Abstract
Citrus bacterial canker (CBC) caused by Xanthomonas citri subsp. citri (Xcc), is the most devastating of the citrus diseases worldwide. During our study, we found that Essential oils (EOs) of some citrus cultivars are effective on Xcc. Therefore, it prompted us to determine the plant metabolites responsible for the antibacterial properties. We obtained EOs from some locally cultivated citrus by using a Clevenger apparatus and their major constituents were identified by gas chromatography/mass spectrometry (GC-MS). The effect of Citrus aurantium, C. aurantifolia, Fortunella sp. EOs and their major constituents were evaluated against Xcc-KVXCC1 using a disk diffusion assay. Minimal inhibitory and bactericidal concentration of the EOs and their constituents were determined using the broth microdilution method. C. aurantium, C. aurantifolia Eos, and their major constituents including citral, linalool, citronellal, geraniol, α-terpineol, and linalyl acetate indicated antibacterial effects against Xcc. The C. aurantifolia EO and citral showed the highest antibacterial activity among the tested EOs and constituents with inhibition zones of 15 ± 0.33 mm and 16.67 ± 0.88 mm, respectively. Synergistic effects of the constituents were observed between α-terpineol-citral, citral-citronellal, citral-geraniol, and citronellal-geraniol by using a microdilution checkerboard assay. Transmission electron microscopy revealed that exposure of Xcc cells to citral caused cell wall damage and altered cytoplasmic density. We introduced C. aurantifolia and C. aurantium EOs, and their constituents citral, α-terpineol, citronellal, geraniol, and linalool as possible control agents for CBC.
Keywords: Citrus aurantifolia, C. aurantium, citral, synergistic effect, transmission electron microscopy
1. Introduction
Citrus bacterial canker (CBC) is one of the most devastating citrus diseases which limits the cultivation of citrus plants in some tropical areas. The disease is caused by Xanthomonas citri subsp. citri (Xcc). The disease symptoms appear as crater-like lesions with a raised margin and sunken center surrounded by a yellow halo [1,2,3]. In severe infections, defoliation, twig dieback, badly blemished fruit, premature fruit drop, and reduction in quantity and quality of the fruits have been observed [4,5,6]. Different factors such as warmth, humidity, rainfall, wind, and leaf miners could affect the development and distribution of the disease. Several strategies including sanitation, resistant varieties, windbreaks, along with garden and plant treatment with bactericides have been proposed in order to control CBC [1,7,8]. Today, the use of bactericides are the most effective solution to manage CBC [8]. In order to increase public awareness regarding environmental problems associated with pesticides and antibiotics, a search for natural compounds to manage plant diseases seems inevitable [9,10]. Biological control by EOs could be an appropriate alternative for pesticides. These natural substances are aromatic, rarely colored, lipid soluble, and also soluble in organic solvents. They have been identified as safe constituents for prevention and therapy of plant-pathogenic microorganisms [9,11,12]. Antimicrobial properties of EOs are a result of the pivotal role of ketones, phenols and terpenes [13,14,15]. The antibacterial effect of EOs is associated with disturbance in coordinated ion flow of the cytoplasmic membrane via enhancement of membrane permeability or lipid depolymerisation. They also transform the structural makeup of the fatty acids, polysaccharides, proteins, and phospholipid layers in the membrane and cell wall of mitochondria. Some vital processes of the cells, such as energy conversion processes, nutrient processing, synthesis of macromolecules, and secretion of many growth regulators were impaired by EOs [13,14,16]. Fisher and Phillips have shown morphological changes of the blend effect of C. sinensis and C. bergamia against Enterococcus faecium and E. faecalis with the use of transmission electron microscopy (TEM) [17]. The citrus’s EO including lemon, bitter orange and kumkuat are widely used in medicine, cosmetic products, agriculture, and food industries [18]. Asnaashari et al. [19] introduced d-limonene (28.27%), α-terpineol (19.61%), p-cymene (8.58%), β-pinene (5.70%), 4-terpineol (4.76%), and linalool (2.39%) as major constituents of C. aurantifolia EO. In a survey conducted by Monsef-Esfahani et al. [20], the major constituents of C. aurantium were identified as geraniol (26.6%), α-terpineol (20.7%), linalool (15.4%) and benzene acetaldehyde. Several studies have reported the antibacterial effect of citrus EOs such as C. aurantium C. sinensis, C. limon, C. reticulata, and C. aurantifolia [21,22,23,24]. The geographical region can affect the EO composition and their antibacterial properties. Therefore, the purpose of this study was to explore the potential role of locally cultivated citrus EOs and their major constituents on growth of Xcc. Furthermore, we evaluated the role of citral as the most effective antibacterial constituent by TEM.
2. Results
2.1. Antibacterial Activity Assays
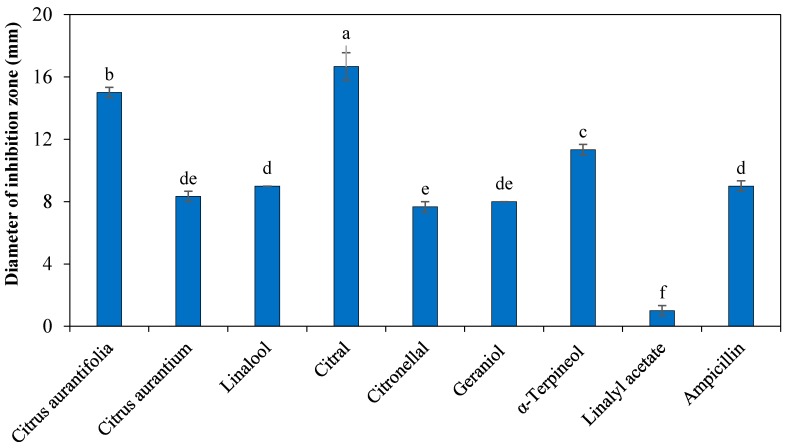
The effect of C. aurantium, C. aurantifolia, Fortunella sp. EOs, and their major constituents were investigated against Xcc-KVXCC1 using a disk diffusion assay. A broad variation in the antibacterial properties of EO and their constituents against Xcc-KVXCC1 were observed. Our results indicated that C. aurantium, C. aurantifolia EOs, and their major constituents including citral, linalool, citronellal, geraniol, α-terpineol, and linalyl acetate have antibacterial effects against Xcc-KVXCC1 (Figure 1). Antibacterial activity was not observed for Fortunella sp., limonene, geranyl acetate and trans-caryophllene. The C. aurantifolia and citral showed the highest inhibition zones of 15 ± 0.33 mm and 16.67 ± 0.88 mm, respectively. Furthermore, citral, α-terpineol and C. aurantifolia EO showed more antibacterial activity than ampicillin. On the other hand, linalyl acetate (1 ± 0.33 mm) demonstrated the weakest inhibitory effects against Xcc-KVXCC1.
Figure 1.
Antibacterial effect of essential oils and their constituents against Xcc-KVXCC1 isolates of Xanthomonas citri subsp. citri. Different letters indicate significant differences according to Duncan analysis using SPSS software (p = 0.05). All data represent means ± standard error of the mean (SEM) from 3 independent experiments. All data represent means ± standard error of the mean (SEM) from 3 independent experiments.
