Abstract
Background: Prior studies suggest that the relationship between hypothyroidism and mortality is dependent on underlying cardiovascular risk. Little is known about the association of hypothyroidism with hospitalization risk, and how these associations are modified by cardiovascular status.
Methods: This study examined the association of thyroid status, defined by serum thyrotropin (TSH), with hospitalization risk among patients who received care at a large university-based tertiary care center between 1990 and 2015. Thyroid status was categorized as hypothyroidism versus euthyroidism (TSH >4.7 vs. 0.3–4.7 mIU/L, respectively). The relationship between thyroid status and hospitalization risk stratified by cardiovascular status was examined using multivariable Cox models.
Results: Among 52,856 patients who met eligibility criteria, 49,791 (94.2%) had euthyroidism and 3065 (5.8%) had hypothyroidism. In analyses stratified by congestive heart failure (CHF) status, compared to euthyroidism, hypothyroidism was associated with higher risk of hospitalization in those with CHF but slightly lower risk in those without CHF (adjusted hazard ratio [aHRs] = 1.86 [confidence interval (CI) 1.17–2.94] and HR = 0.95 [CI 0.92–0.99], respectively; p = 0.006). In sensitivity analyses accounting for death as a competing event, underlying coronary artery disease modified the hypothyroidism–hospitalization relationship, such that stronger associations were observed among those with versus without coronary artery disease. In competing risk analyses, hypothyroidism was associated with higher versus lower risk of hospitalization among those with versus without cerebrovascular disease, respectively.
Conclusions: Hypothyroidism is associated with higher hospitalization risk among patients with underlying cardiovascular disease. Future studies are needed to determine whether correction of thyroid status with replacement therapy ameliorates hospitalization risk in this population.
Keywords: : thyroid, thyrotropin, hypothyroidism, hospitalization, heart failure
Introduction
Hypothyroidism is a highly prevalent condition, affecting approximately 4–10% of the general population (1–3). Despite its pervasiveness, prior studies of the association between hypothyroidism and hard outcomes such as hospitalization risk and mortality have yielded conflicting findings (4–12). Given that hypothyroidism has been associated with various cardiac derangements (i.e., impaired cardiac contractility, altered cardiac conduction, increased systemic vascular resistance, dyslipidemia, accelerated atherosclerosis, neuro-hormonal activation, and ventricular remodeling), it has been hypothesized that the relationship between hypothyroidism and adverse outcomes may be dependent on an individual's underlying cardiac risk (6,13–15). For example, in a study of 14,879 participants from the Third National Health and Nutrition Examination Survey (NHANES III), it was shown that hypothyroidism was associated with higher mortality in participants with heart failure, but not in those with normal cardiac status (6). However, little is known about whether the inter-relationship between thyroid functional disease and hospitalization risk may differ according to underlying cardiovascular status.
There has also been increasing interest in whether race and ethnicity may modify the association of hypothyroidism and outcomes (6,16). Large population-based studies in the United States have shown that both non-Hispanic black and Hispanic populations have lower median thyrotropin (TSH) levels than non-Hispanic whites, suggesting that there may be different physiologic set points for thyroid status according to race/ethnicity (2). While one study has shown that hypothyroidism is associated with higher mortality risk in black participants versus those of other racial backgrounds, there remains a paucity of data examining whether there is a differential relationship between thyroid dysfunction and outcomes across race and ethnicity (6).
Thus, to better inform the field, a study was conducted to examine whether the association between hypothyroidism and hospitalization risk is modified by (i) underlying cardiovascular status and (ii) race/ethnicity among a racially and ethnically diverse cohort of patients receiving care from a large university-based tertiary care center with detailed laboratory data and extended follow-up over which to observe outcomes.
Methods
Source cohort
A historical cohort study was conducted utilizing data from the University of California Los Angeles (UCLA) Health System electronic medical records with detailed patient-level information on socio-demographics, comorbidities, laboratory tests, and clinical events, including hospitalizations. Patients were included in the study provided that they underwent a serum TSH measurement over the period January 1, 1990–March 30, 2015, were aged ≥18 years at the time of baseline serum TSH measurement, and had available race and ethnicity data. The study was approved by the UCLA Institutional Review Committee.
In total, 54,340 patients met the aforementioned criteria with follow-up data. Given that the primary subject of interest was the examination of hypothyroidism versus euthyroidism with hospitalization risk, patients with hyperthyroidism—defined as baseline serum TSH levels less than the lower limit of the normal reference range (TSH <0.3 mIU/L)—were excluded (n = 1484). After exclusions, 52,856 patients remained in the final analytic cohort.
