Abstract
Background: In an era of rising differentiated thyroid cancer incidence, the rate and impact of neck reoperation may inform the intensity of earlier interventions and surveillance. This study sought to define predictors of neck reoperation and to assess its impact on survival.
Methods: Using the California Cancer Registry linked to the California Office of Statewide Health Planning and Development records, a retrospective cohort study was performed of 24,230 patients with total or near-total thyroidectomy for papillary or follicular thyroid cancer between 1991 and 2008 and follow-up through 2013. The primary outcome was neck reoperation 91 days to 5 years after the initial thyroid surgery. Using logistic and Cox proportional hazards regression, the impact of sociodemographics, tumor staging, and hospital thyroid cancer surgery volume on neck reoperation and survival was determined.
Results: Neck reoperation was identified in 1231 (5.1%) patients in increasing odds from 1991 to 2008. In multivariable models, male sex, papillary thyroid cancer, and advancing tumor stage were associated with neck reoperation. Among men, neck reoperation was associated with Asian/Pacific Islander (odds ratio [OR] = 1.44 [confidence interval (CI) 1.07–1.94]) race/ethnicity. Among women, neck reoperation was associated with younger age (15–34 years; OR = 1.50 [CI 1.17–1.92] versus ≥55 years), and Asian/Pacific Islander (OR = 1.24 [CI 1.02–1.51]) or Hispanic (OR = 1.20 [CI 1.00–1.44]) race/ethnicity. After controlling for baseline characteristics, neck reoperation predicted worse thyroid cancer–specific survival (hazard ratio = 4.26 [CI 3.50–5.19]). The effect differed between men and women, and was most pronounced among women who received radioiodine in initial treatment (hazard ratio = 8.32 [CI 6.14–11.27]).
Conclusions: Neck reoperation is becoming increasingly frequent and is strongly predictive of mortality. Advancing tumor stage, Asian/Pacific Islander race/ethnicity, male sex, as well as younger age and Hispanic ethnicity among women predict a higher risk for neck reoperation and subsequent mortality, reflecting a higher risk of persistent or more biologically aggressive disease.
Keywords: : thyroid neoplasms, survival, reoperation, demography, thyroidectomy
Introduction
For the last several decades, the incidence of differentiated thyroid cancer has risen substantially across the world (1). In many countries, this incidence increase has been the result of the introduction of new diagnostic medical technologies and screening programs. In the United States, it has been estimated that 70–80% of differentiated thyroid cancer diagnoses in women and 45% in men have been the result of increased diagnosis (2). However, several lines of evidence suggest that the rising incidence in the United States is not completely explained by the increasing use of diagnostic procedures (1). First, the rate of large thyroid cancers that would be clinically apparent without diagnostic imaging has risen dramatically (3). Second, thyroid cancer rates appear to be rising at similar pace in populations with different use of and access to medical care (4). Third, while thyroid cancer–specific mortality in the United States is low, death rates have been rising on average 0.7% each year over 2004–2013 (5).
In California, worse thyroid cancer–specific survival was previously observed among young men, and sex-specific differences in survival were seen among young patients with differentiated thyroid cancer based on older age, African American or Hispanic race/ethnicity, residence in low socioeconomic status (SES) neighborhoods, and residence in nonmetropolitan areas (6). Additionally, it was identified that younger patients with differentiated thyroid cancer are more likely to undergo a total thyroidectomy and receive radioiodine as part of their initial treatment (7). Differentiated thyroid cancer, especially papillary thyroid cancer, has a propensity to spread to regional lymph nodes, necessitating a neck reoperation when it becomes apparent after initial therapy is complete. Patients with papillary thyroid cancer in California who require a neck reoperation experience worse thyroid cancer–specific survival (8). While guidelines incorporate an assessment of the risk for all forms of structural disease recurrence into management recommendations (9), this study sought to identify the clinical and demographic subgroups of differentiated thyroid cancer patients who are specifically at increased risk for neck reoperation.
This study utilized the large diverse population of cancer patients captured by the cancer registry and hospitalization data in California to identify clinical and demographic subgroups of patients at risk for neck reoperation and the impact of these factors and neck reoperation on survival. It was hypothesized that neck reoperation rates after initial total thyroid cancer surgery would differ among sociodemographic groups of patients and that these differences would impact thyroid cancer–specific mortality.
Methods
Setting and subjects
Patients diagnosed with invasive, first primary thyroid cancer in 1991–2008 were identified from the California Cancer Registry (CCR)—the largest population-based cancer registry in the United States. The CCR contains demographic, diagnostic, and initial treatment information for every reportable cancer diagnosed among residents of the state. Using the record linkage number (an encrypted form of the social security number) and sex, new thyroid cancer cases were linked to hospital and ambulatory surgery discharges from the State of California Office of Statewide Health Planning and Development (OSHPD). Since 1990, OSHPD has maintained records of all patients hospitalized in non-federal hospitals in the state, called the Patient Discharge Database (PDD). Beginning in 2005, an Ambulatory Surgery (AS) database of all hospital-associated AS facilities has also been mandated. Facilities in PDD and AS are required to report up to 25 diagnoses and up to 20 procedures associated with each hospitalization, coded using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) in the PDD and Current Procedural Terminology (CPT) in the AS. Each procedure code has an associated date. In addition to diagnostic and procedure information, demographic information including age, sex, race/ethnicity, and insurance coverage, type of admission (PDD only), and disposition is collected in both PDD and AS.
This study identified patients from the CCR with papillary or follicular thyroid cancer who had undergone thyroid cancer surgery. The International Classification of Diseases for Oncology, Third Edition (ICD-O-3), histology codes 8050, 8260, and 8340-8344 defined papillary thyroid cancers. Histology codes 8290, 8330–8332, and 8335 defined follicular cancers. Thyroidectomy was defined using ICD-9-CM and CPT codes for total or near-total thyroidectomy in OSHPD (PDD or AS) or most extensive surgery at initial treatment from CCR (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy). Patients with two lobectomies within 90 days in OSHPD were classified as having total or near-total thyroidectomy. Date of thyroidectomy was identified as the OSHPD procedure date of thyroidectomy or the recorded CCR date. Patients without a total or near-total thyroidectomy were excluded because the intensity of subsequent neck surveillance of those who undergo partial thyroidectomy for thyroid cancer is likely distinct.
