Abstract
Transient ischemic attack (TIA) is an ischemic episode of neurologic dysfunction characterized by a spontaneous clinical resolution of symptoms within 24 hours. Mechanisms of this remarkable recovery are not yet well understood. In patients with permanent brain injury caused by a stroke cortical levels of γ-Aminobutyric acid (GABA) are decreased. In this study, we aimed to investigate, whether similar alterations of cortical GABA are also present in patients with TIA. Ten first-time TIA patients with temporary unilateral motor symptoms from upper limb and 10 control subjects underwent Magnetic Resonance Spectroscopy (MRS) with SPECIAL technique. GABA:creatine (GABA:CR) ratios were measured in the hand area of the primary motor cortex in both hemispheres. GABA:CR ratios were significantly lower in the symptomatic hemisphere of TIA patients when compared with healthy subjects. Whether reduced GABA is induced directly by transient ischemia or is a secondary compensatory mechanism, which facilitate re-establishment of normal function remains to be elucidated. Further research investigating our findings in larger samples will aid in understanding of the clinical significance of GABA alterations in TIA patients. GABA MRS may provide vital information about mechanisms involved in recovery after transient ischemia, which may have crucial importance for development of new neuroprotective strategies in stroke.
Keywords: Neuroscience, Neurology, Medical imaging
1. Introduction
In stroke, neurophysiological and imaging studies demonstrate substantial changes in intracortical circuits [1], γ-Aminobutyric acid (GABA) activity [2] and metabolism [3]. Functional recovery after permanent cerebral ischemia involves early alterations in brain activity [1, 4] and it has been suggested that especially GABA activity may have an important role in post stroke recovery [5], as decreased GABA levels correlate with better improvements in motor function after rehabilitation [6]. Whether similar alterations of GABAergic activity are also involved in a spontaneous recovery after transient ischemic attack (TIA) has yet to be elucidated.
Only a few studies have investigated alterations of brain activity and metabolism in TIA patients. Paired-pulse Transcranial Magnetic Stimulation (TMS) to the affected hemisphere has demonstrated reduced intracortical inhibition [7] and enhanced facilitation [7, 8] after TIA. Magnetic Resonance Spectroscopy (MRS) performed within 3 days after onset of TIA symptoms have demonstrated signs of metabolic damage with decreased N-Acetylaspartic Acid (NAA) and increased lactate in both hemispheres [9]. Interestingly, drug challenge with a GABAA agonist lead to reoccurrence of symptoms in TIA patients [10], thus the regulation of GABAergic inhibition may play a role in remission of symptoms after TIA.
Recently, a new MRS technique called SPECIAL (spin-echo full-intensity acquired localized) has been developed that enables detection of several metabolites in the brain, including GABA and glutamate (GLU) [11, 12]. To date no study has employed this method in patients with TIA.
We hypothesized that GABA levels are decreased in the hand area of the primary motor cortex (M1) in TIA patients suffering from transient paralysis of the upper limb when compared to healthy subjects.
2. Methods
2.1. Study design
Study was approved by the Central Denmark Region Committees on Health Research Ethics and have been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
TIA was defined as a sudden focal neurologic deficit of presumed vascular origin lasting less than 24h (WHO definition). Ten TIA patients (5 males, aged 69 years (55–78)) were recruited from Department of Neurology at Aarhus University Hospital. Inclusion criteria: TIA with unilateral upper limb motor symptoms lasting at least 10 min. Exclusion criteria: prior stroke or TIA, history of other neurological disease and contraindications to Magnetic Resonance Imaging (MRI).
A control group consisted of 10 age-matched, right-handed healthy participants (6 males, aged 66 years (58–73)). All subjects gave informed consent prior to participation in the study.
2.2. Clinical evaluations
TIA patients underwent diagnostic work-up including a neurological examination, a ABCD2 score [13], a clinical MRI and carotid artery imaging.
2.3. Magnetic resonance spectroscopy
All participants were scanned using a 3T Trio system (Siemens, Erlangen, Germany) with T1 MPRAGE (TR/TE = 2420/4.6 ms, 1mm isotropic resolution), T2 FLAIR and magnetic resonance spectroscopy SPECIAL (spin-echo full-intensity acquired localized) technique [11, 12].
A T1 MPRAGE scan was used for identification of the precentral knob [14], which was the region of interest. SPECIAL data with water suppression (TR/TE = 4000/8.5 ms, 146 averages) were acquired from 2 × 2 × 2 cm voxels placed in both hemispheres, pseudo randomly starting either ipsilateral or contralateral to the affected limb.