Exposure ascertainment
The exposure of interest was thyroid status defined by baseline serum TSH level. The study sought to examine the association between baseline thyroid status and hospitalization risk, and how these associations are modified by cardiovascular status and race/ethnicity. Patients with TSH levels within the assay reference range (TSH 0.3–4.7 mIU/L) were considered euthyroid, whereas patients with TSH levels higher than the upper limit of the normal reference range were considered hypothyroid (TSH >4.7 mIU/L). The same TSH assay was used for the duration of the study. Patients receiving thyroid hormone supplementation or antithyroid therapy were categorized according to their baseline TSH level, as a patient with a high TSH level despite receipt of thyroid hormone supplementation was considered to be biochemically hypothyroid.
Outcome ascertainment
The primary outcome of interest was all-cause hospitalization, which was defined as a composite of both inpatient hospital admissions and/or emergency room visits within the UCLA Medical Centers. At-risk time began the day after the baseline TSH measurement. Patients were censored for death or at the end of the study period (March 30, 2015).
Comorbidities, race, and ethnicity
Comorbidities were defined using the International Classification of Diseases ninth revision (ICD-9) codes for congestive heart failure (CHF), coronary artery disease (CAD), cerebrovascular disease (CVD), diabetes, hypertension, and hyperlipidemia (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy). Race/ethnicity data were self-reported. Race was categorized as white, black, Asian, or other. Ethnicity was categorized as Hispanic versus non-Hispanic.
Statistical analysis
Baseline characteristics of patients according to thyroid status were compared using two-sample t-tests, Wilcoxon rank sum tests, and chi-square tests according to data type. The associations between thyroid status and hospitalization risk were estimated using multivariable Cox proportional hazards models adjusted for age, sex, race, ethnicity, body mass index, diabetes, hypertension, hyperlipidemia, CHF, CAD, and CVD (17,18). The proportional hazards assumption was confirmed graphically and through Schoenfeld residual testing.
To determine whether the association of thyroid status and hospitalization risk is modified by cardiovascular status, subgroup analyses were conducted across categories of baseline CHF, CAD, CVD, and cardiovascular medication use (Supplementary Table 2). In secondary analyses, the association of thyroid status and hospitalization risk was then examined across subgroups of race (white, black, Asian, other) and ethnicity (Hispanic vs. non-Hispanic). Statistical significance of subgroup analyses was assessed by likelihood ratio testing comparing models with and without the corresponding two-way factor-by-exposure cross-product terms (e.g., CHF × hypothyroidism). An p-value of <0.05 was considered statistically significant.
Given that thyroid status has been associated with higher death risk in prior studies (5), mortality may be a competing risk such that a death event hinders the observation of the event of interest (e.g., hospitalization risk) or alters the likelihood that this event occurs (19). Given that standard Cox regression analyses do not account for competing risks, sensitivity analyses were also conducted using the cumulative incidence function. Maximum likelihood estimates of the regression coefficients were obtained by the Newton–Raphson algorithm. Due to the bias in the Kaplan–Meier method of estimating the survivor function with competing risk cases, the cumulative incidence function (20) was introduced to handle the marginal failure sub-distribution of a given cause. In SAS v9.4 (SAS Institute, Inc., Cary, NC), PROC PHREG was performed in the subgroup analysis by specifying the EVENTCODE = option in the MODEL statement. To specify the maximum likelihood estimates of coefficients, PROC PHREG was implemented with the Newton–Raphson algorithm (21). There were no missing covariate data for the multivariable analyses, including age, sex, race, ethnicity, comorbidities, and medication use. This study was conducted under STROBE guidelines.
Results
Study population
Among 52,856 patients who met eligibility criteria (Supplementary Fig. S1), 49,791 (94.2%) were euthyroid and 3065 (5.8%) were hypothyroid. The baseline characteristics of the cohort are shown in Table 1. Compared to euthyroid patients, hypothyroid patients tended to be of older age, female sex, white race, and Hispanic ethnicity. Hypothyroid patients also had higher prevalence of diabetes and greater use of thyroid hormone supplementation as well as antithyroid medication. The median (IQR) and minimum–maximum values of TSH in the euthyroid group were 1.7 mIU/L (1.2–2.4 mIU/L) and 0.3–4.68 mIU/L, respectively. The median (IQR) and minimum–maximum values of TSH in the hypothyroid group were 6.0 mIU/L (5.1–8.2 mIU/L) and 4.7–204.0 mIU/L, respectively.
Table 1.