Neck reoperation
The primary endpoint of this study was neck reoperation, which was defined from OSHPD as a neck lymph node surgery 91 days to 5 years after the initial thyroid surgery. This time period was chosen to capture clinically evident persistent or recurrent disease that is most likely to be dependent on initial treatment and/or aggressive biology, and is consistent with prior studies (8). Specific ICD-9-CM (PDD) and CPT (AS) codes were used to identify neck lymph node surgeries of interest (Supplementary Table S2).
Tumor, sociodemographic, and treatment variables
Pathologic data (T stage, extrathyroidal extension, N stage, and M stage), demographic data (age, race/ethnicity, and sex), and administration of radioiodine as part of initial treatment were obtained from the CCR. Neighborhood socioeconomic status in the CCR is a multicomponent index of U.S. Census characteristics (education, occupation, unemployment, household income, poverty, rent, and house values) based on residential census-block group at diagnosis (10). Comorbidities, excluding cancer, were captured up to two years prior to the thyroid cancer diagnosis date and identified using the Elixhauser index (11). Comorbidities were categorized as no admissions in PDD within the two prior years, no comorbidities, one or two comorbidities, and three or more comorbidities. Hospital volume for thyroid cancer surgery was measured by identifying all thyroid cancers diagnosed in CCR during 1988–2012, identifying all admissions with thyroidectomy (total or partial) for these patients, and then sorting hospitals by frequency of thyroidectomies. Hospitals were recategorized dynamically by five-year eras. Given the rising numbers of thyroidectomies performed during the study, high-volume thyroidectomy hospitals were defined as the top 10% of hospitals during each era (12).
Statistical analyses
Chi-square tests were used for unadjusted comparisons between patients with and without neck reoperation. Then, multivariable logistic regression was performed to identify demographic, tumor, and hospital characteristics associated with neck reoperation. Finally, multivariable Cox proportional hazards models were developed for thyroid cancer–specific and overall survival, including demographic, tumor, and hospital characteristics. Variables were included in the multivariable logistic and survival models if they were significant at p < 0.05 in univariable analyses, and neck reoperation was treated as a time-dependent variable. The study looked for specific interactions between neck reoperation and key tumor/initial treatment characteristics (extrathyroidal tumor extension and radioiodine administration as part of initial treatment). For deceased patients, survival time was measured in days from the date of diagnosis to the date of death from thyroid cancer for thyroid cancer–specific survival or to the date of death from any cause for overall survival. Patients who died from other causes were censored at the time of death for analyses of thyroid cancer–specific survival. Because thyroid cancer is a biologically distinct disease for males and females, all regression models were stratified by sex. Results are presented as adjusted odds ratios (OR) or hazard ratios (HR) and confidence intervals (CI). Interactions were considered significant if p < 0.05, and the proportionality assumption was tested using Schoenfeld residuals. All analysis was performed using SAS v9.4 (SAS Institute, Cary, NC). These data analyses were performed under a research protocol approved by the University of California, Davis, Institutional Review Board and the California's Health and Human Services Agency Committee for the Protection of Human Subjects.
Results
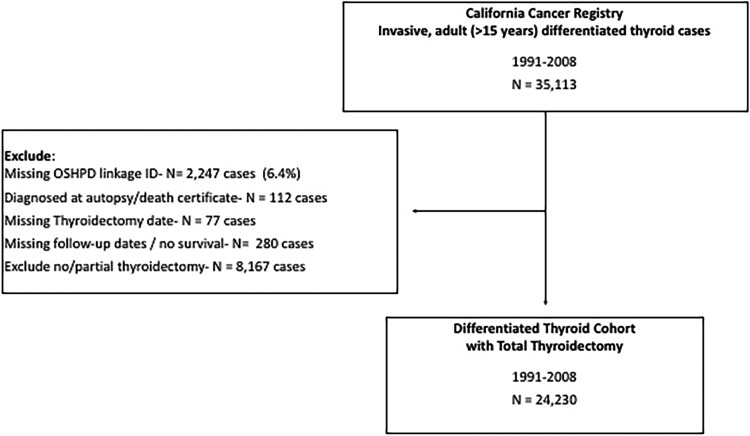
The study identified 35,113 patients aged ≥15 years when diagnosed with invasive, first primary differentiated thyroid cancer from the CCR during 1991–2008 with follow-up through 2013. Diagnoses through 2008 were considered to allow for a minimum of five years of follow-up from initial diagnosis for neck reoperation in OSHPD. After including only patients who underwent total or near-total thyroidectomy and excluding patients with missing identifiers for linkage to OSHPD, autopsy diagnosis, and missing follow-up, a total of 24,230 patients were included for analyses (Fig. 1).
FIG. 1.
Derivation of analysis cohort of differentiated thyroid cancer patients treated with total thyroidectomy, California, 1991–2008.
The primary endpoint of neck reoperation from 91 days to 5 years after initial surgery was observed in 5.1% of patients (Table 1). Neck reoperation was more common in the first two years but could be detected throughout the first five years after diagnosis using the linked databases (Supplementary Fig. S1). In unadjusted analyses, patients were more likely to have a neck reoperation with papillary histology, higher stage, male sex, and more recent (2000–2008) diagnosis. Radioiodine was administered to 60.6% of patients diagnosed in 1991–1999 and to 59.1% of patients diagnosed in 2000–2008 (p = 0.03). Hispanics and Asian/Pacific Islanders had higher rates of neck reoperations than black and non-Hispanic white populations. There were 169 (2.8%) neck reoperations among patients with microcarcinomas (tumor size <1 cm). Patients with node-positive disease (21.9%) and extrathyroidal extension (20.1%) at diagnosis were the most likely to require subsequent neck reoperation.
