Abstract
Background: The prevalence and impact of allergic and immune-mediated food disorders in pediatric acute-onset neuropsychiatric syndrome (PANS) are mostly unknown.
Objective: We sought to explore the prevalence of atopic dermatitis (AD), asthma, allergic rhinitis (AR), IgE-mediated food allergies (FAs), and other immune-mediated food disorders requiring food avoidance in patients with PANS. In addition, to further understand the extent of food restriction in this population, we investigated the empiric use of dietary measures to improve PANS symptoms.
Methods: Pediatric patients in a PANS Clinic and Research Program were given surveys regarding their caregiver burdens, allergic and food-related medical history, and whether food elimination resulted in perception of improvement of PANS symptoms. A review of health records was conducted to confirm that all responses in the survey were concordant with documentation of each patient's medical chart.
Results: Sixty-nine (ages 4–20 years) of 80 subjects who fulfilled PANS criteria completed the surveys. Thirteen (18.8%) had AD, 11 (15.9%) asthma, 33 (47.8%) AR, 11 (15.9%) FA, 1 (1.4%) eosinophilic gastrointestinal disorders, 1 (1.4%) food protein-induced enterocolitis syndrome, 3 (4.3%) milk protein-induced proctocolitis syndrome, and 3 (4.3%) celiac disease. Thirty subjects (43.5%) avoided foods due to PANS; elimination of gluten and dairy was most common and was associated with perceived improvement of PANS symptoms (by parents). This perceived improvement was not confirmed with objective data.
Conclusions: The prevalence of allergic and immune-mediated food disorders in PANS is similar to the general population as reported in the literature, with the exception of AR that appears to be more prevalent in our PANS cohort. More research will be required to establish whether diet or allergies influence PANS symptoms.
Keywords: : allergies, celiac, elimination, food, immunologic, pediatric neuropsychiatric disorder
Introduction
Pediatric acute-onset neuropsychiatric syndrome (PANS) is a disorder characterized by an abrupt onset of obsessive-compulsive disorder (OCD), restricted food intake, and other neuropsychiatric disturbances.1,2 Symptoms can be severe and exert a heavy burden on the entire family. The pathogenesis of PANS is not well understood, although it is postulated, for certain subtypes, to occur secondary to lymphocytic responses against microbial pathogens that are autoreactive to the basal ganglia and the surrounding brain tissues.3–5 Many patients with PANS have concurrent autoimmune/inflammatory diseases, with the most common being arthritis (postinfectious, enthesitis-related, psoriatic, or spondyloarthritis) and autoimmune thyroiditis.6 These patients have higher antinuclear antibodies, antihistone antibodies, and antithyroid antibodies than expected in the general population.6 Symptoms appear to improve from anti-inflammatory and immunosuppressive therapies.7–11 A recent experimental model found that sera from children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS), a subtype of PANS, bound cholinergic interneurons significantly higher than the <50% binding seen with matched healthy controls.12 These observations support the notion that exaggerated immune responses play a pathophysiologic role.2,4–13 However, there appears to be a wide heterogeneity of the disease. For instance, a subgroup of patients can develop this disorder and/or relapse during or after infections, such as acute sinusitis or Streptococcal tonsillitis.14,15 Antimicrobial treatment has been observed to alleviate symptoms in some patients with PANS,14,16,17 but the use of antimicrobials in this patient population remains controversial. The underlying cause of PANS and potential triggers are not fully understood.
Currently, the impact and occurrence of TH2-mediated allergic diseases in the PANS population are largely unknown. Characterizing the prevalence of allergic diseases is important since these conditions likely further augment the morbidity and psychosocial burden on patients with PANS.
