Summary
Cobalt diselenide (CoSe2) has been demonstrated to be an efficient and economic electrocatalyst for oxygen evolution reaction (OER) both experimentally and theoretically. However, the catalytic performance of up-to-now reported CoSe2-based OER catalysts is still far below commercial expectation. Herein, we report a hybrid catalyst consisting of CoSe2 nanosheets grafted on defective graphene (DG). This catalyst exhibits a largely enhanced OER activity and robust stability in alkaline solution (overpotential at 10 mA cm−2: 270 mV; Tafel plots: 64 mV dec−1). Both experimental evidence and density functional theory calculations reveal that the outstanding OER performance of this hybrid catalyst can be attributed to the synergetic effect of exposed cobalt atoms and carbon defects (electron transfer from CoSe2 layer to defect sites at DG). Our results suggest a promising way for the development of highly efficient and low-cost OER catalysts based on transition metal dichalcogenides.
Subject Areas: Catalysis, Chemistry, Energy Materials
Graphical Abstract
Highlights
-
•
A hybrid catalyst with in-plane CoSe2/defective graphene heterostructures
-
•
The catalyst exhibits an excellent and stable oxygen evolution reaction (OER) activity
-
•
Enhanced OER performance is due to the synergy of exposed cobalt atoms and carbon defects
Catalysis; Chemistry; Energy Materials
Introduction
The oxygen evolution reaction (OER) plays an important role in various energy storage technologies, such as H2 production from water splitting and rechargeable metal-air batteries (Hunter et al., 2016, Suen et al., 2017, Xia et al., 2016, Xu et al., 2016). However, the inherent sluggish OER kinetics, involving a four-electron transfer associated with O-H bond breaking and O-O bond formation, significantly inhibits the large-scale electrochemical water splitting (Dau et al., 2010, Suntivich et al., 2011). Although precious metal oxides (such as RuO2 and IrO2) have currently been employed as the state-of-the-art catalysts to overcome the drawback of very low OER kinetics, the high cost and limited resources seriously impede their widespread application (Li and Dai, 2014). As a consequence, it is crucial to explore highly efficient and inexpensive alternative catalysts that are made of geologically abundant elements.
Recently, many economical cobalt-based catalysts, including metal oxides (Masa et al., 2014), phosphates (Zhou et al., 2016), perovskite (Fabbri et al., 2015), hydro(oxy)oxides (Wan et al., 2017), and chalcogenides (Hao et al., 2017, Gao et al., 2017) have been developed and exhibited remarkable OER performance. Notably, CoSe2, with a t2g6eg1 electronic configuration close to the optimal eg filling for OER was considered as an ideal candidate (Kong et al., 2014, Gao et al., 2012). However, the originally synthesized pure CoSe2 catalysts still showed unsatisfied OER activity, inferior to that of the corresponding oxides or hydro(oxy)oxides. Thus, with the purpose of optimizing the OER electrocatalytic performance of CoSe2, tremendous efforts have been made to develop diverse strategies: (1) creating strong chemical and electrical coupling by constructing hybrid structures with metals, metal oxides, or carbon materials (Zheng et al., 2015a, Zheng et al., 2015b, Gao et al., 2014, Zhao et al., 2017a, Zhao et al., 2017b); (2) increasing the number of active sites by reducing the thickness into atomic scale and introducing more defects (Liu et al., 2014, Liang et al., 2015); and (3) rationally engineering the electrical conductivity by doping metal cations or non-metal heteroatoms (Zhao et al., 2017a, Zhao et al., 2017b, Fang et al., 2017). The search for new nanocomposites based on CoSe2, which are comparable to nonprecious metal catalysts, continues (Gao et al., 2017). Specifically, modification of pure CoSe2 by increasing its number of active sites or improving its electrical conductivity could not effectively regulate the electronic configuration of cobalt to optimize OER catalytic activity of each active site, which accordingly restrained the overall performance. To solve the problem, hybridizing with other functional materials offered a promising way. It had been demonstrated that electron-deficient cobalt with high valence could facilitate transforming O∗ to OOH∗ (key intermediates in OER process) (Wan et al., 2017). Therefore, if foreign materials grafted with CoSe2 can induce electron donation from cobalt to the foreigners, the OER performance should be significantly promoted. However, in most previously reported hybrid OER catalysts based on CoSe2, electrons usually transferred from grafting materials to CoSe2 host, contrary to our assumption, thus encouraging us to develop new hybrid material systems for optimal performance gains.
Non-doped graphene with structural defects has recently received growing attention due to its excellent performance in electrocatalysis (Tang et al., 2016, Jia et al., 2016, Liu et al., 2017, Yan et al., 2017). Incorporation of different types of defects into the sp2 structure of graphene can effectively break the integrity of π conjugation to enhance the electrocatalytic activity (Tang and Zhang, 2017). Besides, the defects on defective graphene (DG) can also offer additional efficient anchor sites for directly coupling other functional metal-based species, such as Mn-Co nanoparticles and Ni-Fe double layered hydroxide (LDH) nanosheets, through strong chemical interaction at two-phase interface (Yan et al., 2016, Jia et al., 2017). These hybrid catalysts can exhibit much better electrochemical performance than individual components due to their unique structural features such as highly exposed active sites on 2D nanolayers, conductive graphene substrates, as well as enhanced proton coupling and electron transport between the components. Particularly, in the case of Ni-Fe LDH nanosheets hybridized with DG, density functional theory (DFT) calculations had confirmed the accumulation of holes on Ni-Fe LDH nanosheets and that of electrons at the defect sites in DG (Jia et al., 2017). In this regard, properly integrating pre-synthesized 2D CoSe2 nanosheets onto DG may be an effective way to modulate the interfaces and chemical and electronic structures to access optimized OER energetics.