2.2. GC-MS Analysis
EOs of C. aurantium, C. aurantifolia, and Fortunella sp. were obtained from leaves via a hydrodistillation method. The three EOs were analyzed by gas a chromatography/mass spectrometry (GC-MS) system and their chemical compositions were identified based on the comparison of the substance’s mass spectrum, with NIST mass spectra library stored in the GC–MS database and Adams literature (Table 1).
Table 1.
Chemical composition of the essential oils of Citrus aurantifolia, C. aurantium and Fortunella sp.
| No. | Citrus aurantifolia | Citrus aurantium | Fortunella sp. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RI | Constituent | Percent | RI | Constituent | Percent | RI | Constituent | Percent | |
| 1 | 935 | α-pinene | 0.3 | 973 | sabinene | 2.1 | 952 | camphene | 0.2 |
| 2 | 978 | β-pinene | 3.4 | 978 | β-pinene | 1.7 | 1100 | linalool | 0.6 |
| 3 | 1028 | limonene | 47.2 | 992 | β-myrcene | 2.3 | 1168 | borneol | 0.9 |
| 4 | 1039 | cis-β-ocimene | 0.5 | 1028 | limonene | 2.3 | 1188 | α-terpineol | 0.4 |
| 5 | 1100 | linalool | 6.7 | 1042 | cis-β-ocimene | 2.3 | 1259 | trans-geraniol | 0.1 |
| 6 | 1133 | cis-limonene oxide | 0.2 | 1086 | α-terpinolene | 0.2 | 1335 | δ-elemene | 9.4 |
| 7 | 1142 | trans-limonene oxide | 0.1 | 1100 | linalool | 25.9 | 1351 | α-cubebene | 0.6 |
| 8 | 1154 | citronellal | 4.9 | 1188 | α-terpineol | 9.6 | 1377 | α-copaene | 1 |
| 9 | 1201 | (4Z)-decanal | 0.1 | 1250 | geraniol | 1.7 | 1392 | β-elemene | 5 |
| 10 | 1227 | nerol | 0.1 | 1257 | linalyl acetate | 43.7 | 1417 | trans-caryophyllene | 7 |
| 11 | 1250 | geraniol | 9.8 | 1367 | neryl acetate | 0.8 | 1440 | β-gurjunene | 6 |
| 12 | 1341 | citral | 5.2 | 1386 | geranyl acetate | 2.5 | 1445 | aromadendrene | 0.3 |
| 13 | 1357 | citronellyl acetate | 0.3 | 1417 | trans-caryophyllene | 0.1 | 1448 | α-humulene | 2.5 |
| 14 | 1367 | neryl acetate | 2.1 | 1448 | α-humulene | 0.2 | 1481 | germacrene d | 17.4 |
| 15 | 1386 | geranyl acetate | 9.3 | 1508 | α-farnesene | 0.1 | 1487 | β-selinene | 1.6 |
| 16 | 1417 | trans-caryophyllene | 3.9 | 1725 | farnesol | 0.1 | 1492 | δ-selinene | 0.5 |
| 17 | 1419 | cis-α-bergamotene | 0.3 | 1490 | valencene | 7.3 | |||
| 18 | 1448 | α-humulene | 0.4 | 1507 | (E)-α-farnesene | 0.4 | |||
| 19 | 1458 | (E)-β-farnesene | 0.7 | 1509 | ϒ-cadinene | 2 | |||
| 20 | 1582 | caryophyllene oxide | 0.8 | 1519 | δ-cadinene | 1.9 | |||
| 21 | 1677 | (Z)-nerolidyl acetate | 0.1 | 1549 | elemol | 7.9 | |||
| 22 | 1725 | (2E,6Z)-farnesol | 0.1 | 1553 | germacrene B | 8.5 | |||
| 23 | 1563 | (E)-nerolidol | 1.5 | ||||||
| 24 | 1581 | spathulenol | 1.2 | ||||||
| 25 | 1590 | globulol | 2.1 | ||||||
| 26 | 1624 | 10-epi-γ-eudesmol | 1.3 | ||||||
| 27 | 1649 | β-eudesmol | 2.5 | ||||||
| 28 | 1693 | α-eudesmol | 6.7 | ||||||
| total | 96.5 | 95.6 | 96.8 | ||||||
A total of 23, 16 and 28 EO constituents of C. aurantifolia, C. aurantium and Fortunella sp. were identified, representing 96.5%, 95.6%, and 96.8% of them, respectively. The main chemical constituents of the C. aurantifolia EO were limonene (47.2%), geraniol (9.8%), geranyl acetate (9.3%), linalool (6.7%), citral (5.2%), citronellal (4.9%), and trans-caryophyllene (3.9%). According to the result presented in Table 1, linalyl acetate (43.7%), linalool (25.9%), and α-terpineol (9.6%) were the prime constituents of C. aurantium. Germacrene D (17.4%), germacrene B (8.5%), elemol (7.9%), valencene (7.3%), trans-caryophyllene (7%), α-eudesmol (6.7%), and β-gurjunene (6%) were found to be the major constituents of Fortunella sp.
2.3. Determination of MIC and MBC
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values of EOs and their constituents with bactericidal properties were determined and shown in Table 2. Different values of MIC for treatments against Xcc-KVXCC1 were observed. The MIC value for the EOs and their constituents ranged between 0.5 and 8.5. The lowest MIC value was related to the EO of C. aurantifolia and citral with 0.5 mg/mL and 0.375 mg/mL, respectively. In addition, the lowest and highest obtained MBC values for citral and linalyl acetate were 0.725 mg/mL and 14.5 mg/mL.
Table 2.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) value of EOs and their main constituents against Xcc-KVXCC1 isolates of Xanthomonas citri subsp. citri.
| Essential Oil/Compound | MIC mg/mL | MBC mg/mL |
|---|---|---|
| Citrus aurantifolia | 0.5 b | 0.9 b |
| Citrus aurantium | 1.3 g | 2 g |
| α-terpineol | 0.625 c | 1.1 c |
| citral | 0.375 a | 0.725 a |
| citronellal | 1 f | 1.41 f |
| geraniol | 0.9 e | 1.325 e |
| linalool | 0.85 d | 1.225 d |
| linalyl acetate | 8.5 h | 14.5 h |
Means with a different letter in a row are statistically significant at 1% level.
2.4. Synergist Assay
To investigate in vitro synergistic interaction of combinations of α-terpineol, citral, citronellal, geraniol, and linalool a microdilution checkerboard method was used. According to the obtained results, the synergism effect between α-terpineol-citral, citral-citronellal, citral-geraniol, and citronellal-geraniol were observed, and no antagonistic effect was showed between the tested constituents. In our study, the highest level of synergistic effect was related to a combination between citral-geraniol with 0/313 FIC index (Table 3).
Table 3.