Patients with PANS have been reported to restrict food intake for various reasons, among them dysphagia, fear of choking or vomiting, rejection of certain flavors, smells or textures, obsessive compulsive symptoms such as fear of spoiled, contaminated, or poisoned food, and body image concerns. These patients and their families have also reported restricting intake of certain foods when they have felt that those foods seemed to have led to exacerbations of psychiatric symptoms.13 Such deteriorations after food intake can occur in other psychiatric conditions including anxiety, bipolar disorder, and autism spectrum disorder (ASD).17,18 Food reactions associated with arthritis flares and escalation of PANS symptoms have been reported13 but are likely rare. The mechanisms by which a food protein leads to inflammatory reactions and subsequent neuropsychiatric symptoms are not known at this time. Furthermore, the effects of dietary modification in neuropsychiatric disorders are unknown and highly controversial.19
Owing to the severity of psychiatric symptoms, and burden on the family, many families/clinicians empirically try food elimination diets. Unfortunately, implementation of dietary limitation itself may have widespread negative implications, including nutritional deficiencies, neurocognitive and growth impairments, and reductions in quality of life. Food elimination in patients with PANS is especially concerning since it can compound food-restrictive behaviors already present in patients with PANS.20 Furthermore, concern over food triggers may further escalate food-avoidance behaviors, compounding the risk of coexisting immune-mediated food disorders such as food allergies (FAs) that can result in anaphylaxis, eczema exacerbations triggered by certain foods, eosinophilic gastrointestinal disorders including eosinophilic esophagitis, and celiac disease (CD).20
Therefore, this study aims to characterize the practice of food elimination diets among patients with PANS, as well as the prevalence of allergic diseases and immune-mediated food disorders. We hypothesize that an earlier age of onset of PANS, a high caregiver burden, and multiple IgE-mediated FAs may increase the odds of parents instituting empiric food elimination diets to improve their child's PANS symptoms.
Methods
Study population and the survey
Patients who were <21 years of age and met PANS criteria were recruited to this study.1 All aspects of the study were authorized by the Stanford University School of Medicine Institutional Review Board (Stanford, CA). Subjects and respective guardians were fully informed of the objectives of our research and patient care clinic and the risks involved in sharing their confidential private health information among our institution's Health Insurance Portability and Accountability Act-trained staff before signing assent and consent forms. Patients with coexisting neurobehavioral disorders, including ASD, were excluded. Detailed medical history, psychiatric history, and physical examination were performed to verify health status and study inclusion. The subjects and their parents also filled out a questionnaire during their initial clinic visit day to assess their overall caregiver burden (Supplementary File SI; Supplementary Data available online at www.libertpub.com/ped).21 Subsequently, they were sent a confidential online survey secured by encryption and programmed with branching logic to obtain a thorough history of physician-diagnosed allergic diseases and immune-mediated food disorders (Supplementary File SII). Parents were asked whether elimination of foods from the diet was attempted and whether they believed this led to improvement of PANS symptoms. E-mail invitations to complete the survey were initially sent with weekly reminders for 6 months.
Nonresponders and chart review
For patients who were sent but did not complete the survey, an extensive medical chart review was performed using our electronic health record (EHR) system to obtain information regarding history of allergic diseases, food disorders, and food elimination patterns diagnosed by physicians in our group, their primary physicians, allergists, and gastroenterologists. EHRs from both Stanford and community providers were included in the review (using the Care Everywhere functionality of our Epic EHR system). Data regarding parental perception of the effects of food elimination for PANS were only available through the survey.
Review of survey responses
After completion, each subject's survey was reviewed by a licensed allergist to ensure congruence of responses. If certain responses appeared equivocal, subjects were contacted, and further chart review was conducted for clarification before finalization of each survey.
Statistical analysis
Continuous demographic information is presented as group medians and 95th confidence, which were compared using the Mann–Whitney U test. Frequencies are shown in proportions and compared using the Fisher's exact test. Stepwise multivariate logistic regression was performed with the forward selection method and food elimination as the dependent variable. P < 0.05 between groups was considered significant. Statistical procedures were completed using Stata 14 (StataCorp LP, College Station, TX) and JMP (SAS Institute, Cary, NC) software applications.
Results
Participant features
Eighty children initially fulfilled the inclusion criteria and were sent the survey (Table 1). Sixty-nine subjects completed the survey, while 11 did not, resulting in an 86.3% response rate. To check for the possibility of nonresponse bias, the following variables were evaluated, and no group differences were found between those who completed the survey versus those who did not: demographic characteristics, allergic history, and caregiver burden inventory (CGBI) scores (Table 1). The remainder of the results in this study presents the detailed responses provided by the 69 participants who completed the survey.
Table 1.