Baseline Characteristics According to Thyroid Functional Status
| Euthyroid (n = 49,791) | Hypothyroid (n = 3065) | p-Value | |
|---|---|---|---|
| TSH, mIU/L | |||
| Median | 1.7 | 6.0 | <0.001 |
| IQR | 1.2–2.4 | 5.1–8.2 | |
| Min–max | 0.3–4.7 | 4.7–204.0 | |
| Age (years), M ± SD | 52 ± 17 | 57 ± 1 | <0.001 |
| Sex, % | |||
| Male | 36 | 34 | <0.001 |
| Female | 64 | 67 | |
| BMI (kg/m2), M ± SD | 26.6 ± 6.0 | 26.8 ± 6.3 | = 0.04 |
| Race, % | |||
| White | 76 | 82 | <0.001 |
| Black | 9 | 5 | |
| Asian | 13 | 12 | |
| Other | 2 | 2 | |
| Ethnicity, % | |||
| Hispanic | 9 | 11 | <0.001 |
| Non-Hispanic | 92 | 89 | |
| Diabetes, % | |||
| Yes | 4 | 6 | <0.001 |
| No | 96 | 94 | |
| Hypertension, % | |||
| Yes | 3 | 3 | = 0.55 |
| No | 98 | 97 | |
| Hyperlipidemia, % | |||
| Yes | 2 | 1 | = 0.20 |
| No | 98 | 99 | |
| CHF, % | |||
| Yes | 0.7 | 0.8 | = 0.37 |
| No | 99.3 | 99.2 | |
| CAD, % | |||
| Yes | 0.6 | 0.7 | = 0.68 |
| No | 99.4 | 99.3 | |
| CVD, % | |||
| Yes | 1.1 | 1.7 | = 0.003 |
| No | 98.9 | 98.3 | |
| AFib, % | |||
| Yes | 1.48 | 2.87 | <0.001 |
| No | 98.52 | 97.73 | |
| Thyroid hormone supplementation use, % | |||
| Yes | 5.5 | 20.4 | <0.001 |
| No | 94.5 | 79.6 | |
| Antithyroid medication use, % | |||
| Yes | 0.1 | 0.4 | = 0.01 |
| No | 99.9 | 99.6 | |
| Prior history of thyroid disease, % | |||
| Yes | 0.83 | 4.21 | <0.001 |
| No | 99.17 | 95.79 | |
| Prior thyroidectomy/RAI ablation, % | |||
| Yes | 0.61 | 1.86 | <0.001 |
| No | 99.39 | 98.14 |
TSH, thyrotropin; IQR, interquartile range; SD, standard deviation; BMI, body mass index; CHF, congestive heart failure; CAD, coronary artery disease; CVD, cerebrovascular disease; AFib, atrial fibrillation; RAI, radioactive iodine.
Hypothyroidism and hospitalization risk according to underlying cardiovascular status
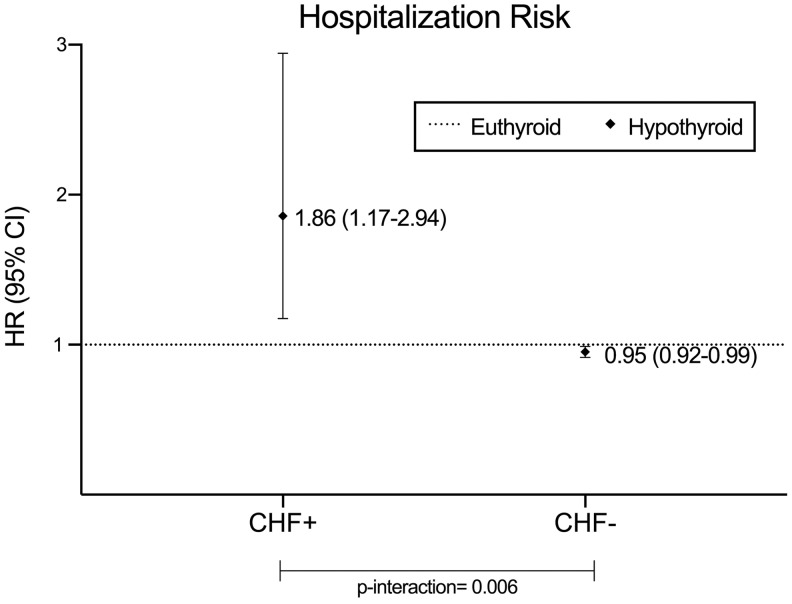
Among the overall cohort, there were a total of 13,023 hospitalization events (Supplementary Table S3), and the median (IQR) follow-up period was 5.61 years (2.63–8.78 years). In adjusted analyses, underlying CHF status was found to modify the association between hypothyroidism and hospitalization risk (p = 0.006; Fig. 1 and Table 2). In patients with CHF, hypothyroidism was associated with a significantly higher risk of hospitalization (reference: euthyroidism): adjusted hazard ratio (aHR) = 1.86 [confidence interval (CI) 1.17–2.94]. However, among those without CHF, hypothyroidism was associated with a slightly lower risk of hospitalization (aHR = 0.95 [CI 0.92–0.99]).