Table 1.
Baseline Characteristics of California Differentiated Thyroid Cancer Patients with Total or Near-Total Thyroidectomy by Neck Reoperation (91 Days–5 Years), 1991–2008
| Neck reoperation | No neck reoperation | ||||
|---|---|---|---|---|---|
| n | % | n | % | p-Value | |
| All | 1231 | 100 | 22,999 | 100 | |
| Sex | |||||
| Male | 391 | 31.7 | 5085 | 22.1 | <0.001 |
| Female | 842 | 68.3 | 17,914 | 77.9 | <0.001 |
| Race/ethnicity | |||||
| NH white | 625 | 50.8 | 13,447 | 58.5 | <0.001 |
| NH African American | 24 | 1.9 | 785 | 3.4 | 0.005 |
| Hispanic | 342 | 27.8 | 5157 | 22.4 | <0.001 |
| NH Asian/Pacific Islander | 231 | 18.8 | 3397 | 14.8 | <0.001 |
| Other/unknown | 9 | 0.7 | 213 | 0.9 | 0.48 |
| Age at diagnosis (years) | |||||
| 15–24 | 130 | 10.6 | 1424 | 6.2 | <0.001 |
| 25–34 | 247 | 20.1 | 4141 | 18.0 | 0.07 |
| 35–44 | 277 | 22.5 | 5767 | 25.1 | 0.04 |
| 45–54 | 228 | 18.5 | 5324 | 23.1 | <0.001 |
| 55–64 | 160 | 13.0 | 3332 | 14.5 | 0.14 |
| 65–74 | 137 | 11.1 | 2015 | 8.8 | 0.005 |
| ≥75 | 52 | 4.2 | 996 | 4.3 | 0.96 |
| Year of diagnosis | |||||
| 1991–1999 | 280 | 22.7 | 8262 | 35.9 | <0.001 |
| 2000–2008 | 951 | 77.3 | 14,737 | 64.1 | <0.001 |
| Histology | |||||
| Papillary | 1178 | 95.7 | 20,791 | 90.4 | <0.001 |
| Follicular | 53 | 4.3 | 2208 | 9.6 | <0.001 |
| N stage at diagnosis | |||||
| N0 | 265 | 21.5 | 13,072 | 56.8 | <0.001 |
| N1 | 677 | 55.0 | 4623 | 20.1 | <0.001 |
| Unknown | 289 | 23.5 | 2159 | 9.4 | 0.15 |
| M stage at diagnosis | |||||
| M0 | 877 | 71.2 | 17,406 | 75.7 | <0.001 |
| M1 | 34 | 2.8 | 352 | 1.5 | <0.001 |
| Unknown | 320 | 26.0 | 1474 | 6.4 | 0.001 |
| Tumor size | |||||
| <1 cm | 169 | 13.7 | 5936 | 25.8 | <0.001 |
| 1–2 cm | 320 | 26.0 | 6592 | 28.7 | 0.04 |
| 2–3 cm | 289 | 23.5 | 4393 | 19.1 | <0.001 |
| 3–4 cm | 139 | 11.3 | 2181 | 9.5 | 0.03 |
| >4 cm | 221 | 18.0 | 2278 | 9.9 | <0.001 |
| Unknown | 93 | 7.6 | 1619 | 7.0 | 0.50 |
| Extrathyroidal extension | |||||
| No | 615 | 50.0 | 17,393 | 75.6 | <0.001 |
| Yes | 571 | 46.4 | 4293 | 18.7 | <0.001 |
| Unknown | 45 | 3.7 | 1313 | 5.7 | 0.002 |
| Comorbidities at diagnosisa | |||||
| No admission ≤2 years of diagnosis | 753 | 61.2 | 11,349 | 49.3 | <0.001 |
| 0 | 180 | 14.6 | 6419 | 27.9 | <0.001 |
| 1–2 | 240 | 19.5 | 4252 | 18.5 | 0.39 |
| ≥3 | 58 | 4.7 | 979 | 4.3 | 0.45 |
| Treatment | |||||
| Radioiodine therapy | 929 | 75.5 | 13,513 | 58.8 | <0.001 |
| Neighborhood socioeconomic status | |||||
| Low | 595 | 48.3 | 11,317 | 49.2 | 0.51 |
| High | 619 | 50.3 | 11,466 | 49.9 | 0.72 |
| Unknown | 17 | 1.4 | 216 | 0.9 | 0.12 |
| Residence at time of diagnosis | |||||
| Urban | 1193 | 96.9 | 22,054 | 95.9 | 0.07 |
| Rural | 38 | 3.1 | 930 | 4.0 | 0.09 |
| Unknown | 15 | 0.1 | 0.37 | ||
| Marital status | |||||
| Never married | 337 | 27.4 | 4944 | 21.5 | <0.001 |
| Previously married | 127 | 10.3 | 2,923 | 12.7 | 0.01 |
| Married | 744 | 60.4 | 14,758 | 64.2 | 0.009 |
| Unknown | 23 | 1.9 | 374 | 1.6 | 0.52 |
| Insurance coverage | |||||
| No insurance/self-pay | 26 | 2.1 | 403 | 1.8 | 0.35 |
| Private insurance | 869 | 70.6 | 15,398 | 67.0 | 0.009 |
| Medicaid/government | 81 | 6.6 | 1682 | 7.3 | 0.33 |
| Medicare | 172 | 14.0 | 2159 | 9.4 | <0.001 |
| Unknown | 83 | 6.7 | 3357 | 14.6 | <0.001 |
| Hospital thyroidectomy volumeb | |||||
| High | 501 | 40.7 | 9072 | 39.4 | 0.37 |
| Low | 611 | 49.6 | 11,144 | 48.5 | 0.42 |
| Unknown | 119 | 9.7 | 2783 | 12.1 | 0.01 |
| Vital status | |||||
| All cause | 229 | 18.6 | 2524 | 11.0 | <0.001 |
| Thyroid cancer specific | 148 | 12.0 | 631 | 2.7 | <0.001 |
Exlihauser comorbidity index diagnosis or two years prior to diagnosis, cancer was excluded as a comorbidity.