Clinical Characteristics of All 80 Subjects Given the Survey
| All (n = 80) | Completed surveys (n = 69) | Not completed surveys (n = 11) | P | |
|---|---|---|---|---|
| Age (years) | 12.0 (5.3–19.6) | 12.0 (5.1–19.8) | 11.8 (6.7–17.0) | 0.900 |
| Gender (F/M) | 32/48 (40.0%/60.0%) | 27/42 (39.1%/60.9%) | 5/6 (45.5%/54.5%) | 0.747 |
| Age of onset of PANS (years) | 8.0 (2.1–15.5) | 7.6 (1.7–15.8)a | 9.6 (4.5–13.7) | 0.403 |
| CGBI score | 37 (0.3–78.9) | 37 (0–81) | 26 (1–70) | 0.520 |
| History of IgE-mediated FAs | 12 (15.0%) | 11 (15.9%) | 1 (9.1%) | 1.000 |
| Prescribed epinephrine autoinjector | 8 (9.9%) | 7 (10.1%) | 1 (9.1%) | 1.000 |
| History of AD | 34 (42.5%) | 32 (46.4%) | 2 (18.2%) | 0.106 |
| History of asthma | 18 (22.5%) | 16 (23.2%) | 2 (18.2%) | 1.000 |
| History of AR | 44 (55.0%) | 39 (56.5%) | 5 (45.5%) | 0.531 |
| History of any of the mentioned allergic diseases | 61 (76.3%) | 55 (79.7%) | 6 (54.5%) | 0.120 |
| History of food elimination for PANS | 32 (40.0%) | 30 (43.5%) | 2 (18.2%) | 0.185 |
Data are medians and 95% interpercentile ranges, or proportions (percentages).
Data available for 66 subjects.
AD, atopic dermatitis; AR, allergic rhinitis; CGBI, caregiver burden inventory; F, female; FAs, food allergies; M, male; PANS, pediatric acute-onset neuropsychiatric syndrome.
Prevalence of atopic dermatitis, asthma, and allergic rhinitis in PANS
Of the 32 subjects (46.4%) who had a history of atopic dermatitis (AD), 9 (28.1%) subjects reported that foods seemed to trigger or exacerbate the AD (Tables 1 and 2). Of the 19 (59.4%) of patients whose AD resolved, the median age of onset of AD was 1.0 [95% interpercentile range (IPR): 0–5.0] years old, and median age of resolution was 5.0 years (95% IPR: 0.6–12.0), giving a duration of AD of 4.0 (95% IPR: 0.6–11.0) years. Thirteen of 69 subjects (18.8%) had ongoing AD, and foods were a trigger of AD flares in 2 (2.9%) of these participants.
Table 2.
Allergic Characteristics of the 69 Subjects Who Completed the Survey
| Completed questionnaires (n = 69) | |
|---|---|
| Age of onset of AD (years) (n = 32) | 2.0 (0–10.0) |
| Subjects with AD exacerbated by foods | 9/32 (28.1%) |
| Subjects whose AD resolved | 19/32 (59.4%) |
| Age of resolution of AD (years) (n = 19) | 5.0 (0.6–12.0) |
| Duration of AD (years) (n = 19) | 4.0 (0–11) |
| Subjects with ongoing AD | 13/69 (18.8%) |
| Subjects with ongoing AD exacerbated by foods | 2/69 (2.9%) |
| Age of onset of asthma (years) (n = 16) | 3.5 (0.6–8.0) |
| Subjects whose asthma resolved | 5/16 (31.3%) |
| Age of resolution of asthma (years) (n = 5) | 6.0 (3.0–10.0) |
| Duration of asthma (years) (n = 5) | 5.0 (2.0–9.0) |
| Subjects with ongoing asthma | 11/69 (15.9%) |
| Age of onset of AR (years) | 4.0 (0–10.0) |
| Subjects whose AR resolved | 6/69 (15.4%) |
| Age of resolution of AR (years) (n = 6) | 10.0 (8.0–17.0) |
| Duration of AR (years) (n = 6) | 9.0 (7.0–16.0) |
| Subjects with ongoing AR | 33/69 (47.8%) |
Data are medians and 95% interpercentile ranges, or proportions (percentages).
AD, atopic dermatitis; AR, allergic rhinitis.
Of the 16 subjects (23.2%) who had a history of asthma, the median age of onset was 3.5 (95% IPR: 0.6–8.0) years old. Asthma resolved for 5 participants (31.3%) at a median age of 6.0 (95% IPR: 3.0–10.0) years old, and the duration of asthma was 5.0 (95% IPR: 2.0–9.0) years. Eleven of 69 subjects (15.9%) had ongoing asthma.
Thirty-nine participants (56.5%) had a history of allergic rhinoconjunctivitis (AR). The median age of onset of AR was 4.0 (95% IPR: 0.0–6.0) years old. For 6 subjects (15.4%), the AR lasted for a median duration of 9.0 (95% IPR: 7.0–16.0) years such that the median age of resolution was 10.0 (95% IPR: 8.0–17.0) years, and 33 of 69 subjects (47.8%) had ongoing AR.