Herein, we report that exfoliated ultrathin CoSe2 nanosheets can be grafted with DG to form a CoSe2@DG hybrid OER catalyst by a facile hydrothermal method. Benefiting from the synergistic effect between CoSe2 and DG substrate, the as-prepared CoSe2@DG nanocomposite exhibits a remarkable performance toward OER. The OER overpotential at a current density of 10 mA cm−2 is only 270 mV and a high current density of 129 mA cm−2 is obtained at an overpotential of 350 mV, which can be ranked as the top-performing one among the hitherto reported CoSe2-based OER catalysts. In addition, the CoSe2@DG electrode also demonstrates an outstanding stability after a long-term cycle measurement. This work demonstrated that the CoSe2@DG will be a very promising nonprecious metal-based OER catalyst.
Results
Synthesis and Characterization of CoSe2@DG Composite Catalysts
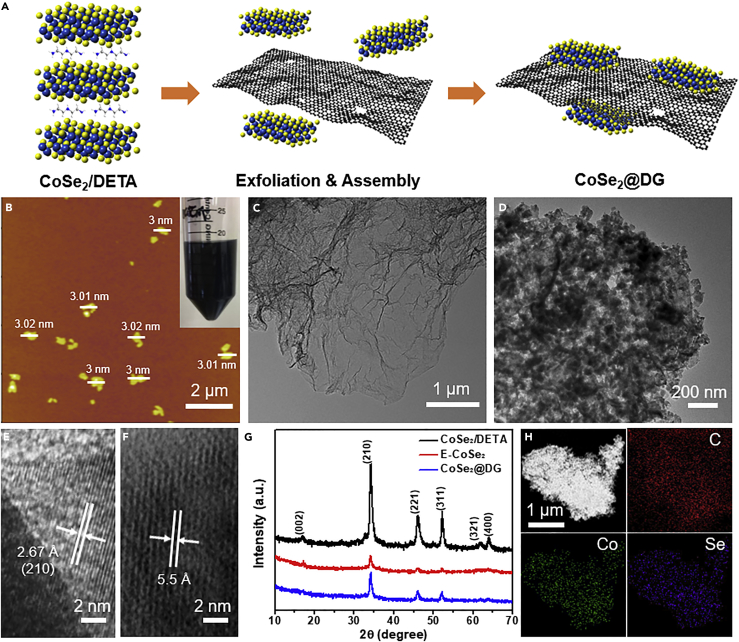
As illustrated in Figure 1A, the whole synthesizing process includes two steps: (1) ultrasound induced liquid exfoliation of pre-synthesized lamellar CoSe2/DETA (diethylenetriamine) intermediate (Liu et al., 2014) and (2) assembling ultrathin CoSe2 nanosheets with DG via a simple hydrothermal route. For the first step, a homogeneously dispersed black suspension was obtained after the ultrasonic exfoliation process, which could keep stable for a long time without any precipitate (Figure 1A, inset). Morphology characterization (scanning electron micrographs and transmission electron microscopic [TEM] images in Figures S1 and S2) clearly confirmed the formation of ultrathin CoSe2 nanosheets (denoted as E-CoSe2). X-ray diffraction (XRD) patterns showed weaker characteristic peaks when compared with pristine CoSe2/DETA, which further demonstrated the well-done exfoliation (Figure 1G, red). The thickness of the exfoliated nanosheets was measured to be around 3 nm from the atomic force microscopic image, corresponding to about 5 layers (a single CoSe2 layer along the lattice parameter is ∼0.61 nm) (Figure 1B) (Gao et al., 2009). Subsequently, these CoSe2 ultrathin sheets could be tightly overlain on the DG in a certain mass ratio (typically 9:1) by a hydrothermal treatment process at 60°C for 24 hr. TEM images revealed that the smooth and near-transparent surface of DG sheets became extremely rougher and opaque, indicating the successful grafting of CoSe2 onto DG (Figures 1C and 1D). The high-resolution TEM image in Figure 1E showed the lattice fringe with a spacing of 2.67 Å, which can be assigned to the (210) plane of cubic-phase CoSe2. The interlayer distance could be estimated to be ∼5.5 Å, smaller than that of pristine CoSe2/DETA, which was attributed to the removal of DETA molecules during the exfoliation process (Figure 1F). Further experiments revealed that the mass ratio of CoSe2/DG showed a considerable influence on the morphology of the products (Figure S3). When decreasing the mass ratio to 3:1 or 6:1, only partially and non-uniformly covered CoSe2@DG hybrids could be obtained. In addition, DG support also plays an important role in the synthesis. To demonstrate it, CoSe2@G (G presents pristine graphene) and CoSe2@NG (NG represents nitrogen-doped G) samples were fabricated for comparison using the same method. As illustrated in the TEM images, aggregated CoSe2 only anchored on part of G or NG sheet surface, in contrast to the entirely covered morphology for CoSe2@DG (Figure S4). This indicates that DG is more suitable as the support for CoSe2 nanosheet dispersion and anchoring, effectively preventing the aggregation of CoSe2 nanosheets, which should be beneficial for the catalytic performance enhancement.
