Highlights
-
•
Report on safety and clinical feasibility of a novel liquid fiducial marker for radiotherapy in lung cancer patients.
-
•
No serious safety or toxicity issues related to implantation procedure, radiotherapy or follow-up period.
-
•
Visible in ultrasound, kV X-ray, CT and ConeBeam CT images.
-
•
Slow degradation and no migration of the marker in follow up CT images.
-
•
Clinically feasible for use as a fiducial marker in radiotherapy of lung cancer patients.
Keywords: Liquid fiducial marker, Image-guided radiotherapy, NSCLC, Endoscopic ultrasound, EBUS
Abstract
Safety and clinical feasibility of injecting a novel liquid fiducial marker for use in image guided radiotherapy in 15 patients with non-small cell lung cancer are reported. No major safety or toxicity issues were encountered. Markers present at start of radiotherapy remained visible in cone beam computed tomography and fluoroscopy images throughout the treatment course and on computed tomography images during follow-up (0–38 months). Marker volume reduction was seen until 9 months after treatment, after which no further marker breakdown was found. No post-treatment migration or marker related complications were found.
Introduction
Image guidance has improved the precision of lung cancer radiotherapy (RT) [1], [2]. Radio-opaque markers (fiducial markers) can be used as surrogates for the target position when image contrast is poor [3]. Thoracic implantation of fiducial markers is described in several studies [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. Depending on tumour location, the markers can be inserted percutaneously [10], [11], [12] or endoscopically [13], [14], [15]. Percutaneous implantation carries a higher risk of pneumothorax [7], [10], [11], [12], while markers placed endoscopically in the bronchi can dislodge and migrate [6].
Liquid fiducial markers can be injected using standard endoscopic equipment, and allows for larger flexibility in marker size and performing several marker injections in an uninterrupted procedure. Previous studies of liquid markers in thoracic RT have showed limitations in radio-opacity [16], [17] or have limited data on stability throughout the RT course [18]. Furthermore, data on the post-treatment stability (migration) of fiducial markers in lungcancer patients has only been sparsely reported [6], [19].
BioXmark (Nanovi Radiotherapy A/S, Lyngby, Denmark) is a liquid fiducial marker that changes viscosity after injection. It consists of sucrose acetate isobutyrate (SAIB), an iodinated analogue of SAIB and ethanol. Upon injection into soft tissue, the ethanol starts diffusing out of the matrix, causing an increase in marker viscosity, resulting in a sticky gel-like marker after a few hours. The marker degrades into non-toxic compounds by hydrolysis over an expected period of years. Pre-clinical phantom and animal studies have demonstrated the marker to be safe and fully biocompatible as well as having good contrast in ultrasound, 2D and 3D kV X-ray images [20], [21], [22]. Our group has previously reported on the stability and visibility of BioXmark throughout the treatment course of patients with non-small cell lung cancer (NSCLC) referred for definitive RT [23]. In this short communication we report on safety, marker visibility and stability during the injection procedure and through a follow-up period of three years.
Materials & methods
The study was an open label, clinical feasibility study. It was approved by the local ethics committee (H-1-2013-133), the Danish National Board of Health (Application #2013113675) and was registered on clinicaltrials.gov (NCT02447900).
Patients with NSCLC referred for concurrent chemo-radiotherapy (66 Gy in 33 fractions) with no iodine allergy were eligible for inclusion.
The marker injection was performed within a two-week window between the first induction cycle of chemotherapy and the RT planning procedure. Two experienced pulmonologists performed the marker injections as an outpatient procedure. Marker injection sites were primary tumour (if possible) and endoscopically reachable pathologic mediastinal lymphnodes. During sedation, the marker was injected using a 22G needle using either a conventional video-bronchoscope, an endobronchial ultrasound bronchoscope (EBUS) or an oesophageal endoscopic ultrasound (EUS) device. Marker injection was monitored with real-time ultrasound guidance (all but one patients) or C-arm fluoroscopy. Doppler ultrasound was used to minimize the risk of endovascular marker injection. The injection volume was limited per protocol to a maximum of 0.5 ml per site and four sites per patient. Sedation and post-procedure monitoring were performed per local guidelines for interventional endoscopic procedures.