Prevalence of immune-mediated food disorders in patients with PANS
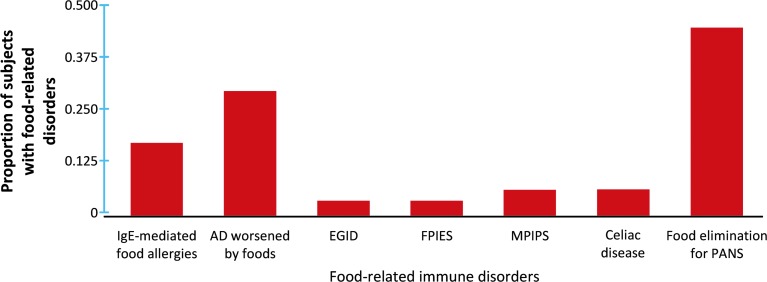
Out of 69 participants, 11 subjects (15.9%) had a history of IgE-mediated FAs and 7 subjects (10.1%) were carrying a prescribed epinephrine autoinjector, 1 (1.4%) had eosinophilic esophagitis, 1 (1.4%) had food protein-induced enterocolitis syndrome, 3 (4.3%) had milk protein-induced proctocolitis syndrome (MPIPS), and 3 (4.3%) were had CD requiring adherence to a gluten-free diet (Table 1 and Fig. 1). To further understand the impact of foods in this population, patients were asked whether they were voluntarily avoiding foods in an attempt to improve their PANS symptoms, and 30 subjects (43.5%) reported that they were indeed implementing such dietary modifications due to parental concerns that foods may worsen PANS symptoms. The prevalence of these disorders was not mutually exclusive and there were several patients who had different combinations of these food disorders at the same time.
FIG. 1.
Distribution of subjects avoiding foods due to an immune-mediated food disorder and self-imposed food elimination trial. Immunoglobulin E, IgE; AD, atopic dermatitis; EGID, eosinophilic gastrointestinal disorders including eosinophilic esophagitis; FPIES, food protein-induced enterocolitis syndrome; MPIPS, milk protein-induced protocolitis syndrome; PANS, pediatric acute-onset neuropsychiatric syndrome.
Types of foods eliminated from the diet in patients with PANS
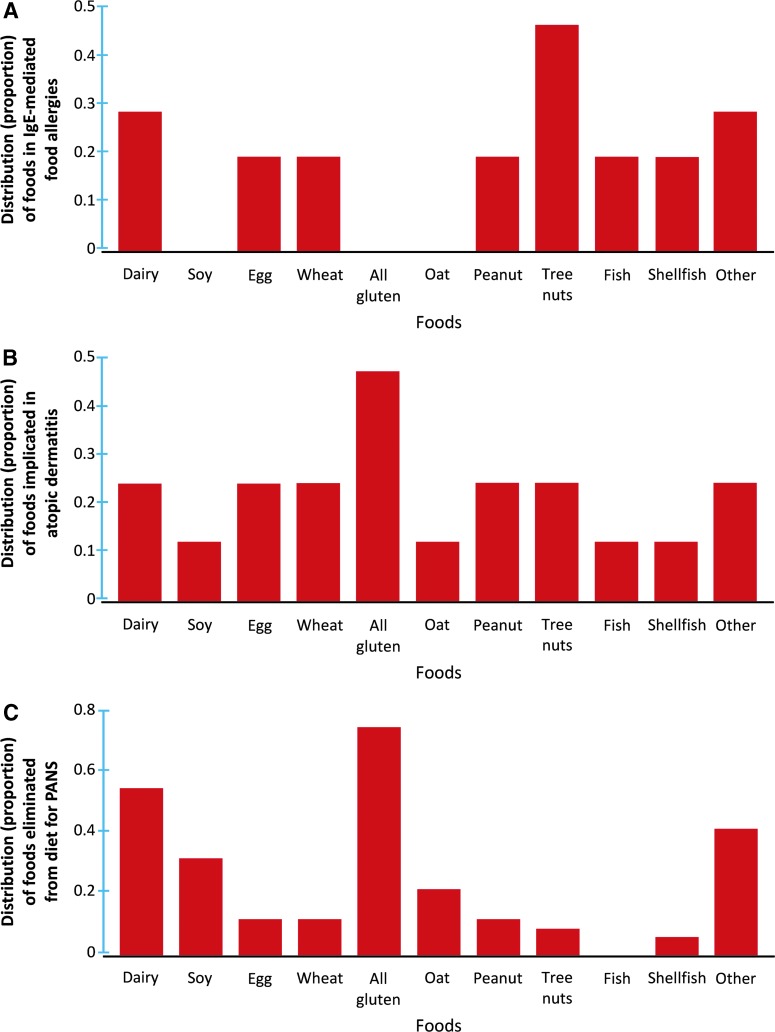
Out of 11 subjects with a history of IgE-mediated FAs, the most common food allergens involved were tree nuts (n = 5, 45.5%), followed by dairy (n = 3, 27.2%), wheat only (n = 2, 18.2%), peanut (n = 2, 18.2%), egg (n = 2, 18.2%), fish (n = 2, 18.2%), shellfish (n = 2, 18.2%), and other foods (sesame, banana, and celery; n = 3, 27.3%) (Fig. 2A). A wide gamut of other foods was suspected to worsen AD for the 9 participants with AD, most commonly all gluten-containing products (n = 4, 44.4%) (Fig. 2B). It is important to note that several patients had variable combinations of foods associated with their FA and AD.