Figure 1.
Schematic Illustration of the Synthesis and Characterization of As-prepared CoSe2@DG Composites
(A) Schematic illustration of the fabrication of CoSe2@DG composites.
(B) AFM image of exfoliated CoSe2 nanosheets. Inset: homogeneously dispersed exfoliated CoSe2 nanosheets suspension.
(C and D) TEM images of pristine DG (C) and CoSe2@DG composites (D).
(E and F) High-resolution TEM images of CoSe2@DG composites.
(G) XRD patterns of CoSe2/DETA lamellar intermediates, exfoliated CoSe2 nanosheets, and CoSe2@DG composites.
(H) Scanning transmission electron microscope (STEM)-energy-dispersive X-ray spectrum elemental mapping images of CoSe2@DG composites.
To further investigate the microstructure and composition of as-prepared CoSe2@DG composites, a series of measurements were carried out. XRD pattern of CoSe2@DG nanocomposites still exhibited the featured diffraction peaks of CoSe2, suggesting that no phase change occurred during the synthesis process (Figure 1G, blue). The intensity of all the peaks was a bit stronger than that of exfoliated ultrathin CoSe2 nanosheets, but still much weaker than that of original CoSe2/DETA, indicating only a small part of ultrathin nanosheets reassembled. In addition, the energy-dispersive X-ray spectrum and elemental mapping indicated the existence of elements C, Co, Se and their uniform distribution in the hybrids, also confirming the formation of CoSe2@DG composites (Figures 1H and S5). The Co loading amount in as-made CoSe2@DG, CoSe2@G, and CoSe2@NG composites was obtained according to the thermogravimetric analysis as 31.6, 28.9, 29.5 wt%, respectively (Figure S6). Based on this, we further calculated the number of moles (n) of Co element in different catalysts deposited on the glass carbon electrode (CoSe2@DG: 0.132 μmol; CoSe2@G: 0.121 μmol; CoSe2@NG: 0.124 μmol), which could be used to gain the turnover frequency (TOF) value for the evaluation of their intrinsic OER efficiency.
OER Performance of CoSe2@DG Catalysts-Coated Glass Carbon Electrode
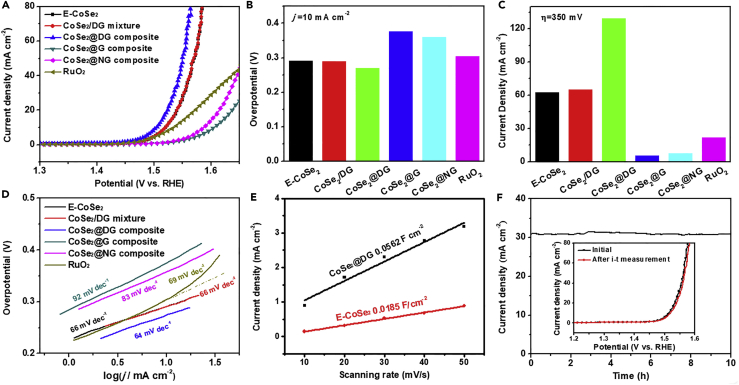
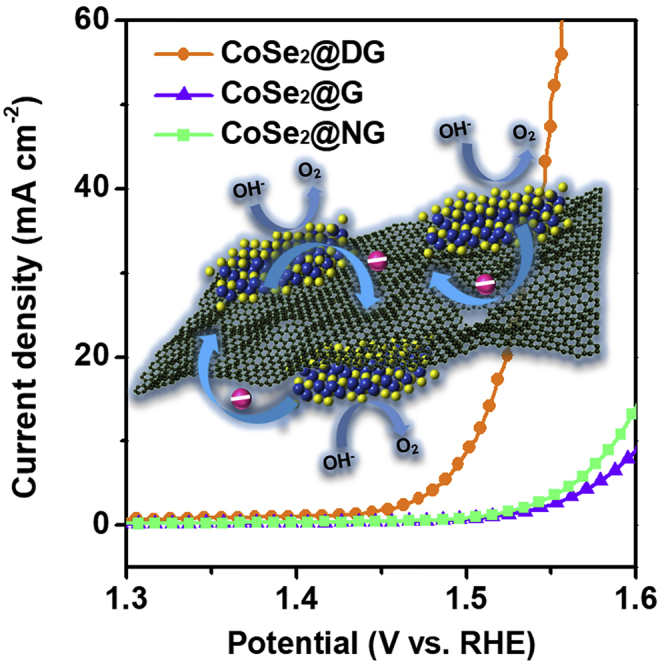
Using a standard three-electrode system, the electrocatalytic OER properties of different samples were evaluated in an O2-saturated 1 M KOH aqueous solution (4OH−→O2 + 2H2O + 4e−). The as-prepared catalysts were uniformly cast on a glassy carbon electrode with a loading of 0.2 mg cm−2 and used as a working electrode. The OER activities of CoSe2@DG composites prepared with different mass ratios (3:1, 6:1, and 9:1) were first studied to determine the optimal synthetic condition. As shown in Figure S7, CoSe2@DG-9-1 exhibited the best OER activity among the three products, with the smallest overpotential at 10 mA cm−2, the largest current density at an overpotential of 0.35 V, and the fastest kinetics. The results should be attributed to their morphological difference mentioned above. Reassembly of CoSe2 nanosheets in CoSe2@DG-3-1 and CoSe2@DG-6-1 samples significantly inhibited their OER performance. If not specially specified, the CoSe2@DG, CoSe2@NG, and CoSe2@G products are all synthesized in the mass ratio of 9:1 in this research. Subsequently, the OER activities for other comparative samples, such as CoSe2 nanosheets coupling with different graphene (NG, G), exfoliated CoSe2 nanosheet (E-CoSe2), CoSe2 nanosheets physically mixed with DG (CoSe2/DG), and the OER benchmark catalyst (RuO2), were also measured. Figure 2A shows polarization curves recorded by linear sweep voltammetry (LSV) at a slow scan rate of 5 mV s−1 with a continuous rotating speed of 1,600 rpm after internal resistance (iR) correction. Among the five tested catalysts, CoSe2@DG displayed the highest intrinsic OER activity. To reach a current density of 10 mA cm−2, CoSe2@DG required an overpotential of only 270 mV, which was 35, 22, 20, 106, and 83 mV smaller than that of