Image acquisition for RT treatment planning included a FDG PET/Computed tomography (CT), a respiratory correlated 4DCT, and three consecutive CT scans in voluntary deep inspiration breath-hold (DIBH). Intravenous contrast enhancement was used in the FDG-PET/CT and in the 4DCT scans. All patients were planned and treated with volumetric arc therapy technique (VMAT) (Varian Medical System, Palo Alto, CA, USA) in either DIBH or free breathing. Daily cone beam CT (CBCT) with registration on the tumour was used for image guidance. Orthogonal 2D X-ray fluoroscopies were acquired after treatment fractions 2, 16 and 30 for analysis of marker visibility.
Treatment and device related toxicity was registered at baseline, halfway through- and at the end of the RT course using CTCAE v. 4.0. After completion of treatment, all patients entered a follow-up procedure, consisting of a diagnostic CT scan and a clinical evaluation every three months until 18 months and thereafter at six-month intervals until five years of follow-up or death. In case of progression, the follow-up interval was changed to fit with the intervals of treatment. Measurement of marker volume and opacity was performed on the planning CT, treatment CBCT and on follow-up CT scans (3,6,9,12,18,24,36 months).
Results
Fifteen patients were included in the study. Baseline patient characteristics are shown in Table 1.
Table 1.
Baseline patient characteristics.
| Patient characteristics | n |
|---|---|
| Gender | |
| Male/Female | 9/6 |
| Age | |
| Median (range) [years] | 64 (53–78) |
| WHO performance status | |
| 0/1 | 6/9 |
| Charlson comorbidity score | |
| Median (range) | 6 (4–7) |
| Clinical stage | |
| IIIA/IIIB/IVa | 5/9/1 |
| Pathology | |
| Squamous cell carcinoma | 6 |
| Adenocarcinoma | 7 |
| Other | 2 |
| Location of primary tumour | |
| Right upper lobe | 6 |
| Right lower lobe | 4 |
| Left upper lobe | 1 |
| Left lower lobe | 3 |
| Central | 1 |
| Gross tumour volume | |
| Median (range) [cm3] | 136 (30–522) |
M1a, contralateral lung metastasis diagnosed at planning.
A total of 35 markers were injected in 15 patients, 11 into or near the primary tumour (T) and 24 into the hilar or mediastinal lymph nodes (N). Two marker injections into endobronchial tumours were performed by visual guidance using a conventional video-bronchoscope, the remaining injection procedures were performed using EUS and EBUS endoscopes with real-time monitoring of the injected volume (Fig. 1). One to four markers were injected per patient. Lymph node markers were injected into PET-positive mediastinal and hilar lymph nodes. For details see Rydhög et al. [23].
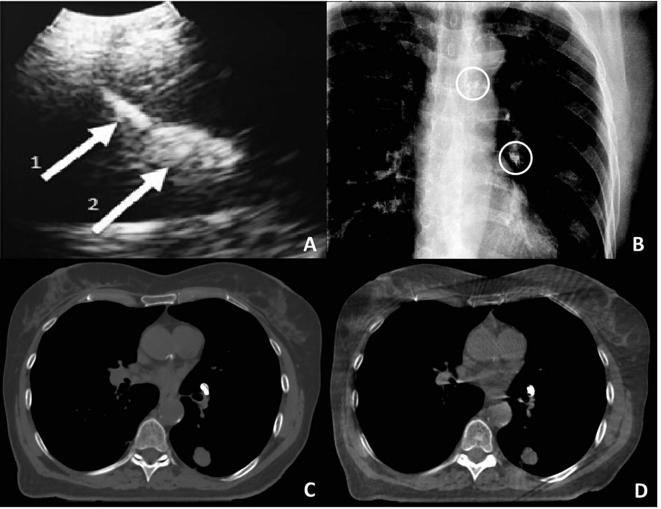
Fig. 1.
Example of ultrasound guided injection (EBUS probe) of liquid marker in a pig (A). Arrow 1 marks the needle tip and arrow 2 the liquid marker (A). B–D) Images of a patient with liquid markers injected into lymph node stations 4 L and 10 L: Anterior-posterior fluoroscopy projection with the markers encircled (B), axial plane from a computed tomography (C) and a cone beam computed tomography (D).
Two patients did not receive RT. One patient was diagnosed with multiple lung metastases on the planning PET/CT. Another patient died between marker injection and RT start. A subsequent autopsy including a post mortem CT-scan found the injected marker in situ at the injection site. The autopsy report concluded acute heart failure as the cause of death.