High-volume thyroidectomy hospital defined as the top 10% of hospitals performing thyroidectomies.
NH, non-Hispanic.
In the overall multivariable logistic regression model, males were more likely to require neck reoperation (OR = 1.29 [CI 1.13–1.48]). Although there were differences in the predictors of reoperation between men and women, papillary histology, larger tumor size, more advanced nodal disease at diagnosis, and the presence of extrathyroidal tumor extension were predictors of neck reoperation among both men and women (Table 2). Similarly, the provision of radioiodine with initial treatment was associated with higher odds of neck reoperation. Asian/Pacific Islanders of both sexes had higher odds of neck reoperation, while Hispanic women had higher odds. The odds of neck reoperation were higher in younger women, but age was not a factor among men. Women residing in lower SES neighborhoods or who received their initial thyroid surgery in high-volume hospitals had lower odds of neck reoperation. After accounting for other tumor and patient factors, an earlier era of diagnosis was strongly associated with reduced odds of neck reoperation among both men and women.
Table 2.
Multivariable Logistic Regression Models for Neck Reoperation by Sex for California Differentiated Thyroid Cancers with Total or Near-Total Thyroidectomy, 1991–2008
| Males | Females | |||||
|---|---|---|---|---|---|---|
| OR | CI | p | OR | CI | p | |
| Race/ethnicity | ||||||
| NH white | REF | — | — | REF | — | — |
| NH African American | 0.96 | [0.43–2.14] | 0.92 | 0.74 | [0.44–1.24] | 0.25 |
| Hispanic | 1.18 | [0.89–1.58] | 0.24 | 1.20 | [1.00–1.44] | 0.05 |
| NH Asian/PI | 1.44 | [1.07–1.94] | 0.02 | 1.24 | [1.01–1.50] | 0.04 |
| Other/unknown | 1.34 | [0.38–4.68] | 0.65 | 0.71 | [0.31–1.64] | 0.42 |
| Age at diagnosis (years) | ||||||
| 15–34 | 0.93 | [0.65–1.33] | 0.69 | 1.50 | [1.17–1.92] | 0.001 |
| 35–44 | 0.82 | [0.58–1.16] | 0.26 | 1.26 | [0.99–1.61] | 0.06 |
| 45–54 | 1.16 | [0.86–1.58] | 0.33 | 0.88 | [0.68–1.14] | 0.33 |
| ≥55 | REF | — | — | REF | — | — |
| Year of diagnosis | ||||||
| 1991–1993 | 0.34 | [0.16–0.73] | 0.006 | 0.68 | [0.41– 1.13] | 0.14 |
| 1994–1996 | 0.34 | [0.20–0.57] | <0.001 | 0.50 | [0.37–0.67] | <0.001 |
| 1997–1999 | 0.67 | [0.46–0.99] | 0.04 | 0.45 | [0.35–0.59] | <0.001 |
| 2000–2002 | 0.82 | [0.59–1.13] | 0.23 | 0.45 | [0.35–0.58] | <0.001 |
| 2003–2005 | 0.94 | [0.70–1.26] | 0.66 | 0.84 | [0.70–1.02] | 0.07 |
| 2006–2008 | REF | — | — | REF | — | — |
| Histology | ||||||
| Papillary | 1.82 | [1.12–2.98] | 0.02 | 1.59 | (1.10, 2.31) | 0.01 |
| Follicular | REF | — | — | REF | — | — |
| N stage | ||||||
| N0 | REF | — | — | REF | — | — |
| N1 | 2.86 | [2.22–3.69] | <0.001 | 3.61 | [3.05–4.26] | <0.001 |
| Unknown | 1.83 | [1.20–2.79] | 0.005 | 2.01 | [1.51–2.67] | <0.001 |
| M stage | ||||||
| M0 | REF | — | — | REF | — | — |
| M1 | 0.57 | [0.32–1.05] | 0.07 | 0.93 | [0.56—1.53] | 0.77 |
| Unknown | 1.17 | [0.75–1.81] | 0.49 | 1.18 | [0.88–1.58] | 0.28 |
| Tumor size | ||||||
| <1 cm | REF | — | — | REF | — | — |
| 1–2 cm | 0.93 | [0.64–1.35] | 0.69 | 1.32 | [1.04–1.67] | 0.02 |
| 2–3 cm | 1.14 | [0.78–1.65] | 0.50 | 1.72 | [1.35–2.21] | <0.001 |
| 3–4 cm | 1.62 | [1.08–2.42] | 0.02 | 1.49 | [1.10–2.02] | 0.01 |
| >4 cm | 2.05 | [1.43–2.96] | <0.001 | 2.19 | [1.64–2.93] | <0.001 |
| Unknown | 1.25 | [0.75–2.10] | 0.39 | 1.96 | [1.40–2.75] | <0.001 |
| Extrathyroidal extension | ||||||
| No | REF | — | — | REF | — | — |
| Yes | 2.19 | [1.73–2.78] | <0.001 | 2.34 | [1.99–2.74] | <0.001 |
| Unknown | 1.69 | [0.88–3.24] | 0.11 | 1.28 | [0.79–2.09] | 0.31 |
| Comorbidities at diagnosisa | ||||||
| No admission ≤2 years after diagnosis | 1.28 | [0.89–1.83] | 0.18 | 1.38 | [1.12–1.70] | 0.002 |
| 0 | REF | — | — | REF | — | — |
| 1–2 | 1.37 | [0.92–2.04] | 0.12 | 1.37 | [1.07–1.75] | 0.01 |
| ≥3 | 0.99 | [0.56–1.77] | 0.98 | 1.38 | [0.92–2.06] | 0.12 |
| Treatment | ||||||
| No radioiodine | REF | — | — | REF | — | — |
| Radioiodine | 1.66 | [1.29–2.14] | <0.001 | 1.62 | [1.37–1.92] | <0.001 |
| Marital status | ||||||
