Abstract
We report the use of voriconazole troughs to achieve appropriate therapeutic levels in treatment of a cutaneous Scedosporium apiospermum infection. Following heart transplantation, a 63-year-old immunocompromised patient presented with post-traumatic nodular lesions on his right shin. Pathology showed fungal yeasts with culture revealing Scedosporium apiospermum. According to therapeutic drug monitoring, initial voriconazole treatment was subtherapeutic requiring increased dosing until appropriate therapeutic trough levels were attained, and resolution of the fungal infection was achieved.
Keywords: Cutaneous Scedosporium apiospermum, Voriconazole, Trough, Transplant, Immunocompromised
1. Introduction
In recent years, Scedosporium apiospermum has gained recognition as a causative agent of infection in immunocompromised hosts, notably transplant recipients [1], [2], [3]. Even in immunocompetent hosts, traumatic inoculation can result in localized cutaneous infections [4]. Diagnosis can prove difficult owing to clinical and morphological similarity with other fungal species, namely Aspergillus and Fusarium [1], [4]. Accurate diagnosis is essential, as this opportunist is highly antifungal-resistant often requiring unconventional antifungal treatment [1], [2], [4], [5].
In this report, we present a case of cutaneous Scedosporium apiospermum infection treated with voriconazole. Therapeutic drug monitoring revealed subtherapeutic voriconazole levels requiring repeated dosage increases. Once therapeutic voriconazole levels were achieved, the patient's infection successfully resolved. A previous study reported successful use of voriconazole drug monitoring to treat lung scedosporiosis [6]. However, to our knowledge, this is the only reported case of cutaneous S. apiospermum infection utilizing voriconazole troughs to achieve therapeutic antifungal treatment.
2. Case
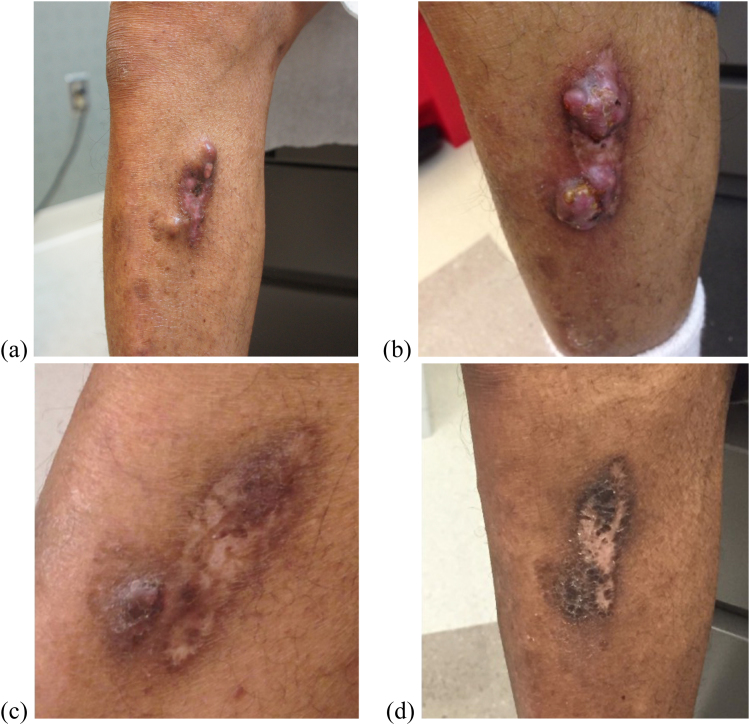
A 63-year-old African American male with past medical history significant for heart transplantation, on mycophenolic acid, prednisone, and tacrolimus immunosuppressant therapy, presented to dermatology on Day 0 for disseminated cutaneous zoster. The patient also reported sustaining an injury to his right shin two weeks prior, appearing on Day 0 as non-specific nodules and tumors (Fig. 1a).
Fig. 1.
Progression of cutaneous S. apiospermum infection. (a) Day 0 (b) Day 168 (c) Day 350 (d) Day 516.