One marker-related event was registered in relation to the injection procedure: patient 5 had a marker placed in an endobronchial tumour using a video-bronchoscope. The marker did not appear on the planning CT images, but a new and slightly PET positive, hyperdense area was observed in the phrenico-costal sinus of the ipsilateral lung. It was suspected that the marker was accidently injected into the pleural cavity. The patient had no symptoms during or after the procedure. To minimise the risk of endovascular injection all subsequent injections were monitored with Doppler ultrasound.
A technical aspect was reported during marker injection in the second patient. Contamination of the working channel with a residual drop of marker liquid caused the needle to adhere to the working channel damaging the fiberoptic part of the endoscope. Procedures for needle loading were updated and no other equipment related complications were reported.
No further complications were observed in relation to the injection procedure, during the RT period or in the follow-up period. A total of 98 adverse events, including 53 serious adverse events were reported during the study period. Apart from the events described above, none were related to the marker.
Thirty-two markers (10 T and 22 N) were injected in the thirteen patients receiving RT. The two markers injected into endobronchial tumours by visual guidance did not appear on the planning CT images. One T marker was too dispersed to act as a fiducial marker. The markers (0.1–0.2 ml) injected into lymph nodes were generally more well-defined compared to the larger volume markers (0.20–0.30 mL) injected in the primary tumours which adopted a more complex 3D shape. The 29 markers suitable as fiducial markers remained visible throughout the RT course. The injected markers displayed high Hounsfield Unit (HU) values with a mean >900 on CT and CBCT images, and all marker sizes were identifiable on fluoroscopy [23] and CBCT (Fig. 1, Fig. 2) throughout the full treatment course.
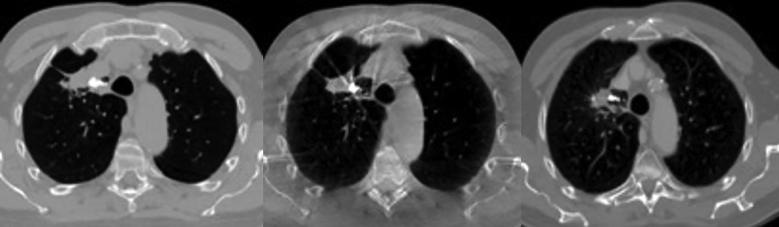
Fig. 2.
Example of liquid fiducial marker (300 µL) implanted in a primary tumour. Axial images of the planning CT (left), the CBCT at fraction 32 (middle) and follow-up CT 21 months after treatment (right).
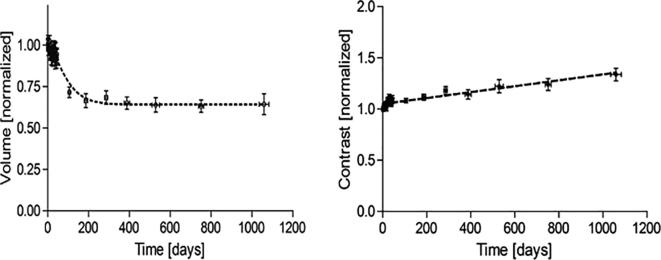
Median follow-up time was 34 months (0–38 months) at data lock December 31, 2017. In all follow-up scans performed the markers remained visible and in-situ relative to the implantation site by manual assessment. (Fig. 2). The measurements of marker volume during and after treatment showed a reduction in marker size of approximately 35% after 9 months, followed by a steady state with no significant size reduction between the 12 and 36 month scans. Marker opacity was constant during the treatment and follow up period (Fig. 3).
Fig. 3.
Normalized values of marker volume (left) and –contrast (right) during follow up, showing a steady state in marker volume after 9 months and stable marker contrast values throughout the period.
Discussion
Our study is the first to examine the BioXmark liquid fiducial marker long term stability and safety during follow-up in a human population. We found no serious adverse events in relation to the injection procedure or during treatment and follow-up.
An advantage of the liquid fiducial marker is that it can be injected with standard endoscopic (EUS/EBUS) equipment with 22–25 gauge needles compared to the 18–19 gauge needles used for placement of most solid metal fiducial markers [6], [11]. The liquid formulation also allows for the placement of several markers in one session without needing to “reload” the endoscopy needle between each implantation as is most often the case with solid metal markers [24]. The size of the liquid fiducial marker can be customised by varying the injected volume. Preclinical studies, where the marker was injected into phantoms, showed that a volume of as little as 10 μl was sufficient for the marker to appear visible in the CBCT images (data not published). However, a larger volume is needed for the marker to appear visible on the 2D kV/fluoroscopy images [20]. The visibility and positional stability of the injected markers remained constant throughout the RT course, as did the volume of the marker [23].