| Never married | 1.05 | [0.78–1.40] | 0.75 | 1.21 | [1.02–1.45] | 0.03 |
| Previously married | 1.23 | [0.83–1.83] | 0.30 | 0.85 | [0.66–1.08] | 0.19 |
| Married | REF | — | — | REF | — | — |
| Unknown marital status | 1.22 | [0.49–3.05] | 0.67 | 1.46 | [0.86–2.46] | 0.16 |
| Neighborhood socioeconomic status | ||||||
| High | REF | — | — | REF | — | — |
| Low | 0.94 | [0.74–1.18] | 0.59 | 0.84 | [0.72–0.98] | 0.03 |
| Unknown | 2.01 | [0.99–4.06] | 0.05 | 0.50 | [0.20–1.25] | 0.14 |
| Health insurance | ||||||
| No insurance/self-pay | 0.40 | [0.12–1.33] | 0.14 | 1.36 | [0.86–2.14] | 0.19 |
| Private insurance | REF | — | — | REF | — | — |
| Medicaid/government | 0.87 | [0.54–1.41] | 0.58 | 0.69 | [0.52–0.93] | 0.01 |
| Medicare | 1.83 | [1.32–2.53] | <0.001 | 1.29 | [0.97–1.72] | 0.08 |
| Unknown | 0.97 | [0.53–1.77] | 0.92 | 0.46 | [0.30–0.71] | <0.001 |
| Hospital thyroidectomy volumeb | ||||||
| Low | REF | — | — | REF | — | — |
| High | 1.07 | [0.85—1.35] | 0.54 | 0.85 | [0.73–0.99] | 0.04 |
| Unknown | 0.61 | [0.40–0.93] | 0.02 | 0.84 | [0.65–1.07] | 0.15 |
Exlihauser comorbidity index diagnosis or two years prior to diagnosis, cancer was excluded as a comorbidity.
High-volume thyroidectomy hospital defined as the top 10% of hospitals performing thyroidectomies.
OR, odds ratio; CI, confidence interval; REF, reference group.
In the adjusted Cox proportional hazards survival model, neck reoperation was associated with a higher hazard of thyroid cancer–specific mortality (HR = 4.26 [CI 3.50–5.19]), and men had worse outcomes than women (HR = 1.44 [CI 1.24–1.68]). Older age and more advanced tumor characteristics predicted a higher hazard of thyroid cancer–specific death. There were differences in the stratified Cox proportional hazards survival model between men and women (Table 3). In men, neck reoperation was associated with nearly threefold worse thyroid cancer–specific survival (HR = 2.90 [CI 2.13–3.96]), and the provision of radioiodine in initial treatment was associated with a reduced hazard for thyroid cancer death (HR = 0.60 [CI 0.47–0.75]). Among women, the overall association of neck reoperation on thyroid cancer–specific survival (HR = 6.29 [CI 4.86–8.14]) differed based on the receipt of radioiodine at initial treatment (p = 0.0039). The magnitude of the association of neck reoperation and thyroid cancer–specific mortality was higher among women who received radioiodine as part of their initial treatment course (HR = 8.32 [CI 6.14–11.27]) than among women who did not (HR = 3.64 [CI 2.28–5.80]). African American men had better thyroid cancer–specific survival than non-Hispanic white men. African American women had worse outcomes compared to non-Hispanic white women. The volume of thyroid surgeries performed at the initial treating hospital was not associated with survival. Findings in Cox proportional hazards models for overall survival were similar (Supplementary Table S3).
Table 3.
Multivariable Cox Proportional Hazard Models for Thyroid Specific Mortality Among California Differentiated Thyroid Cancers with Total or Near-Total Thyroidectomy, 1991–2008
| Males | Females | |||||
|---|---|---|---|---|---|---|
| HR | CI | p | HR | CI | p | |
| Neck reoperation | ||||||
| No | REF | — | — | REF | — | — |
| Yes | 2.90 | [2.13–3.96] | <0.001 | 6.29 | [4.86–8.14] | <0.001 |
| Initial treatment | ||||||
| No radioiodine | REF | — | — | REF | — | — |
| Radioiodine | 0.60 | [0.47–0.75] | <0.001 | 0.88 | [0.73–1.07] | 0.22 |
| Race/ethnicity | ||||||
| NH white | REF | — | — | REF | — | — |
| NH African American | 0.43 | [0.21–0.89] | 0.02 | 1.62 | [1.02–2.56] | 0.04 |
| Hispanic | 0.95 | [0.70–1.28] | 0.72 | 1.28 | [1.00–1.64] | 0.05 |
| NH Asian/Pacific Islander | 0.82 | [0.58–1.15] | 0.24 | 1.21 | [0.94–1.56] | 0.13 |
| Other/unknown | 1.28 | [0.17–9.46] | 0.81 | 0.96 | [0.30–3.07] | 0.95 |
| Age at diagnosis (years) | ||||||
| 15–34 | 0.05 | [0.03–0.11] | <0.001 | 0.01 | [0.00–0.02] | <0.001 |
| 35–44 | 0.12 | [0.07–0.21] | <0.001 | 0.05 | [0.03–0.08] | <0.001 |
| 45–54 | 0.32 | [0.21–0.49] | <0.001 | 0.13 | [0.09–0.20] | <0.001 |