FIG. 1.
Hypothyroidism and hospitalization among patients with versus without congestive heart failure (CHF). Analyses adjusted for age, sex, race, ethnicity, body mass index, diabetes, hypertension, hyperlipidemia, CHF, coronary artery disease, and cerebrovascular disease.
Table 2.
Association Between Hypothyroidism with Hospitalization Risk, Stratified by Underlying Cardiovascular Disease Status
| Unadjusted | Adjusted | Adjusted (including amiodarone) | ||||||
|---|---|---|---|---|---|---|---|---|
| # of events | Risk time | HR [CI] | p | HR [CI] | p | HR [CI] | p | |
| CHF | ||||||||
| Yes | 124 | 3.75 | 1.22 [1.02–1.43] | 0.001 | 1.86 [1.17–2.94] | 0.006 | 1.23 [1.08–1.39] | 0.002 |
| No | 12899 | 4.77 | 0.82 [0.72–0.92] | 0.95 [0.92–0.99] | 0.87 [0.65–1.02] | |||
| CAD | ||||||||
| Yes | 144 | 4.31 | 1.41 [1.28–1.68] | 0.53 | 1.08 [0.61–1.89] | 0.81 | 1.07 [0.74–1.38] | 0.62 |
| No | 12879 | 4.76 | 0.73 [0.64–0.84] | 0.96 [0.92–0.99] | 0.93 [0.86–1.16] | |||
| CVD | ||||||||
| Yes | 202 | 4.14 | 1.15 [1.01–1.31] | 0.24 | 0.84 [0.59–1.18] | 0.56 | 0.91 [0.72–1.26] | 0.39 |
| No | 12821 | 4.77 | 0.9 [0.82–0.99] | 0.96 [0.92–1.00] | 1.11 [1.0–1.22] | |||
| Cardiovascular medication use | ||||||||
| Yes | 184 | 3.73 | 1.16 [0.98–1.41] | 0.39 | 1.05 [0.81–1.34] | 0.43 | 1.13 [1.03–1.24] | 0.11 |
| No | 7993 | 4.78 | 0.89 [0.81–0.97] | 0.96 [0.92–0.99] | 0.89 [0.69–1.09] | |||
Analyses adjusted for age, sex, race, ethnicity, body mass index, diabetes, hypertension, hyperlipidemia, CHF, CAD, and CVD.
The study also examined whether other preexisting cardiovascular diseases modified the association between hypothyroidism and hospitalization risk in adjusted analyses (Table 2). There did not appear to be effect modification of the association between hypothyroidism and hospitalization risk according to underlying CAD status (p = 0.81). In analyses stratified by CVD status, there appeared to be a trend toward an association between hypothyroidism and lower risk of hospitalization among those without underlying CVD (aHR = 0.84 [CI 0.59–1.18]) but not in those with CVD (aHR = 0.96 [CI 0.92–0.99]). However, interaction testing did not show statistical significance (p = 0.56), indicating that these differences were no more than what was explained by chance. Similarly, in analyses stratified by cardiovascular medication use, there appeared to be an association between hypothyroidism and lower risk of hospitalization among those without medication use (aHR = 0.96 [CI 0.92–0.99]) but not in those with medication use (aHR = 1.05 [CI 0.81–1.34]). However, interaction testing did not show statistical significance (p = 0.43), indicating that these differences were no more than what is explained by chance.
Hypothyroidism and hospitalization risk according to sociodemographic characteristics
The study also examined whether patients' socio-demographic characteristics modified the association between hypothyroidism and hospitalization risk in adjusted analyses (Table 3). It was found that age modified the association between hypothyroidism and hospitalization risk (p < 0.001). In younger patients (aged <60 years), hypothyroidism was associated with a significantly lower risk of hospitalization (aHR = 0.89 [CI 0.85–0.94]). In older patients (aged ≥60 years), there was a trend toward an association between hypothyroidism and higher hospitalization risk, although this did not reach statistical significance (aHR = 1.05 [CI 0.99–1.11]).
Table 3.