2.4. MR spectroscopy analysis
Spectroscopy data were analyzed by one of the authors (TS) who was blinded to group allocation and clinical information. Raw data was pre-processed in MATLAB (Natick, MA USA) and motion distorted averages were rejected, with subsequent correction of frequency and phase drift using the FID-A processing toolkit [15]. Processed spectra were analyzed using LCModel, ver 6.3-1H [16]. Linewidth (LW - below 8 Hz) and Cramer-Rao lower bounds (CRLB) (below 20%) were used as criteria of spectral quality and quantification reliability. Concentrations of metabolites were expressed in reference to total creatine (CR). Furthermore, to evaluate changes relative to the neuronal content, GABA and GLU were expressed in reference to NAA. The amount of grey matter within the voxels T1 images was determined using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/).
2.5. Statistical analysis
Group data are presented as mean ± SD. Data was analyzed using parametric statistics and within group differences between hemispheres were compared using paired t test, and between group comparisons were analyzed with unpaired t test.
3. Results
3.1. Participants
Patients characteristics are presented in Table 1. Three of the 10 TIA subjects had an acute ischemic lesion visible on the Diffusion Weighted Imaging (DWI) scan, however, none of these lesions were located in the primary motor area. None of the patients had significant carotid artery stenosis. All patients were scanned with MRS within 8 days after TIA.
Table 1.
Patients characteristics. Abbreviations: M/F, male/female; R/L, right/left; DWI, Diffusion Weighted Imaging; TIA, Transient Ischemic Attack; MRS, Magnetic Resonance Spectroscopy.
| Patient | Sex M/F |
Age, years | Affected hemisphere | Duration of symptoms, min | Positive DWI | Time from TIA to MRS, days | ABCD2 score |
|---|---|---|---|---|---|---|---|
| 1 | M | 55 | R | ≥60 | + | 7 | 5 |
| 2 | M | 76 | L | 10–59 | + | 4 | 5 |
| 3 | F | 75 | R | ≥60 | + | 4 | 5 |
| 4 | M | 63 | R | ≥60 | − | 8 | 6 |
| 5 | M | 71 | R | ≥60 | − | 6 | 5 |
| 6 | F | 78 | L | ≥60 | − | 6 | 5 |
| 7 | F | 72 | R | 10–59 | − | 5 | 4 |
| 8 | F | 65 | L | ≥60 | − | 5 | 5 |
| 9 | F | 68 | L | 10–59 | − | 8 | 4 |
| 10 | F | 66 | R | 10–59 | − | 4 | 5 |
3.2. Magnetic resonance spectroscopy
Spectra from one TIA and one healthy subject were rejected due to a LW > 8, giving 9 subjects in each group with bilateral spectra. There was no significant difference for GABA:CR, GABA:NAA, GLU:Cr and GLU:NAA between the two hemispheres in healthy subjects. Since six of the ten TIA patients had symptoms from the right hemisphere we compared the affected side with the right hemisphere of control subjects and the unaffected hemisphere with the left hemisphere of control subjects.
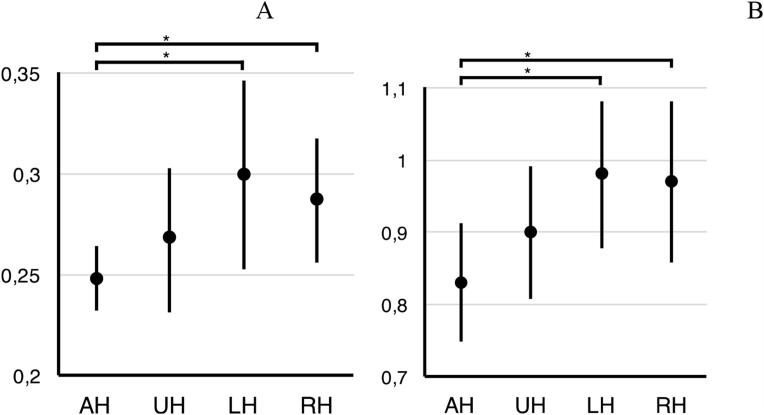
In the affected hemisphere of patients, the GABA:CR ratio (0.25 ± 0.01) was significantly lower as compared to control subjects (0.29 ± 0.03), t(16) = −3.5, p = 0.003 (Fig. 1A). There was no significant difference between GABA:CR ratio in the unaffected hemisphere of TIA patients (0.27 ± 0.04) and the control subjects (0.3 ± 0.05) (p = 0.11). Comparing the affected and unaffected hemisphere in the TIA group, GABA:CR ratio tended to be lower in the affected hemisphere (p = 0.08).
Fig. 1.
GABA:CR (A) and GLU:CR (B) ratios in the affected and unaffected hemisphere of TIA patients and in the left and right hemisphere of healthy subjects. AH, affected hemisphere; UH, unaffected hemisphere; LH, left hemisphere; RH, right hemisphere. Data presented as mean ± SD, *p < 0.01.
