Abstract
Various thiazolidine-2,4-dione derivatives 3a-l possessing indole moiety were designed, synthesized using appropriate conventional heating as well as microwave irradiation methods. All the synthesized compounds were characterized physically and spectrally. The compounds were evaluated for in vitro antibacterial activity, in vitro antioxidant activity and in vivo hypoglycemic activity in relation to the standard drugs. Most of the new compounds exhibited moderate activity and some showed considerable activity. Molecular docking studies were carried out using AutoDock software and revealed that compound 3b has significant binding interaction with PPARγ receptor compared with the standard ligand Rosiglitazone.
Keyword: Pharmaceutical chemistry
1. Introduction
Diabetes mellitus is one of the life threatening causes found in the majority of the countries in the world due to impaired carbohydrate, protein and lipid metabolism. Thiazolidinediones (TZDs) are a novel class of hypoglycemic agents for the management of NIDDM (Non-insulin dependent diabetes mellitus); initially they were identified as antidiabetic drugs known to sensitize tissues to insulin. A deficient insulin discharge which translates into impaired glucose use is a characteristic feature of diabetes mellitus and results in hyperglycemia [1].
TZDs normalize elevated glucose levels in blood and therefore are used in the controlling type 2 diabetes. TZDs have high affinity towards Peroxisomal Proliferator Activated Receptor gamma type (PPARγ) receptors and act as insulin sensitizers at PPARγ receptors. Moreover, they stimulate peripheral adiposity increasing the free fatty acids uptake, which leads to decline the fat stored in muscles, liver and deposits of visceral fat. TZDs improve insulin sensitivity in liver, muscle and fat tissues and thus counteract insulin resistance. Ciglitazone is the first synthesized thiazolidinedione derivative having antihyperglycemic activity in the insulin resistant animal models, but it was withdrawn because of low potency and appearance of cataracts, anemia and oedema in animals. Troglitazone was failed to survive due to liver toxicity. Pioglitazone and Rosiglitazone are currently in clinical use (Fig. 1). These are also having drawbacks like hepatotoxicity [2], oedema, haematological toxicity and body weight gain problems [3].
Fig. 1.
Structures of Pioglitazone, Rosiglitazone, Ciglitazone and Troglitazone.
Recent studies with various thiazolidinedione derivatives were developed as they possess a wide variety of biological activities such as antimicrobial activity [4, 5], antihyperglycemic activity [6, 7], anti-inflammatory [8, 9], hypolipepidemic [10, 11], in vitro aldose reductase inhibitory activity [12], protein tyrosine phosphatase 1B inhibitory activity [13], 15-hydroxy prostaglandin dehydrogenase inhibitors [14], activators of PPARγ receptors [15], cytotoxic activity on different cell lines [16], antitubercular activity [17], antioxidant activity [18].
Nowadays microwave irradiated reaction techniques are broadly used in the development of organic compounds with or without presence of solvents because of the simplicity in reaction handling, the eco-friendly nature and high yields [19]. Microwave irradiation method can quickly increase the temperature, uniform heat transfer into the reaction, improve the yield and reduce the formation of by-products or the decomposition of products, in comparison to the conventional synthetic reactions [20, 21]. These considerations led us to develop novel bioactive TZDs substituted at 3rd position and 5th position using both conventional heating and microwave irradiated methods. Synthesized compounds were screened for antibacterial, antioxidant, antidiabetic activities and molecular docking studies were carried out on designed ligands to observe better efficacy property and binding interaction at the target site.
2. Materials and methods
2.1. Chemicals and instruments
All the chemicals (reagents and solvents) were purchased from commercial suppliers (Merck grade) and they were used further without purification. Raga's scientific microwave synthesis system (RGSSIRR model) with different power levels from 140 W to 700 W was used for microwave irradiation. Melting points were determined by using electrical melting point apparatus and were uncorrected. Progress and completion of the reaction was monitored by using commercially available pre-coated TLC plates (E. Merck 0.25 mm silica gel 60GF-254), spots were visualized by exposing the dry plates under UV-light and in iodine vapours. IR spectra were recorded (λmax in cm−1) on Bruker analyzer FT-IR spectrophotometer using KBr pressed pellet technique. 1H-NMR and 13C-NMR spectra were recorded on Bruker AMX-400 MHz spectrometer (chemical shifts in δ, ppm) in DMSO-d6 solvent using internal standard TMS. The mass spectra of the compounds were recorded on Agilent LC-MSD. Elemental analysis for C, H and N was carried out using elemental analyzer.
2.2. Chemistry
2.2.1. Synthesis of 1,3-thiazolidine-2,4-dione (1)
Conventional heating method: A solution of chloroacetic acid (1.89 g, 20 mmol) in water (5 mL) was added into a stirring solution of thiourea (1.52 g, 20 mmol) in a three-necked round bottom flask. The reaction mixture was stirred until white precipitate was formed. Concentrated solution of HCl (6 mL) was added dropwise slowly into the reaction mixture by a fitted dropping funnel. A reflux condenser is connected in the middle of the flask. The reaction mixture was heated at 100–110 °C for 10–12 hrs and then cooled down to room temperature. The resulting suspension was filtered off and the precipitate was well washed with water to remove the traces of HCl. The product was further purified by recrystallization from ethanol.
Microwave irradiation method: A mixture of chloroacetic acid (0.95 g, 10 mmol) and thiourea (0.76 g, 10 mmol) in water (3 mL) was added into Raga's scientific microwave synthesis reaction vessel. The vessel was sealed and the reaction mixture was stirred for about 1 hr at room temperature under ventilation. Conc. HCl (3 mL) was added into the reaction mixture, which was, then, irradiated by 280W power at 120 °C for 6 min. The reaction mixture was cooled to room temperature and the resulting solid was filtered off, well washed with water, dried and recrystallized from ethanol.
Yield 78.42% (conventional synthesis), 90.25% (MWI synthesis), white crystalline powder, mp 124–126 °C, Rf value is 0.61 from TLC of chloroform and methanol (9:1). IR [KBr ѵ cm−1]: 3321.46 (—NH—), 1718.95 (C O), 1776.94 (C O), 1303.29 (C—N), 2968.89 (C—H), 626.69 (C—S). 1H-NMR [400 MHz, δ, ppm, DMSO-d6]: 12.01 (1H, s, NH), 4.13 (2H, s, CH2). 13C-NMR [400 MHz, δ, ppm, DMSO-d6]: 173.8, 173.0, 35.8. ESI-MS: m/z (M+) 117.
2.2.2. Synthesis of 5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (2)
Conventional heating method: Thiazolidine-2,4-dione (1.17 g, 0.01 mol) 1 was added in a solution of indole-3-carboxaldehyde (1.45 g, 0.01 mol) in toluene (8 mL). Catalytic amount of piperidine (0.4 mL) was added to reaction mixture and the resulting mixture was refluxed for about 5–6 hrs at 110–120 °C using an oil bath. Upon completion of the reaction, monitored by TLC, the reaction mixture was allowed to cool to room temperature. 1M HCl and cold water was added to reaction mixture. The resulting solid was filtered and washed with cold water and dry toluene, then dried and further purified by recrystallization from ethanol.
Microwave irradiation method: Piperidine (0.4 mL) was added to a solution of thiazolidine-2,4-dione (1.17 g, 0.01 mol) and indole-3-carboxaldehyde (1.45 g, 0.01 mol) in toluene (8 mL) The reaction mixture was placed in Raga's scientific microwave synthesis reaction vessel, which was connected with a water condenser. The reaction mixture was irradiated at 350W for about 8 min at 120 °C. After completion of the reaction, the mixture was cooled and diluted with ice-water (15 mL), filtered, washed with cold water and dry toluene. The product was further purified by recrystallization from ethanol.
Yield 70.48% (conventional synthesis), 84.94% (MWI synthesis), yellow powder, mp186-188 °C, Rf value is 0.58 from TLC of benzene and ethyl acetate (8:2), IR [KBr ѵ cm−1]: 3320.08 (—NH—), 3288.49 (—NH—), 1687.44 (C O), 1718.98 (C O), 1352.89 (C—N), 3018.25 (C—H), 624.81 (C—S), 1687.44 (C C). 1H-NMR [400 MHz, δ, ppm, DMSO-d6]: 12.31 (1H, s, TZD-NH-), 12.14 (1H, s, indole-NH-), 8.06 (1H, s, CH— methylene), 7.18–7.90 (5H, d & t, indole-H). 13C-NMR [400 MHz, δ, ppm, DMSO-d6]: 167.6, 167.2, 136.2, 128.5, 126.7, 124.4, 123.0, 121.0, 118.2, 116.2, 112.3, 110.4. ESI-MS: m/z (M+) 244.
2.2.3. General procedure for synthesis of 3-[(substituted phenylamino)methyl]-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (3a-3l)
Conventional heating method: Formaldehyde (0.01 mol) was added in a solution of 5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (2) (0.005 mol) in DMF. The reaction mixture was stirred at room temperature for about 30 min. The solution of aryl amine (0.005 mol) in DMF was added to the above reaction mixture and then catalytic amount of conc. HCl (3–5 drops) was added. The reaction mixture was refluxed for 10–14 h at 120 °C and then, cooled at 2–8 °C for about 24 hrs. The reaction mixture was poured into crushed ice and the resulting solid was filtered, washed with cold water and dry toluene. The product was dried and recrystallized from ethanol. Completion of the reaction was monitored by TLC using an eluent, a mixture of solvents, n-hexane:ethylacetate (9:1).