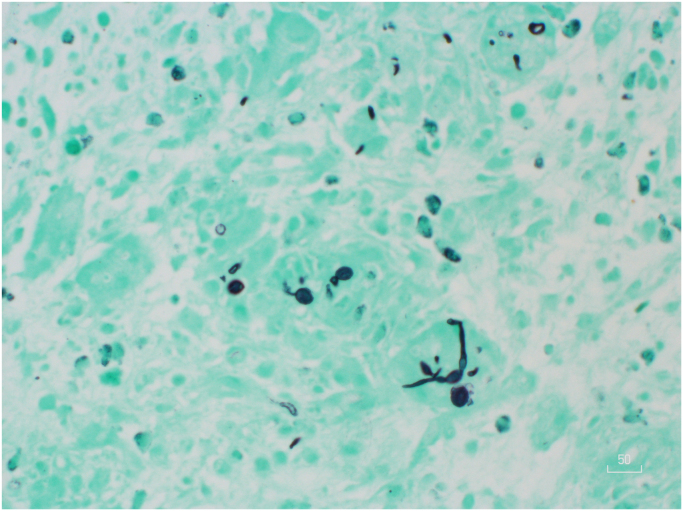
The patient returned for follow-up on Day 27 with healing cutaneous zoster lesions; however, the wound on his right shin was larger. A biopsy of the right shin revealed granulomatous inflammation and abscesses with numerous fungal yeasts and structures suggestive of pseudohyphae when stained with Grocott's methenamine silver (GMS) (Fig. 2). Subsequent fungal culture, processed by Clinical Pathology Laboratories in Austin, Texas using the lab's standard procedures*, revealed a Scedosporium apiospermum fungal infection for which the patient was referred to infectious disease. Treatment with this team began on Day 42 at which time the lesion was approximately 5 cm in length with two nodules at the superior and inferior borders. The lesion was freely movable above the underlying tibia, suggesting no bony involvement. At this time voriconazole 200 mg PO BID was prescribed. Therapeutic drug monitoring was also ordered due to the non-linear and idiosyncratic pharmacokinetics of voriconazole combined with its augmenting effects on tacrolimus levels, an immunosuppressant taken by the patient after his heart transplantation.
Fig. 2.
GMS stain showing numerous fungal yeasts and structures suggestive of pseudohyphae.
At his follow-up on Day 77, the patient reported tolerating the medication well; however, the wound was healing slowly. A voriconazole trough collected on Day 82 revealed a level of 0.1 mcg/mL, far below the therapeutic range of 1.0–5.5 mcg/mL [7]. The voriconazole dose was subsequently raised to 250 mg BID on Day 105 and again to 300 mg BID on Day 133 after trough levels continued to be low at 0.3 mcg/mL.
Delays in treatment were encountered due to multiple issues including patient being lost to follow-up and drug cost and consequently the skin lesion showed no improvement during this time (Fig. 1b). A voriconazole trough at the 300 mg dose was not collected until Day 250 which revealed a level of 0.6 mcg/mL. The dosage was increased to 400 mg BID on Day 259 and finally to a maximum dose of 500 mg BID on Day 308. By Day 350 marked improvement was noted in the size and elevation of the nodules (Fig. 1c). In the following months several voriconazole troughs were taken, many of which were in the therapeutic range reaching the maximal value of 2.0 mcg/mL. By Day 392 the cutaneous nodules were decreased in size and by Day 516 the skin lesions were essentially resolved (Fig. 1d). At this time, the patient was advised to continue treatment for an additional 3 months to achieve 6 months of therapy at therapeutic range.
*Clinical Pathology Laboratories tissue sample fungal procedure: Ground tissue is plated on the following media: sabouraud dextrose, sabouraud dextrose with clindamycin and cycloheximide, and brain heart infusion agar with gentamicin and chloramphenicol. Fungal structures are observed using lactophenol cotton blue tape preparation. No genetic analysis was performed.