FIC index EO of constituents against Xcc-KVXCC1 isolates of Xanthomonas citri subsp. citri.
| Compound | FIC Index | Activity |
|---|---|---|
| α-terpineol-citral | 0.44 | Synergistic |
| α-terpineol-citronellal | 1 | Indifferent |
| α-terpineol-geraniol | 0.625 | Additive |
| α-terpineol-linalool | 1 | Indifferent |
| citral-citronellal | 0.5 | Synergistic |
| citral-geraniol | 0.313 | Synergistic |
| citral-linalool | 0.625 | Additive |
| citronellal-geraniol | 0.625 | Additive |
| citronellal-linalool | 0.5 | Synergistic |
| geraniol-linalool | 0.625 | Additive |
2.5. Ultrastructural Changes of KVXCC1
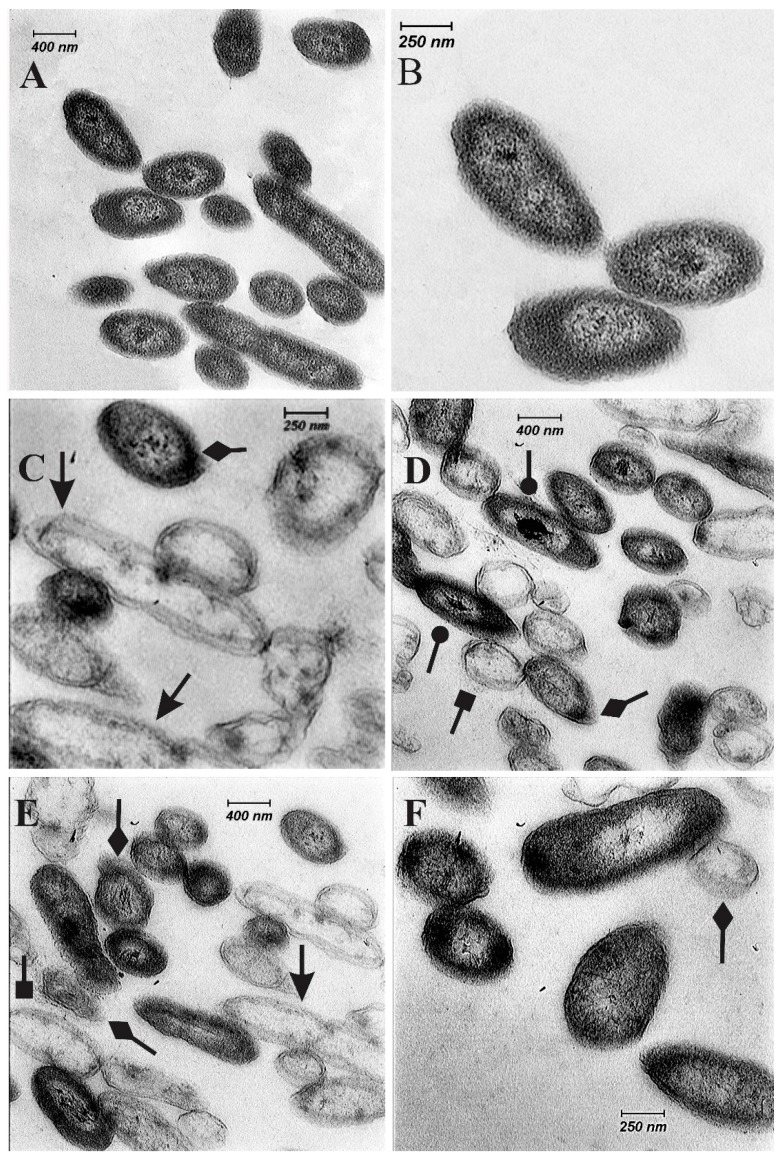
Untreated cells of Xcc-KVXCC1 in a TEM study did not show any changes in the cell structure. The cells indicated obvious unified cell structure including a plasma membrane, cell wall, nuclear substances, and uniform cytoplasm (Figure 2A,B). While transformations such as complete destruction of the cell, cell wall damage, colored nuclear area, alteration in cytoplasm density, and swelling in cells treated with citral constituent were observed (Figure 2D–F).
Figure 2.
Transmission electron micrographs of Xcc-KVXCC1 isolate of Xanthomonas citri subsp. citri exposed to MIC value of citral for 4 h. (A,B), control with complete cell wall; (C) bacterial cells exposed to streptomycin sulfate (25 mg·mL−1); (D–F), bacterial cells exposed to citral with apparent the complete destruction of the cell (arrows), damage of cell wall (arrows with lozenge), alteration in cytoplasm density (arrows with square), swelling and colored nuclear area (arrows with circle).
2.6. Time-Kill Assay
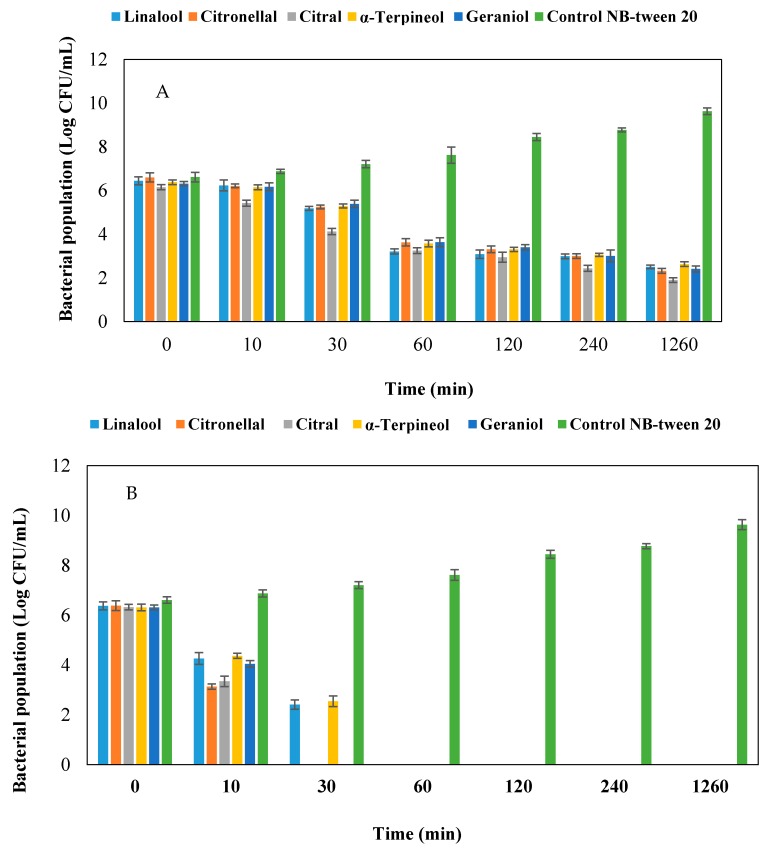
To analyze the killing rate of EO constituents, time-kill assays were used at values of MIC, 2.5× MIC and 4× MIC. The killing curves of EO constituents are presented in Figure 3. Linalool and α-terpineol at 2.5× MIC value killed the bacterial cell during the first hour. While others constituents led to cell death after 0.5 h of incubation. All the constituents murdered bacterial cells after 10 min at 4× MIC concentration.