The low pneumothorax risk in our study agrees with previous studies where fiducial markers were implanted in lung tissue or mediastinum using endoscopic techniques [8], [13], [15], [25], [26]. Three of 32 (9%) markers in patients receiving RT were lost or not suitable as fiducial markers at the planning CT images. In comparison, in studies of endoscopically implanted solid metal fiducial markers, the marker loss varies from 0–2% (coil shaped markers) to 25–53% (linear markers) and 21% (sphere-shaped markers) [25], [26], [27], [28].
While there are multiple studies of solid metal fiducial markers in RT of lung cancer patients [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], the experience with liquid fiducial markers in this group of patients is limited. Two other liquid based fiducial markers have been clinically tested: TraceIT (Augmenix, Waltham, MA) and Lipiodol (Lipiodol® Ultra-Fluide, Guerbet Laboratorie, Villepinte, France). TraceIT is a hydrogel-based radiopaque marker developed for use in surgery and RT [16]. Studies with TraceIT in human cadavers and patients with oesophageal cancer demonstrated radio-opacity of around 300 HU and visibility in CT, MRI and ultrasound images, but not in 2D kV X-ray images [16], [17]. Lipiodol is an oil-based contrast agent and has been reported used in RT of four patients with lung cancer [18] Lipiodol deposits were injected via EBUS and electromagnetic navigational bronchoscopy (ENB) techniques and were visible on ultrasound, 2D kV X-ray and CT images. Three of the four patients received radiotherapy, but no data on the marker performance during the RT course was reported.
As the present study was a first in man study, a detailed analysis on marker degradation during follow up was undertaken. From preclinical studies, the time until full degradation was estimated at up to three years. The measurements showed degradation of the marker until approximately 9 months, after which a steady state with no degradation was seen. In the preclininical animal studies, histology examinations after 28, 90 and 365 days revealed the formation of a thin fibrous capsule around the marker, possibly slowing down the hydrolysis reaction responsible for the breakdown of the marker. The HU values of the marker were constant throughout the study period, confirming degradation happens on the marker surface rather than being a homogenous degradation.
Since marker positional stability is only crucial during treatment and no post treatment marker related complications has previously been reported in lung cancer patients, information on possible marker migration in this group of patients is limited. In the lipiodol study follow up CT images showed marker material present at the implantation site after up to 8 months post RT [18]. Another study where solid metal fiducial markers was implanted in 10 lung cancer patients, showed no marker migration in follow-up CT images up to 18 months post-treatment [29]. In the present study, no marker migration relative to the injection site was found in CT images up to 36 months after end of treatment.
In conclusion, the liquid fiducial marker in this study (BioXmark) was safe to endoscopically inject into primary tumours and lymph nodes in patients with locally advanced NSCLC and provided adequate visibility and stability when acting as fiducial markers for image guided radiotherapy. The marker was partially degraded during a three year follow up. No post-RT marker migration or complications were found.
Conflicts of interest
None.
Acknowledgment
This work has been funded by the Danish Council for Strategic Research (Nanoguide, casenr. 0603-00442B).