Microwave irradiation method: To a solution of 5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (2) (0.005 mol) in of DMF (3 mL), formaldehyde (0.01 mol) was added and the reaction mixture stirred for about 20–30 min at room temperature. The solution of aryl amine (0.005 mol) in DMF and catalytic amount of conc. HCl (3–5 drops) were added to the above reaction mixture. The resulting mixture was placed in Raga's scientific microwave synthesis reaction vessel and was irradiated at 420W for about 8–12 min at 120 °C. The reaction mixture was cooled and diluted with ice cold water and the resulting solid was filtered, washed with cold water and dry toluene. The product was further purified by recrystallization from boiling ethanol.
2.2.3.1. 3-(phenylaminomethyl)-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (3a)
IR [KBr ѵ cm−1]: 3394.73 (—NH—), 3222.36 (—NH—), 1720.82 (C O), 1757.43 (C O), 1353.93 (C—N), 2921.84 (C—H), 3115.36 ( C-H), 616.05 (C—S), 1672.14 (C C). 1H-NMR [400 MHz, δ, ppm, DMSO-d6]: 12.10 (1H, s, indole-NH-), 7.91 (1H, s, CH— methylene), 6.68–7.66 (10H, d & t, phenyl-H and indole-H), 4.88–4.91 (2H, d, —CH2—NH—), 3.99–4.10 (1H, t, —CH2—NH—). ESI-MS: m/z (M+) 349.
2.2.3.2. 3-[(4-chlorophenyl)aminomethyl]-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (3b)
IR [KBr ѵ cm−1]: 3320.09 (—NH—), 3223.39 (—NH—), 1719.32 (C O), 1777.35 (C O), 1351.97 (C—N), 2971.21 (C—H), 3021.23 ( C—H), 612.51 (C—S), 1675.42 (C C), 872.88 (C—Cl). 1H-NMR [400 MHz, δ, ppm, DMSO-d6]: 12.04 (1H, s, indole-NH-), 8.29 (1H, s, CH— methylene), 6.78–8.04 (9H, d & t, phenyl-H and indole-H), 4.81–4.83 (2H, d, —CH2—NH—), 4.01–4.10 (1H, t, —CH2—NH—). 13C-NMR [400 MHz, δ, ppm, DMSO-d6]: 167.5, 167.2, 149.7, 146.1, 138.2, 129.5, 128.5, 126.7, 1246.4, 123.0, 121.0, 118.2, 116.1, 112.3, 111.4, 110.4, 65.6. ESI-MS: m/z (M+) 383.
2.2.3.3. 3-[(2-chlorophenyl)aminomethyl]-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (3c)
IR [KBr ѵ cm−1]: 3323.39 (—NH—), 3221.32 (—NH—), 1719.69 (C O), 1764.35 (C O), 1357.91 (C—N), 2911.25 (C—H), 3070.50 ( C—H), 623.11 (C—S), 1690.32 (C C), 879.19 (C—Cl). 1H-NMR [400 MHz, δ, ppm, DMSO-d6]: 12.30 (1H, s, indole-NH-), 8.06 (1H, s, CH— methylene), 7.18–7.90 (9H, d & t, phenyl-H and indole-H), 4.29–4.33 (1H, t, —CH2—NH—), 3.96–3.98 (2H, d, —CH2—NH—).
2.2.3.4. 3-[(4-fluorophenyl)aminomethyl]-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (3d)
IR [KBr ѵ cm−1]: 3338.07 (—NH—), 3215.60 (—NH—), 1742.56 (C O), 1723.14 (C O), 1330.43 (C—N), 2974.02 (C—H), 3038.51 ( C—H), 635.12 (C—S), 1629.31 (C C), 1237.07 (C—F). 1H-NMR [400 MHz, δ, ppm, DMSO-d6]: 12.31 (1H, s, indole-NH-), 8.06 (1H, s, CH— methylene), 7.18–7.90 (9H, d & t, phenyl-H and indole-H), 4.54–4.56 (2H, d, —CH2—NH—), 4.06–4.10 (1H, t, —CH2—NH—). 13C-NMR [400 MHz, δ, ppm, DMSO-d6]: 167.6, 167.2, 156.7, 144.2, 143.2, 136.2, 128.6, 126.7, 124.4, 123.0, 121.0, 118.3, 116.1, 112.3, 111.3, 110.4, 65.9. ESI-MS: m/z (M+) 367.
2.2.3.5. 3-[(4-bromophenyl)aminomethyl]-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (3e)
IR [KBr ѵ cm−1]: 3375.76 (—NH—), 3238.86 (—NH—), 1754.58 (C O), 1763.85 (C O), 1354.35 (C—N), 2965.65 (C—H), 3075.45 ( C—H), 628.33 (C—S), 1659.35 (C C), 526.56 (C—Br). 1H-NMR [400 MHz, δ, ppm, DMSO-d6]: 12.30 (1H, s, indole-NH-), 8.06 (1H, s, CH— methylene), 7.18–7.89 (9H, d & t, phenyl-H and indole-H), 5.14 (1H, s, —CH2—NH—), 4.32–4.34 (2H, d, —CH2—NH—).
2.2.3.6. 3-[(3-nitrophenyl)aminomethyl]-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (3f)
IR [KBr ѵ cm−1]: 3382.25 (—NH—), 3265.74 (—NH—), 1785.52 (C O), 1747.45 (C O), 1348.65 (C—N), 2987.64 (C—H), 3065.65 (=C—H), 632.21 (C—S), 1663.33 (C C), 1533.26 & 1363.89 (—NO2). 1H-NMR [400 MHz, δ, ppm, DMSO-d6]: 12.30 (1H, s, indole-NH-), 8.06 (1H, s, CH— methylene), 7.18–7.90 (9H, s, d & t, phenyl-H and indole-H), 5.28 (1H, s, —CH2—NH—), 4.10–4.12 (2H, d, —CH2—NH—). 13C-NMR [400 MHz, δ, ppm, DMSO-d6]: 169.1, 167.2, 149.5, 144.3, 143.3, 135.2, 127.2, 126.0, 125.4, 123.9, 121.1, 117.3, 116.6, 114.3, 112.3, 111.0, 110.4, 108.5, 66.9. ESI-MS: m/z (M+) 394.
2.2.3.7. 3-[(4-nitrophenyl)aminomethyl]-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (3g)
IR [KBr ѵ cm−1]: 3376.82 (—NH—), 3275.52 (—NH—), 1766.56 (C O), 1753.45 (C O), 1339.41 (C—N), 2967.56 (C—H), 3085.12 ( C—H), 628.52 (C—S), 1669.78 (C C), 1539.45 & 1348.23 (—NO2). 1H-NMR [400 MHz, δ, ppm, DMSO-d6]: 12.50 (1H, s, indole-NH-), 8.09 (1H, s, CH— methylene), 7.10–7.90 (9H, d & t, phenyl-H and indole-H), 5.32 (1H, s, —CH2—NH—), 4.32–4.34 (2H, d, —CH2—NH—).
2.2.3.8. 3-[(2,4-dinitrophenyl)aminomethyl]-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (3h)
IR [KBr ѵ cm−1]: 3365.46 (—NH—), 3256.42 (—NH—), 1774.22 (C O), 1765.52 (C O), 1328.78 (C—N), 2964.54 (C—H), 3104.28 ( C—H), 618.45 (C—S), 1668.65 (C C), 1545.25 & 1339.56 (—NO2). 1H-NMR [400 MHz, δ, ppm, DMSO-d6]: 11.78 (1H, s, indole-NH-), 8.29 (1H, s, CH— methylene), 6.78–8.04 (8H, s, d & t, phenyl-H and indole-H), 4.81–4.83 (2H, d, —CH2—NH—), 4.01–4.10 (1H, t, —CH2—NH—). 13C-NMR [400 MHz, δ, ppm, DMSO-d6]: 175.6, 166.2, 151.2, 144.8, 137.5, 136.8, 134.6, 130.7, 128.9, 127.6, 123.4, 121.9, 121.5, 119.9, 118.6, 115.6, 111.5, 110.5, 63.8. ESI-MS: m/z (M+) 439.
2.2.3.9. 3-[(3-methylphenyl)aminomethyl]-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (3i)
IR [KBr ѵ cm−1]: 3368.85 (—NH—), 3248.45 (—ENH—), 1726.09 (C O), 1765.60 (C O), 1352.44 (C—N), 2965.50 (C—H), 3075.90 ( C—H), 622.46 (C—S), 1673.20 (C C). 1H-NMR [400 MHz, δ, ppm, DMSO-d6]: 11.25 (1H, s, indole-NH-), 8.12 (1H, s, CH— methylene), 6.59–7.88 (9H, s, d & t, phenyl-H and indole-H), 4.26 (1H, s, —CH2—NH—), 4.12–4.13 (2H, d, —CH2—NH—), 2.21 (3H, s, phenyl-CH3).
