM maps are available via the following accession numbers: PDB 6BUA, EMD-7291, EMD-7292 (apo-dmDcr-2RIII); PDB 6BU9, EMD-7290 (dmDcr-2RIII•52 BLT dsRNA•ATP-γS complex).
References and Notes
- 1.Ha M, Kim VN, Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15, 509–524 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Wilson RC, Doudna JA, Molecular mechanisms of RNA interference. Annual Review of Biophysics 42, 217–239 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okamura K, Lai EC, Endogenous small interfering RNAs in animals. Nat Rev Mol Cell Biol 9, 673–678 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aliyari R et al. , Mechanism of induction and suppression of antiviral immunity directed by virus-derived small RNAs in Drosophila. Cell Host Microbe 4, 387–397 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynt A, Liu N, Martin R, Lai EC, Dicing of viral replication intermediates during silencing of latent Drosophila viruses. Proc Natl Acad Sci USA 106, 5270–5275 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabin LR et al. , Dicer-2 processes diverse viral RNA species. PLoS ONE 8, e55458 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fairman-Williams ME, Guenther U-P, Jankowsky E, SF1 and SF2 helicases: family matters. Curr Opin Struct Biol 20, 313–324 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad S, Hur S, Helicases in antiviral immunity: Dual properties as sensors and effectors. Trends Biochem Sci 40, 576–585 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YS et al. , Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 117, 69–81 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Tsutsumi A, Kawamata T, Izumi N, Seitz H, Tomari Y, Recognition of the pre-miRNA structure by Drosophila Dicer-1. Nat Struct Mol Biol 18, 1153–1158 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Wang X-H et al. , RNA interference directs innate immunity against viruses in adult Drosophila. Science (New York, NY) 312, 452–454 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann JA, Imler J-L, Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat Immunol 7, 590–597 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Marques JT et al. , Functional specialization of the small interfering RNA pathway in response to virus infection. PLoS Pathog 9, e1003579 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q et al. , R2D2, a bridge between the initiation and effector steps of the Drosophila RNAi pathway. Science (New York, NY) 301, 1921–1925 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Welker NC et al. , Dicer’s helicase domain discriminates dsRNA termini to promote an altered reaction mode. Mol Cell 41, 589–599 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha NK, Trettin KD, Aruscavage PJ, Bass BL, Drosophila Dicer-2 cleavage is mediated by helicase- and dsRNA termini-dependent states that are modulated by Loquacious-PD. Mol Cell 58, 406–417 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cenik ES et al. , Phosphate and R2D2 restrict the substrate specificity of Dicer-2, an ATP-driven ribonuclease. Mol Cell 42, 172–184 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu Y et al. , Human virus-derived small RNAs can confer antiviral immunity in mammals. Immuni 46, 992–1004.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Li Y et al. , Induction and suppression of antiviral RNA interference by influenza A virus in mammalian cells. Nat Microbiol 2, 16250 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Kolb FA, Brondani V, Billy E, Filipowicz W, Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J 21, 5875–5885 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau P-W, Potter CS, Carragher B, MacRae IJ, Structure of the human Dicer-TRBP complex by electron microscopy. Structure 17, 1326–1332 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau P-W et al. , The molecular architecture of human Dicer. Nat Struct Mol Biol 19, 436–440 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor DW et al. , Substrate-specific structural rearrangements of human Dicer. Nat Struct Mol Biol 20, 662–670 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo D et al. , Structural insights into RNA recognition by RIG-I. Cell 147, 409–422 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowalinski E et al. , Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell 147, 423–435 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Takahasi K et al. , Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol Cell 29, 428–440 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Myong S et al. , Cytosolic viral sensor RIG-I is a 5’-triphosphate-dependent translocase on double-stranded RNA. Science (New York, NY) 323, 1070–1074 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linder P, Jankowsky E, From unwinding to clamping - the DEAD box RNA helicase family. Nat Rev Mol Cell Biol 12, 505–516 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Kolb FA, Jaskiewicz L, Westhof E, Filipowicz W, Single processing center models for human Dicer and bacterial RNase III. Cell 118, 57–68 (2004). [DOI] [PubMed] [Google Scholar]