Association Between Hypothyroidism with Hospitalization Risk, Stratified by Demographics
| Unadjusted | Adjusted | Adjusted (including Amiodarone) | ||||||
|---|---|---|---|---|---|---|---|---|
| # of Events | Risk Time | HR [CI] | p | HR [CI] | p | HR [CI] | p | |
| Age, years | ||||||||
| <60 | 19,384 | 5.76 | 0.94 [0.93–0.96] | <0.001 | 0.89 [0.85–0.94] | <0.001 | 0.92 [0.89–0.94] | <0.001 |
| ≥60 | 33,472 | 5.43 | 1.14 [1.12–1.17] | 1.05 [0.99–1.11] | 1.12 [1.10–1.14] | |||
| Sex | ||||||||
| Male | 19,089 | 5.48 | 1.14 [1.12–1.16] | 0.06 | 0.99 [0.93–1.06] | 0.28 | 1.16 [1.13–1.19] | 0.11 |
| Female | 33,767 | 5.73 | 0.94 [0.92–0.96] | 0.94 [0.90–0.99] | 0.84 [0.82–0.86] | |||
| Race | ||||||||
| Black | 4778 | 5.71 | 0.96 [0.93–1.01] | 0.07 | 0.89 [0.75–1.07] | 0.57 | 0.99 [0.98–1.0] | 0.66 |
| Non-black | 48,078 | 5.64 | 1.03 [0.98–1.07] | 0.96 [0.92–1.00] | 1.01 [0.97–1.1] | |||
| Ethnicity | ||||||||
| Hispanic | 4557 | 5.74 | 0.98 [0.95–1.01] | 0.26 | 0.96 [0.85–1.09] | 0.83 | 0.98 [0.94–1.02] | 0.36 |
| Non-Hispanic | 48,299 | 5.63 | 1.14 [1.12–1.16] | 0.96 [0.92–1.00] | 1.06 [0.98–1.14] | |||
Analyses adjusted for age, sex, race, ethnicity, body mass index, diabetes, hypertension, hyperlipidemia, congestive heart failure, coronary artery disease, and cerebrovascular disease.
In analyses stratified by sex, while there appeared to be a trend toward an association between hypothyroidism and lower risk of hospitalization among female patients (aHR = 0.94 [CI 0.90–0.99]) but not in male patients (aHR = 0.99 [CI 0.93–1.06]). However, interaction testing did not show statistical significance (p = 0.28). Similarly, in analyses stratified by race, while there appeared to be a trend toward an association between hypothyroidism and lower risk of hospitalization among non-black patients (aHR = 0.96 [CI 0.92–1.00]) but not in black patients (aHR = 0.89 [CI 0.5–1.07]), interaction testing did not show statistical significance (p = 0.57). Finally, in analyses stratified by ethnicity, while there appeared to be a trend toward an association between hypothyroidism and lower risk of hospitalization among non-Hispanic patients (aHR = 0.96 [CI 0.92–1.00]) but not in Hispanic patients (aHR = 0.96 [CI 0.85–1.09]), interaction testing did not show statistical significance (p = 0.83).
Competing risk analyses
Sensitivity analyses were also conducted of the association between hypothyroidism and hospitalization risk stratified by underlying cardiovascular disease status in which all-cause death was accounted for as a competing risk. Similar to the primary analyses, when strata of CHF status were examined, there was an association between hypothyroidism and higher risk of hospitalization among those with underlying CHF (aHR = 2.26 [CI 1.77–2.75]) but not among those without CHF (aHR = 1.06 [CI 0.92–1.23]), although interaction testing narrowly missed statistical significance (p = 0.07). In contrast to the primary analyses, it was found that preexisting CAD modified the association between hypothyroidism and hospitalization risk such that point estimates of the hypothyroidism–hospitalization risk association were even stronger among those with CAD (aHR = 3.32 [CI 3.21–3.50]) versus those without CAD (aHR = 1.20 [CI 1.02–1.38]; p < 0.001). It was also found that underlying CVD modified the association between hypothyroidism and hospitalization risk such that hypothyroidism was associated with higher risk of hospitalization among those with CVD (aHR = 1.98 [CI 1.79–2.16]) and lower risk among those without CVD (aHR = 0.68 [CI 0.50–0.87]; p < 0.001). Finally, it was also observed that cardiovascular medication use modified the association between hypothyroidism and hospitalization risk such that hypothyroidism was associated with lower risk of hospitalization among those without medication use (aHR = 0.96 [CI 0.92–0.99]) but not in those with medication use (aHR = 1.05 [CI 0.81–1.34]; p = 0.04).