2.2.3.10. 3-[(4-methylphenyl)aminomethyl]-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (3j)
IR [KBr ѵ cm−1]: 3376.65 (—NH—), 3250.60 (—NH—), 1758.11 (C O), 1743.55 (C O), 1364.43 (C—N), 2959.05 (C—H), 3082.44 ( C—H), 620.42 (C—S), 1669.08 (C C). 1H-NMR [400 MHz, δ, ppm, DMSO-d6]: 12.00 (1H, s, indole-NH-), 8.14 (1H, s, CH— methylene), 6.89–7.99 (9H, d & t, phenyl-H and indole-H), 4.52 (1H, s, —CH2—NH—), 4.10–4.11 (2H, d, —CH2—NH—), 2.33 (3H, s, phenyl-CH3). 13C-NMR [400 MHz, δ, ppm, DMSO-d6]: 175.1, 167.4, 145.2, 142.6, 137.4, 129.8, 129.4, 128.9, 127.6, 122.4, 121.8, 119.8, 118.9, 115.6, 111.2, 110.7, 65.8, 25.8. ESI-MS: m/z (M+) 363.
2.2.3.11. 3-[(4-methoxyphenyl)aminomethyl]-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (3k)
IR [KBr ѵ cm−1]: 3356.46 (—NH—), 3255.21 (—NH—), 1732.45 (C O), 1754.22 (C O), 1348.64 (C—N), 2956.85 (C—H), 3063.74 ( C—H), 634.32 (C—S), 1126.38 (C—O—C). 1H-NMR [400 MHz, δ, ppm, DMSO-d6]: 12.03 (1H, s, indole-NH-), 8.15 (1H, s, CH— methylene), 6.87–7.84 (9H, d & t, phenyl-H and indole-H), 4.42 (1H, s, —CH2—NH—), 3.90–4.01 (2H, d, —CH2—NH), 3.65 (3H, s, —OCH3). ESI-MS: m/z (M+) 379.
2.2.3.12. 3-[(4-hydroxyphenyl)aminomethyl]-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (3l)
IR [KBr ѵ cm−1]: 3582.84 (—OH), 3376.65 (—NH—), 3263.44 (—NH—), 1746.14 (C O), 1753.13 (C O), 1374.28 (C—N), 2965.08 (C—H), 3078.78 ( C—H), 624.33 (C—S), 1658.54 (C C). 1H-NMR [400 MHz, δ, ppm, DMSO-d6]: 11.93 (1H, s, indole-NH-), 10.54 (1H, s, phenyl-OH), 8.02 (1H, s, CH— methylene), 6.95–7.98 (9H, d & t, phenyl-H and indole-H), 4.35 (1H, s, —CH2—NH—), 4.00–4.11 (2H, d, —CH2—NH—). 13C-NMR [400 MHz, δ, ppm, DMSO-d6]: 173.5, 165.2, 147.9, 144.0, 141.7, 135.5, 130.8, 127.4, 122.8, 121.5, 120.4, 119.8, 117.4, 113.4, 112.4, 110.8, 64.3. ESI-MS: m/z (M+) 365.
2.3. Biological evaluation
2.3.1. In vitro antibacterial activity
Minimum inhibitory concentrations (MIC) of the compounds were measured by two-fold serial dilution method [22, 23, 24] for screening the in vitro antibacterial activity against gram positive bacteria (Staphylococcus aureus: MTCC-1134, Bacillus subtilis: MTCC-1144) and gram negative bacteria (Escherichia coli: MTCC-1089, Pseudomonas aeruginosa: MTCC-424). Test compounds and reference standard Ampicillin were dissolved in DMSO at a concentration of 1280 μg/mL. Further dilutions were made using DMSO only, tested at a concentration of 640, 320, 160, 80, 40, 20 μg/mL and DMSO as a control. Drug solution was added to the each tube containing 5 mL sterilized nutrient broth medium. MIC tests were carried out in nutrient broth with inoculums of (1–2) × 106 Colony Forming Unit/ml (CFU/mL) bacterial strains. The test compounds and standard of nutrient broth serial tube dilutions inoculated with each bacterial strain were incubated at 37 ± 2 °C for 18–24 hrs.
2.3.2. In vitro antioxidant activity evaluation
DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging assay and hydrogen peroxide scavenging assay methods were carried out to evaluate the in vitro antioxidant activity.
2.3.2.1. DPPH free radical scavenging assay method
The use of DPPH free radical scavenging assay [25, 26] provides a simple and speedy way to estimate antioxidant property by spectrophotometer and it is useful to evaluate different compounds at a time. DPPH (2,2-diphenyl-1-picrylhydrazyl) is stable free radical. Methanolic solution of DPPH is used to estimate the antioxidant activity of numerous synthetic compounds. Interaction of antioxidant compound with DPPH, both transfer electron or hydrogen atom to DPPH, neutralizing its free radical nature and converted to 2,2-diphenyl-1-picrylhydrazine. The scavenging activity of the compound was indicated by degree of discoloration. At 517 nm, the change in absorbance was used to measure antioxidant activity. DPPH solution in methanol (0.002%) was prepared and 1.0 ml of this solution was added to 3.0 ml of the test solutions in DMSO at different concentrations (50, 100, 300 and 500 μg/mL). The mixture was shaken well and was incubated at 37 °C for 30 minutes; the absorbance was measured at 517 nm. A blank was prepared without adding test solution. Ascorbic acid in methanol at various concentrations (50, 100, 300 and 500 μg/mL) was used as standard. The experiment was repeated triplicate. The percentage inhibition capability of scavenging the DPPH radical was calculated using the following equation:
Where A0 is the absorbance of the control reaction (containing all reagents except the test solution) and A1 is the absorbance of the test solution. Ascorbic acid was used as positive controls.
2.3.2.2. Hydrogen peroxide scavenging assay method
The ability of synthesized compounds to scavenge hydrogen peroxide was determined according to the literature procedures [27, 28, 29]. 40 mM concentration of hydrogen peroxide was prepared in phosphate buffer (pH 7.4). At 230 nm, the concentration of hydrogen peroxide was determined using a spectrophotometer. Different concentrations of the test compounds (50, 100, 300, and 500 μg/mL) in 3.4 ml of phosphate buffer were added to 0.6 ml of 40 mM hydrogen peroxide solution. The absorbance of reaction mixture was measured at 230 nm against a blank solution consisting of phosphate buffer without hydrogen peroxide. The experiment was repeated triplicate and the percentage scavenging of hydrogen peroxide by the test samples and standard compound was calculated as follows:
Where A0 is the absorbance of the control reaction (containing all reagents except the test solution) and A1 is the absorbance of the test solution. Ascorbic acid was used as positive control.
2.3.3. In vivo hypoglycemic activity evaluation
All the synthesized compounds were screened for in vivo hypoglycemic activity using Alloxan induced wister albino rats by tail tipping method [30, 31]. Wister albino rats of either sex having 160–200 g weight were taken for this study. Rats were purchased from Sainadh Agencies- Laboratory animal suppliers, Hyderabad. All the rats were acclimatized for one week to the laboratory conditions before commencing the experiments and fed with pellet and tap water ad libitum. At room temperature the animals were housed in the polypropylene cages for 12 hrs/12 hrs dark and light cycle. Acclimatized animals were kept fasting for 24 hrs with water ad libitum and Alloxan monohydrate was administered at120 mg/kg i.p. in normal saline. The animals were given ad libitum after one hour of Alloxan administration. To overcome the early hypoglycemic phase 5% dextrose solution was given in feeding bottle for a day. The blood glucose levels were monitored after alloxination by withdrawing a drop of blood from tail vein by Tail tipping method. The blood glucose levels as well as biochemical parameters were measured using digital Accu-Chek active digital glucose monitoring system and Robonik biochemical analyzer respectively.
After 72 hrs, the rats having blood glucose levels beyond 150 mg/dL were selected for the study and divided into six groups. The quantity of thiazolidine-2,4-dione derivatives equivalent to average human intake 200 mg/kg was calculated for single dose 36 mg/kg for acute study. The test compounds were administered orally by mixing with CMC-0.25% solution. Glibenclamide was administered as standard drug at 500 μg/kg body weight. All samples were administered at a dose of 35 mg/kg body weight for acute study. The blood samples were withdrawn and analyzed for blood glucose level at different time intervals 0 hr, 1 hr, 2 hr, 4 hr, 6 hr and 8 hr respectively. Based on the results of acute study few samples were selected for chronic study and they were administered at a dose of 35 and 70 mg/kg body weight. After 30 minutes of the administration of the dose the blood glucose level was measured and decrease in blood glucose was calculated on 7th day and 15th day. The effect of samples 3b, 3d, 3g and 3h on insulin, urea, creatinine, lipid profiles, HDL, LDL and VLDL levels in control and in Alloxan induced diabetic rats in serum or plasma were studied on Day 15.
2.4. Molecular docking studies
The choice of protein for docking studies is based upon numerous factors such as structure should be determined by X-ray diffraction, it should have a resolution between 2.0 to 2.5A°, contain a co-crystallized ligand and the selected protein 3D structure should not have any protein breaks [32]. The co-crystal structure of the target receptor was obtained from the protein data bank (http://www.rcsb.org/pdb) PDB ID: 2PRG having resolution of 2.3A°. Then it was prepared for docking by removing all the heteroatoms, nonreceptor atoms, water and other ions, etc. Molecular docking was performed on the designed compounds 3a-l as potential PPARγ agonists [33]. PPARγ receptor is the major target for some of the antidiabetic drugs consisting of thiazolidine-2,4-dione nucleus [34]. The docking procedure was applied on a set of designed ligands within the region of 2PRG active site using AutoDock 4.2.6 software. Based on the validations and hydrogen bond interactions of various substituents, they were considered for the evaluation. It was done to understand the kind of interactions that occurred between various substituted thiazolidine-2,4-diones with 2PRG binding site region. The active site was considered as a rigid molecule, while the ligands were treated as being flexible. Series of compounds 3a-l were modeled by using ChemDraw Ultra 8.0 software and converted into suitable 3D model, subjected to energy minimization using molecular mechanics. The energy minimized structures are required for molecular docking and for the preparation of corresponding pdb files. Docking studies were preformed on prepared ligands to predict the binding energy at the region of 2PRG active site to find out the possible locations for the ligand in active site region of the receptor. Using default parameters Grid based docking studies were carried out and docking was performed on all the designed compounds using standard ligand Rosiglitazone.