There were no significant differences in the amount of grey matter in affected hemisphere of TIA patients (25 ± 4%) and healthy controls (27% ± 3%), t(14) = −1.17, p = 0.26 and consequently, GABA:CR ratios were not corrected for tissue volume estimates.
In the affected hemisphere of TIA patients, the GABA:NAA ratio (0.2 ± 0.01) was significantly lower than in the healthy controls (0.22 ± 0.02), t(16) = −3.34, p = 0.004.
After exclusion of the 3 TIA patients with positive DWI the GABA:CR remained lower (0.25 ± 0.02) in the affected hemisphere as compared to the healthy controls (0.29 ± 0.03), t(13) = −2.55, p = 0.02.
The mean GLU:CR ratio in the affected hemisphere of TIA patients (0.83 ± 0.08) was significantly lower when compared to the controls (mean 0.97 ± 0.1), t(16) = −3.15, p = 0.0061 (Fig. 1B), also when the three patients with positive DWI were removed from the analysis (p = 0.02). The significant difference was also observed when the mean GLU:NAA ratio in the affected hemisphere TIA (0.67 ± 0.08) was compared with the healthy controls (0.75 ± 0.05), t(16) = −2.69, p = 0.016.
There was no significant difference in GLU:CR ratios between the affected hemisphere and unaffected hemisphere of TIA patients (p = 0.18), as well there was no significant difference between the mean GLU:CR ratio from the unaffected hemisphere of TIA patients and the controls (p = 0.09).
4. Discussion
The main finding of our study is the lower GABA levels in the symptomatic hemisphere in TIA patients compared to healthy subjects even in patients without ischemia on DWI. We believe that this is the first study to assess the GABA levels using MRS in TIA patients.
Findings of the study are in line with previous TMS [7, 8] showing that GABA mediated inhibition is decreased following TIA. Whether reduced GABA is induced directly by ischemia or is a secondary compensatory mechanism remains to be elucidated. However, as TIA symptoms can be re-induced by a GABA agonist (midazolam) [10], this suggests that a reduced inhibition contribute to preserve a normal function in the affected hemisphere. This is also consistent with studies showing an association between GABA reduction and enhanced recovery [6].
In addition to reduced GABA levels, our data show significantly reduced glutamate levels in the affected hemisphere, which stands in contrast to the study by Edwards et al [8], where enhanced intracortical facilitation (ICF) in the affected hemisphere was found. However, this discrepancy may be caused by differences in MRS assessed glutamatergic activity and TMS measures of excitability [17], as MRS glutamate measures do not correlate with paired-pulse ICF [18]. Another hypothetical explanation could be derived from experimental models of transient ischemia, where in mice excitotoxic glutamate is released from neurons after ischemia and thereafter cleared by astrocytes and transformed to glutamine, which ultimately results in a reduction of glutamate [19, 20].
In contrast to previous studies, where NAA was reduced in the affected hemisphere after TIA [9], we have only found a tendency to lower NAA in the affected hemisphere. This could be due to our small sample size or, alternatively to differences in the timing of MRS. In the previous study patients were investigated within 3 days after onset of symptoms whereas we investigated up to 8 days after onset of symptoms, when normalization of NAA levels may have occurred.
Our study has some limitations. Only a small group of patients were recruited, as we aimed to include a homogenous group of patients with motor symptoms of the upper limb only. Furthermore, to ensure a correct diagnosis the symptoms had to last for at least 10 minutes. The small sample size did not allow a comparison of patients with and without DWI positive lesions. Since all subjects were scanned only once, it remains unknown whether the GABA levels normalized later or were permanently reduced.
5. Conclusion
We demonstrated decreased GABA levels within the primary motor cortex in patients shortly after their first TIA. More research is needed to corroborate our findings and to establish the role and importance of GABA changes in tissue exposed to ischemia.
Declarations
Author contribution statement
Krystian Figlewski, Tobias Stærmose, Jakob U. Blicher: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Henning Andersen, Jørgen Feldbæk Nielsen: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Paul von Weitzel-Mudersbach: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Bevica Foundation.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We wish to thank Dr. Jamie Near from McGill University, Montreal, Canada for providing the SPECIAL sequence.