Figure 3.
Time-kill kinetics of EO constituents against Xcc-KVXCC1 Xanthomonas citri subsp. citri at (A). MIC value and (B). 2.5× MIC value. All data represent means ± standard error of the mean (SEM) from three independent experiments.
3. Discussion
CBC is one of the most destructive citrus diseases in citrus-producing areas. However, the disease management strategies including sanitation; resistant varieties, windbreaks, antibiotics, copper bactericides, and leafminer control could not completely eliminate the disease. The limitation in using bactericides resources and probability of appearance resistance to the bactericides (copper) prompted us to search for new bactericides with no side-effect on human health. For this purpose, we selected some citrus cultivars which were resistant to CBC. The EOs were obtained from the leaves of these cultivars and their constituents were identified using GC-MS analysis [9,11,12,25].
According to our results obtained by the disk diffusion assay, the EOs and constituents of C. aurantifolia, C. aurantium, citral, α-terpineol, geraniol, linalool, citronellal, and linalyl acetate indicated antibacterial activity. Chudasama and Thaker [9] showed that the Cinnamomum cassia EO has the highest antibacterial activity against Xcc with growth inhibition zones of 59 mm, while C. aurantium inhibited the growth of Xcc to a lesser amount of 8 ± 1 mm. In another study, Taiwo et al. [26] demonstrated antibacterial effect of C. aurantifolia and Tithonia diversifolia on clinical bacteria. They showed that the C. aurantifolia EO inhibited the growth of Staphylococcus sp., Escherichia coli, Klebsiella sp., Proteus sp., and Pseudomonas sp. [26].
Several researchers have described the antibacterial activity of citral, linalool, α-terpineol, citronellal, geraniol, and linalyl acetate against different pathogens [25,27,28,29,30,31]. The antibacterial effect of citral against E. coli K12, Listeria innocua, and L. monocytogenes have been reported by Belda-Galbis et al. [32] and Silva-Angulo et al. [33]. However, there are few reports about the antibacterial effects of EOs constituents against phytopathogenic bacteria, especially Xcc. [29]. The mode of action of citral against bacteria and fungi has been attributed to its reaction with the DNA, outer membrane, plasma membrane, and cell wall damage [28,29,34,35]. In the current study, we have shown the effect of citral on the plasma membrane, cell wall, and the nuclear substances (Figure 2).
The antibacterial effect of linalool was shown against Gram-positive and Gram-negative bacterial strains. Silva et al. [33] suggested, that linalool (terpene-alcohol) could cause disruption of the negatively charged Gram negative bacterial outer membrane. Geraniol has antibacterial activity against E. coli ATCC 25922, P. mirabilis ATCC 12453, K. pneumoniae ATCC 700603, P. aeruginosa ATCC 27853, and S. aureus ATCC 29213 [36]. Li et al. [37] indicated antibacterial effect of α-terpineol on growth of E. coli. Geraniol and α-terpineol are monoterpene alcohols. It may be through interaction with the membrane of cells that geraniol permeates into the interior of the cell and causes disturbance in the function of the bacteria [36]. Li et al. [37], by using of TEM, showed that during different time points, α-terpineol caused various change in the cell structure of E. coli such as plasmolysis, irregular cell shape, cell size decrease, cytoplasm lost, unequal division, and vacuolization of cells. Antibacterial activity of linalyl acetate attributed to a perturbation of the lipid fractions of bacterial plasma-membranes and the changes in membrane permeability [13].
We did not observe antibacterial activity of the geranyl acetate, limonene, and trans-caryophllene against Xcc-KVXCC. Limonene is in the terpene group. Based on the report of Nazzaro et al. [38], the terpene group had low or no antibacterial activity. For example, limonene had no antibacterial effect on Pseudomonas mirabilis and P. aeruginosa, while it had antibacterial effect on several pathogens [25,39,40].
By using GC-MS analysis, we identified 23, 16 and 28 molecules as major constituents of C. aurantifolia, C. aurantium, and Fortunella sp., respectively. Limonene (47.2%) constituted the highest percentage of C. aurantifolia EO. This result was in good agreement with other studies [22,39,40]. Similar to Babazadeh Darjazi [41] and Sarrou et al. [42], our data indicated that linalyl acetate (43.7%), linalool (25.9%), and α-terpineol (9.6%) were the major constituents of the C. aurantium EO, while these results were in contrast with the findings of Ellouze et al. [23], Caccioni et al. [43] and Rahimi et al. [44]. The EO obtained from the leaves of Fortunella sp. were found to contain 28 constituents with germacrene D (17.4%), germacrene B (8.5%), elemol (7.9%), valencene (7.3%), trans-caryophyllene (6.9%), α-eudesmol (6.7%), and β-gurjunene (6%) as the main constituents. The data obtained was broadly consistent with several studies [45,46]. The observed difference between the compound percentages and type of each EO’s compound (the type, number and percentage of constituents) depends on plant tissues such as leaves, peel and flower, developmental stage, extraction methods, temperature variation, different genotype, climatic conditions, season’s variation, relative humidity, and soil composition [13,23,42,44].
In the present study, the MICs for the selected EOs and their constituents were between 0.5 mg/mL and 8.5 mg/mL. Frassinetti et al. [24] evaluated the antimicrobial activity of EOs from Citrus spp. against ten strains of gram positive and negative bacteria. They showed that the MIC of the EOs against bacterial strains ranged between 15 µg/mL and 250 µg/mL and the lowest amount of MIC belonged to C. lemon against Xcc. In the study of Shi et al. [47], the MIC value of citral against Cronobacter sakazakii strains that was determined ranged from 0.27 mg/mL to 0.54 mg/mL. The citral in comparison with most EOs constituents is known to be a stronger antibacterial constituent. Park et al. [48] reported the MIC and MBC values of linalool and α-terpineol against periodontopathogens between 0.1 to 1.6 mg/mL. Furthermore, MIC of BIOLL+, a commercial extract obtained from citrus fruits, has a range from 10 ppm to 80 ppm for strains of Brachyspira hyodysenteriae, Salmonella enterica, and E. coli [16]. Li et al. [49] reported a MIC value of amicarthiazol against three amicarthiazol-resistant mutants and wild-type of Xcc between 400 µg/mL to 100 µg/mL.
In the present study, the synergistic effects among α-terpineol-citral, citral-citronellal, citral-geraniol, and citronellal-geraniol were observed (Figure 4). The combination of citral with α-terpineol, citronellal, and geraniol induced a synergistic activity against Xcc-KVXCC1 and in combination with linalool caused an additive effect. Many researchers have reported a synergistic relationship between citral-ε-PL, piperacillin-cinnamon bark oil, savory oil-chloramphenicol, savory oil-tetracycline, geraniol-chloramphenicol, and piperacillin-lavenderfor E. coli strains [36,47,50]. Using a combination of EO constituents could lead to a reduction in the effective dose of constituents and expand the antibacterial spectrum [51,52].