3. Results and discussion
3.1. Chemistry
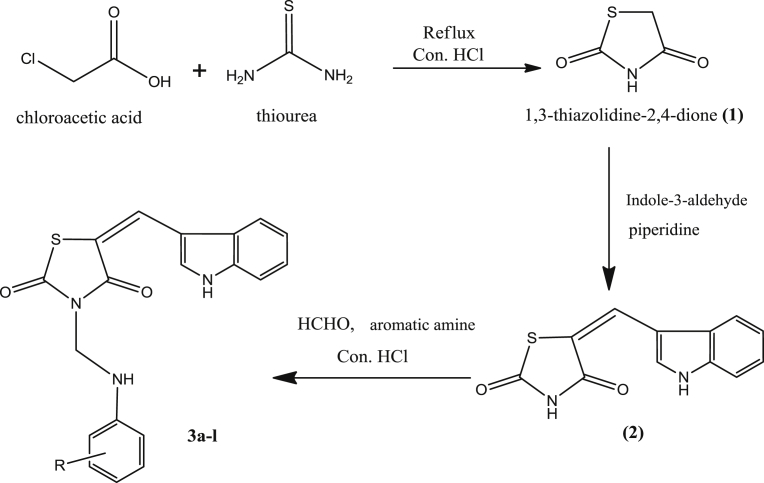
Initially, thiazolidine-2,4-dione (1) was synthesized conventionally according to the literature procedure [35, 36]. Thiazolidine-2,4-dione (1) was condensed with indole-3-aldehyde to form 5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione (2), under Knoevenagel reaction conditions [37]. Compound 2 was then coupled with formaldehyde and substituted aromatic amines under Mannich reaction conditions [38, 39] to afford the desired final derivatives 3a-l, depicted in Fig. 2. The titled compounds were also prepared by microwave-assisted irradiation techniques according to the literature procedures [40, 41, 42, 43] with different power levels. All the compounds were characterized physically and most of the compounds were characterized spectrally. The physical characterization data, the comparative study of conventional and microwave irradiation methods with respect to their percentage yield and reaction time were given in Table 1.
Fig. 2.
Scheme of synthesis.
Table 1.
Physical characterization data of synthesized compounds 3a-l.
| Compd. | R | M.p. (°C) | Molecular formula | M.w. | % yield (Reaction tine) |
Elemental analysis (%) |
|
|---|---|---|---|---|---|---|---|
| Conventional | Microwave | C, H, N-Calculated (found) | |||||
| 3a | H | 210–212 | C19H15N3O2S | 349.41 | 72.45 (10 hrs) | 81.56 (8 min) | 65.31(65.27), 4.33(4.25), 12.03(11.93) |
| 3b | 4-chloro | 224–226 | C19H14ClN3O2S | 383.05 | 60.30 (12 hrs) | 75.46 (10 min) | 59.45(59.38), 3.68(3.56), 10.95(10.89) |
| 3c | 2-chloro | 218–220 | C19H14ClN3O2S | 383.05 | 74.05 (11 hrs) | 85.36 (9 min) | 59.45(59.36), 3.68(3.51), 10.95(10.85) |
| 3d | 4-fluoro | 228–230 | C19H14FN3O2S | 367.40 | 68.50 (12 hrs) | 79.25 (10 min) | 62.11(62.02), 3.84(3.76), 11.44(11.35) |
| 3e | 4-bromo | 198–200 | C19H14BrN3O2S | 428.30 | 77.30 (12 hrs) | 86.94 (12 min) | 53.28(53.15), 3.29(3.18), 9.81(9.74) |
| 3f | 3-nitro | 240–242 | C19H14N4O4S | 394.40 | 65.88 (14 hrs) | 80.24 (9 min) | 57.86(57.68), 3.58(3.39), 14.21(14.10) |
| 3g | 4-nitro | 236–238 | C19H14N4O4S | 394.40 | 80.50 (12 hrs) | 89.64 (10 min) | 57.86(57.70), 3.58(3.42), 14.21(14.15) |
| 3h | 2,4-dinitro | 200–202 | C19H13N5O6S | 439.40 | 72.30 (13 hrs) | 88.46 (10 min) | 51.94(51.85), 2.98(2.85), 15.94(15.85) |
| 3i | 3-methyl | 256–258 | C20H17N3O2S | 363.43 | 70.85 (12 hrs) | 87.48 (8 min) | 66.10(66.01), 4.71(4.62), 11.56(11.42) |
| 3j | 4-methyl | 220–222 | C20H17N3O2S | 363.43 | 69.75 (10 hrs) | 76.28 (9 min) | 66.10(66.03), 4.71(4.59), 11.56(11.45) |
| 3k | 4-methoxy | 234–236 | C20H17N3O3S | 379.43 | 68.60 (12 hrs) | 82.65 (12 min) | 63.31(63.20), 4.52(4.49), 11.07(10.99) |
| 3l | 4-hydroxy | 204–206 | C19H15N3O3S | 365.41 | 70.42 (11 hrs) | 89.46 (10 min) | 62.45(62.28), 4.14(4.05), 11.50(11.42) |
3.2. Biological evaluation efficacy
3.2.1. In vitro antibacterial efficacy
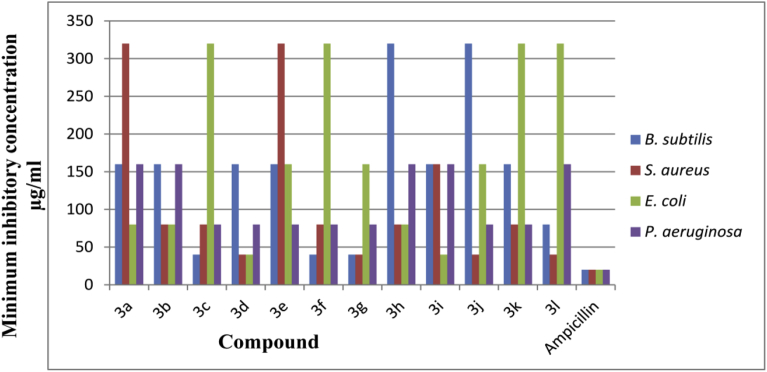
All the synthesized compounds were evaluated for their in vitro antibacterial activity against the both gram positive and gram negative bacteria. The MIC of each test compound was recorded as the lowest concentration in the tubes with no growth i.e. no turbidity of inoculated bacterial strains. The MIC values were determined by using serial dilution technique in nutrient broth medium by observing the presence or absence of turbidity. The lowest concentration that completely inhibits macroscopic growth was determined and MICs were reported. Antibacterial activity results of synthesized test compounds and Ampicillin as reference standard were depicted in Table 2. The comparative antimicrobial activity of the synthesized compounds was given in Fig. 3. All the tested compounds showed MIC values between 320-40 μg/mL. In vitro antibacterial evaluation states that compounds 3c, 3f, 3g shown good activity against B. subtilis at 40 μg/mL while compound 3d, 3g, 3l shown good activity against S. aureus at 40 μg/mL. Compounds 3d, 3i shown good activity against E. coli and P. aeruginosa at 40 μg/mL.
Table 2.
In vitro antibacterial activity of compounds 3a-l.
| Compounds | MIC values of tested compounds (μg/mL) against |
|||
|---|---|---|---|---|
| Gram positive bacteria |
Gram negative bacteria |
|||
|
B. subtilis MTCC 1134 |
S. aureus MTCC 1144 |
E. coli MTCC 1089 |
P. aeruginosa MTCC 424 |
|
| 3a | 160 | 320 | 80 | 160 |
| 3b | 160 | 80 | 80 | 160 |
| 3c | 40 | 80 | 320 | 40 |
| 3d | 160 | 40 | 40 | 40 |
| 3e | 160 | 320 | 160 | 40 |
| 3f | 40 | 80 | 320 | 80 |
| 3g | 40 | 40 | 160 | 80 |
| 3h | 320 | 80 | 80 | 40 |
| 3i | 160 | 160 | 40 | 40 |
| 3j | 320 | 40 | 160 | 80 |
| 3k | 160 | 80 | 320 | 160 |
| 3l | 80 | 40 | 320 | 160 |
| Ampicillin | 20 | 20 | 20 | 20 |
Fig. 3.
Comparative antibacterial activity of the synthesized compounds.
3.2.2. In vitro antioxidant efficacy
Antioxidant activity of the synthesized compounds 3a-l was performed by using DPPH (2,2-diphenyl-1-picrylhydrazyl) scavenging free radical activity assay method and hydrogen peroxide method in comparison with ascorbic acid as reference standard. The % inhibition of DPPH scavenging activity and the % inhibition of H2O2 scavenging activity along with their IC50 values was calculated and the results were given in Tables 3 and 4.
Table 3.