FIG. 2.
Distributions of foods removed from the diet due to IgE-mediated FAs (A), exacerbation of AD (B), and impact on PANS symptoms (C). Immunoglobulin E, IgE; AD, atopic dermatitis; FAs, food allergies; PANS, pediatric acute-onset neuropsychiatric syndrome.
For the 30 participants who were empirically eliminating foods from their diets in an attempt to improve PANS symptoms, the most common foods avoided were all gluten-containing products (n = 22, 73.3%), followed by dairy (n = 16, 53.3%), other foods (most commonly food additives, sugars, and corn; n = 12, 40.0%), soy (n = 9, 30.0%), oat only (n = 6, 20.0%) (the other grains such as barley and rye were not individually avoided), peanut (n = 3, 10.0%), egg (n = 3, 10.0%), wheat only (n = 3, 10.0%), tree nuts (n = 2, 6.7%), and shellfish (n = 1, 3.3%) (Fig. 2C).
In total, 38 subjects (55.1%) had food-restrictive behaviors as a part of their PANS symptoms. Eleven of these 38 (15.9% of all participants) were also diagnosed with an immune-mediated food disorder requiring further food avoidance, whereas 16 of 38 (23.2% of all participants) empirically eliminated foods. Seven subjects (10.1% of all participants) with disordered eating as a component of their PANS had additional immune-mediated food disorder and limited their diets empirically. There was no association between pre-existing immune-mediated food issues with PANS-related food-restrictive behaviors.
Perceived improvement from food elimination
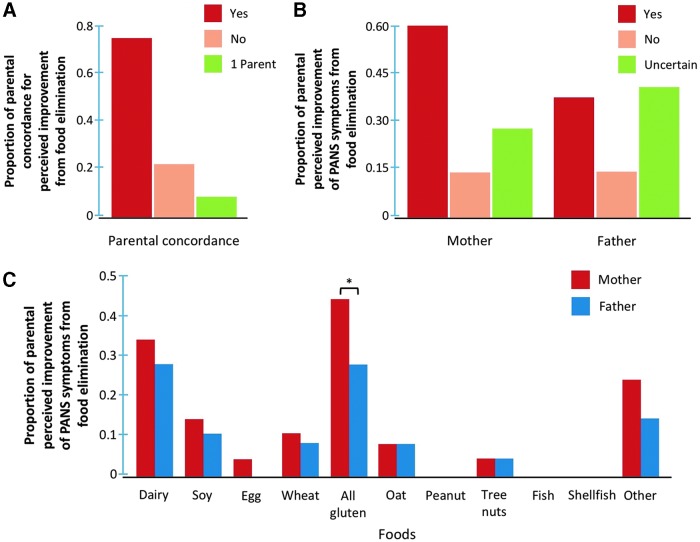
Parents were asked whether empiric elimination of foods from their child's diet improved their child's PANS symptoms. Of the 30 patients who engaged in food elimination diets, 2 subjects (6.7%) were raised by single parents who perceived that self-imposed elimination of food additives and corn appeared to have positively impacted PANS symptoms (Fig. 3A). Of the remaining 28 patients, parents of 22 patients (73.3%) had complete concordance with each other regarding their perception of improvement from avoiding foods, whereas there were disagreements among parents of 6 patients (20.0%) (Fig. 3A). Eighteen mothers (60.0%) and 11 fathers (36.7%) felt that PANS symptoms improved after food elimination, whereas 4 parents (13.3%) did not perceive any changes with dietary modifications, and 8 mothers (26.7%) and 12 fathers (40.0%) were uncertain (Fig. 3B).