3. Discussion
Scedosporium apiospermum is a highly antifungal resistant, opportunistic pathogen. This organism was previously considered the asexual form Pseudallescheria boydii; however, recent DNA sequence analysis show that these are two distinct species [8]. S. apiospermum infections present in various ways: 1) localized disease due to trauma 2) cavity colonization, and 3) systemic, invasive disease. In immunocompetent hosts, inoculation is typically trauma induced and results in a localized infection, specifically a subcutaneous mycetoma [1]. Most localized infections involve the feet or lower extremities. A mycetoma begins as a firm, small, painless nodule and progressively softens and ulcerates. In immunocompromised patients, such as the one reported in this case, the fungus produces scattered hyphae while in immunocompetent individuals the fungus will produce a grain formation [1], [9]. Localized cutaneous infections are rare, but most often occur in the immunosuppressed and solid organ transplant recipients [4], [5]. Invasive, systemic infections typically occur in immunocompromised hosts and victims of near drownings [1].
Due to antifungal resistance, treatment options for S. apiospermum infections are limited. Compared to other antifungals, such as amphotericin B and itraconazole, voriconazole shows superior activity and lower MIC values in the treatment of S. apiospermum [10], [11]. Furthermore, a large retrospective study including over 100 patients supports the use of voriconazole for the treatment of this species [12]. Voriconazole has successfully treated cutaneous, subcutaneous, and disseminated S. apiospermum infections [13], [14], [15]. While voriconazole is widely accepted as an appropriate treatment option for S. apiospermum, the appropriate dosing is less clear. Currently, the recommended dose according to the manufacturer is 200 mg PO BID; however, this dose often results in low serum concentrations and in such cases an increased dose is required to reach therapeutic levels [7]. When an appropriate dose is determined, antifungal therapy should continue until no signs and symptoms of infection persist, often requiring several months or even years of treatment.
In addition to antifungal treatment, surgical debridement is highly recommended due to improved outcomes [2]. For this patient, debridement was seriously considered, and plastic surgery was consulted. However, this option was not initially pursued due to the infection's proximity to the tibia which would require skin grafting to an area with slow healing rates. Thankfully, an increased dose of voriconazole resolved the infection and surgical intervention was not necessary.
For invasive Scedosporium infections, current guidelines suggest monitoring serum voriconazole levels five to seven days into therapy. The therapeutic range of voriconazole is 1.0–5.5 mcg/mL and, for sub-therapeutic trough concentrations, it is recommended to increase the dose of voriconazole [16]. Serum voriconazole trough levels were taken to both evaluate therapeutic serum levels and interactions with other medications, namely the calcineurin inhibitor tacrolimus. Voriconazole is an inhibitor of the CYP450 system and will thus increase levels of substances metabolized through this system. Consequently, during treatment with voriconazole, the patient's dose of tacrolimus was monitored and lowered with each increase in antifungal dosage in close collaboration with a transplant pharmacist.
Treatment of this patient was prolonged due to continuous sub-therapeutic concentrations of voriconazole; however, continued analysis via serum trough concentrations allowed the treatment team to find a dose that adequately controlled the infection. Thus, this case presents a strong argument for the use of voriconazole trough levels in all S. apiospermum infections treated with this antifungal.
Acknowledgements
None.
Acknowledgments
Conflict of interest
There are none.
Ethical form
Please note that this journal requires full disclosure of all sources of funding and potential conflicts of interest. The journal also requires a declaration that the author(s) have obtained written and signed consent to publish the case report from the patient or legal guardian(s).
The statements on funding, conflict of interest and consent need to be submitted via our Ethical Form that can be downloaded from the submission site www.ees.elsevier.com/mmcr. Please note that your manuscript will not be considered for publication until the signed Ethical Form has been received.
Contributor Information
McKenna E. Boyd, Email: mckenna.boyd@bcm.edu.
Vagish Hemmige, Email: vahemmig@montefiore.org.