| 55–64 | 0.51 | [0.34–0.77] | 0.001 | 0.43 | [0.32–0.59] | <0.001 |
| 65–74 | 0.82 | [0.57–1.17] | 0.27 | 0.63 | [0.49–0.82] | <0.001 |
| ≥75 | REF | — | — | REF | — | — |
| Year of diagnosis | ||||||
| 1991–1993 | 2.00 | [1.13–3.54] | 0.02 | 2.14 | [1.28–3.59] | 0.004 |
| 1994–1996 | 1.87 | [1.18–2.94] | 0.007 | 1.82 | [1.24–2.69] | 0.002 |
| 1997–1999 | 1.75 | [1.13–2.70] | 0.01 | 1.99 | [1.39–2.84] | <0.001 |
| 2000–2002 | 1.08 | [0.70–1.67] | 0.72 | 1.35 | [0.93–1.95] | 0.12 |
| 2003–2005 | 1.16 | [0.76–1.78] | 0.49 | 1.08 | [0.75–1.57] | 0.68 |
| 2006–2008 | REF | — | — | REF | — | — |
| Histology | ||||||
| Papillary | 0.64 | [0.47–0.88] | 0.006 | 0.52 | [0.41–0.66] | <0.001 |
| Follicular | REF | — | — | REF | — | — |
| N stage at diagnosis | ||||||
| N0 | REF | — | — | REF | — | — |
| N1 | 1.25 | [0.94–1.67] | 0.13 | 1.72 | [1.35–2.20] | <0.001 |
| Unknown | 1.63 | [1.14–2.31] | 0.007 | 1.68 | [1.27–2.23] | <0.001 |
| M stage at diagnosis | ||||||
| M0 | REF | — | — | REF | — | — |
| M1 | 6.21 | [4.48–8.61] | <0.001 | 3.16 | [2.33–4.28] | <0.001 |
| Unknown | 1.01 | [0.66–1.55] | 0.97 | 1.05 | [0.74–1.48] | 0.80 |
| Tumor size | ||||||
| <1 cm | REF | — | — | REF | — | — |
| 1–2 cm | 1.42 | [0.79–2.57] | 0.24 | 1.22 | [0.76–1.96] | 0.42 |
| 2–3 cm | 2.37 | [1.36–4.11] | 0.002 | 2.09 | [1.33–3.29] | 0.002 |
| 3–4 cm | 3.32 | [1.89–5.83] | <0.001 | 3.62 | [2.27–5.76] | <0.001 |
| >4 cm | 5.06 | [3.03–8.45] | <0.001 | 4.47 | [2.86–6.99] | <0.001 |
| Unknown | 3.92 | [2.23–6.89] | <0.001 | 4.01 | [2.51–6.39] | <0.001 |
| Extrathyroidal extension | ||||||
| No | REF | — | — | REF | — | — |
| Yes | 3.64 | [2.75–4.80] | <0.001 | 4.32 | [3.39–5.49] | <0.001 |
| Unknown | 2.28 | [1.38–3.75] | 0.001 | 2.37 | [1.52–3.71] | <0.001 |
| Comorbidities at diagnosisa | ||||||
| No admission ≤2 years of diagnosis | 1.80 | [1.21–2.68] | 0.004 | 1.20 | [0.88–1.64] | 0.25 |
| 0 | REF | — | — | REF | — | — |
| 1–2 | 1.37 | [0.89–2.10] | 0.15 | 1.44 | [1.04–2.00] | 0.03 |
| ≥3 | 2.26 | [1.30–3.92] | 0.004 | 2.09 | [1.40–3.12] | <0.001 |
| Neighborhood socioeconomic status | ||||||
| Low | 1.56 | [1.23–1.98] | <0.001 | 0.99 | [0.81–1.21] | 0.92 |
| High | REF | — | — | REF | — | — |
| Marital status | ||||||
| Never married | 1.02 | [0.73–1.44] | 0.90 | 0.91 | [0.65–1.26] | 0.56 |
| Previously married | 0.92 | [0.63–1.35] | 0.67 | 1.18 | [0.95–1.47] | 0.13 |
| Married | REF | — | — | REF | — | — |
| Unknown marital status | 1.39 | [0.61–3.17] | 0.43 | 1.73 | [0.97–3.10] | 0.07 |
| Health insurance at diagnosis | ||||||
| No insurance/self-pay | 0.88 | [0.38–2.07] | 0.77 | 1.45 | [0.75–2.79] | 0.27 |
| Private | REF | — | — | REF | — | — |
| Medicaid/government | 1.16 | [0.73–1.84] | 0.54 | 1.53 | [1.07–2.19] | 0.02 |
| Medicare | 1.14 | [0.83–1.56] | 0.42 | 1.08 | [0.83–1.40] | 0.55 |
| Unknown insurance | 0.53 | [0.34–0.82] | 0.005 | 0.69 | [0.47–1.03] | 0.07 |
| Hospital thyroidectomy volumeb | ||||||
| Low | REF | — | — | REF | — | — |
| High | 1.06 | [0.82–1.36] | 0.66 | 0.92 | [0.74–1.13] | 0.42 |
| Unknown | 1.30 | [0.90–1.88] | 0.16 | 1.09 | [0.79–1.51] | 0.58 |
Exlihauser comorbidity index diagnosis or two years prior to diagnosis, cancer was excluded as a comorbidity.
High-volume thyroidectomy hospital defined as the top 10% of hospitals performing thyroidectomies.
HR, hazard ratio.
Discussion
Using linked cancer registry and hospitalization/ambulatory care databases in California, this study found that the rate of neck reoperation in the first five years after total thyroidectomy for differentiated thyroid cancers (diagnosed during 1991–2008) is rising and that neck reoperation is associated with worse thyroid cancer–specific and overall survival. The data confirm others that report an adverse impact of neck reoperation on thyroid cancer–specific survival (8,13), and expands on them by identifying a rise in neck reoperation rates, as well as specific clinical and demographic subgroups that are at increased risk for neck reoperation after controlling for other tumor and sociodemographic variables.