In vitro antioxidant activity evaluation of samples against DPPH radicals.
| Compound | % inhibition (DPPH scavenging) at different concentrations |
IC50 μg/mL |
|||
|---|---|---|---|---|---|
| 50 μg/mL | 100 μg/mL | 300 μg/mL | 500 μg/mL | ||
| 3a | 33.75 ± 0.42 | 36.04 ± 0.18 | 55.83 ± 0.26 | 74.79 ± 0.32 | 145.21 ± 0.09 |
| 3b | 52.26 ± 1.25 | 58.63 ± 0.92 | 69.33 ± 0.45 | 78.84 ± 0.58 | 52.36 ± 0.12 |
| 3c | 35.28 ± 0.44 | 48.22 ± 1.01 | 51.06 ± 0.63 | 60.71 ± 0.52 | 187.49 ± 0.11 |
| 3d | 51.33 ± 0.36 | 59.83 ± 0.42 | 67.33 ± 0.36 | 76.29 ± 0.38 | 56.36 ± 0.07 |
| 3e | 48.26 ± 0.11 | 55.23 ± 0.68 | 65.85 ± 1.05 | 73.75 ± 0.84 | 67.60 ± 0.15 |
| 3f | 28.33 ± 0.24 | 42.08 ± 0.46 | 44.38 ± 0.58 | 59.58 ± 0.24 | 331.13 ± 0.21 |
| 3g | 53.78 ± 0.66 | 59.28 ± 0.32 | 69.86 ± 0.86 | 78.52 ± 0.23 | 50.11 ± 0.14 |
| 3h | 45.46 ± 0.24 | 56.92 ± 0.36 | 62.83 ± 0.56 | 71.00 ± 0.42 | 76.91 ± 0.32 |
| 3i | 34.26 ± 0.44 | 40.23 ± 0.65 | 52.65 ± 0.46 | 63.71 ± 1.03 | 200.91 ± 0.25 |
| 3j | 36.04 ± 0.36 | 41.46 ± 0.26 | 45.63 ± 0.26 | 47.08 ± 0.42 | 438.53 ± 0.45 |
| 3k | 38.26 ± 0.15 | 45.26 ± 0.32 | 56.52 ± 0.13 | 68.25 ± 0.52 | 128.82 ± 0.62 |
| 3l | 43.82 ± 0.42 | 51.25 ± 0.41 | 60.41 ± 0.33 | 67.65 ± 0.84 | 97.72 ± 0.22 |
| Ascorbic acid | 55.36 ± 0.18 | 60.32 ± 0.24 | 70.85 ± 0.42 | 80.32 ± 0.12 | 46.99 ± 0.15 |
All the values are expressed as Mean ± SEM, n = 3.
Table 4.
In vitro antioxidant activity evaluation of samples against H2O2 radicals.
| Compound | % inhibition (H2O2 scavenging) at different concentrations |
IC50 μg/mL |
|||
|---|---|---|---|---|---|
| 50 μg/mL | 100 μg/mL | 300 μg/mL | 500 μg/mL | ||
| 3a | 40.87 ± 0.35 | 43.62 ± 0.42 | 49.8 ± 0.28 | 59.25 ± 0.36 | 203.23 ± 0.33 |
| 3b | 67.98 ± 0.58 | 80.91 ± 0.12 | 81.85 ± 0.44 | 85.54 ± 1.01 | 26.79 ± 0.14 |
| 3c | 64.27 ± 0.56 | 69.78 ± 0.91 | 74.28 ± 0.75 | 83.46 ± 0.72 | 34.83 ± 0.41 |
| 3d | 70.93 ± 0.42 | 80.31 ± 0.23 | 82.87 ± 0.36 | 86.31 ± 0.22 | 26.48 ± 0.22 |
| 3e | 74.98 ± 0.84 | 78.26 ± 0.12 | 85.45 ± 0.45 | 88.24 ± 1.02 | 24.15 ± 0.13 |
| 3f | 41.25 ± 0.24 | 58.31 ± 0.22 | 74.25 ± 0.42 | 88.93 ± 0.22 | 45.91 ± 0.25 |
| 3g | 49.26 ± 0.35 | 54.28 ± 0.47 | 60.45 ± 0.32 | 74.61 ± 0.65 | 73.96 ± 0.16 |
| 3h | 65.40 ± 0.42 | 76.25 ± 0.24 | 79.50 ± 0.26 | 82.51 ± 0.38 | 30.62 ± 0.09 |
| 3i | 60.60 ± 1.10 | 65.46 ± 0.43 | 72.28 ± 0.75 | 78.44 ± 0.56 | 42.26 ± 0.25 |
| 3j | 60.25 ± 0.36 | 75.56 ± 0.22 | 77.25 ± 0.24 | 80.12 ± 0.18 | 34.35 ± 0.16 |
| 3k | 55.24 ± 0.56 | 61.43 ± 0.42 | 72.25 ± 1.04 | 79.28 ± 0.66 | 46.23 ± 0.17 |
| 3l | 60.45 ± 0.44 | 66.24 ± 0.25 | 75.26 ± 0.33 | 82.14 ± 0.48 | 37.84 ± 0.45 |
| Ascorbic acid | 69.47 ± 0.32 | 82.55 ± 0.26 | 83.46 ± 0.12 | 87.65 ± 0.16 | 24.94 ± 0.16 |
All the values are expressed as Mean ± SEM, n = 3.
3.2.2.1. DPPH scavenging efficacy
DPPH assay results states that, the compounds 3b, 3d and 3g were found to be shown significant antioxidant activity with IC50 values 52.36 ± 0.12, 56.36 ± 0.07 and 50.11 ± 0.14 μg/mL respectively when compared with standard Ascorbic acid IC50 value 46.99 ± 0.15 μg/mL. The compounds 3e and 3h exhibited moderate activity with the IC50 values 67.60 ± 0.15 and 76.91 ± 0.32 μg/mL respectively.
3.2.2.2. Hydrogen peroxide efficacy
Hydrogen peroxide assay results revealed the compounds 3b, 3d and 3e were found to exhibit significant antioxidant activity with the IC50 values 26.79 ± 0.14, 26.48 ± 0.22 and 24.15 ± 0.13 μg/mL respectively when compared with standard Ascorbic acid IC50 value 24.94 ± 0.16. The compounds 3c, 3h and 3j exhibited moderate activity with the IC50 values 34.83 ± 0.41, 30.62 ± 0.09 and 34.35 ± 0.16 μg/mL respectively.
3.2.3. In vivo hypoglycaemic efficacy
Study protocols related to in vivo hypoglycaemic activities were approved by the Institutional Animal Ethics Committee under the supervision of Committee for the Purpose of Control and Supervision of Experiments on Animals, New Delhi bearing registration number 1847/PO/Re/S/16/CPCSEA. Blood glucose levels, body weight and serum biochemical parameters were expressed as mean ± standard error of mean (SEM). The values were analyzed by one-way analysis of variance (ANOVA) followed by Dunnet's ‘t’ test. The acute study data of all the synthesized compounds were depicted in Table 5 in relation to the standard drug Glibenclamide. The compounds 3b, 3d, 3g and 3h have shown significant hypoglycaemic activity. Chronic study analysis results were depicted in Table 6 revealed that compound 3b and 3h at 70 mg/kg body weight possess significant activity. On day 15, effect of compounds 3b, 3d, 3g and 3h on insulin, urea, creatinine, lipid profiles, HDL, LDL and VLDL levels in control and Alloxan induced diabetic rats in serum or plasma were placed in Table 7, revealed that the compounds shows significant to moderate reduction.
Table 5.
Effect of synthesized compounds 3a-l on blood glucose level in Alloxan induced diabetic rats (Acute Study).
| Compound | Mean ± SEM of blood glucose level mg/dL |
|||||
|---|---|---|---|---|---|---|
| 0 hr | 1 hr | 2 hr | 4 hr | 6 hr | 8 hr | |
| Normal | 122.22 ± 2.4 | 124.12 ± 1.46 | 123.5 ± 5.11 | 120.54 ± 3.22 | 122.5 ± 4.22 | 120.33 ± 2.3 |
| Standard | 383.8 ± 14.28 | 222.8 ± 8.05** | 180.3 ± 6.92 | 120.42 ± 9.86* | 93.6 ± 4.95 | 85.42 ± 2.53* |
| 3a | 313.3 ± 5.46 | 288.3 ± 4.41 | 259.3 ± 7.23 | 242.33 ± 4.33** | 250.7 ± 6.57* | 282.7 ± 2.34 |
| 3b | 305.3 ± 5.46* | 290.3 ± 7.32 | 200.3 ± 9.29** | 145.33 ± 1.76 | 102 ± 5.78* | 90.58 ± 4.73 |
| 3c | 339.3 ± 4.06 | 315 ± 2.89 | 298.7 ± 3.53* | 275 ± 5.78 | 285 ± 2.89 | 301.7 ± 6.02** |
| 3d | 316 ± 6.51** | 297.3 ± 6.37* | 195.3 ± 6.02 | 142 ± 8.67 | 105.3 ± 6.02** | 95 ± 2.89 |
| 3e | 317.3 ± 6.18 | 300.7 ± 5.21** | 276.7 ± 4.41 | 249.3 ± 8.70* | 263.3 ± 6.02 | 285.0 ± 2.89 |
| 3f | 320.0 ± 2.00* | 303.3 ± 6.02 | 276.7 ± 3.53** | 250.0 ± 2.89 | 281.7 ± 6.02 | 300.0 ± 5.30* |
| 3g | 309.0 ± 5.51* | 282.3 ± 4.37** | 200.3 ± 4.22 | 168.01 ± 7.65* | 128.7 ± 6.02** | 100.02 ± 2.89** |
| 3h | 306.0 ± 2.08 | 280.3 ± 3.85** | 208.3 ± 3.39 | 155.6 ± 3.48** | 110.3 ± 6.02 | 94.7 ± 4.41 |
| 3i | 333.0 ± 5.87** | 311.3 ± 5.21 | 292.7 ± 6.37 | 264.0 ± 5.87** | 285.0 ± 2.89* | 301.7 ± 6.02 |
| 3j | 316.7 ± 2.41 | 301.3 ± 5.24* | 273.3 ± 6.02 | 243.00 ± 3.22** | 266.7 ± 6.02 | 288.0 ± 2.65 |
| 3k | 311.4 ± 5.42* | 302.61 ± 2.16 | 289.45 ± 4.11* | 265.32 ± 8.12 | 279.65 ± 2.35 | 295.44 ± 3.51** |
| 3l | 319.12 ± 4.15 | 310.52 ± 3.05 | 283.64 ± 4.22** | 272.62 ± 6.42 | 284.61 ± 3.15 | 301.82 ± 4.56* |
Standard Drug: Glibenclamide; Statistical analysis is done by One-way ANOVA followed by Dunnet's ‘t’ test; **P < 0.01 (considered as significant), *P < 0.001.