commercial RuO2, E-CoSe2, CoSe2/DG mixture, CoSe2@G, and CoSe2@NG, respectively (Figure 2B). The current density at an overpotential of 350 mV was 129 mA cm−2, which was 6, 2.1, 2, 23.7, and 16.5 times higher than that of RuO2, E-CoSe2, CoSe2/DG mixture, CoSe2@G, and CoSe2@NG, respectively (Figure 2C). Meanwhile, the intrinsic activity of different catalysts was assessed by TOF, assuming all the Co element was catalytically active (Figure S8). Compared with RuO2, E-CoSe2, CoSe2@G, and CoSe2@NG catalysts, the CoSe2@DG hybrids exhibited obviously larger TOF value of 0.024 s−1 at an overpotential of 270 mV, indicating the significant role of interaction between CoSe2 and DG toward OER in our study. As all the active sites were presumed to come from CoSe2, comparative experiment was also offered to confirm that DG was inert in OER relative to CoSe2 (Figure S9). Furthermore, the kinetic parameters of OER for the five catalysts were also calculated (Figure 2D). CoSe2@DG had a Tafel slope of about 64 mV dec−1, similar to that of E-CoSe2 (66 mV dec−1), CoSe2/DG mixture (66 mV dec−1), and RuO2 (69 mV dec−1), but much smaller than that of the other samples (83 mV dec−1 for CoSe2@NG and 92 mV dec−1 for CoSe2@G). The above OER measurement was done in 1 M KOH solution. However, in some cases, OER catalysts were reported to assess their activity in 0.1 M KOH solution (Liu et al., 2014). Therefore, to achieve a more accurate comparison of the OER performance between our synthesized CoSe2@DG hybrid catalyst and other nonprecious metal catalysts, we also measured its OER LSV curves in 0.1 M KOH solution and found that it only required an overpotential of 304 mV to afford a current density of 10 mA cm−2 (Figure S10). In fact, the activity of CoSe2@DG has outperformed those of most CoSe2-based OER catalysts reported to date and is comparable to those of other state-of-the-art nonprecious metal catalysts (Table S1). To gain further insight of the OER kinetics occurring on such CoSe2@DG hybrid catalysts, electrical impedance spectroscopy was performed. The CoSe2@DG composites exhibited a much smaller charge transfer resistance of 42 Ω than that of E-CoSe2 of 88 Ω (Figure S11). Moreover, the double layer capacitance (Cdl) was obtained through the cyclic voltammetry measurement, which was considered as an alternative method to estimate the electrochemical active surface areas (ECSAs) (Figures 2E and S12). The CoSe2@DG composites show a Cdl of 28.1 mF cm−2, much larger than that of E-CoSe2 nanosheets (9.3 mF cm−2), indicating that the effective combination of CoSe2 and DG leads to an increased ECSA and more exposed active sites.
Figure 2.
OER Performance of CoSe2@DG Composite Catalysts
(A) OER LSV curves of different catalysts in O2-saturated 1 M KOH solution.
(B) Overpotential required for j = 10 mA cm−2.
(C) Current densities at an overpotential of 350 mV.
(D) The corresponding Tafel plots.
(E) Scan rate dependence of the current density difference for exfoliated CoSe2 nanosheets and CoSe2@DG composites. The linear slope is equivalent to twice the double-layer capacitance Cdl.
(F) Continuous amperometric i-t measurement at a constant overpotential of 305 mV. The inset shows the polarization curves before and after i-t measurement.
To assess the stability of the conditioned CoSe2@DG electrode, the continuous amperometric i-t measurement was employed under a constant overpotential of 305 mV. Figure 2F displayed that the current density maintained at ∼ 31 mA cm−2 during 10 hr and the polarization curve after i-t measurement showed negligible current loss, demonstrating the high electrocatalytic durability of CoSe2@DG. Various postmortem characterizations reveal no obvious change in the structure of CoSe2@DG catalysts after OER stability test, further evidencing their remarkable catalytic robustness in alkaline medium (Figure S13).