FIG. 3.
The mothers and fathers of each subject were queried as to whether there was perceived improvement from their self-imposed food elimination practices, and there was complete agreement by 73.3% between parents, 20% discordance in reporting between parents, and 6.7% were raised by single parents (A, B). Distribution of foods that was followed by improvement of PANS symptoms after elimination (C). There was a significantly higher proportion of mothers reporting a positive impact from self-imposed restriction of all gluten-containing products than fathers. *P < 0.05. PANS, pediatric acute-onset neuropsychiatric syndrome.
The self-imposed elimination of all gluten-containing products or dairy from the diet was the 2 most common foods reported to have improved PANS symptoms (Fig. 3C). A significantly higher proportion of mothers (n = 13, 43.4%) believed that avoidance of all gluten-containing products reduced PANS symptoms compared with fathers (n = 8, 26.7%) (P < 0.05).
Logistic regression and odds ratios of food elimination for PANS
The relationships between early age of PANS onset, CGBI score, and IgE-mediated FAs and food elimination of any food (30 subjects) and ≥3 foods (15 subjects) were investigated using logistic regression and are presented in Table 3. Increased CBGI had a small association with elimination of any food [odds ratio (OR): 1.04, 95% confidence interval (CI): 1.01–1.07] and elimination of ≥3 foods (OR: 1.04, 95% CI: 1.04–1.07). Those who did not implement a food elimination diet had a median CGBI score of 32 (95% CI: 0–75), whereas those who avoided any food and ≥3 foods had median scores of 47 (95% CI: 0–87) and 54 (95% CI: 15–79), respectively.
Table 3.
Logistic Regression and Odds Ratios of Food Elimination for Pediatric Acute-Onset Neuropsychiatric Syndrome
| ORs and 95% CIs of any food elimination for PANS | P | ORs and 95% CIs of elimination of ≥3 foods for PANS | P | |
|---|---|---|---|---|
| Age of onset of PANSa | 0.92 (0.79–1.07) | 0.298 | 0.92 (0.77–1.09) | 0.335 |
| CGBI score | 1.04 (1.01–1.07) | 0.005b | 1.04 (1.01–1.07) | 0.022b |
| History of IgE-mediated FAs | 2.07 (0.48–8.83) | 0.328 | 1.84 (0.40–8.38) | 0.430 |
Data are ORs and 95% CIs.
Data available for 66 subjects.
P < 0.05.
CGBI, caregiver burden inventory; CI, confidence interval; FAs, food allergies; OR, odds ratio; PANS, pediatric acute-onset neuropsychiatric syndrome.
Discussion
This is the first study to investigate the prevalence of various allergic diseases and explore the impact of food elimination in PANS. Overall, we observed that the frequency of allergic disorders is similar to the general population, with the exception of AR that appears to be more prevalent in patients with PANS than in the general population. Seven of our 69 participants in our cohort, or 10.1% (95% IPR: 5%–19.5%), were carrying a prescribed epinephrine autoinjector for IgE-mediated FAs as compared with the 8% (95% CI: 7.6%–8.3%) estimated in the literature for the entire United States.22 There was 18.8% (95% IPR: 5%–19.5%: 11.4%–29.6%) with AD in our cohort versus 13.3% (95% CI: 12.7%–13.8%) for the general population as recently reported by other authors, 15.9% (95% IPR: 9.1%–26.3%) versus 9.7% (95% CI: 12.7%–13.8%) for asthma, and 47.8% (95% IPR: 36.5%–59.4%) versus 18% (95% CI: 17.7%–18.2%) for AR, respectively.23,24 These gross comparisons suggest that the proportions of patients with PANS with these allergic and immune-mediated food disorders were generally at least as high, and in the case of AR much higher, as those that have been reported in the literature for the general population.22–24 The prevalence of empiric food elimination diets in our PANS population is high—between 18% (based on chart review) and 43% (based on parent survey).
We observed a slightly but statistically significantly higher CGBI score among caregivers of patients on elimination diets than among caregivers of patients not on an elimination diet. There are 2 possibilities that explain this: (1) confounding by indication (parents caring for children who are more ill and/or not responding to therapies may be more desperate for relief and more likely to try unproven therapies) and (2) the elimination diet may add to family burden.