Table 6.
Effect of compounds 3a, 3d, 3f and 3j on fasting blood glucose level and body weight in Alloxan induced diabetic rats (Chronic Study 15 days).
| Compound | Blood glucose in mg/dL |
Body weight in gm |
||||
|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 15 | Day 0 | Day 7 | Day 15 | |
| Standard | 308.3 ± 6.51 | 214.3 ± 1.15 | 147.0 ± 4.36 | 193 ± 7.00 | 189.3 ± 3.79 | 192.0 ± 5.29 |
| 3b (35 mg/kg bw) | 316.7 ± 1.70 | 252.3 ± 3.06** | 199.3 ± 5.03 | 195.3 ± 3.06 | 200.7 ± 1.53 | 192.3 ± 3.06** |
| 3b (70 mg/kg bw) | 313 ± 4.58 | 221.3 ± 5.03* | 157 ± 6.24 | 201 ± 3.61* | 197 ± 1.00* | 195.3 ± 2.52 |
| 3d (35 mg/kg bw) | 311.67 ± 2.19** | 262.33 ± 1.77 | 212.00 ± 4.17 | 194.33 ± 1.45 | 200.67 ± 0.88** | 192.33 ± 1.77 |
| 3d (70 mg/kg bw) | 314.33 ± 2.24 | 261.33 ± 2.91 | 197 ± 3.61** | 205.00 ± 3.22 | 196.67 ± 0.67 | 195.33 ± 2.03 |
| 3g (35 mg/kg bw) | 311.67 ± 2.19 | 260.33 ± 6.65 | 207.00 ± 3.61 | 204.57 ± 2.50* | 196.63 ± 0.32 | 190.33 ± 1.02* |
| 3g (70 mg/kg bw) | 314.33 ± 2.61 | 255.67 ± 2.03** | 192.67 ± 3.18 | 204.67 ± 3.53 | 196.67 ± 1.30 | 195.33 ± 2.11 |
| 3h (35 mg/kg bw) | 312.33 ± 2.03* | 271.00 ± 2.00 | 218.00 ± 1.16** | 195.67 ± 1.33** | 200.67 ± 0.60* | 192.33 ± 1.77 |
| 3h (70 mg/kg bw) | 314.33 ± 2.61 | 227.67 ± 2.85 | 165.00 ± 3.52** | 194.33 ± 2.19 | 204.33 ± 1.20 | 195.33 ± 2.03** |
Table 7.
Effect of compounds 3b, 3d, 3g and 3h on insulin, urea, creatinine, lipid profiles, HDL, LDL and VLDL levels in control and Alloxan induced diabetic rats in serum or plasma on Day 15.
| Compound | Insulin (μIU/mL) | Urea (mg/dL) | Creatinine (mg/dL) | Total cholesterol (mg/dL) | Triglyceride (mg/dL) | Free fatty acids (mg/dL) | HDL-cholesterol (mg/dL) | LDL-cholesterol (mg/dL) | VLDL-cholesterol (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 16.3 ± 0.68 | 17.7 ± 0.2 | 0.71 ± 0.12 | 87.16 ± 6.12 | 13.29 ± 1.08 | 65.21 ± 4.12 | 45.16 ± 3.61 | 23.67 ± 1.67 | 19.72 ± 1.21 |
| Diabetic control | 6.9 ± 0.26 | 37.2 ± 1.6 | 1.21 ± 0.16 | 258.13 ± 19.98 | 45.17 ± 3.11 | 132.22 ± 9.92 | 22.68 ± 1.81 | 79.66 ± 4.95 | 47.51 ± 3.79 |
| Diabetic + Glibenclamide (500 μg/kg) | 11.3 ± 0.12 | 20.1 ± 0.98 | 0.78 ± 0.32 | 80 ± 0.26 | 28.60 ± 1.35 | 68.21 ± 4.12 | 22.30 ± 1.52 | 25.67 ± 1.67 | 20.50 ± 0.25 |
| Diabetic + sample 3b (35 mg/kg bw) | 10.3 ± 0.51 | 29.4 ± 1.6 | 0.96 ± 0.27 | 91.32 ± 7.12 | 16.72 ± 1.62 | 59.65 ± 5.16 | 41.67 ± 3.05 | 41.56 ± 4.12 | 28.91 ± 2.07 |
| Diabetic + sample 3b (70 mg/kg bw) | 14.3 ± 0.26 | 21.1 ± 2.5 | 0.85 ± 0.19 | 85.65 ± 7.73 | 18.94 ± 1.92 | 64.12 ± 7.07 | 40.12 ± 3.01 | 32.14 ± 2.71 | 25.71 ± 1.86 |
| Diabetic + sample 3d (35 mg/kg bw) | 12.3 ± 0.21 | 27.4 ± 0.25 | 0.86 ± 0.32 | 95.26 ± 0.50 | 36.60 ± 1.85 | 70.65 ± 2.56 | 35.67 ± 2.12 | 40.21 ± 1.56 | 19.38 ± 0.25 |
| Diabetic + sample 3d (70 mg/kg bw) | 16.3 ± 0.46 | 19.1 ± 1.06 | 0.65 ± 0.26 | 90.23 ± 1.63 | 58.40 ± 2.05 | 85.12 ± 1.76 | 40.12 ± 1.52 | 35.14 ± 1.27 | 14.97 ± 0.72 |
| Diabetic + sample 3g (35 mg/kg bw) | 10.5 ± 0.12 | 20.1 ± 0.98 | 0.68 ± 0.32 | 95.26 ± 0.50 | 46.60 ± 1.55 | 69.65 ± 1.56 | 38.67 ± 2.32 | 38.21 ± 1.32 | 20.50 ± 0.25 |
| Diabetic + sample 3g (70 mg/kg bw) | 15.3 ± 0.36 | 25.4 ± 0.32 | 0.89 ± 0.23 | 90.23 ± 1.63 | 58.40 ± 2.05 | 83.23 ± 1.26 | 42.12 ± 2.01 | 35.14 ± 1.25 | 16.23 ± 0.34 |
| Diabetic + sample 3h (35 mg/kg bw) | 10.3 ± 1.32 | 29.4 ± 1.23 | 0.96 ± 0.32 | 91.26 ± 0.50 | 45.60 ± 1.25 | 62.65 ± 1.56 | 35.67 ± 1.32 | 40.21 ± 0.26 | 22.30 ± 0.42 |
| Diabetic + sample 3h (70 mg/kg bw) | 12.3 ± 2.26 | 22.1 ± 2.47 | 0.78 ± 0.23 | 102.23 ± 1.63 | 50.40 ± 1.85 | 72.23 ± 1.26 | 45.12 ± 0.26 | 32.14 ± 1.32 | 18.52 ± 0.26 |
3.3. Molecular docking results
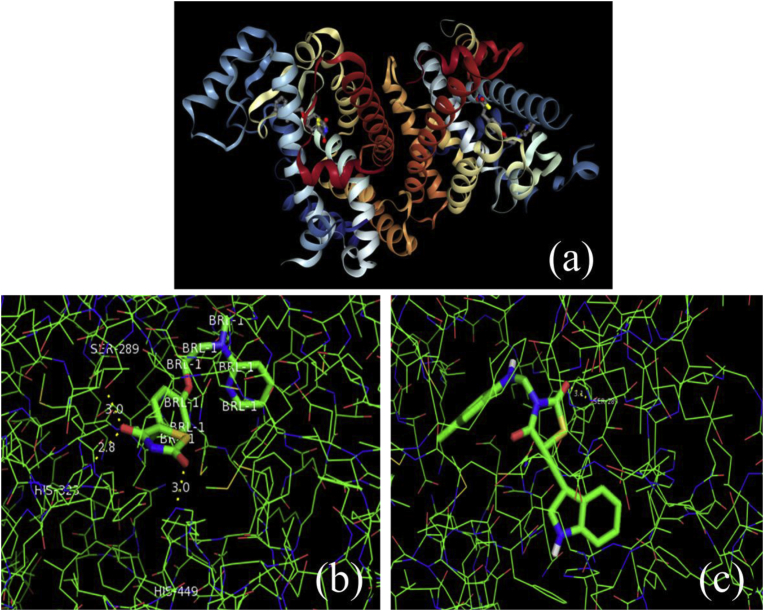
In this study, all the designed compounds were subjected to docking to explore their binding mode at PPARγ receptor. Biological target PPARγ receptor was downloaded from the protein data bank PDB ID- 2PRG. AutoDock molecular docking technique was employed to dock the designed compounds against PPARγ receptor (PDB ID-2PRG) to trace the interaction between various compounds and PPARγ receptor. Non-polar hydrogen atoms were removed from the receptor and their partial charges were added to the corresponding carbon atoms. PPARγ receptor agonist Rosiglitazone was used as a reference ligand. The docking study has been conducted to predict the binding mode and to rationalize the observed biological activity. Molecular docking was performed using recently updated version AutoDock docking engine 4.2.6 software. Default settings were used for all the calculations. The interactions between the receptor protein and ligands were studied in Pymol 1.7.4.5. The binding energy (kcal/mol) with hydrogen bonds, number of hydrogen bonds, hydrogen bond length and amino acid residues interacted were identified. The binding energy values revealed that most of the compounds had good binding affinity toward the PPARγ receptor and the computed values were depicted in Table 8. The interaction of Rosiglitazone at the active site of the receptor has showed binding energy of −8.26 kcal/mol and forms three H-bonds with His449, His323 and Ser289. Fig. 4 shown the 3D structure of PPARγ receptor, docking complex of PPARγ protein-2PRG against Rosiglitazone and compound 3b. The compound 3b shown promising binding affinity i.e. −9.65 kcal/mol and forms two H-bonds with Ser289 and Gln286.