Catalytic Mechanism by Synergistic Effect of CoSe2 with DG
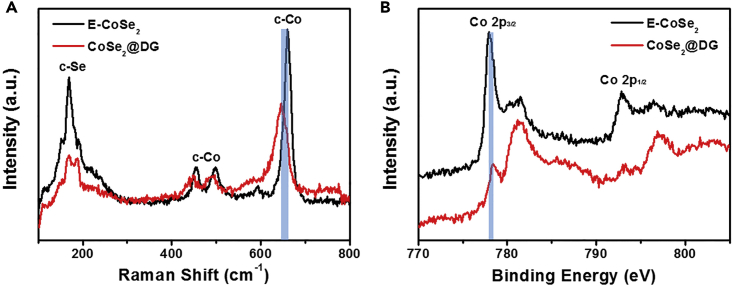
It is imperative to gain a comprehensive understanding of the intrinsic reason for the improved OER performance after the effective coupling of CoSe2 and DG. To achieve this goal, both experimental and theoretical methods were used. First, the strong interactions between CoSe2 and DG in the composites were demonstrated experimentally by Raman scattering and X-ray photoelectron spectroscopic (XPS) measurements, indicating that the synthetic effect should play a vital role for OER enhancement (Figure 3). As shown in Figure 3A, both E-CoSe2 and CoSe2@DG samples exhibited four characteristic peaks corresponding to the cubic CoSe2 (Campos et al., 2002). Notably, the Raman peak at ∼660 cm−1 shifted to lower wave number after incorporating CoSe2 with DG, suggesting the charge transfer between CoSe2 nanosheets and DG substrates, which resembled previous observation in the case of CeO2/CoSe2 and Au cluster/CoSe2 composites (Zheng et al., 2015a, Zheng et al., 2015b, Zhao et al., 2017a, Zhao et al., 2017b). In addition, we also compared the Raman spectra of DG and CoSe2@DG and found that the characteristic D band (1,344 cm−1) and G band (1,574 cm−1) became much weaker after anchoring CoSe2 on DG (Figure S14) (Banhart et al., 2011). This change can be ascribed to the relatively low amount of DG in the CoSe2@DG composite (prepared in the mass ratio of 9:1 for CoSe2 and DG as depicted above). To further confirm the interaction between CoSe2 layer and defects on DG, XPS of E-CoSe2 and CoSe2@DG were carried out. The Co3+ cations were generally believed to be the real reason for the decent OER activity, which could facilitate the formation of O−OH (key intermediates in OER) and then further be oxidized to produce O2 (Chen et al., 2016, Yeo and Bell, 2011). As shown in the high-resolution Co 2p spectra, there existed obvious characteristic peaks of Co2+ and Co3+ cations, which was in accordance with that of CoSe2 materials reported previously (Figure 3B) (Li et al., 2017). Of note, the electron binding energy of Co 2p in CoSe2@DG composites showed an ∼0.4 eV increase compared with pure E-CoSe2, which indicated the effective electron transfer from CoSe2 to DG (Gao et al., 2012, Zheng et al., 2015a, Zheng et al., 2015b). Both Raman spectra and XPS results demonstrated that the synergetic effect between CoSe2 and DG could change the electron configuration in the composites, which consequently promoted their OER performance. Moreover, perhaps someone will wonder whether the hydrothermal process has positive influence on the CoSe2 nanosheets for the improved OER activity of final CoSe2@DG composites. To exclude it, hydrothermal-treated CoSe2 (H-CoSe2) without DG was also prepared (Figure S15). LSV curves illustrated that H-CoSe2 showed decreased OER activity than original E-CoSe2, further proving that the synergetic effect of CoSe2 and DG was the cause of the enhanced OER performance for CoSe2@DG composites.
Figure 3.
Study of Interaction between CoSe2 and DG in the Composite Catalysts
(A and B) Raman spectra (A) and high-resolution Co 2p XPS (B) of exfoliated CoSe2 nanosheets and CoSe2@DG composites.