References
- 1.Korreman S., Persson G., Nygaard D., Brink C., Juhler-Nøttrup T. Respiration-correlated image guidance is the most important radiotherapy motion management strategy for most lung cancer patients. Int J Radiat Oncol Biol Phys. 2012;83(4):1338–1343. doi: 10.1016/j.ijrobp.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann L., Ingerslev M., Marquard M., Ahmed A. Anatomical landmarks accurately determine interfractional lymph node shifts during radiotherapy of lung cancer patients. Radiother Oncol. 2015;116(1):64–69. doi: 10.1016/j.radonc.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt M.L., Hoffmann L., Knap M.M., Rasmussen T.R., Folkersen B.H., Toftegaard J. Cardiac and respiration induced motion of mediastinal lymph node targets in lung cancer patients throughout the radiotherapy treatment course. Radiother Oncol. 2016;121(1):52–58. doi: 10.1016/j.radonc.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Patel A., Khalsa B., Lord B., Sandrasegaran K., Lall C. Planting the seeds of success: CT-guided gold seed fiducial marker placement to guide robotic radiosurgery. J Med Imaging Radiat Oncol. 2013;57(2):207–211. doi: 10.1111/j.1754-9485.2012.02445.x. [DOI] [PubMed] [Google Scholar]
- 5.Schaake E.E., Belderbos J.S.A., Buikhuisen W.A., Rossi M.M.G., Burgers J.A., Sonke J.J. Mediastinal lymph node position variability in non-small cell lung cancer patients treated with radical irradiation. Radiother Oncol. 2012;105(2):150–154. doi: 10.1016/j.radonc.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Bhagat N., Fidelman N., Durack J.C., Collins J., Gordon R.L., Laberge J.M., Kerlan R.K. Complications associated with the percutaneous insertion of fiducial markers in the thorax. Cardiovasc Intervent Radiol. 2010;33(6):1186–1191. doi: 10.1007/s00270-010-9949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Mey J., Van de Steene J., Vandenbroucke F., Verellen D., Trappeniers L., Meysman M. Percutaneous placement of marking coils before stereotactic radiation therapy of malignant lung lesions. J Vasc Interv Radiol. Jan. 2005;16(1):51–56. doi: 10.1097/01.RVI.0000142599.48497.6B. [DOI] [PubMed] [Google Scholar]
- 8.Shirato H., Harada T., Harabayashi T., Hida K., Endo H., Kitamura K. Feasibility of insertion/implantation of 2.0-mm-diameter gold internal fiducial markers for precise setup and real-time tumor tracking in radiotherapy. Int J Radiat Oncol. May 2003;56(1):240–247. doi: 10.1016/s0360-3016(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 9.Nuyttens J.J., Prévost J.-B., Praag J., Hoogeman M., Van Klaveren R.J., Levendag P.C. Lung tumor tracking during stereotactic radiotherapy treatment with the CyberKnife: Marker placement and early results. Acta Oncol. Jan. 2006;45(7):961–965. doi: 10.1080/02841860600902205. [DOI] [PubMed] [Google Scholar]
- 10.Yousefi S., Collins B.T., Reichner C.A., Anderson E.D., Jamis-Dow C., Gagnon G. Complications of thoracic computed tomography-guided fiducial placement for the purpose of stereotactic body radiation therapy. Clin Lung Cancer. 2007;8(4):252–256. doi: 10.3816/CLC.2007.n.002. [DOI] [PubMed] [Google Scholar]
- 11.Kothary N., Heit J.J., Louie J.D., Kuo W.T., Loo B.W., Koong A. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. J Vasc Interv Radiol. 2009;20(2):235–239. doi: 10.1016/j.jvir.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Persson G.F., Josipovic M., Nygaard D.E., Von Der Recke P., Aznar M., Juhler-nøttrup T. Percutaneously implanted markers in peripheral lung tumours: report of complications. Acta Oncol. 2013;52:2–7. doi: 10.3109/0284186X.2013.764009. [DOI] [PubMed] [Google Scholar]
- 13.Harley D.P., Krimsky W.S., Sarkar S., Highfield D., Aygun C., Gurses B. Fiducial marker placement using endobronchial ultrasound and navigational bronchoscopy for stereotactic radiosurgery: an alternative strategy. Ann Thorac Surg. 2010;89(2):368–373. doi: 10.1016/j.athoracsur.2009.09.048. discussion 373–4. [DOI] [PubMed] [Google Scholar]