Discussion
In a large, racially/ethnically diverse cohort of patients receiving care at a large university-based academic center, it was found that underlying cardiovascular status was a potent modifier of the association between hypothyroidism and risk of hospitalization. In the primary analyses, it was found that compared to euthyroid status, hypothyroidism was associated with a 1.9-fold higher risk of hospitalization among those with CHF and a slightly lower risk of hospitalization among those without CHF. In sensitivity analyses that accounted for death as a competing risk, it was additionally found that hypothyroidism had an even stronger association with hospitalization risk among those with versus without CAD (3.3- vs. 1.2-fold higher risk, respectively). It was also found that hypothyroidism was associated with a nearly twofold higher risk of hospitalization among those with CVD, but slightly lower risk among those without CVD. These associations were observed independent of confounders such as socio-demographic characteristics, comorbidity burden, and body mass index.
To the authors' knowledge, this is the first large, population-based study to examine whether hypothyroidism is associated with hospitalization risk according to underlying cardiovascular status. While prior studies of hypothyroidism and mortality have shown mixed findings, there has been a tendency toward positive versus negative studies among cohorts of higher versus low-to-average cardiovascular risk (6,8,13–15,22). Furthermore, NHANES III data have shown that the hypothyroidism–mortality association was dependent on participants' self-reported CHF status (6). Given the short-term effects that thyroid hormone perturbations may have upon health status (e.g., arrhythmia and systolic and diastolic function), a decision was made to examine hospitalization risk as an equally relevant outcome. In light of the high healthcare costs and resource utilization associated with hospitalization, as well as broad-scale efforts at the governmental and policy-level to reduce inpatient admissions (particularly 30-day admissions for heart failure and myocardial infarction), further studies are needed to determine whether correction of thyroid dysfunction may reduce hospitalization risk in high cardiovascular risk populations (23–28).
The findings suggest a decreased risk of hospitalization among those <60 years old, and hypothyroidism was associated with a significantly decreased risk of hospitalization. It is possible that this observation may be due to the fact that symptoms of hypothyroidism are less pronounced in younger individuals, and thus this group would present to an emergency room less often. In contrast, we did not observe a differential relationship between hypothyroidism and hospitalization risk on the basis of race or ethnicity in this diverse Southern California–based cohort. The findings contrast with those of an earlier NHANES III study showing a differential association between hypothyroidism and mortality risk among black versus non-black participants. It is possible that these distinctions across studies may be explained by heterogeneous study populations, disparate short- versus long-term effects of hypothyroidism upon health status, and differential care in university- versus community-based settings (24–30). Future studies are needed to explore whether the prognostic implications of thyroid dysfunction differ according to racial/ethnic background, as well as their potential underpinnings (e.g., genetic/biologic, geographic, sociocultural, and economic factors).
The strengths of this study include its examination of a large, racially/ethnically diverse cohort with detailed longitudinal data on comorbidities, laboratory tests, medications, and hospitalization risk; definition of cardiovascular risk using objective measures (i.e., ICD-9 codes and cardiovascular medication prescription data as opposed to self-reported health status); and rigorous analytic approaches, including comprehensive adjustment for potential confounders of the hypothyroidism–hospitalization risk association and competing risk methods. However, several limitations of the study bear mention. First, the definition of hypothyroidism did not additionally consider triiodothyronine (T3) and thyroxine (T4) levels due to sparse measurements concurrent with TSH. However, it should be noted that compared to TSH as the most sensitive and specific single metric of thyroid status, T3 and T4 levels are more likely to be influenced by mild to moderate nonthyroidal illness, respectively, and low TSH levels may be observed with severe, critical illness, TSH levels at this spectrum were not considered in the analyses (31–37). Second, it is also possible that the study did not capture thyroid functional tests and hospitalizations that occurred outside of the university-based center. However, misclassification on this basis would not be expected to differ according to exposure status (i.e., non-differential misclassification), thus rendering the findings to be conservative. Third, data on cause-specific hospitalization were lacking to elucidate potential pathways between thyroid status and hospitalization risk. Fourth, the study examined TSH levels measured at a single point in time (i.e., baseline) in lieu of repeated measurements. However, longitudinal examination of TSH trends in the general population has shown minimal variations over time (i.e., variation of 0.5 mIU/L when measured monthly over a one-year period) (29,30). In addition, the most common scenario for TSH fluctuation would be TSH falling in response to systemic illness, in which this potential bias should favor patients with higher TSH levels and render conservative the observed association between hypothyroidism and hospitalization risk. Lastly, as with all observational studies, the possibility of residual confounding cannot be excluded.
In summary, this study has shown for the first time that hypothyroidism is associated with higher risk of hospitalization among patients with underlying cardiovascular disease (e.g., CHF, CAD, and CVD). Future studies are needed to corroborate findings, elucidate underlying mechanisms, and determine whether correction of TSH perturbations with thyroid hormone replacement therapy ameliorates hospitalization risk.