Table 8.
Binding energy and amino acid residues interacted by the compounds 3a-l with the target PPARγ protein PDB ID – 2PRG.
| Compound | Binding energy (kcal/mol) | No. of H bonds | H-bond length | Amino acid residues interacted |
|---|---|---|---|---|
| Rosiglitazone | −8.26 | 3 | 3.01, 2.82, 3.11 | His449, His323, Ser289 |
| 3a | −7.85 | 2 | 3.25, 2.56 | Arg288, Ser289 |
| 3b | −9.65 | 2 | 3.10, 3.28 | Ser289, Gln286 |
| 3c | −7.99 | 3 | 2.72, 2.42, 2.41 | Lys296, Leu268, Met348 |
| 3d | −8.76 | 2 | 3.10, 2.32 | Thr246, His449 |
| 3e | −8.85 | 2 | 2.80, 2.51 | Met329, Leu268 |
| 3f | −9.05 | 2 | 3.11, 2.16 | Met348, Tyr473 |
| 3g | −8.45 | 2 | 2.91, 1.95 | Tyr396, His449 |
| 3h | −9.04 | 2 | 3.16, 2.91 | HIS449, LYS367 |
| 3i | −7.22 | 3 | 2.15, 3.25, 1.98 | Leu298, Ser289, Met329 |
| 3j | −9.42 | 2 | 2.54, 3.20 | Gln286, Cys255 |
| 3k | −7.68 | 2 | 2.53, 1.94 | Ser289, Leu292 |
| 3l | −6.48 | 2 | 1.97, 2.24 | Ser289, His449 |
Fig. 4.
Molecular docking studies at PPARγ protein. (a) Structure of PPARγ protein from PDB ID- 2PRG. (b) Docking complex of PPARγ protein (PDB ID- 2PRG) with Rosiglitazone. (c) Docking complex of PPARγ protein (PDB ID- 2PRG) with compound 3b.
4. Conclusion
A Series of 3-substituted-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione derivatives were developed by incorporating different aromatic amines, using conventional and microwave irradiation methods and compared. The results of microwave irradiation technique indicated drastic fall of reaction time and improvement in percentage yield in comparison with traditional conventional synthesis. All the compounds were characterized physically and most of the compounds were characterized spectrally by FT-IR, 1H-NMR, 13C-NMR and mass spectroscopy. In vitro antibacterial evaluation indicated that, compound 3g has shown good activity against gram positive bacteria (B. subtilis and S. aureus) at 40 μg/mL while compound 3i has shown good activity against gram negative bacteria (E. coli and P. aeruginosa) at the same concentration. In vitro antioxidant results stated that, compound 3b and 3d were found to exhibit significant antioxidant activity in both DPPH assay and hydrogen peroxide assay methods. In vivo hypoglycemic activity evaluation revealed that, the compounds 3b and 3h have shown promising hypoglycaemic activity in acute study as well as in chronic study. Molecular docking studies revealed that, compound 3b shown highest binding affinity at PPARγ receptor protein. All these results indicate that the novel synthesized TZDs may be beneficial compounds.
Declarations
Author contribution statement
K. Srikanth Kumar: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A. Lakshmana Rao: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
M.V. Basaveswara Rao: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are thankful to Management of V. V. Institute of Pharmaceutical Sciences, Gudlavalleru, Andhra Pradesh for providing necessary facilities to carry out the research work.
References
- 1.Gregory R.B., Greg A., Beverly B., Christine B., Bork B., Bryan F.B., Michele E., Jiaping G., Prasad K., Robert J.S., Herbert F.S., Mary W., David D.X. The effect of 1,3-diaryl-[1H]-pyrazole-4-acetamides on glucose utilization in ob/ob mice. J. Med. Chem. 2001;44:2601–2611. doi: 10.1021/jm010032c. [DOI] [PubMed] [Google Scholar]
- 2.Willson T.M., Brown P.J., Sternbach D.D., Henke B.R. The PPARs: from orphan receptors to drug discovery. J. Med. Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 3.Trisha M.O.S., Johannes B.P. Thiazolidinediones and type 2 diabetes: new drug for an old disease. Med. J. Aust. 2002;176:381–386. doi: 10.5694/j.1326-5377.2002.tb04461.x. [DOI] [PubMed] [Google Scholar]
- 4.Bamakanta G., Simachal D. Recent advances and potential antimicrobial activities of thiazolidinone derivatives: a review. Asian J. Res. Chem. 2014;7:446–457. [Google Scholar]
- 5.Neeru M., Prasad D.N. Synthesis and antimicrobial evaluation of N-substituted-5- benzylidene-2,4-thiazolidinedione derivatives, Iran. J. Pharm. Sci. Summer. 2012;8:209–214. [Google Scholar]
- 6.Partha N., Fredrick J.L., Satyanarayana M., Jin C., Debendranath D., Maya G., Bishwajit N., Somesh D.S., Lesley B.P. Synthesis and structure–activity relationship studies of cinnamic acid-based novel thiazolidinedione antihyperglycemic agents. Bioorg. Med. Chem. 2003;11:4059–4067. doi: 10.1016/s0968-0896(03)00393-6. [DOI] [PubMed] [Google Scholar]
- 7.Hiroshi K., Mitsuharu N., Hiroki T., Nobuharu G. Hybridization of non-sulfonylurea insulin secretagogue and thiazolidinedione-derived insulin sensitizer. Bioorg. Med. Chem. Lett. 2000;10:2453–2456. doi: 10.1016/s0960-894x(00)00491-1. [DOI] [PubMed] [Google Scholar]
- 8.Pattan S.R., Reddy V.V.K., Pawar P.D., Khade A.B., Desai N.S., Bhat A.R., Taranalli A.D. Synthesis and anti-inflammatory evaluation of (4-phenylamino)benzylidene (thiazolidine-2,4-Dione) derivatives. Indian Drugs. 2007;44:253–256. [Google Scholar]
- 9.Pattan M.Y., Amal, White M.S., Erika B.V., Ibrahim M.E.A., Andis K. Synthesis and biological evaluation of novel pyrazolyl-2,4-thiazolidinediones as anti-inflammatory and neuroprotective agents. Bioorg. Med. Chem. 2010;18:2019–2028. doi: 10.1016/j.bmc.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Anji Reddy K., Lohray B.B., Bhushan V., Sekar Reddy A., Rao Mamidi N.V.S., Papi Reddy P., Saibaba V., Jaipal Reddy N., Suryaprakash A., Parimal M., Reeba K.V., Rajagopalan R. Novel antidiabetic and hypolipidemic agents. 5-Hydroxyl versus benzyloxy containing chroman derivatives. J. Med. Chem. 1999;42:3265–3278. doi: 10.1021/jm9805541. [DOI] [PubMed] [Google Scholar]
- 11.Hong W.L., Bok Y.K., Joong B.A., Sung K.K., Jung H.L., Jae S.S., Soon K.A., Sang J.L., Seung S.Y. Molecular design, synthesis, and hypoglycemic and hypolipidemic activities of novel pyrimidine derivatives having thiazolidinedione. Eur. J. Med. Chem. 2005;40:862–874. doi: 10.1016/j.ejmech.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Rosanna M., Rosaria O., Rosella C., Dietmar R., Barbara M., Christian L., Thierry L. Synthesis, induced-fit docking investigations and in vitro aldose reductase inhibitory activity of non-carboxylic acid containing 2,4-thiazolidinedione derivatives. Bioorg. Med. Chem. 2008;16:5840–5852. doi: 10.1016/j.bmc.2008.04.072. [DOI] [PubMed] [Google Scholar]
- 13.Zengtao W., Zhiguo L., Woojung L., Su-Nam K., Goo Y., Seung H.C. Design, synthesis and docking study of 5-(substitutedbenzylidene)thiazolidine-2,4-dione derivatives as inhibitors of protein tyrosine phosphatase 1B. Bioorg. Med. Chem. Lett. 2014;24:3337–3340. doi: 10.1016/j.bmcl.2014.05.099. [DOI] [PubMed] [Google Scholar]