References
- 1.Guarro J., Kantarcioglu A.S., Horré R., Rodriguez-Tudelas J., Estrella M.C., Berenguer J., Sybren de Hoog S. Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med. Mycol. 2006;44:295–327. doi: 10.1080/13693780600752507. [DOI] [PubMed] [Google Scholar]
- 2.Husain S., Muñoz P., Forrest G., Alexander B.D., Somani J., Brennan K., Wagener M.M., Singh N. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin. Infect. Dis. 2005;40(1):89–99. doi: 10.1086/426445. [DOI] [PubMed] [Google Scholar]
- 3.Nucci M. Emerging moulds: fusarium, Scedosporium and Zygomycetes in transplant recipients. Curr. Opin. Infect. Dis. 2003;16:607–612. doi: 10.1097/00001432-200312000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Miele P.S., Levy C.S., Smith M.A. Primary cutaneous fungal infections in solid organ transplantation: a case series. Am. J. Transpl. 2002;2:678–683. doi: 10.1034/j.1600-6143.2002.20716.x. [DOI] [PubMed] [Google Scholar]
- 5.Uenotsuchi T., Moroi Y., Urabe K. Cutaneous Scedosporium apiospermum infection in an immunocompromised patient and review of the literature. Acta Derm. Venereol. 2005;85:156–159. doi: 10.1080/00015550410024553. [DOI] [PubMed] [Google Scholar]
- 6.Ogata R., Hagiwara E., Shiihara J., Ogura T., Takahashi H., Kamei K. A case of lung scedosporiosis successfully treated with monitoring of plasma voriconazole concentration level. Nihon Kokyuki Gakkai Zasshi J. Jpn. Respir. Soc. 2011;49:388–392. (in Japanese) [PubMed] [Google Scholar]
- 7.Pascual A., Calandra T., Bolay S., Buclin T., Bille J., Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 2008;46(2):37–40. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 8.Gilgado F., Cano J., Gené J., Guarro J. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. J. Clin. Microbiol. 2005;43(10):4930–4942. doi: 10.1128/JCM.43.10.4930-4942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ichikawa T., Saiki M., Tokunaga S., Saida T. Scedosporium apiospermum skin infection in a patient with nephrotic syndrome. Acta Derm. Venereol. 1997;77:172–173. doi: 10.2340/0001555577172173. [DOI] [PubMed] [Google Scholar]
- 10.Cuenca-Estrella M., Ruiz-Diez B., Martinez-Suarez J.V., Monzon A., Rodriguez-Tudela J.L. Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J. Antimicrob. Chemother. 1999;43(1):149–151. doi: 10.1093/jac/43.1.149. [DOI] [PubMed] [Google Scholar]
- 11.Meletiadis J., JFGM Meis, Mouton J.W. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 2002;46(1):62–68. doi: 10.1128/AAC.46.1.62-68.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troke P., Aguirrebengoa K., Arteaga C., Ellis D., Heath C.H., Lutsar I., Rovira M., Nguyen Q., Slavin M., Chen S.C., Global Scedosporium Study Group Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob. Agents Chemother. 2008;52(5):1743–1750. doi: 10.1128/AAC.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girmenia C., Giovanni L., Monaco M., Martino P. Use of Voriconazole in treatment of Scedosporium apiospermum infection: case report. J. Clin. Microbiol. 1998;36(5):1436–1438. doi: 10.1128/jcm.36.5.1436-1438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stur-Hofmann K., Stos S., Saxa-Enenkel M., Rappersberger K. Primary cutaneous infection with Scedosporium apiospermum successfully treated with voriconazole. Mycoses. 2011;54(4):201–204. doi: 10.1111/j.1439-0507.2009.01799.x. [DOI] [PubMed] [Google Scholar]
- 15.Azofra M.M., Somovilla J.L.P., Porras M.C., Carrillo L.H., Pérez R.D. Use of intralesional voriconazole for the treatment of cutaneous Scedosporium apiospermum infection. Clin. Infect. Dis. 2010;51(2):255–257. doi: 10.1086/653683. [DOI] [PubMed] [Google Scholar]
- 16.Vfend® package insert. Roerig, a Division of Pfizer, Inc., New York, NY. 〈http://media.pfizer.com/files/products/uspi_vfend.pdf〉.