Supplementary Material
Acknowledgments
The authors are supported by research grants from the NIH, including K23-HD068552 (A.M.L.) and K23-DK102903 (C.M.R.). Portions of the data have been presented as an abstract at the 86th Annual Meeting of the American Thyroid Association, September 21–25, 2016, Denver, CO.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F. 1995. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 43:55–68 [DOI] [PubMed] [Google Scholar]
- 2.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. 2002. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- 3.Walsh JP, Bremner AP, Feddema P, Leedman PJ, Brown SJ, O'Leary P. 2010. Thyrotropin and thyroid antibodies as predictors of hypothyroidism: a 13-year, longitudinal study of a community-based cohort using current immunoassay techniques. J Clin Endocrinol Metab 95:1095–1104 [DOI] [PubMed] [Google Scholar]
- 4.Baraona F, Gurvitz M, Landzberg MJ, Opotowsky AR. 2013. Hospitalizations and mortality in the United States for adults with Down syndrome and congenital heart disease. Am J Cardiol 111:1046–1051 [DOI] [PubMed] [Google Scholar]
- 5.Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J, Collaboration TS. 2010. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 304:1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhee CM, Curhan GC, Alexander EK, Bhan I, Brunelli SM. 2013. Subclinical hypothyroidism and survival: the effects of heart failure and race. J Clin Endocrinol Metab 98:2326–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boekholdt SM, Titan SM, Wiersinga WM, Chatterjee K, Basart DC, Luben R, Wareham NJ, Khaw KT. 2010. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf) 72:404–410 [DOI] [PubMed] [Google Scholar]
- 8.Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. 2006. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 295:1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez AC, Jhund PS, Stott DJ, Gullestad L, Cleland JG, van Veldhuisen DJ, Wikstrand J, Kjekshus J, McMurray JJ. 2014. Thyroid-stimulating hormone and clinical outcomes: the CORONA trial (controlled rosuvastatin multinational study in heart failure). JACC Heart Fail 2:35–40 [DOI] [PubMed] [Google Scholar]
- 10.Frey A, Kroiss M, Berliner D, Seifert M, Allolio B, Güder G, Ertl G, Angermann CE, Störk S, Fassnacht M. 2013. Prognostic impact of subclinical thyroid dysfunction in heart failure. Int J Cardiol 168:300–305 [DOI] [PubMed] [Google Scholar]
- 11.Gencer B, Collet TH, Virgini V, Bauer DC, Gussekloo J, Cappola AR, Nanchen D, den Elzen WP, Balmer P, Luben RN, Iacoviello M, Triggiani V, Cornuz J, Newman AB, Khaw KT, Jukema JW, Westendorp RG, Vittinghoff E, Aujesky D, Rodondi N, Collaboration TS. 2012. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation 126:1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selmer C, Olesen JB, Hansen ML, von Kappelgaard LM, Madsen JC, Hansen PR, Pedersen OD, Faber J, Torp-Pedersen C, Gislason GH. 2014. Subclinical and overt thyroid dysfunction and risk of all-cause mortality and cardiovascular events: a large population study. J Clin Endocrinol Metab 99:2372–2382 [DOI] [PubMed] [Google Scholar]
- 13.Iervasi G, Molinaro S, Landi P, Taddei MC, Galli E, Mariani F, L'Abbate A, Pingitore A. 2007. Association between increased mortality and mild thyroid dysfunction in cardiac patients. Arch Intern Med 167:1526–1532 [DOI] [PubMed] [Google Scholar]
- 14.McQuade C, Skugor M, Brennan DM, Hoar B, Stevenson C, Hoogwerf BJ. 2011. Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: a PreCIS database study. Thyroid 21:837–843 [DOI] [PubMed] [Google Scholar]
- 15.Molinaro S, Iervasi G, Lorenzoni V, Coceani M, Landi P, Srebot V, Mariani F, L'Abbate A, Pingitore A. 2012. Persistence of mortality risk in patients with acute cardiac diseases and mild thyroid dysfunction. Am J Med Sci 343:65–70 [DOI] [PubMed] [Google Scholar]
- 16.Surks MI, Boucai L. 2010. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab 95:496–502 [DOI] [PubMed] [Google Scholar]
- 17.Rhee CM, Alexander EK, Bhan I, Brunelli SM. 2013. Hypothyroidism and mortality among dialysis patients. Clin J Am Soc Nephrol 8:593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee CM, Kim S, Gillen DL, Oztan T, Wang J, Mehrotra R, Kuttykrishnan S, Nguyen DV, Brunelli SM, Kovesdy CP, Brent GA, Kalantar-Zadeh K. 2015. Association of thyroid functional disease with mortality in a national cohort of incident hemodialysis patients. J Clin Endocrinol Metab 100:1386–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noordzij M, Leffondré K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ. 2013. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant 28:2670–2677 [DOI] [PubMed] [Google Scholar]