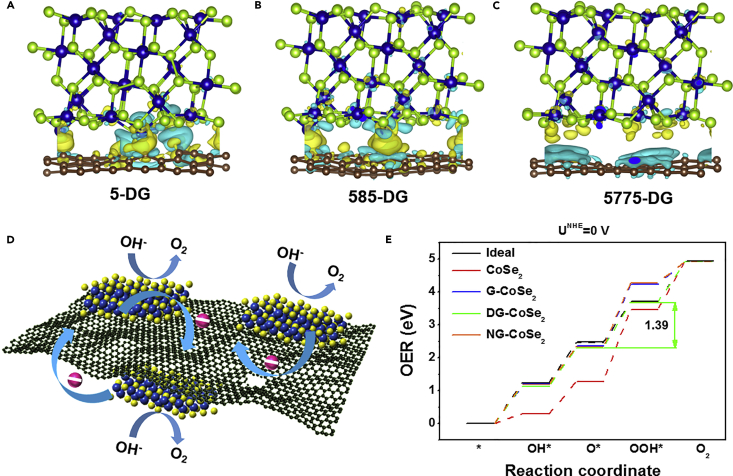
Based on above experimental data, we have attributed the enhanced OER activity after coupling CoSe2 nanosheets and DG to the effective charge transfer from CoSe2 layer to DG. To further demonstrate it, we conducted a series of DFT calculations (see computational details in the Supplemental Information). In a previous study, we have confirmed that different defect types (denoted as DG-5, DG-585, and DG-5775 defect) on DG serve as highly efficient catalytically active sites (Jia et al., 2016). Therefore, we chose the three defect types as the computational models of DG in this part. Figures 4A–4C and S16 showed the geometries of the fully relaxed CoSe2@DG-5, CoSe2@DG-585, CoSe2@DG-5775, CoSe2@NG, and CoSe2@G composites in side view and their charge density differences. It could be seen clearly that the electrons were inclined to redistribute around the 5 and 585 defect sites, resulting in the charge depletion on CoSe2 layer, which favors OER (Figure 4D). In contrast, the electron transfer in the other three models was negligible. The calculation results on the charge transfer between the two components were consistent with our experimental data. Moreover, we also calculated the Gibbs free energy differences for OER occurring on CoSe2@DG, CoSe2@NG, CoSe2@G composites, and pristine CoSe2 (Figure 4E). The ideal barrier (1.23 eV) for each step was also included for comparison (black thick line). The overpotential was determined by the largest Gibbs free energy barrier. For the pristine CoSe2, CoSe2@G, CoSe2@DG, and CoSe2@NG composites, the third step (O∗ is transformed to OOH∗) is the rate-determining step. As observed, the CoSe2@DG composite possessed the lowest Gibbs free energy with a barrier of 1.39 eV among the models, indicating its best OER activity. Both experimental and theoretical results demonstrate that the charge depletion on CoSe2 induced by the electron transfer from CoSe2 to DG is the cause of the outstanding OER performance of the CoSe2@DG catalysts.
Figure 4.
Understanding of OER Catalytic Mechanism on CoSe2@DG Catalysts
(A–C) The side views of 3D charge density difference plot for the interfaces between a defective graphene sheet [DG-5 (A), DG-585 (B), DG-5775 (C)] and CoSe2 layer are calculated by DFT. Yellow and cyan isosurfaces represent charge accumulation and depletion in the 3D space with an isosurface value of 0.002 e Å−3. Brown, green, and blue balls represent C, Se, and Co atoms, respectively.
(D) The schematic of the probable OER mechanism of CoSe2@DG composites is presented based on the DFT calculation results. The pink spheres represent electrons.
(E) The free energy diagram for oxygen evolution on pristine CoSe2, CoSe2@G, CoSe2@DG, and CoSe2@NG composites. The ideal catalyst with a barrier of 1.23 eV for each step is also included for comparison (black thick line).
Discussion
In summary, we have successfully grafted exfoliated CoSe2 nanosheets onto the DG to prepare a new CoSe2@DG composite by a hydrothermal process. The hybrid catalyst displays excellent OER performance with a small overpotential of ∼270 mV at a current density of 10 mA cm−2, large anodic current density of ∼129 mA cm−2 at an overpotential of 350 mV, and good durability in alkaline solution, which is superior to most reported CoSe2-based OER catalysts. Based on our experimental analysis and DFT calculations, the improved OER activity can be attributed to the synergistic effect (charge redistribution) between CoSe2 nanosheets and DG substrates. This work will raise promising possibilities for designing advanced OER electrocatalysts via synergistically coupling nonprecious metal-based materials with DG, which is highly desirable for energy conversion technologies.
Limitations of Study
This work proposes a promising avenue to enhance catalytic performance by effective hybridization of carbon defects and foreign materials. Although demonstrated theoretically, the fabrication of a certain type of carbon defect is now experimentally unavailable, limiting the further optimization of catalytic energetics for such composite catalysts.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors appreciate the funding support from the National Natural Science Foundation of China (Grants 51732011, 21431006), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (Grant 21521001), Key Research Program of Frontier Sciences, CAS (Grant QYZDJSSW-SLH036), the National Basic Research Program of China (Grant 2014CB931800), the Users with Excellence and Scientific Research Grant of Hefei Science Center of CAS (2015HSC-UE007, 2015SRG-HSC038), the Australian Research Council (ARC DP170103317), the China Postdoctoral Science Foundation (Grant BH2060000037), and the Fundamental Research Funds for the Central Universities (Grant WK2060190057), the authors also would like to thank the Australian National Fabrication Facility (ANFF-Q) for the AFM test.
Author Contributions
X.W. and L.Zhuang contributed equally to this work. X.W., L.Zhuang, X.Yao, and S.Y. conceived and designed the experiments. M.G., Z.Z., X.Yao, and S.H.Y. supervised the project. X.W., L.Zhuang, Y.J., L.Zhang, and X.Yan performed the synthesis experiments, material characterization, and OER measurement. T.H. and A.D. performed the DFT calculation. All authors discussed the results and commented on the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: September 28, 2018
Footnotes
Supplemental Information includes Transparent Methods, 16 figures, and 1 table and can be found with this article online at https://doi.org/10.1016/j.isci.2018.08.013.
Contributor Information
Xiangdong Yao, Email: x.yao@griffith.edu.au.
Shu-Hong Yu, Email: shyu@ustc.edu.cn.