- 30.MacRae IJ et al. , Structural basis for double-stranded RNA processing by Dicer. Science (New York, NY) 311, 195–198 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Park J-E et al. , Dicer recognizes the 5’ end of RNA for efficient and accurate processing. Nature 475, 201–205 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian Y et al. , A phosphate-binding pocket within the platform-PAZ-connector helix cassette of human Dicer. Mol Cell 53, 606–616 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colmenares SU, Buker SM, Buhler M, Dlakić M, Moazed D, Coupling of double-stranded RNA synthesis and siRNA generation in fission yeast RNAi. Mol Cell 27, 449–461 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Ma E, MacRae IJ, Kirsch JF, Doudna JA, Autoinhibition of human dicer by its internal helicase domain. J Mol Biol 380, 237–243 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kandasamy SK, Fukunaga R, Phosphate-binding pocket in Dicer-2 PAZ domain for high-fidelity siRNA production. Proc Natl Acad Sci USA 113, 14031–14036 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Q et al. , Virus discovery by deep sequencing and assembly of virus-derived small silencing RNAs. Proc Natl Acad Sci USA 107, 1606–1611 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trettin KD, Sinha NK, Eckert DM, Apple SE, Bass BL, Loquacious-PD facilitates Drosophila Dicer-2 cleavage through interactions with the helicase domain and dsRNA. Proc Natl Acad Sci USA 114, E7939–E7948 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou R et al. , Processing of Drosophila endo-siRNAs depends on a specific Loquacious isoform. RNA 15, 1886–1895 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinha NK, Bass BL, Overexpression and purification of Dicer and accessory proteins for biochemical and structural studies. Methods 126, 54–65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose SD et al. , Functional polarity is introduced by Dicer processing of short substrate RNAs. Nucleic Acids Res 33, 4140–4156 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fukunaga R, Colpan C, Han BW, Zamore PD, Inorganic phosphate blocks binding of pre-miRNA to Dicer-2 via its PAZ domain. EMBO J 33, 371–384 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noland CL, Ma E, Doudna JA, siRNA repositioning for guide strand selection by human Dicer complexes. Mol Cell 43, 110–121 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goubau D et al. , Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5’-diphosphates. Nature 514, 372–375 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mastronarde DN, Automated electron microscope tomography using robust prediction of specimen movements. Journal of Structural Biology 152, 36–51 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Zheng SQ et al. , MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Meth 14, 331–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang K, Gctf: Real-time CTF determination and correction. Journal of Structural Biology 193, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang G et al. , EMAN2: An extensible image processing suite for electron microscopy. Journal of Structural Biology 157, 38–46 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Scheres SHW, RELION: implementation of a Bayesian approach to cryo-EM structure determination. Journal of Structural Biology 180, 519–530 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai X-C, Rajendra E, Yang G, Shi Y, Scheres SHW, Sampling the conformational space of the catalytic subunit of human γ-secretase. eLife 4 (2015), doi: 10.7554/eLife.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE, The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10, 845–858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pettersen EF et al. , UCSF chimera - A visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Goddard TD, Huang CC, Ferrin TE, Visualizing density maps with UCSF Chimera. Journal of Structural Biology 157, 281–287 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Emsley P, Lohkamp B, Scott WG, Cowtan K, Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Afonine PV et al. , Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr 68, 352–367 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu S, Zhang Y, MUSTER: Improving protein sequence profile-profile alignments by using multiple sources of structure information. Proteins 72, 547–556 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Webb B, Sali A, Comparative Protein Structure Modeling Using MODELLER 86, 2.9.1–2.9.37 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Jiang F et al. , Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature 479, 423–427 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawaoka J, Jankowsky E, Pyle AM, Backbone tracking by the SF2 helicase NPH-II. Nat Struct Mol Biol 11, 526–530 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Armougom F et al. , Expresso: automatic incorporation of structural information in multiple sequence alignments using 3D-Coffee. Nucleic Acids Res 34, W604–W608 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marchler-Bauer A et al. , CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res 39, D225–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gan J et al. , Structural insight into the mechanism of double-stranded RNA processing by ribonuclease III. Cell 124, 355–366 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Du Z, Lee JK, Tjhen R, Stroud RM, James TL, Structural and biochemical insights into the dicing mechanism of mouse Dicer: a conserved lysine is critical for dsRNA cleavage. Proc Natl Acad Sci USA 105, 2391–2396 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwon SC et al. , Structure of Human DROSHA. Cell 164, 81–90 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Robert X, Gouet P, Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42, W320–4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishino T, Komori K, Tsuchiya D, Ishino Y, Morikawa K, Crystal structure and functional implications of Pyrococcus furiosus hef helicase domain involved in branched DNA processing. Structure 13, 143–153 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA, cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat Meth 14, 290–296 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.