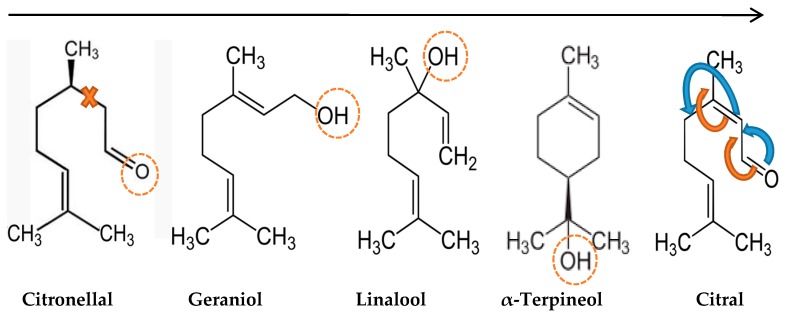
Figure 4.
Schematic overview of the chemical structure of major essential oils. The straight black arrow shows the toxicity of the constituents subsequently. The curved red and blue arrows indicates different positions for double bands which makes two different isomers for citral. The circle dashed lines demonstrate the hydroxyl groups which probably attach to the bacteria. The cross red lines shows no possibility for citronellal to make any isomer.
In a time killing assay, reduction in bacterial population size was different. The highest population reduction rate at MIC was in the range of log 6.15 to log 1.9 of citral constituent. While, the lowest population reduction rate was attributed α-terpineol. In this study, the highest population reduction size occurred at zero to one hour, and the killing effect of constituents had a descending trend. By increasing the concentration of the constituents, the time required for killing of the bacteria has decreased.
4. Materials and Methods
4.1. Bacterial Strains, Culture Conditions, and Chemical Constituents
The antibacterial effects of citrus EOs were screened on a hypervirulent strain of Xcc obtained from Kerman province, Iran (Xcc-KVXCC1). The bacteria was grown on NA (Nutrient Agar) at 28 °C for 24–48 h, unless stated otherwise. The bacterial cell concentration was synchronized to an optical density (OD) of 0.45 (1 × 108 CFU/mL) at 600 nm in Nutrient Broth (NB). The EO constituents including geranyl acetate, linalyl acetate, limonene, trans-caryophllene, linalool, citronellal, citral, α-terpineol, and geraniol were prepared from Sigma-Aldrich (Brussels, Belgium).
4.2. Essential Oils Extraction
The leaf samples of C. aurantifolia, Fortunella sp. and C. aurantium were collected from the plants grown at Ramsar greenhouses during the fruiting stage in the summer of 2014. All samples were washed with distilled water and dried out for 20 days at room temperature. EOs were extracted from leaf samples with hydrodistillation methods [53]. Fifty grams of crushed leaf samples were mixed with 500 mL of deionized water in a round-bottomed flask. Then the EO’s extraction was performed by using a Clevenger apparatus for 3 h. The extracted EOs were dried over anhydrous sodium sulfate and stored in refrigerator at 4 °C.
4.3. Disk Diffusion Assay
This method was used for primary screening of antibacterial properties of three EOs (C. aurantium, C. aurantifolia, and Fortunella sp.) and their major constituents against Xcc-KVXCC1 isolate [54]. A hundred microliter of the bacterial cultures (1 × 108 CFU/mL) in NB were inoculated for 24 h to the NA media to prepare homogenous culture plates. Then blank paper discs (6 mm) smeared with 10 mg of each of the three EOs and nine constituents. Subsequently, plates were incubated at 28 °C for 48 h and thereafter the diameters of bacterial growth inhibition zones around discs were measured. Ampicillin (100 mg/mL) and distilled water were used as positive and negative controls, respectively. The experiments were done in triplicate.
4.4. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
MIC is defined as the lowest concentration of an antimicrobial agent which prevents the growth of bacteria after 24 h. The MIC of C. aurantium, C. aurantifolia, and their major constituents including citral, linalool, citronellal, α-terpineol, and geraniol were determined using microdilution and macro-dilution methods [54,55,56]. The overnight culture of Xcc-KVXCC1 strain in NB medium was prepared and the bacterial populations were adjusted to an optical density (OD) of 0.4 at 600 nm including 108 CFU/mL bacteria. The 70 μL aliquots of NB medium were poured into the wells of ninety-six well tissue culture plates. Then 70 μL of NB medium including each of the EOs, or their tested constituents, were added to the wells at concentrations ranging from 30,000 µg/mL to 14 µg/mL. Finally, 10 μL of the bacterial suspensions were added to each well and the plates were incubated at 28 °C in a rotary shaker at 120 rpm.
To determine MBC, 30 μL of each well were cultured on the surface of NA medium and incubated for 48 h at 28 °C. The complete absence of bacterial growth was considered as MBC. To discover the exact MIC and MBC concentrations, a serial dilution in 5 mL falcon tubes including NB medium was performed between MIC and MBC concentrations that were obtained by a micro-dilution method. The experiment was performed in triplicate.
4.5. GC-MS Analysis
GC analyses were performed using a gas chromatograph (Thermo Quest 2000, Lancashire, UK) equipped with a DB-5 fused silica column (30 m × 0.2 mm, film thickness 0.32 μm). The analyzed condition of EOs consisted of: injection temperature: 260 °C, interface heating: 300 °C, ion source heating: 200 °C, EL mode: 70 eV, scan range: 41–450 amu and scan time 0.50 s. The GC-MS oven temperature was 55–120 °C (3 °C/min), 120–200 °C (4 °C/min), 200–220 °C (6 °C/min) and 220 for 5 min [42]. Percentages were calculated by electronic integration of FID, peak areas without the use of a response factor correction. GC/MS analyses were carried out on a Saturn-3400 GC-MS system (Thermo Quest Finningan, Lancashire, UK) equipped with a DB-5 fused silica column (30 m × 0.2 mm, film thickness 0.32 μm); with similar temperature programmed as in GC, transfer line temperature 260 °C, carrier gas He with a linear velocity of 31.5 cm/s, split ratio 1/60, ionization energy 70 eV.
The constituents of the essential oils were identified by calculation of their retention indices under temperature-programmed conditions on a DB-5 column under the same chromatographic conditions. Identification of individual compounds was made by comparison of their mass spectra with those of the internal reference mass spectra library or with authentic compounds and confirmed by comparison of their retention indices with authentic compounds or with those reported in the literature [57]. For quantification purposes, relative area percentages obtained by FID were used without the use of correction factors.
4.6. Identification of Synergistic Effects between EOs Constituents
The microdilution checkerboard method on 96-well plates was used to evaluate synergism effects of EO constituents (α-terpinolene, linalool, citronellal, geraniol, and citral) [58]. Seventy microliter of each dilution (2× MIC, 1× MIC, 1/2, 1/4, 1/8, 1/16, 1/32, and 1/64 MIC) were dispensed to each row, and then 70 μL of another constituent added to each row of the wells in the direction perpendicular to the previous constituents in different dilutions. Finally, 10 μL of NB media containing 1 × 108 CFU/mL was added to each well. The plates were incubated at 28 °C on a rotary shaker at 125 rpm for 24 h. All treatments were triplicated. A fractional inhibitory concentration index (FICI) of the dual combination of EO constituents were calculated by using the following formula:
Interaction of the combination of two substances was defined as a synergistic effect, if the FIC index was ≤0.5, additively if 0.5 < FICI < 1, indifferently if 1 < FICI ≤ 4, and antagonistically if FICI > 4.