Supplemental Information
References
- Banhart F., Kotakoski J., Krasheninnikov A.V. Structural defects in graphene. ACS Nano. 2011;5:26–41. doi: 10.1021/nn102598m. [DOI] [PubMed] [Google Scholar]
- Campos C.E.M., de Lima J.C., Grandi T.A., Machado K.D., Pizani P.S. Structural studies of cobalt selenides prepared by mechanical alloying. Physica B. 2002;324:409–418. [Google Scholar]
- Chen W., Liu Y.Y., Li Y.Z., Sun J., Qiu Y.C., Liu C., Zhou G.M., Cui Y. In situ electrochemically derived nanoporous oxides from transition metal dichalcogenides for active oxygen evolution catalysts. Nano Lett. 2016;16:7588–7596. doi: 10.1021/acs.nanolett.6b03458. [DOI] [PubMed] [Google Scholar]
- Dau H., Limberg C., Reier T., Risch M., Roggan S., Strasser P. The mechanism of water oxidation: from electrolysis via homogeneous to biological catalysis. ChemCatChem. 2010;2:724–761. [Google Scholar]
- Fabbri E., Nachtegaal M., Cheng X., Schmidt T.J. Superior bifunctional electrocatalytic activity of Ba0.5Sr0.5Co0.8Fe0.2O3-δ/Carbon composite electrodes: insight into the local electronic structure. Adv. Energy Mater. 2015;5:1402033. [Google Scholar]
- Fang L., Li W., Guan Y., Feng Y., Zhang H., Wang S., Wang Y. Tuning unique peapod-like Co(SxSe1–x)2 nanoparticles for efficient overall water splitting. Adv. Funct. Mater. 2017 [Google Scholar]
- Gao M.-R., Yao W.-T., Yao H.-B., Yu S.-H. Synthesis of unique ultrathin lamellar mesostructured CoSe2−amine (protonated) nanobelts in a binary solution. J. Am. Chem. Soc. 2009;131:7486–7487. doi: 10.1021/ja900506x. [DOI] [PubMed] [Google Scholar]
- Gao M.-R., Xu Y.-F., Jiang J., Zheng Y.-R., Yu S.-H. Water oxidation electrocatalyzed by an efficient Mn3O4/CoSe2 nanocomposite. J. Am. Chem. Soc. 2012;134:2930–2933. doi: 10.1021/ja211526y. [DOI] [PubMed] [Google Scholar]
- Gao M.-R., Cao X., Gao Q., Xu Y.-F., Zheng Y.-R., Jiang J., Yu S.-H. Nitrogen-doped graphene supported CoSe2 nanobelt composite catalyst for efficient water oxidation. ACS Nano. 2014;8:3970–3978. doi: 10.1021/nn500880v. [DOI] [PubMed] [Google Scholar]
- Gao M.-R., Zheng Y.-R., Jiang J., Yu S.-H. Pyrite-type nanomaterials for advanced electrocatalysis. Acc. Chem. Res. 2017;50:2194–2204. doi: 10.1021/acs.accounts.7b00187. [DOI] [PubMed] [Google Scholar]
- Hao J., Yang W., Peng Z., Zhang C., Huang Z., Shi W. A nitrogen doping method for CoS2 electrocatalysts with enhanced water oxidation performance. ACS Catal. 2017;7:4214–4220. [Google Scholar]
- Hunter B.M., Gray H.B., Müller A.M. Earth-abundant heterogeneous water oxidation catalysts. Chem. Rev. 2016;116:14120–14136. doi: 10.1021/acs.chemrev.6b00398. [DOI] [PubMed] [Google Scholar]
- Jia Y., Zhang L., Du A., Gao G., Chen J., Yan X., Brown C.L., Yao X. Defect graphene as a trifunctional catalyst for electrochemical reactions. Adv. Mater. 2016;28:9532–9538. doi: 10.1002/adma.201602912. [DOI] [PubMed] [Google Scholar]
- Jia Y., Zhang L., Gao G., Chen H., Wang B., Zhou J., Soo M.T., Hong M., Yan X., Qian G. A heterostructure coupling of exfoliated Ni–Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting. Adv. Mater. 2017 doi: 10.1002/adma.201700017. [DOI] [PubMed] [Google Scholar]
- Kong D., Wang H., Lu Z., Cui Y. CoSe2 nanoparticles grown on carbon fiber paper: an efficient and stable electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2014;136:4897–4900. doi: 10.1021/ja501497n. [DOI] [PubMed] [Google Scholar]
- Li Y., Dai H. Recent advances in zinc-air batteries. Chem. Soc. Rev. 2014;43:5257–5275. doi: 10.1039/c4cs00015c. [DOI] [PubMed] [Google Scholar]
- Li W., Gao X., Xiong D., Wei F., Song W.-G., Xu J., Liu L. Hydrothermal synthesis of monolithic Co3Se4 nanowire electrodes for oxygen evolution and overall water splitting with high efficiency and extraordinary catalytic stability. Adv. Energy Mater. 2017 [Google Scholar]
- Liang L., Cheng H., Lei F., Han J., Gao S., Wang C., Sun Y., Qamar S., Wei S., Xie Y. Metallic single-unit-cell orthorhombic cobalt diselenide atomic layers: robust water-electrolysis catalysts. Angew. Chem. Int. Ed. 2015;54:12004–12008. doi: 10.1002/anie.201505245. [DOI] [PubMed] [Google Scholar]