- 20.Zhou B, Latouche A, Rocha V, Fine J. 2011. Competing risks regression for stratified data. Biometrics 67:661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. 1999. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509 [Google Scholar]
- 22.de Jongh RT, Lips P, van Schoor NM, Rijs KJ, Deeg DJ, Comijs HC, Kramer MH, Vandenbroucke JP, Dekkers OM. 2011. Endogenous subclinical thyroid disorders, physical and cognitive function, depression, and mortality in older individuals. Eur J Endocrinol 165:545–554 [DOI] [PubMed] [Google Scholar]
- 23.Crosson FJ. Report to the Congress: Medicare Payment Policy. Available at: www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf (accessed March30, 2018)
- 24.Bradley EH, Curry L, Horwitz LI, Sipsma H, Wang Y, Walsh MN, Goldmann D, White N, Piña IL, Krumholz HM. 2013. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circ Cardiovasc Qual Outcomes 6:444–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. 2010. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA 303:1716–1722 [DOI] [PubMed] [Google Scholar]
- 26.Kociol RD, Peterson ED, Hammill BG, Flynn KE, Heidenreich PA, Piña IL, Lytle BL, Albert NM, Curtis LH, Fonarow GC, Hernandez AF. 2012. National survey of hospital strategies to reduce heart failure readmissions: findings from the Get With the Guidelines—Heart Failure registry. Circ Heart Fail 5:680–687 [DOI] [PubMed] [Google Scholar]
- 27.Bradley EH, Curry L, Horwitz LI, Sipsma H, Thompson JW, Elma M, Walsh MN, Krumholz HM. 2012. Contemporary evidence about hospital strategies for reducing 30-day readmissions: a national study. J Am Coll Cardiol 60:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernheim SM, Grady JN, Lin Z, Wang Y, Savage SV, Bhat KR, Ross JS, Desai MM, Merrill AR, Han LF, Rapp MT, Drye EE, Normand SL, Krumholz HM. 2010. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure. Update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes 3:459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soldin OP. Measuring serum thyroid-stimulating hormone, thyroid hormones, thyroid-directed antibodies, and transport proteins. In: Braverman LE, Cooper DS, eds. Werner and Ingbar's The Thyroid. Tenth edition Lippincott Williams and Wilkins, Philadelphia, PA, 2013, pp 279–297 [Google Scholar]
- 30.Andersen S, Pedersen KM, Bruun NH, Laurberg P. 2002. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab 87:1068–1072 [DOI] [PubMed] [Google Scholar]
- 31.Schectman JM, Pawlson LG. 1990. The cost-effectiveness of three thyroid function testing strategies for suspicion of hypothyroidism in a primary care-setting. J Gen Intern Med 5:9–15 [DOI] [PubMed] [Google Scholar]
- 32.Surks MI, Chopra IJ, Mariash CN, Nicoloff JT, Solomon DH. 1990. American Thyroid Association guidelines for use of laboratory tests in thyroid disorders. JAMA 263:1529–1532 [PubMed] [Google Scholar]
- 33.Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, LiVosli VA, Niccoli-Sire P, John R, Ruf J, Smyth PP, Spencer CA, Stockigt JR; Guidelines Committee, National Academy of Clinical 2003. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 13:3–126 [DOI] [PubMed] [Google Scholar]
- 34.Stockigt JR. 1996. Guidelines for diagnosis and monitoring of thyroid disease: nonthyroidal illness. Clin Chem 42:188–192 [PubMed] [Google Scholar]
- 35.Hamblin PS, Dyer SA, Mohr VS, Le Grand BA, Lim CF, Tuxen DV, Topliss DJ, Stockigt JR. 1986. Relationship between thyrotropin and thyroxine changes during recovery from severe hypothyroxinemia of critical illness. J Clin Endocrinol Metab 62:717–722 [DOI] [PubMed] [Google Scholar]
- 36.Faber J, Kirkegaard C, Rasmussen B, Westh H, Busch-Sørensen M, Jensen IW. 1987. Pituitary-thyroid axis in critical illness. J Clin Endocrinol Metab 65:315–320 [DOI] [PubMed] [Google Scholar]
- 37.Peeters RP, van der Geyten S, Wouters PJ, Darras VM, van Toor H, Kaptein E, Visser TJ, Van den Berghe G. 2005. Tissue thyroid hormone levels in critical illness. J Clin Endocrinol Metab 90:6498–6507 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.