Abstract
SECISBP2 is an essential factor in selenoprotein synthesis, and its mutations result in a multiorgan syndrome, including abnormal thyroid hormone metabolism. A 10-year-old obese Turkish boy born to consanguineous parents presented with high thyroxine, low triiodothyronine, high reverse triiodothyronine, and normal or slightly elevated thyrotropin. He also had attention-deficit disorder and muscle weakness but no delay in growth or bone age. Sequencing of genomic DNA revealed a novel c.800_801insA, p.K267Kfs*2 mutation, homozygous in the proband and heterozygous in both parents and his brother. Studies showed reduction in several selenoproteins in serum and fibroblasts.
Keywords: : SECISBP2, SBP2, selenium, selenoprotein, deiodinase, thyroid hormone metabolism defect
Introduction
Humans express 25 selenoproteins. SECISBP2 interacts with the selenoprotein insertion sequence elements in the 3′-untranslated regions of selenoprotein mRNAs to recode the UGA codon as a selenocysteine. Selenoproteins have diverse biological functions, including the control of cellular redox homeostasis (glutathione peroxidases), regulation of protein folding and degradation (SEP15), and thyroid hormone metabolism (deiodinases). SECISBP2 gene mutations were found to be associated with multiorgan defects, including abnormal thyroid hormone metabolism, myopathy, hearing loss, and male infertility (1,2). Here, a case is described with a novel homozygous SECISBP2 gene mutation that presented with abnormal thyroid function tests (TFTs) and muscle weakness.
Case History
The proband was born to first cousins of Turkish origin. Pregnancy, delivery at 37 weeks, birth weight, and early development were normal. He was 10 years old when one of the authors (G.C.) was consulted to evaluate the patient for obesity. He had leg weakness, fatigued easily, and had attention-deficit disorder with poor school performance. At 11 years and 5 months, his weight was 67.7 kg (SDS +2.02), his height was 148.2 cm (SDS −0.45), his body mass index (BMI) was 29.5 kg/m2 (SDS +2.19), and his bone age was 11 years. Tanner stage was III, with a testes volume of 10 mL. His mother's and father's BMIs were 29.6 and 28.4 kg/m2, respectively. Right-eye ptosis was present since infancy. He had no goiter (volume 1.7 mL on ultrasound; SDS −1.92). Neurological examination revealed Gowers' sign, indicating weakness of the proximal lower-limb muscles. Muscle biopsy was normal, though electromyography revealed myogenic involvement of the proximal upper and lower extremities, and magnetic resonance imaging (MRI) showed fatty infiltration of the muscles. His IQ score was 91. His audiogram and echocardiogram were normal.
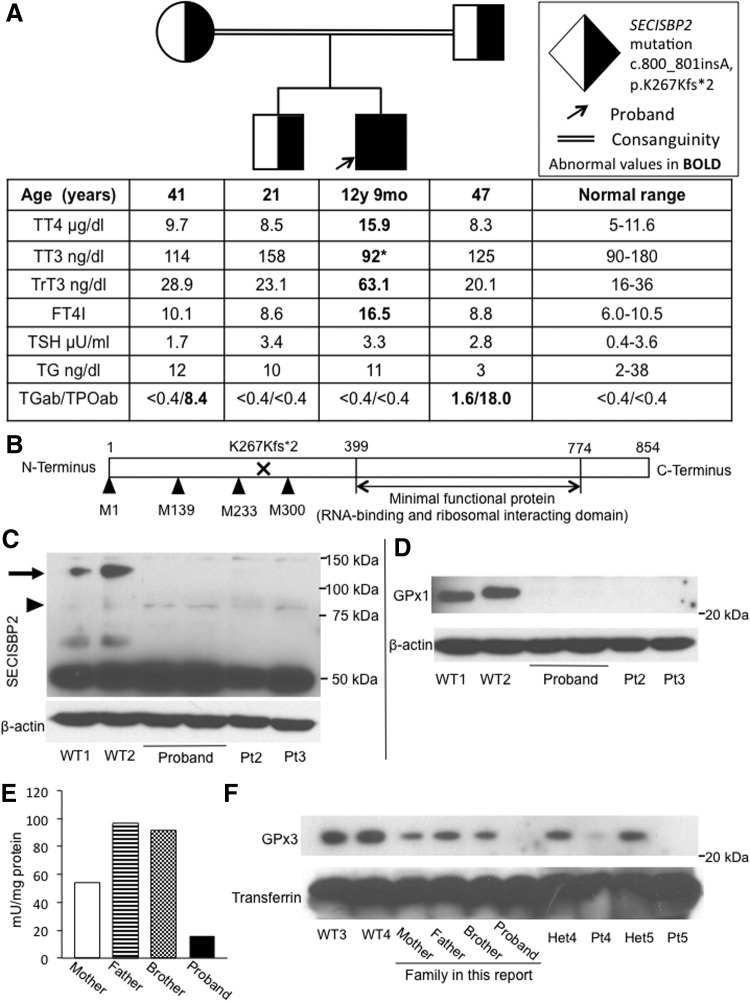
TFTs revealed elevated free thyroxine (T4), low free triiodothyronine (T3), elevated reverse T3, and normal or slightly elevated thyrotropin levels (Fig. 1A). His HbA1c was 5.6%, but his plasma glucose on oral glucose tolerance test (OGTT) was 80, 220, and 150 mg/dL at 0, 60, and 120 minutes, respectively. Neither of his parents had diabetes mellitus, and an OGTT was not performed in them. Serum selenium (Se) was low at 23 μg/dL (reference range 63–160 μg/dL).