4.7. Time-Kill Assay
A time-kill assay of EO constituents was carried out at 1× MIC, 2.5× MIC, and 4× MIC using the method of Gerits et al. [59]. The Xcc-KVXCC1 strain was inoculated to NB at 105 CFU/mL and incubated in orbital shakerat 28 °C for 24 h. Subsequently, a 100 μL aliquot was removed from each sample at different time points (0, 10, 30, 60, 120, 240, and 1260 min) and serially diluted in MgSO4 (10 mM). The number of viable cells was determined by the plate count technique. The distilled water and inoculated NB without EO constituents was used as the control. The experiments were performed in triplicate.
4.8. Preparation of Cells for TEM
In order to prepare Xcc-KVXCC1 cells for evaluating the citral effect on bacteria, overnight cultures of Xcc-KVXCC1 at 28 °C in NB was prepared for transmission electron microscopy (Leo 912 AB) analyses [12]. The citral was added to the bacterial culture at the MIC value. The bacterial suspensions were centrifuged at 6000 rpm for 7 min and cell pellets were pre-fixed on glutaraldehyde solution 2.5% (with cacodylate buffer 0.1 mol/L) for 24 h at −4 °C. Then samples were washed three times for 10 min with cacodylate buffer and post-fixed on 1.0% osmium tetroxide. The samples, which were washed on cacodylate buffer for 15, 30, and 60 min, were dehydrated in a graded ethanol series (30, 50, 70, 80, and 100%). Later, samples were embedded in propylene oxide and resin in ratios of 1:3, 1:1, 3:1, and pure resin for 12, 4, 12 and 24 h, the sample blocks were polymerized in an oven at 60 °C, for 48 h. Then sections of 75 nm by ultramicrotomy (Ultracut R, Leica, Wetzlar, Germany) were prepared and stained with 1% uranyl acetate and lead (II) nitrate. In this study, no-treated bacterial cells or streptomycin were used as negative and positive control, respectively.
5. Conclusions
Working on antibacterial and synergistic combinations in order to increase efficiency and prevent the development of resistance in bacteria against bactericides will help to control the disease caused by pathogens. C. aurantifolia and C. aurantium showed stronger antibacterial activity against the Xcc-KVXCC1 strain. The main constituents of EOs including citral, linalool, α-terpineol, and geraniol have shown proper antibacterial effect. Many studies have investigated synergistic effects of antibiotics and EOs against different pathogens. So far, no studies have been done about synergistic effect of the constituents α-terpineol, citral, citronellal, geraniol, and linalool against Xcc. In our study, synergistic, additive, and indifferent effects were showed, but no antagonistic effects were observed among treatments. Based on the present study, we introduced C. aurantifolia and C. aurantium EOs and constituents citral, α-terpineol, citronellal, geraniol, and linalool as possible control agents for CBC.
Acknowledgments
This study was supported by the Ferdowsi University of Mashhad with project number 3/31641 approved on 06/08/2014.
Author Contributions
H.M.-N., S.T., and P.T. wrote the manuscript and prepared the figures. M.G. collaborated in preparing of citrus cultivars and plant materials. S.T. revised and edited the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Graham J.H., Gottwald T.R., Cubero J., Achor D.S. Xanthomonas axonopodis pv. citri: Factors affecting successful eradication of citrus canker. Mol. Plant Pathol. 2004;5:1–15. doi: 10.1046/j.1364-3703.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 2.Pruvost O., Goodarzi T., Boyer K., Soltaninejad H., Escalon A., Alavi S., Javegny S., Boyer C., Cottyn B., Gagnevin L. Genetic structure analysis of strains causing citrus canker in Iran reveals the presence of two different lineages of Xanthomonas citri pv. citri pathotype A. Plant Pathol. 2015;64:776–784. doi: 10.1111/ppa.12324. [DOI] [Google Scholar]
- 3.Picchi S., Takita M., Coletta-Filho H., Machado M., Souza A. N-acetylcysteine interferes with the biofilm formation, motility and epiphytic behaviour of Xanthomonas citri subsp. citri. Plant Pathol. 2015;65:561–569. doi: 10.1111/ppa.12430. [DOI] [Google Scholar]
- 4.Leduc A., Traoré Y., Boyer K., Magne M., Grygiel P., Juhasz C., Boyer C., Guerin F., Wonni I., Ouedraogo L. Bridgehead invasion of a monomorphic plant pathogenic bacterium: Xanthomonas citri pv. citri, an emerging citrus pathogen in Mali and Burkina Faso. Environ. Microbiol. 2015;17:4429–4442. doi: 10.1111/1462-2920.12876. [DOI] [PubMed] [Google Scholar]
- 5.Deng Z., Xu L., Li D., Long G., Liu L., Fang F., Shu G. Screening citrus genotypes for resistance to canker disease (Xanthomonas axonopodis pv. citri) Plant Breed. 2010;129:341–345. doi: 10.1111/j.1439-0523.2009.01695.x. [DOI] [Google Scholar]
- 6.Escalon A., Javegny S., Vernière C., Noël L.D., Vital K., Poussier S., Hajri A., Boureau T., Pruvost O., Arlat M. Variations in type III effector repertoires, pathological phenotypes and host range of Xanthomonas citri pv. citri pathotypes. Mol. Plant Pathol. 2013;14:483–496. doi: 10.1111/mpp.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mustafa M., Imran M., Rasool A., Azeem M., Riaz A., Afzal M. Evaluation of commercial citrus cultivars for resistance to citrus leaf miner and its management. J. Entomol. Zool. Stud. 2014;2:213–216. [Google Scholar]
- 8.Ali A., Haider M.S., Hanif S., Akhtar N. Assessment of the antibacterial activity of Cuscuta pedicellata Ledeb. Afr. J. Biotechnol. 2014;13:430–433. [Google Scholar]
- 9.Chudasama K.S., Thaker V.S. Screening of potential antimicrobial compounds against Xanthomonas campestris from 100 essential oils of aromatic plants used in India: An ecofriendly approach. Arch. Phytopathol. Plant Prot. 2012;45:783–795. doi: 10.1080/03235408.2011.595967. [DOI] [Google Scholar]
- 10.Machial C. M., Shikano I., Smirle M., Bradbury R., Isman M.B. Evaluation of the toxicity of 17 essential oils against Choristoneura rosaceana (Lepidoptera: Tortricidae) and Trichoplusia ni (Lepidoptera: Noctuidae) Pest Manag. sci. 2010;66:1116–1121. doi: 10.1002/ps.1988. [DOI] [PubMed] [Google Scholar]
- 11.Samavi S., Hassanzadeh N., Faghihi M., Danesh Y.R. Effects of thyme (zaatar) essential oil and some chemical compounds in the control of citrus bacterial canker in Iran. J. Plant Pathol. 2009;91:691–696. [Google Scholar]