Although appropriate dietary adjustments can help patients with FAs, unnecessary dietary restrictions may be harmful, especially among patients who are restricting due to psychiatric symptoms. Excessive food limitations could lead to nutritional deficiencies, interfere with intellectual and physical development, and reduce quality of life.20 Patients with food avoidance due to multiple different reasons will require close monitoring for appropriate growth, and in certain situations, some children with PANS may benefit from formal consultation with a dietitian to ensure that nutritional demands are adequately met.20
Future studies implementing double-blind placebo-controlled food challenges will be needed to determine whether food elimination programs guided by healthcare professionals can be effective in modifying PANS symptoms, which are unknown at this time. Currently, findings are controversial as to whether dietary changes can help alleviate psychiatric manifestations, such as for OCD, ASD, and schizoaffective disorders.18,19,25 For those with neuropsychiatric disorders who experience an improvement from limiting certain foods, 1 proposed underlying mechanism based on animal experimental models may include the influence of foods on the gastrointestinal microbiota that can modulate circulating metabolites, hormones, and immunologic signaling pathways.18,19,25 Ultimately, these factors can alter the microbiome–gut–brain axis and affect one's mood, stress response, and other aspects of neurocognitive behaviors.18,19,25
The primary limitation of this study is that survey results were based on subjective recounting of events by patients and families at 1 moment in time rather than following objective findings longitudinally, although the data were strengthened by subsequent medical chart review to minimize self-reporting bias. There was also a lack of a non-PANS-matched control group and this study only used indirect gross comparisons with prevalence data previously reported in the literature. Further prospective studies of allergic diseases in patients with PANS, particularly laboratory or clinical evaluation of food sensitivities and/or allergies, complemented by a control group without PANS, will be needed to confirm the findings from this study.26
Although the parents of 36.7%–60% of the patients engaged in food elimination diets believed that food elimination helped to alleviate their child's PANS symptoms, the parents of 26.7%–40.0% of patients were not certain whether empiric avoidance of foods was beneficial. None of the patients included in the study had IgE-mediated allergies to all glutens, oat, rye, or barley. Two out of 11 (18.2%) with IgE-mediated FAs reported that their FA was due to wheat, but whether this diagnosis was made on the basis of allergy testing, food challenges, or medical history alone is uncertain. Therefore, additional investigations should include double-blind placebo-controlled food challenges or reintroductions, the gold-standard testing modality for the diagnosis of many food disorders.26,27
In summary, the frequencies of AD, asthma, AR, and immune-mediated food disorders in patients with PANS are similar to otherwise healthy children. Many families, especially those with a higher caregiver burden score, empirically avoided foods in an attempt to improve PANS symptoms, and a substantial fraction reported subjective improvement. This finding is preliminary and will need to be confirmed using a study design to control for placebo effects. The high burden of PANS symptoms may drive clinicians and families to try food elimination diets given the paucity of treatment studies in this disorder. Our finding that a high proportion of families with a child suffering from PANS engage in a food elimination diet highlights the importance for more research in this area.