FIG. 1.
(A) Pedigree of the family, indicating the genotype of each individual and the results of thyroid function tests, aligned with the symbol of each family member. Note that in addition, the parents have autoimmune thyroid disease. *Low triiodothyronine (T3) value for the patient's age. (B) Graphic representation of the SECISBP2 protein: the positions of the methionines used as alternative translation initiation sites are indicated. Western blot for (C) SECISBP2 and (D) GPX1, in extracts of cultured skin fibroblasts from the proband and controls, consisting of unrelated individuals without SECISBP2 mutations (WT) and patients with biallelic SECISBP2 mutations (Pt): Pt2 is homozygous for SECISBP2 p.R540Q (1) and Pt3 is compound heterozygous for SECISBP2 p.Q782* and p.K682fs*1 (5); beta-actin was used as loading control. In (C), the arrow indicates full-length isoform and the arrowhead indicates short isoform of SECISBP2. (E) GPX enzymatic activity in serum of family members. (F) Western blot for GPX3 in serum from family members, normal controls (WT), and patients with biallelic SECISBP2 mutations and heterozygotes family members (Het). Pt4 is homozygous and Het4 is heterozygous for SECISBP2 p.R540Q (1), Pt 5 is homozygous, and Het5 is heterozygous for SECISBP2 p.R128* (3). Transferrin was used as a loading control.
Results
Informed consent was obtained for investigations approved by the Institutional Review Board. The entire coding region of the SECISBP2 gene was sequenced using genomic DNA. A c.800_801insA p.K267Kfs*2 mutation was found; the proband was homozygous, both parents and the brother were heterozygous for the mutation (Fig. 1A). Graphic representation of the SECISBP2 protein shows the location of the mutation relative to the functional domains and the alternative methionine translation initiation sites (Fig. 1B).
Supplementation of 200 μg/day sodium selenite for five months followed by 400 μg/day for three further months increased the serum Se from 23 to 74.5 μg/dL. However, serum T4 remained high and T3 low.
At the age of 12 years and 8 months, his Tanner stage was III, with testes volumes of 12 and 15 mL, and his bone age was 13 years. However, his growth velocity decreased to 3.8 cm/year (−1.46 SDS), and his height curve decreased from the 50th to the 25th percentile (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/thy). Considering the obesity and concurrent reduction in growth velocity, Cushing syndrome was suspected but was ruled out with appropriate testing. MRI of the pituitary and hypothalamus was normal. Although IGF-1 was in the reference range at 315 ng/mL (143–693 ng/mL), provocative testing showed insufficient growth hormone (GH) responses to clonidine and L-DOPA stimulations, with peak GH levels of 4.61 and 1.62 ng/mL, respectively. Sex steroid priming was not done, as he was pubertal with Tanner stage III. GH replacement therapy was not initiated, as the parents did not consent to this.