Differentiated thyroid cancer, especially papillary thyroid cancer, has a propensity to spread to regional lymph nodes. Reoperation for removal of neck lymph nodes after initial thyroid cancer surgery may reflect the intensity of initial surgery (i.e., later management of disease that was present at the time of diagnosis), more intense surveillance, and/or the aggressiveness of tumor biology (i.e., true recurrence of disease not apparent at diagnosis) (14). In an era of rapid increase in the diagnosis of differentiated thyroid cancer (4), the present data demonstrate that repeated intervention on the neck has also risen from an unadjusted rate of 3.3% in 1991–1999 to 6.1% in 2000–2008. These rates are higher than previously reported (8), perhaps because of an expanded list of surgical procedures included in the list of neck reoperations. The rising neck reoperation rate is surprising, given that a large fraction of the rise in thyroid cancer incidence over the same period has been attributed to increased diagnosis (2), suggesting that there has been a concomitant change in the intensity of initial management, surveillance, and/or disease biology. Given that neck reoperation is associated with the risks of hypoparathyroidism, injury to neurovascular structures, and worse survival, the data strongly support attention to the quality of initial presurgical disease assessment and to the quality of post-surgical surveillance (15). Indeed, recent guidelines recommend assessment of the central neck at the time of diagnosis for patients at high risk of nodal metastases and specify risk-stratified surveillance that includes measurement of thyroglobulin, neck ultrasound, and physical examination (9).
As expected, neck reoperation occurred more frequently among patients with advanced tumor stage at diagnosis, including those with extracapsular invasion and nodal involvement. After consideration of stage and demographic factors, neck reoperation was more common among Asian/Pacific Islanders as well as younger and Hispanic women. Previously, it was found that younger patients are more likely to undergo total thyroidectomy as their initial thyroid surgery and to receive radioiodine as part of their initial treatment course (7), supporting the hypothesis that age may impact the intensity of initial therapy. Among women, thyroidectomy at a high-volume hospital was associated with lower odds of neck reoperation, suggesting a possible effect of experience in presurgical evaluation and thyroid cancer surgery in reducing the rates of persistent disease (16). Additionally, lower SES and Medicaid insurance were associated with reduced odds of neck reoperation in women. Thus, factors associated both with the provision of initial surgical care and access to subsequent surveillance may contribute to the differences in neck reoperation rates observed among demographic groups in this study.
Surprisingly, a small but significant (2.8%) rate of neck reoperation after diagnosis of a thyroid microcarcinoma (<1 cm) we identified. It is widely accepted that these tumors are associated with a minimal mortality risk. Consequently, guidelines are moving toward a reduction in the intensity of management or even observation for selected microcarcinomas with other favorable risk factors (9). Nonetheless, the subsequent morbidity of reoperation in patients with these tumors highlights the need for personalized medicine approaches to surveillance and intervention in differentiated thyroid cancer.
It was found that radioiodine administration as part of initial treatment was associated with a higher likelihood of neck reoperation, despite adjustment for multiple tumor and patient-specific factors. This likely reflects unmeasured prognostic variables, either patient or provider based, that are associated with both the provision of radioiodine and neck reoperation. The finding that the radioiodine administration rate declined over time is consistent with a prior study, which showed that the decline is limited to localized tumors <2 cm (17). As advancing tumor stage and radioiodine use were associated with neck reoperation, a decline in radioiodine use among low-risk thyroid cancer patients is unlikely to have impacted the results of this study. Nonetheless, the need for neck reoperation is strongly associated with worse survival, even after controlling for radioiodine administration in both men and women. In women, neck reoperation after administration of radioiodine as part of initial treatment is associated with a greater hazard of thyroid cancer–specific mortality; this interaction was not found in males. The magnitude of this effect suggests that the subgroup of women with a neck reoperation after initial thyroidectomy and radioiodine treatment have a high risk of an aggressive disease phenotype. Thyroid cancer–specific survival outcomes were better in African American men and worse in African American women. Prior studies have connected worse outcomes among minorities to deficiencies in surgical care, including receiving care from inexperienced surgeons (16,18). This study did observe that women receiving their thyroidectomy at high-volume hospitals had lower odds of neck reoperation, but no impact was found of hospital thyroid cancer surgery volume on survival. However, the quality of initial thyroid surgery may be more accurately assessed at the individual surgeon level (12,19), but it is not possible to analyze surgeon-specific neck reoperation rates with these databases. Alternatively, there may be biologic differences in thyroid cancer arising among racial/ethnic groups that impact the rate of persistent or recurrent disease, as have been observed in colorectal and breast cancer (20,21).
There are several limitations of this large retrospective analysis of administrative data. The analysis is limited to patients diagnosed and treated in California. This study focuses on reoperation after total thyroidectomy. The determinants for neck reoperation after less than total thyroidectomy are likely to be distinct and are the subject of a separate ongoing study. Some neck reoperations were missed if they were performed in an ambulatory surgery facility before 2005, prior to the collection of these data by OSHPD. As the numbers of ambulatory surgery operations increased after 2005, the number of missed ambulatory surgery cases prior to 2005 is expected that to be small. Moreover, the rate of neck reoperation rose dramatically, even excluding ambulatory cases after 2005 (data not shown). Out-migration during follow-up may be non-random and will reduce neck reoperation rate estimates. Thus, the rates reported in this study should be considered conservative estimates of the true rate. It was not possible to capture the details of the diagnostic workup prior to initial thyroid surgery and to capture fully the extent of any neck dissection performed along with thyroidectomy. Future studies are needed to understand how the quality of preoperative imaging and the extent initial surgery, including therapeutic neck dissections, impact the rates of neck reoperation for persistent and recurrent differentiated thyroid cancer. Moreover, the specific pathologic details, including variant histologies, are not available in the registry. It is possible that unmeasured prognostic characteristics may impact the need for neck reoperation. Importantly, it was not possible to identify the activity of radioiodine administered, which may impact the need for neck reoperation. Nonetheless, it was found that radioiodine administration at unspecified doses was associated with the likelihood of neck reoperation and an overall improvement in thyroid cancer–specific survival in men.