- 14.Kupelian P.A., Forbes A., Willoughby T.R., Wallace K., Mañon R.R., Meeks S.L. Implantation and stability of metallic fiducials within pulmonary lesions. Int J Radiat Oncol Biol Phys2007;69(3):777–785. doi: 10.1016/j.ijrobp.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 15.Anantham D., Feller-Kopman D., Shanmugham L.N., Berman S.M., DeCamp M.M., Gangadharan S.P. Electromagnetic navigation bronchoscopy-guided fiducial placement for robotic stereotactic radiosurgery of lung tumors: a feasibility study. Chest. 2007;132(3):930–935. doi: 10.1378/chest.07-0522. [DOI] [PubMed] [Google Scholar]
- 16.de Souza Lawrence L., Ford E., Gilbert C., Yarmus L., Meneshian A., Feller-Kopman D. Novel applications of an injectable radiopaque hydrogel tissue marker for management of thoracic malignancies. Chest. Jun. 2013;143(6):1635–1641. doi: 10.1378/chest.12-1691. [DOI] [PubMed] [Google Scholar]
- 17.Machiels M., Van Hooft J., Jin P. Endoscopy/EUS-guided fi ducial marker placement in patients with esophageal cancer : a comparative analysis of 3 types of markers. Gastrointest Endosc. 2015;82(4):641–649. doi: 10.1016/j.gie.2015.03.1972. [DOI] [PubMed] [Google Scholar]
- 18.Rose M., Siva S., Ball D., Irving L.B., Steinfort D.P. Bronchoscopic delivery of lipiodol as a fiducial marker in lung tumors before radiotherapy. J Thorac Oncol. Oct. 2014;9(10):1579–1583. doi: 10.1097/JTO.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 19.Weiss E. Long-term safety and stability of gold coil fiducial markers in non-small-cell lung cancer image-guided radiotherapy. Int J Radiol Radiat Ther. 2017;3(1):1–6. [Google Scholar]
- 20.Rydhog J.S., Jolck R.I., Andresen T.L., af Rosenschold P.M. Quantification and comparison of visibility and image artifacts of a new liquid fiducial marker in a lung phantom for image-guided radiation therapy. Med Phys. 2015;42(6):2818–2826. doi: 10.1118/1.4919616. [DOI] [PubMed] [Google Scholar]
- 21.Jølck R.I., Rydhög J.S., Christensen A.N., Hansen A.E., Bruun L.M., Schaarup-jensen H. Injectable colloidal gold for use in intrafractional 2D image-guided radiation therapy. Adv Healthc Mater. 2015;4:856–863. doi: 10.1002/adhm.201400651. [DOI] [PubMed] [Google Scholar]
- 22.Mortensen S.R., Scherman J.B., Larsen K.R., Jølck R.I., Persson G.F., Hansen A.E. Use of a novel liquid fiducial marker injected with endoscopic ultrasound equipment for use in image guided radiation therapy of thoracic tumors (porcine model) Int J Radiat Oncol Biol Phys. 2014;90(1):S651. [Google Scholar]
- 23.Rydhög J.S., Mortensen S.R., Larsen K.R., Clementsen P., Jølck R.I., Josipovic M. Liquid fiducial marker performance during radiotherapy of locally advanced non small cell lung cancer. Radiother Oncol. 2016;121(1):64–69. doi: 10.1016/j.radonc.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Seides B.J., Egan J.P., French K.D., Kovitz K.L., Desai N.R. Fiducial marker placement for stereotactic body radiation therapy via convex probe endobronchial ultrasound: a case series and review of literature. J Thorac Dis. 2018;10(3):1972–1983. doi: 10.21037/jtd.2018.03.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imura M., Yamazaki K., Shirato H., Onimaru R., Fujino M., Shimizu S. Insertion and fixation of fiducial markers for setup and tracking of lung tumors in radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63(5):1442–1447. doi: 10.1016/j.ijrobp.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder C., Hejal R., Linden P.A. Coil spring fiducial markers placed safely using navigation bronchoscopy in inoperable patients allows accurate delivery of CyberKnife stereotactic radiosurgery. J Thorac Cardiovasc Surg. 2010;140(50):1137–1141. doi: 10.1016/j.jtcvs.2010.07.085. [DOI] [PubMed] [Google Scholar]
- 27.Steinfort D.P., Siva S., Kron T., Chee R.R., Ruben J.D., Ball D.L. Multimodality guidance for accurate bronchoscopic insertion of fiducial markers. J Thorac Oncol. 2014;10(2):324–330. doi: 10.1097/JTO.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 28.Nabavizadeh N., Zhang J., Elliott D.A., Tanyi J.A., Thomas C.R., Fuss M. Electromagnetic navigational bronchoscopy-guided fiducial markers for lung stereotactic body radiation therapy. J Bronchol Interv Pulmonol. 2014;21(2):123–130. doi: 10.1097/LBR.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 29.P. Jackson, D.P. Steinfort, T. Kron, and S. Siva, “Practical Assessment of Bronchoscopically Inserted Fiducial Markers for Image Guidance in Stereotactic Lung Radiotherapy”, 2016. [DOI] [PubMed]