Supplementary Material
Acknowledgments
The authors thank all of the participants, the support from the community, and Joanne Cheung for her assistance on the setup of the surveys and data collection. This study was funded by the PANDAS/PANS Physician Network (PPN) Grant. Jaime S Rosa, MD, PhD, was supported by the National Institutes of Health Training Program in Adult and Pediatric Rheumatology 2 T32 AR050942-06A1.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chang K, Frankovich J, Cooperstock M, et al. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol 2015; 25:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swedo SE, Leckman JF, Rose NR. From research subgroup to clinical syndrome: modifying the PANDAS criteria to describe PANS (pediatric acute-onset neuropsychiatric syndrome). Pediatr Ther 2012; 2:1–8 [Google Scholar]
- 3.Kirvan CA, Swedo SE, Snider LA, et al. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol 2006; 179:173–179 [DOI] [PubMed] [Google Scholar]
- 4.Cutforth T, DeMille MM, Agalliu I, et al. CNS autoimmune disease after Streptococcus pyogenes infections: animal models, cellular mechanisms and genetic factors. Future Neurol 2016; 11:63–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dileepan T, Smith ED, Knowland D, et al. Group A Streptococcus intranasal infection promotes CNS infiltration by streptococcal-specific Th17 cells. J Clin Invest 2016; 126:303–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frankovich J, Thienemann M, Pearlstein J, et al. Multidisciplinary clinic dedicated to treating youth with pediatric acute-onset neuropsychiatric syndrome: presenting characteristics of the first 47 consecutive patients. J Child Adolesc Psychopharmacol 2015; 25:38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown KD, Farmer C, Freeman GM Jr., et al. Effect of early and prophylactic nonsteroidal anti-inflammatory drugs on flare duration in pediatric acute-onset neuropsychiatric syndrome: an observational study of patients followed by an academic community-based pediatric acute-onset neuropsychiatric syndrome clinic. J Child Adolesc Psychopharmacol 2017; 27:619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovacevic M, Grant P, Swedo SE. Use of intravenous immunoglobulin in the treatment of twelve youths with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Child Adolesc Psychopharmacol 2015; 25:65–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown K, Farmer C, Farhadian B, et al. Pediatric acute-onset neuropsychiatric syndrome response to oral corticosteroid bursts: an observational study of patients in an academic community-based PANS clinic. J Child Adolesc Psychopharmacol 2017; 27:629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latimer ME, L'Etoile N, Seidlitz J, et al. Therapeutic plasma apheresis as a treatment for 35 severely ill children and adolescents with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Child Adolesc Psychopharmacol 2015; 25:70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perlmutter SJ, Leitman SF, Garvey MA, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 1999; 354:1153–1158 [DOI] [PubMed] [Google Scholar]
- 12.Frick LR, Rapanelli M, Jindachomthong K, et al. Differential binding of antibodies in PANDAS patients to cholinergic interneurons in the striatum. Brain Behav Immun 2018; 69:304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frankovich J, Thienemann M, Rana S, et al. Five youth with pediatric acute-onset neuropsychiatric syndrome of differing etiologies. J Child Adolesc Psychopharmacol 2015; 25:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahony T, Sidell D, Gans H, et al. Improvement of psychiatric symptoms in youth following resolution of sinusitis. Int J Pediatr Otorhinolaryngol 2017; 92:38–44 [DOI] [PubMed] [Google Scholar]
- 15.Orlovska S, Vestergaard CH, Bech BH, et al. Association of Streptococcal throat infection with mental disorders: testing key aspects of the PANDAS hypothesis in a nationwide study. JAMA Psychiatry 2017; 74:740–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy TK, Brennan EM, Johnco C, et al. A double-blind randomized rlacebo-controlled pilot study of azithromycin in youth with acute-onset obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 2017; 27:640–651 [DOI] [PubMed] [Google Scholar]
- 17.Nadeau JM, Jordan C, Selles RR, et al. A pilot trial of cognitive-behavioral therapy augmentation of antibiotic treatment in youth with pediatric acute-onset neuropsychiatric syndrome-related obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 2015; 25:337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Severance EG, Gressitt KL, Yang S, et al. Seroreactive marker for inflammatory bowel disease and associations with antibodies to dietary proteins in bipolar disorder. Bipolar Disord 2014; 16:230–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buie T. The relationship of autism and gluten. Clin Ther 2013; 35:578–583 [DOI] [PubMed] [Google Scholar]
- 20.Sova C, Feuling MB, Baumler M, et al. Systematic review of nutrient intake and growth in children with multiple IgE-mediated food allergies. Nutr Clin Pract 2013; 28:669–675 [DOI] [PubMed] [Google Scholar]
- 21.Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist 1989; 29:798–803 [DOI] [PubMed] [Google Scholar]
- 22.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 2011; 128:e9–e17 [DOI] [PubMed] [Google Scholar]
- 23.Silverberg JI, Simpson EL, Durkin HG, et al. Prevalence of allergic disease in foreign-born American children. JAMA Pediatr 2013; 167:554–560 [DOI] [PubMed] [Google Scholar]
- 24.Silverberg JI, Braunstein M, Lee-Wong M. Association between climate factors, pollen counts, and childhood hay fever prevalence in the United States. J Allergy Clin Immunol 2015; 135:463–469 [DOI] [PubMed] [Google Scholar]
- 25.Ly V, Bottelier M, Hoekstra PJ, et al. Elimination diets' efficacy and mechanisms in attention deficit hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry 2017; 26:1067–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chinthrajah RS, Tupa D, Prince BT, et al. Diagnosis of food allergy. Pediatr Clin N Am 2015; 62:1393–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubio-Tapia A, Hill ID, Kelly CP, et al. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 2013; 108:656–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.