References
- 1.Liepert J., Storch P., Fritsch A., Weiller C. Motor cortex disinhibition in acute stroke. Clin. Neurophysiol. 2000;111(4):671–676. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y.K., Yang E.J., Cho K., Lim J.Y., Paik N.-J. Functional recovery after ischemic stroke is associated with reduced GABAergic inhibition in the cerebral cortex: a GABA PET study. Neurorehabil. Neural Repair. 2014;28:576–583. doi: 10.1177/1545968313520411. [DOI] [PubMed] [Google Scholar]
- 3.Bivard A., Krishnamurthy V., Stanwell P., Yassi N., Spratt N.J., Nilsson M. Spectroscopy of reperfused tissue after stroke reveals heightened metabolism in patients with good clinical outcomes. J. Cerebr. Blood Flow Metabol. 2014;34(12):1944–1950. doi: 10.1038/jcbfm.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huynh W., Vucic S., Krishnan A.V., Lin C.S.-Y., Hornberger M., Kiernan M.C. Longitudinal plasticity across the neural axis in acute stroke. Neurorehabil. Neural Repair. 2013;27:219–229. doi: 10.1177/1545968312462071. [DOI] [PubMed] [Google Scholar]
- 5.Clarkson A.N., Huang B.S., Macisaac S.E., Mody I., Carmichael S.T. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blicher J.U., Near J., Naess-Schmidt E., Stagg C.J., Johansen-Berg H., Nielsen J.F. GABA levels are decreased after stroke and GABA changes during rehabilitation correlate with motor improvement. Neurorehabil Neural Repair. 2015;29:278–286. doi: 10.1177/1545968314543652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koerner C., Meinck H.-M. Long-lasting motor cortex disinhibition after short transient ischemic attacks (TIAs) in humans. Neurosci. Lett. 2004;361:21–24. doi: 10.1016/j.neulet.2003.12.074. [DOI] [PubMed] [Google Scholar]
- 8.Edwards J.D., Meehan S.K., Levy A.R., Teal P.A., Linsdell M.A., Boyd L.A. Changes in intracortical excitability after transient ischemic attack are associated with ABCD2 score. Stroke. 2011;42:728–733. doi: 10.1161/STROKEAHA.110.602938. [DOI] [PubMed] [Google Scholar]
- 9.Bisschops R.H.C., Kappelle L.J., Mali W.P.T.M., van der Grond J. Hemodynamic and metabolic changes in transient ischemic attack patients: a magnetic resonance angiography and 1H-magnetic resonance spectroscopy study performed within 3 Days of onset of a transient ischemic attack. Stroke. 2002 Jan 1;33:110–115. doi: 10.1161/hs0102.100879. [DOI] [PubMed] [Google Scholar]
- 10.Lazar R.M., Fitzsimmons B.F., Marshall R.S., Mohr J.P., Berman M.F. Midazolam challenge reinduces neurological deficits after transient ischemic attack. Stroke. 2003;34(3):794–796. doi: 10.1161/01.STR.0000056540.04159.F3. [DOI] [PubMed] [Google Scholar]
- 11.Mekle R., Mlynarik V., Gambarota G., Hergt M., Krueger G., Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn. Reson. Med. 2009;61:1279–1285. doi: 10.1002/mrm.21961. [DOI] [PubMed] [Google Scholar]
- 12.Near J., Andersson J., Maron E., Mekle R., Gruetter R., Cowen P. Unedited in vivo detection and quantification of γ-aminobutyric acid in the occipital cortex using short-TE MRS at 3 T. NMR Biomed. 2013;26:1353–1362. doi: 10.1002/nbm.2960. [DOI] [PubMed] [Google Scholar]
- 13.Rothwell P., Giles M., Flossmann E., Lovelock C., Redgrave J., Warlow C. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischemic attack. Lancet. 2005 Jul;366:29–36. doi: 10.1016/S0140-6736(05)66702-5. [DOI] [PubMed] [Google Scholar]
- 14.Yousry T.A., Schmid U.D., Alkadhi H., Schmidt D., Peraud A., Buettner A. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- 15.Simpson R., Devenyi G.A., Jezzard P., Hennessy T.J., Near J. Advanced processing and simulation of MRS data using the FID appliance (FID-A)— an open source, MATLAB-based toolkit. Magn. Reson. Med. 2017;77:23–33. doi: 10.1002/mrm.26091. [DOI] [PubMed] [Google Scholar]
- 16.Provencher S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 17.Stagg C.J. Magnetic Resonance Spectroscopy as a tool to study the role of GABA in motor-cortical plasticity. Neuroimage. 2014;86:19–27. doi: 10.1016/j.neuroimage.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Stagg C.J., Bestmann S., Constantinescu A.O., Moreno L.M., Allman C., Mekle R. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J. Physiol. (London) 2011;589:5845–5855. doi: 10.1113/jphysiol.2011.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthet C., Lei H., Gruetter R., Hirt L. Early predictive biomarkers for lesion after transient cerebral ischemia. Stroke. 2011;42:799–805. doi: 10.1161/STROKEAHA.110.603647. [DOI] [PubMed] [Google Scholar]
- 20.Lei H., Berthet C., Hirt L., Gruetter R. Evolution of the neurochemical profile after transient focal cerebral ischemia in the mouse brain. J. Cerebr. Blood Flow Metabol. 2009;29:811–819. doi: 10.1038/jcbfm.2009.8. [DOI] [PubMed] [Google Scholar]