- Liu Y., Cheng H., Lyu M., Fan S., Liu Q., Zhang W., Zhi Y., Wang C., Xiao C., Wei S. Low overpotential in vacancy-rich ultrathin CoSe2 nanosheets for water oxidation. J. Am. Chem. Soc. 2014;136:15670–15675. doi: 10.1021/ja5085157. [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhao Z., Wang Y., Dou S., Yan D., Liu D., Xia Z., Wang S. In situ exfoliated, edge-rich, oxygen-functionalized graphene from carbon fibers for oxygen electrocatalysis. Adv. Mater. 2017 doi: 10.1002/adma.201606207. [DOI] [PubMed] [Google Scholar]
- Masa J., Xia W., Sinev I., Zhao A., Sun Z., Grützke S., Weide P., Muhler M., Schuhmann W. MnxOy/NC and CoxOy/NC nanoparticles embedded in a nitrogen-doped carbon matrix for high-performance bifunctional oxygen electrodes. Angew. Chem. Int. Ed. 2014;53:8508–8512. doi: 10.1002/anie.201402710. [DOI] [PubMed] [Google Scholar]
- Suen N.-T., Hung S.-F., Quan Q., Zhang N., Xu Y.-J., Chen H.M. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem. Soc. Rev. 2017;46:337–365. doi: 10.1039/c6cs00328a. [DOI] [PubMed] [Google Scholar]
- Suntivich J., May K.J., Gasteiger H.A., Goodenough J.B., Shao-Horn Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science. 2011;334:1383–1385. doi: 10.1126/science.1212858. [DOI] [PubMed] [Google Scholar]
- Tang C., Zhang Q. Nanocarbon for oxygen reduction electrocatalysis: dopants, edges, and defects. Adv. Mater. 2017 doi: 10.1002/adma.201604103. [DOI] [PubMed] [Google Scholar]
- Tang C., Wang H.-F., Chen X., Li B.-Q., Hou T.-Z., Zhang B., Zhang Q., Titirici M.-M., Wei F. Topological defects in metal-free nanocarbon for oxygen electrocatalysis. Adv. Mater. 2016;28:6845–6851. doi: 10.1002/adma.201601406. [DOI] [PubMed] [Google Scholar]
- Wan S., Qi J., Zhang W., Wang W., Zhang S., Liu K., Zheng H., Sun J., Wang S., Cao R. Hierarchical Co(OH)F superstructure built by low-dimensional substructures for electrocatalytic water oxidation. Adv. Mater. 2017 doi: 10.1002/adma.201700286. [DOI] [PubMed] [Google Scholar]
- Xia B.Y., Yan Y., Li N., Wu H.B., Lou X.W., Wang X. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy. 2016;1:15006. [Google Scholar]
- Xu X., Song F., Hu X. A nickel iron diselenide-derived efficient oxygen-evolution catalyst. Nat. Commun. 2016;7:12324. doi: 10.1038/ncomms12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Jia Y., Chen J., Zhu Z., Yao X. Defective-activated-carbon-supported Mn–Co nanoparticles as a highly efficient electrocatalyst for oxygen reduction. Adv. Mater. 2016;28:8771–8778. doi: 10.1002/adma.201601651. [DOI] [PubMed] [Google Scholar]
- Yan D., Li Y., Huo J., Chen R., Dai L., Wang S. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions. Adv. Mater. 2017 doi: 10.1002/adma.201606459. [DOI] [PubMed] [Google Scholar]
- Yeo B.S., Bell A.T. Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2011;133:5587–5593. doi: 10.1021/ja200559j. [DOI] [PubMed] [Google Scholar]
- Zhao S., Jin R., Abroshan H., Zeng C., Zhang H., House S.D., Gottlieb E., Kim H.J., Yang J.C., Jin R. Gold nanoclusters promote electrocatalytic water oxidation at the nanocluster/CoSe2 interface. J. Am. Chem. Soc. 2017;139:1077–1080. doi: 10.1021/jacs.6b12529. [DOI] [PubMed] [Google Scholar]
- Zhao X., Zhang H., Yan Y., Cao J., Li X., Zhou S., Peng Z., Zeng J. Engineering the electrical conductivity of lamellar silver-doped cobalt(ii) selenide nanobelts for enhanced oxygen evolution. Angew. Chem. Int. Ed. 2017;56:328–332. doi: 10.1002/anie.201609080. [DOI] [PubMed] [Google Scholar]
- Zheng Y.-R., Gao M.-R., Gao Q., Li H.-H., Xu J., Wu Z.-Y., Yu S.-H. An efficient CeO2/CoSe2 nanobelt composite for electrochemical water oxidation. Small. 2015;11:182–188. doi: 10.1002/smll.201401423. [DOI] [PubMed] [Google Scholar]
- Zheng Y.-R., Gao M.-R., Yu Z.-Y., Gao Q., Gao H.-L., Yu S.-H. Cobalt diselenide nanobelts grafted on carbon fiber felt: an efficient and robust 3d cathode for hydrogen production. Chem. Sci. 2015;6:4594–4598. doi: 10.1039/c5sc01335f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T., Du Y., Yin S., Tian X., Yang H., Wang X., Liu B., Zheng H., Qiao S., Xu R. Nitrogen-doped cobalt phosphate@nanocarbon hybrids for efficient electrocatalytic oxygen reduction. Energy Environ. Sci. 2016;9:2563–2570. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.