Western blotting of the proband's cultured skin fibroblasts showed lack of full-length SECISBP2 protein (Fig. 1C). Assessment of selenoproteins showed greatly diminished GPX1 protein levels in fibroblasts, similar to other reports in SECISBP2-deficient patients (Fig. 1D) (1,2). The enzymatic activity of GPX in the serum was also markedly decreased (Fig. 1E), with the proband's GPX activity being the lowest compared to the other family members. This was also confirmed at the protein level, with serum GPX3 protein levels being markedly decreased in the proband and diminished in the heterozygous parents and brother (Fig. 1F). This was also the case with biallelic and monoallelic SECISBP2 mutations in two other families (Fig. 1F). These data indicate that the defective expression of full-length SECISBP2 caused a global decrease in selenoprotein synthesis in the proband as in other subjects with SECISBP2 deficiency.
Discussion
This novel homozygous early termination mutation leads to absence of the full-length SECISBP2 protein. However, a shorter isoform starting from methionine 300 containing the C-terminal functional domain is expected to be translated (3) and to provide some residual SECISBP2 function. Different from other SECISBP2 deficient patients, this child had normal height and bone age. However, decreased growth velocity developed at 13 years of age, and provocative testing showed GH deficiency, not previously reported in SECISBP2-deficient patients.
Single patients with increased fat mass and insulin sensitivity as well as recurrent fasting hypoglycemia with low insulin levels have been reported (2). This is attributed in part to decreased GPX1 expression because Gpx1-null mice showed increased insulin sensitivity. In contrast, this patient had impaired glucose tolerance in spite of decreased GPX1. His genetic and environmental background may have affected insulin sensitivity independently of SECISBP2 deficiency.
As in a previous report (4), supplementation of sodium selenite for even longer duration did not alter significantly the serum T4 and T3 abnormalities, despite normalization of the selenium level.
Supplementary Material
Acknowledgments
This work was supported by NIH grant DK110322 (A.M.D.). We thank Miles Tracy for performing the TFTs and Dr. Samuel Refetoff for revision of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Dumitrescu AM, Liao XH, Abdullah MS, Lado-Abeal J, Majed FA, Moeller LC, Boran G, Schomburg L, Weiss RE, Refetoff S. 2005. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet 37:1247–1252 [DOI] [PubMed] [Google Scholar]
- 2.Schoenmakers E, Agostini M, Mitchell C, Schoenmakers N, Papp L, Rajanayagam O, Padidela R, Ceron-Gutierrez L, Doffinger R, Prevosto C, Luan J, Montano S, Lu J, Castanet M, Clemons N, Groeneveld M, Castets P, Karbaschi M, Aitken S, Dixon A, Williams J, Campi I, Blount M, Burton H, Muntoni F, O'Donovan D, Dean A, Warren A, Brierley C, Baguley D, Guicheney P, Fitzgerald R, Coles A, Gaston H, Todd P, Holmgren A, Khanna KK, Cooke M, Semple R, Halsall D, Wareham N, Schwabe J, Grasso L, Beck-Peccoz P, Ogunko A, Dattani M, Gurnell M, Chatterjee K. 2010. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J Clin Invest 120:4220–4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Cosmo C, McLellan N, Liao XH, Khanna KK, Weiss RE, Papp L, Refetoff S. 2009. Clinical and molecular characterization of a novel selenocysteine insertion sequence-binding protein 2 (SBP2) gene mutation (R128X). J Clin Endocrinol Metab 94:4003–4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schomburg L, Dumitrescu AM, Liao XH, Bin-Abbas B, Hoeflich J, Kohrle J, Refetoff S. 2009. Selenium supplementation fails to correct the selenoprotein synthesis defect in subjects with SBP2 gene mutations. Thyroid 19:277–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sillers L, Gönç N, Kandemir N, Alikasifoglu A, Dumitrescu AM, Weiss RE, Refetoff S 2012 Selenoprotein deficiency syndrome caused by novel compound heterozygous mutations in the SBP2 gene. Poster MON-590, 94th Annual Meeting of the Endocrine Society, Houston, TX, June 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.