- 12.Lucas G.C., Alves E., Pereira R.B., Perina F.J., Souza R.M.D. Antibacterial activity of essential oils on Xanthomonas vesicatoria and control of bacterial spot in tomato. Pesq. Agropec. Bras. 2012;47:351–359. doi: 10.1590/S0100-204X2012000300006. [DOI] [Google Scholar]
- 13.Akthar M.S., Degaga B., Azam T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: A review. IBSPR. 2014;2:1–7. [Google Scholar]
- 14.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Golmakani M.T., Moayyedi M. Comparison of heat and mass transfer of different microwave-assisted extraction methods of essential oil from Citrus limon (Lisbon variety) peel. Food Sci. Nutr. 2015;3:506–518. doi: 10.1002/fsn3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Álvarez-Ordóñez A., Carvajal A., Arguello H., Martínez-Lobo F., Naharro G., Rubio P. Antibacterial activity and mode of action of a commercial citrus fruit extract. J. Appl. Microbiol. 2013;115:50–60. doi: 10.1111/jam.12216. [DOI] [PubMed] [Google Scholar]
- 17.Fisher K., Phillips C. The mechanism of action of a citrus oil blend against Enterococcus faecium and Enterococcus faecalis. J. Appl. Microbiol. 2009;106:1343–1349. doi: 10.1111/j.1365-2672.2008.04102.x. [DOI] [PubMed] [Google Scholar]
- 18.Chutia M., Bhuyan P.D., Pathak M., Sarma T., Boruah P. Antifungal activity and chemical composition of Citrus reticulata Blanco essential oil against phytopathogens from North East India. LWT-Food Sci. Technol. 2009;42:777–780. doi: 10.1016/j.lwt.2008.09.015. [DOI] [Google Scholar]
- 19.Asnaashari S., Delazar A., Habibi B., Vasfi R., Nahar L., Hamedeyazdan S., Sarker S.D. Essential Oil from Citrus aurantifolia prevents ketotifen-induced weight-gain in mice. Phytother. Res. 2010;24:1893–1897. doi: 10.1002/ptr.3227. [DOI] [PubMed] [Google Scholar]
- 20.Monsef-Esfahani H.R., Amanzade Y., Alhani Z., Hajimehdipour H., Faramarzi M.A. GC/MS analysis of Citrus aurantium L. hydrolate and its comparison with the commercial samples. Iran. J. Pharm. Res. 2004;3:177–179. [Google Scholar]
- 21.Ammar A.H., Bouajila J., Lebrihi A., Mathieu F., Romdhane M., Zagrouba F. Chemical composition and in vitro antimicrobial and antioxidant activities of Citrus aurantium L. flowers essential oil (Neroli oil) Pak. J. Biol. Sci. 2012;15:1034. doi: 10.3923/pjbs.2012.1034.1040. [DOI] [PubMed] [Google Scholar]
- 22.Costa R., Bisignano C., Filocamo A., Grasso E., Occhiuto F., Spadaro F. Antimicrobial activity and chemical composition of Citrus aurantifolia (Christm.) Swingle essential oil from Italian organic crops. J. Essent. Oil Res. 2014;26:400–408. doi: 10.1080/10412905.2014.964428. [DOI] [Google Scholar]
- 23.Ellouze I., Abderrabba M., Sabaou N., Mathieu F., Lebrihi A., Bouajila J. Season’s variation impact on Citrus aurantium leaves essential oil: Chemical composition and biological activities. J. Food Sci. 2012;77:T173–T180. doi: 10.1111/j.1750-3841.2012.02846.x. [DOI] [PubMed] [Google Scholar]
- 24.Frassinetti S., Caltavuturo L., Cini M., Della Croce C., Maserti B. Antibacterial and antioxidant activity of essential oils from Citrus spp. J. Essent. Oil Res. 2011;23:27–31. doi: 10.1080/10412905.2011.9700427. [DOI] [Google Scholar]
- 25.Shimada T., Endo T., Fujii H., Rodríguez A., Peña L., Omura M. Characterization of three linalool synthase genes from Citrus unshiu Marc. and analysis of linalool-mediated resistance against Xanthomonas citri subsp. citri and Penicilium italicum in citrus leaves and fruits. Plant Sci. 2014;229:154–166. doi: 10.1016/j.plantsci.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Taiwo S., Oyekanmi B., Adesiji Y., Opaleye O., Adeyeba O. In vitro antimicrobial activity of crude extracts of Citrus aurantifolia Linn and Tithonia diversifolia Poaceae on clinical bacterial isolates. Int. J. Trop. Med. 2007;2:113–117. [Google Scholar]
- 27.Belda-Galbis C.M., Leufvén A., Martínez A., Rodrigo D. Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education. Volume 2. Formatex Research Center; Badajoz, Spain: 2013. Quantitative assessment of citral antimicrobial potential at different temperatures; pp. 1257–1264. [Google Scholar]
- 28.Somolinos M., García D., Condón S., Mackey B., Pagán R. Inactivation of Escherichia coli by citral. J. Appl. Microbiol. 2010;108:1928–1939. doi: 10.1111/j.1365-2672.2009.04597.x. [DOI] [PubMed] [Google Scholar]
- 29.Silva-Angulo A.B., Zanini S.F., Rosenthal A., Rodrigo D., Klein G., Martínez A. Comparative Study of the effects of citral on the growth and injury of Listeria innocua and Listeria monocytogenes cells. PLoS ONE. 2015;10:e0114026. doi: 10.1371/journal.pone.0114026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasoul M., Marei G., Abdelgaleil S. Evaluation of antibacterial properties and biochemical effects of monoterpenes on plant pathogenic bacteria. Afr. J. Microbiol. Res. 2012;6:3667–3672. [Google Scholar]
- 31.Zengin H., Baysal A.H. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules. 2014;19:17773–17798. doi: 10.3390/molecules191117773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belda-Galbis C.M., Pina-Pérez M.C., Leufvén A., Martínez A., Rodrigo D. Impact assessment of carvacrol and citral effect on Escherichia coli K12 and Listeria innocua growth. Food Control. 2013;33:536–544. doi: 10.1016/j.foodcont.2013.03.038. [DOI] [Google Scholar]
- 33.Silva F., Ferreira S., Queiroz J.A., Domingues F.C. Coriander (Coriandrum sativum L.) essential oil: Its antibacterial activity and mode of action evaluated by flow cytometry. J. Med. Microbiol. 2011;60:1479–1486. doi: 10.1099/jmm.0.034157-0. [DOI] [PubMed] [Google Scholar]
- 34.Leite M.C.A., Bezerra A.P.D.B., Sousa J.P.D., Guerra F.Q.S., Lima E.D.O. Evaluation of antifungal activity and mechanism of action of citral against Candida albicans. Evid. Based Complement. Altern. Med. 2014;2014 doi: 10.1155/2014/378280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saddiq A.A., Khayyat S.A. Chemical and antimicrobial studies of monoterpene: Citral. Pest. Biochem. Physiol. 2010;98:89–93. doi: 10.1016/j.pestbp.2010.05.004. [DOI] [Google Scholar]