In conclusion, the rate of neck reoperation for thyroid cancer is rising in California and is especially common among men, younger women, and the Asian/Pacific Islander and female Hispanic populations. Neck reoperation is strongly associated with worse survival outcomes, despite adjustment for known prognostic factors, highlighting a subgroup of thyroid cancer patients with more aggressive disease. Taken together, these data argue for enhancing research efforts into personalized medicine approaches to initial thyroid cancer management and subsequent surveillance in an era of rising thyroid cancer incidence.
Supplementary Material
Acknowledgments
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Preventioned in this study was supported by the California cooperative agreement 5NU58DP003862-04/DP003862; the National Cancer Institute Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the authors and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors. During the course of this study, T.S. was supported by the National Cancer Institute of the National Institutes of Health under award number K12CA138464. This study was also supported by the UC Davis Cancer Center Support Grant, P30CA093373-06. This work was presented in part at the 2016 American Society of Clinical Oncology Annual Meeting (Abstract 6093).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kitahara CM, Sosa JA. 2016. The changing incidence of thyroid cancer. Nat Rev Endocrinol 12:646–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. 2016. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med 375:614–617 [DOI] [PubMed] [Google Scholar]
- 3.Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS. 2009. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev 18:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horn-Ross PL, Lichtensztajn DY, Clarke CA, Dosiou C, Oakley-Girvan I, Reynolds P, Gomez SL, Nelson DO. 2014. Continued rapid increase in thyroid cancer incidence in California: trends by patient, tumor, and neighborhood characteristics. Cancer Epidemiol Biomarkers Prev 23:1067–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SEER Cancer Statistics Factsheets: Thyroid Cancer. Available at: http://seer.cancer.gov/statfacts/html/thyro.html (accessed September1, 2017)
- 6.Keegan TH, Grogan RH, Parsons HM, Tao L, White MG, Onel K, Horn-Ross PL. 2015. Sociodemographic disparities in differentiated thyroid cancer survival among adolescents and young adults in California. Thyroid 25:635–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semrad TJ, Semrad AM, Farwell DG, Chen Y, Cress R. 2015. Initial treatment patterns in younger adult patients with differentiated thyroid cancer in California. Thyroid 25:509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young S, Harari A, Smooke-Praw S, Ituarte PH, Yeh MW. 2013. Effect of reoperation on outcomes in papillary thyroid cancer. Surgery 154:1354–1361; discussion 1361–1352 [DOI] [PubMed] [Google Scholar]
- 9.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yost K, Perkins C, Cohen R, Morris C, Wright W. 2001. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 12:703–711 [DOI] [PubMed] [Google Scholar]
- 11.Schoenman JA, Sutton JP, Elixhauser A, Love D. 2007. Understanding and enhancing the value of hospital discharge data. Med Care Res Rev 64:449–468 [DOI] [PubMed] [Google Scholar]
- 12.Loyo M, Tufano RP, Gourin CG. 2013. National trends in thyroid surgery and the effect of volume on short-term outcomes. Laryngoscope 123:2056–2063 [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Higashiyama T, Takamura Y, Kobayashi K, Miya A, Miyauchi A. 2011. Prognosis of patients with papillary thyroid carcinoma showing postoperative recurrence to the central neck. World J Surg 35:767–772 [DOI] [PubMed] [Google Scholar]
- 14.Kouvaraki MA, Lee JE, Shapiro SE, Sherman SI, Evans DB. 2004. Preventable reoperations for persistent and recurrent papillary thyroid carcinoma. Surgery 136:1183–1191 [DOI] [PubMed] [Google Scholar]
- 15.Giordano D, Valcavi R, Thompson GB, Pedroni C, Renna L, Gradoni P, Barbieri V. 2012. Complications of central neck dissection in patients with papillary thyroid carcinoma: results of a study on 1087 patients and review of the literature. Thyroid 22:911–917 [DOI] [PubMed] [Google Scholar]
- 16.Sosa JA, Mehta PJ, Wang TS, Yeo HL, Roman SA. 2007. Racial disparities in clinical and economic outcomes from thyroidectomy. Ann Surg 246:1083–1091 [DOI] [PubMed] [Google Scholar]
- 17.Park KW, Wu JX, Du L, Leung AM, Yeh MW, Livhits MJ. 2018. Decreasing use of radioactive iodine for low-risk thyroid cancer in California, 1999 to 2015. J Clin Endocrinol Metab 103:1095–1101 [DOI] [PubMed] [Google Scholar]
- 18.Epstein AJ, Gray BH, Schlesinger M. 2010. Racial and ethnic differences in the use of high-volume hospitals and surgeons. Arch Surg 145:179–186 [DOI] [PubMed] [Google Scholar]
- 19.Nouraei SA, Virk JS, Middleton SE, Aylin P, Mace A, Vaz F, Kaddour H, Darzi A, Tolley NS. 2017. A national analysis of trends, outcomes and volume-outcome relationships in thyroid surgery. Clin Otolaryngol 42:354–365 [DOI] [PubMed] [Google Scholar]
- 20.Guda K, Veigl ML, Varadan V, Nosrati A, Ravi L, Lutterbaugh J, Beard L, Willson JK, Sedwick WD, Wang ZJ, Molyneaux N, Miron A, Adams MD, Elston RC, Markowitz SD, Willis JE. 2015. Novel recurrently mutated genes in African American colon cancers. Proc Natl Acad Sci U S A 112:1149–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keenan T, Moy B, Mroz EA, Ross K, Niemierko A, Rocco JW, Isakoff S, Ellisen LW, Bardia A. 2015. Comparison of the genomic landscape between primary breast cancer in African American versus white women and the association of racial differences with tumor recurrence. J Clin Oncol 33:3621–3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.