- 14.Ying W., Hsin-Hsiung T., Hoon C. Synthesis and SAR of thiazolidinedione derivatives as 15-PGDH inhibitors. Bioorg. Med. Chem. 2010;18:1428–1433. doi: 10.1016/j.bmc.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Nazreen S., Alam M.S., Hamid H., Yar M.S., Dhulap A., Alam P., Pasha M.A., Bano S., Alam M.M., Haider S., Kharbanda C., Ali Y., Pillai K.K. Thiazolidine-2,4-diones derivatives as PPAR-γ agonists: synthesis, molecular docking, in vitro and in vivo antidiabetic activity with hepatotoxicity risk evaluation and effect on PPAR-γ gene expression. Bioorg. Med. Chem. Lett. 2014;24:3034–3042. doi: 10.1016/j.bmcl.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Vijay P., Kalpana T., Sonali M.M., Rhea M., Ramaa C.S. Synthesis and primary cytotoxicity evaluation of new 5-benzylidene-2,4-thiazolidinedione derivatives. Eur. J. Med. Chem. 2010;45:4539–4544. doi: 10.1016/j.ejmech.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Pattan S., Kedar M., Pattan J., Dengale S., Sanap M., Gharate U., Shinde P., Kadam S. Synthesis and evaluation of some novel 2,4-thiazolidinedione derivatives for antibacterial, antitubercular and antidiabetic activities. Indian J. Chem. 2012;51B:1421–1425. [Google Scholar]
- 18.Mohammad S. Synthesis, characterization of 2,4-thiazolidinedione derivatives and evaluation of their antioxidant activity. J. Drug Deliv. Therapeut. 2013;3:96–101. [Google Scholar]
- 19.Shah J.J., Krishnapriya M. Comparison of conventional and microwave-assisted synthesis of benzotriazole derivatives. Indian J. Pharm. Sci. 2014;76:46–53. [PMC free article] [PubMed] [Google Scholar]
- 20.Mavandadi F., Pilotti A. The impact of microwave-assisted organic synthesis in drug discovery. Drug Discov. Today. 2006;11:165–174. doi: 10.1016/S1359-6446(05)03695-0. [DOI] [PubMed] [Google Scholar]
- 21.Kidwai M. Dry media reactions. Pure Appl. Chem. 2001;73:147–151. [Google Scholar]
- 22.Yun H., Jun Y., Baogen W., Lisa R., Eric E.S. Synthesis and biological evaluation of novel benzimidazoles as potential antibacterial agents. Bioorg. Med. Chem. Lett. 2004;14:1217–1220. doi: 10.1016/j.bmcl.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 23.Hasan K., Riza D., Ersin O., Selami G. Synthesis, antibacterial and antifungal activities of electron-rich olefins derived benzimidazole compounds. IL Farmaco. 2003;58:431–437. doi: 10.1016/S0014-827X(03)00068-5. [DOI] [PubMed] [Google Scholar]
- 24.Indian Pharmacopoeia . Government of India, Ministry of health and family welfare. Ghaziabad: The Indian Pharmacopoeia Commission; 2010. Biological Methods; pp. 49–55. [Google Scholar]
- 25.Sunil K., Dinesh K., Manjusha, Kamal S., Nidhan S., Vashishta B. Antioxidant and free radical scavenging activity of Citrullus colocynthis (L.) Schrad. Methanolic fruit extract. Acta Pharm. 2008;58:215–220. doi: 10.2478/v10007-008-0008-1. [DOI] [PubMed] [Google Scholar]
- 26.Sreejayan N., Rao M.N.A. Free radical scavenging activity of curcuminoids. Drug Res. 1996;46:169–171. [PubMed] [Google Scholar]
- 27.Nabavi S.M., Ebrahimzadeh M.A., Nabavi S.F., Hamidinia A., Bekhradnia A.R. Determination of antioxidant activity, phenol and flavonoid content of Parrotia persica Mey. Pharmacologyonline. 2008;2:560–567. [Google Scholar]
- 28.Elmastas M., Gulcin I., Isildak O., Kufrevioglu O.I., Ibaoglu K., Aboul-Enein H.Y. Radical scavenging activity and antioxidant capacity of Bay leaf extracts. J. Iran. Chem. Soc. 2006;3:258–266. [Google Scholar]
- 29.Ilhami G., Haci A.A., Mehmet C. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem. Pharm. Bull. 2005;53:281–285. doi: 10.1248/cpb.53.281. [DOI] [PubMed] [Google Scholar]
- 30.Anna Pratima G.N., Dipali D., Hemant D.U. Facile synthesis and in vivo hypoglycemic activity of novel 2,4-hiazolidinedione derivatives. Eur. J. Exp. Biol. 2012;2:343–353. [Google Scholar]
- 31.Shashikant R.P., Prajact K., Ashwini P., Ana N., Kittur B.S. Studies on the Synthesis of novel 2,4-thiazolidinedione derivatives with antidiabetic activity. Iran. J. Pharm. Sci. 2009;5(Autumn):225–230. [Google Scholar]
- 32.Madhuri M., Prasad Ch., Vasudeva Rao A. In silico protein-ligand docking studies on thiazolidinediones as potential anticancer agents. Int. J. Comput. Appl. 2014;95:13–16. [Google Scholar]
- 33.Akhiles R., Mahesh R.D., Shilpa S.H., Momin Zarina M.N., Rohini D.D. Synthesis, molecular docking studies and biological evaluation of 5-[4-(substituted) benzylidene or benzyl]thiazolidine-2,4-dione with their oral hypoglycemic activity. Int. Res. J. Pharm. 2013;4:151–157. [Google Scholar]
- 34.Arifa B., Shaheen B., Prasad K.V.S.R.G., Bharathi K. In silico studies on functionalized azaglycine derivatives containing original article 2,4-thiazolidinedione scaffold on multiple targets. Int. J. Pharm. Pharm. Sci. 2017;9:209–215. [Google Scholar]
- 35.Prashantha Kumar B.R., Mukesh S., Santhosh Kumar S., Kuldeep S., Mohan P., Nasir B.R.B., Laxmi A. Synthesis, glucose uptake activity and structure–activity relationships of some novel glitazones incorporated with glycine, aromatic and alicyclic amine moieties via two carbon acyl linker. Eur. J. Med. Chem. 2011;46:835–844. doi: 10.1016/j.ejmech.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Pattan S.R., Suresh Ch., Pujar V.D., Reddy V.V.K., Rasal V.P., Koti B.C. Synthesis and antidiabetic activity of 2-amino-[5'(4-sulphonylbenzylidine)-2,4-thiazolidinedione]-7-chloro-6-fluorobenzothiazole. Indian J. Chem. 2005;44B:2404–2408. [Google Scholar]
- 37.Gilles B., Abed A.A.A.Q., Claes D.E., Izet K., Hans J.N., Serge V.C., Morris S., Tom C. Synthesis and evaluation of thiazolidinedione and dioxazaborocane analogues as inhibitors of AI-2 quorum sensing in Vibrio harveyi. Bioorg. Med. Chem. 2013;21:660–667. doi: 10.1016/j.bmc.2012.11.055. [DOI] [PubMed] [Google Scholar]
- 38.Jiwane S.K., Singh V.K., Namdeo K.P., Prajapati S.K. Synthesis of some novel 2,4-thiazolidinedione derivatives and their biological screening as antidiabetic agents. Asian J. Chem. 2009;21:5068–5072. [Google Scholar]
- 39.Alexander E.R., Underhill E.J. Studies on the mechanism of the mannich reaction. I. Ethylmalonic acid, a methynyl compound. J. Am. Chem. Soc. 1949;17:4014–4019. [Google Scholar]
- 40.Santosh L.G., Namratha B., Nitinkumar S.S., Hiroki S. Microwave-assisted synthesis and evaluation of N-substituted thiazolidine-2,4-dione derivatives as antimicrobial agents. Interact. Med. Chem. 2014;2:1–5. [Google Scholar]
- 41.Prashantha Kumar B.R., Nanjan M.J., Suresh B., Karvekar M.D., Adhikary L. Microwave induced synthesis of the thiazolidine-2,4-dione motif and the efficient solvent free-solid phase parallel syntheses of 5-benzylidene-thiazolidine-2,4-dione and 5-benzylidene-2-thioxo-thiazolidine-4-one compounds. J. Heterocycl. Chem. 2006;43:897–903. [Google Scholar]
- 42.Gabriel M., Ioana I., Adrian P., Laurian V., Dan C.V., Mihaela D., Brindusa T., Ovidiu O. Microwave assisted synthesis of 3,5-disubstituted thiazolidine-2,4-diones with antifungal activity. Design, synthesis, virtual and in vitro antifungal screening. Farmica. 2017;65:414–422. [Google Scholar]
- 43.Kishan D.P., Chhaganbhai N.P., Grishma M.P. Microwave assisted synthesis and antidiabetic activity of novel 5-[4-(substituted)benzylidine]thiazolidine-2,4-dione. Med. Chem. 2016;6:647–651. [Google Scholar]