- 36.Miladinović D., Ilić B., Kocić B., Miladinović M. An in vitro antibacterial study of savory essential oil and geraniol in combination with standard antimicrobials. Nat. Prod. Commun. 2014;9:1629–1632. [PubMed] [Google Scholar]
- 37.Li L., Shi C., Yin Z., Jia R., Peng L., Kang S., Li Z. Antibacterial activity of α-terpineol may induce morphostructural alterations in Escherichia coli. Braz. J. Microbiol. 2014;45:1409–1413. doi: 10.1590/S1517-83822014000400035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nazzaro F., Fratianni F., De Martino L., Coppola R., De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6:1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soković M., Glamočlija J., Marin P.D., Brkić D., van Griensven L.J. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15:7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Espina L., Gelaw T. K., de Lamo-Castellví S., Pagán R., García-Gonzalo D. Mechanism of bacterial inactivation by (+)-limonene and its potential use in food preservation combined processes. PLoS ONE. 2013;8:e56769. doi: 10.1371/journal.pone.0056769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Razzaghi-Abyaneh M., Shams-Ghahfarokhi M., Rezaee M.-B., Jaimand K., Alinezhad S., Saberi R., Yoshinari T. Chemical composition and antiaflatoxigenic activity of Carum carvi L., Thymus vulgaris and Citrus aurantifolia essential oils. Food Control. 2009;20:1018–1024. doi: 10.1016/j.foodcont.2008.12.007. [DOI] [Google Scholar]
- 42.Dongmo P.J., Tatsadjieu L., Tchinda E., Kuate J., Amvam P., Menut C. Essential oils of Citrus aurantifolia from Cameroon and their antifungal activity against Phaeoramularia angolensis. Afr. J. Agric. Res. 2009;4:354–358. [Google Scholar]
- 43.Babazadeh Darjazi B. Comparison of leaf components of sweet orange and sour orange (Citrus sp.) Int. J. Adv. Biol. Biomed. Res. 2013;1:1558–1568. [Google Scholar]
- 44.Sarrou E., Chatzopoulou P., Dimassi-Theriou K., Therios I. Volatile constituents and antioxidant activity of peel, flowers and leaf oils of Citrus aurantium L. growing in Greece. Molecules. 2013;18:10639–10647. doi: 10.3390/molecules180910639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caccioni D.R., Guizzardi M., Biondi D.M., Renda A., Ruberto G. Relationship between volatile components of citrus fruit essential oils and antimicrobial action on Penicillium digitatum and Penicillium italicum. Int. J. Food Microbiol. 1998;43:73–79. doi: 10.1016/S0168-1605(98)00099-3. [DOI] [PubMed] [Google Scholar]
- 46.Rahimi A., Hashemi P., Talei G.R., Borzuei M., Ghiasvand A.R. Comparative analyses of the volatile components of Citrus aurantium L. flowers using ultrasonic-assisted headspace SPME and hydrodistillation combined with GC-MS and evaluation of their antimicrobial activities. Anal. Bioanal. Chem. 2014;1:83–91. [Google Scholar]
- 47.Quijano C.E., Pino J.A. Volatile compounds of kumquat (Fortunella margarita (Lour.) Swingle) leaf oil. J. Essent. Oil Res. 2009;21:194–196. doi: 10.1080/10412905.2009.9700146. [DOI] [Google Scholar]
- 48.Ibrahim N.A., El-Hawary S.S., Mohammed M.M., Farid M.A., Abdel-Wahed N.A., Ali M.A., El-Abd E.A. Chemical composition, antiviral against avian Influenza (H5N1) virus and antimicrobial activities of the essential oils of the leaves and fruits of Fortunella margarita, Lour. Swingle, growing in Egypt. J. Appl. Pharm. Sci. 2015;5:006–012. [Google Scholar]
- 49.Shi C., Song K., Zhang X., Sun Y., Sui Y., Chen Y., Jia Z., Sun H., Sun Z., Xia X. Antimicrobial activity and possible mechanism of action of citral against Cronobacter sakazakii. PLoS ONE. 2016;11:e0159006. doi: 10.1371/journal.pone.0159006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park S.-N., Lim Y.K., Freire M.O., Cho E., Jin D., Kook J.-K. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe. 2012;18:369–372. doi: 10.1016/j.anaerobe.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Li J., Zhou M., Li H., Chen C., Wang J., Zhang Y. A study on the molecular mechanism of resistance to amicarthiazol in Xanthomonas campestris pv. citri. Pest. Manag. Sci. 2006;62:440–445. doi: 10.1002/ps.1187. [DOI] [PubMed] [Google Scholar]
- 52.Yap P.S.X., Krishnan T., Chan K.-G., Lim S. Antibacterial mode of action of Cinnamomum verum bark essential oil, Alone and in combination with piperacillin, against a multi-drug-resistant Escherichia coli Strain. J. Microbiol. Biotechnol. 2015;25:1299–1306. doi: 10.4014/jmb.1407.07054. [DOI] [PubMed] [Google Scholar]
- 53.Charles D.J., Simon J.E. Comparison of extraction methods for the rapid determination of essential oil content and composition of basil. J. Am. Soc. Hortic. Sci. 1990;115:458–462. [Google Scholar]
- 54.Militello M., Settanni L., Aleo A., Mammina C., Moschetti G., Giammanco G., Blàzquez M.A., Carrubba A. Chemical composition and antibacterial potential of Artemisia arborescens L. essential oil. Curr. Microbiol. 2011;62:1274–1281. doi: 10.1007/s00284-010-9855-3. [DOI] [PubMed] [Google Scholar]
- 55.Lapenda J., Silva P., Vicalvi M., Sena K., Nascimento S. Antimicrobial activity of prodigiosin isolated from Serratia marcescens UFPEDA 398. World J. Microbiol. Biotechnol. 2015;31:399–406. doi: 10.1007/s11274-014-1793-y. [DOI] [PubMed] [Google Scholar]
- 56.Bardaweel S.K., Tawaha K.A., Hudaib M.M. Antioxidant, antimicrobial and antiproliferative activities of Anthemis palestina essential oil. BMC Complement. Altern. Med. 2014;14 doi: 10.1186/1472-6882-14-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adams R.P. Identification of Essential oil Components by Gas Chromatography/Mass Spectrometry. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 58.Turgis M., Vu K.D., Dupont C., Lacroix M. Combined antimicrobial effect of essential oils and bacteriocins against foodborne pathogens and food spoilage bacteria. Food Res. Int. 2012;48:696–702. doi: 10.1016/j.foodres.2012.06.016. [DOI] [Google Scholar]
- 59.Gerits E., Blommaert E., Lippell A., O’Neill A.J., Weytjens B., De Maeyer D., Fierro A.C., Marchal K., Marchand A., Chaltin P. Elucidation of the mode of action of a new antibacterial compound active against Staphylococcus aureus and Pseudomonas aeruginosa. PLoS ONE. 2016;11:e0155139. doi: 10.1371/journal.pone.0155139. [DOI] [PMC free article] [PubMed] [Google Scholar]