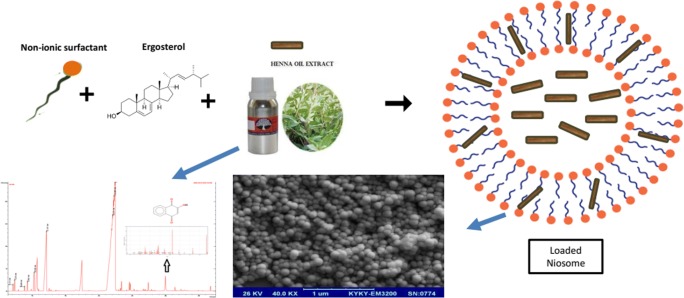
Abstract
Phytochemicals like Lawsone have some drawbacks that stem from their poor solubility. Low solubility in aqueous mediums results in low bioavailability, poor permeability and instability of phytochemical compounds in biological environments. The aim of this study was to design nanoniosomes containing Lawsone (Law) using non-ionic surfactants and cholesterol. Niosomes were prepared by thin film hydration method (TFH). Then, they were loaded with Henna extract (HLaw) and standard Lawsone (SLaw), and two resulted formulations were compared. The henna extract was analyzed by mass gas chromatography. Size, zeta potential, polydispersity index (PDI) and morphology of the loaded formulations were evaluated by dynamic light scattering (DLS) and scanning electron spectroscopy (SEM). The incorporation and release rate of Law from niosome bilayers were evaluated by UV-Vis spectroscopy. In vitro experiments were carried out to evaluate antitumor activity in MCF-7 cell line. The results showed distinct spherical shapes and particle sizes were about 250 nm in diameter and have negative zeta potentials. Niosomes were stable at 4 °C for 2 months. Entrapment efficiently of both formulations was about 70% and showed a sustained release profile. In vitro study exhibited that using of niosome to encapsulating Law can significantly increase antitumor activity of formulation in MCF-7 cell line compared to Law solution (free Law). Thus, niosomes are a promising carrier system for delivery of phytochemical compounds that have poor solubility in biological fluids.
Graphical abstract.
ᅟ
Keywords: Niosome, Lawsone, Solubility, Phytochemicals, Anticancer
Introduction
Nanotechnology approach can increase the stability and also improve the therapeutic efficiency of phytochemicals, such as polyphenols, tannins, alkaloids, terpenoids and herbal extracts. Since ancient times, compounds derived from plants have been a good source of therapeutic drugs [1]. In recent years, there has been growing interest in alternative therapies and therapeutic use of natural materials such as Taxol (a substance originated from the Pacific yew tree) and they have been approved for the clinical treatment of cancer patients [2]. Another example is Plumbagin that is a yellow dye, formally derived from naphthoquinone. Plumbagin has been shown to induce cell cycle arrest and apoptosis in numerous cancer cell lines including melanoma, lung, breast and others [3]. The advantage of using plant derivatives for cancer therapy is in their potential to be used in an edible form and lower side effects than conventional anticancer medicine [4]. However, phytochemicals have some drawbacks that decrease the patient compliance, such as penetrating smelling and nasty taste [5]. Moreover, other factors that reduce their use in remedial are their low solvability, low dissolution rate, high doses required for an therapeutic effect and instability at excessive pH, resulting in lower or no therapeutic efficacy [6–9]. Because of these limitations, an assembled technological approach such as nanocarrier systems is necessary. These nanocarriers develop more efficient and stable formulations that are an important goal for pharmaceutical, nutraceutical, food and cosmetic industries [10].
Persian Henna (Lawsonia inermis) is a bushy and flowering tree that is found in Asia, Australia and Mediterranean coasts of Africa [11]. Crude extracts of Henna and its ingredients have many biological activities such as antibacterial, antioxidant, anti-inflammatory and anticancer properties [12]. The major constituent of Henna plant is Lawsone that is used in the synthesis of some anticancer drugs, like lapachol and dichloroallyl Lawsone [13–15]. Moreover, there are some other chemicals like luteolin, lupeol, isoplumbagin and apigenin, the anticancer effect of which has been approved [16]. Although lawsone presents many medical advantages, its delivery is a problematical challenge due to its hydrophobic nature which results in poor solubility, poor permeability, low bioavailability and instability in biological systems [17–20].
Niosomes have lamellar structures that are built by a mixture of a non-ionic surfactant and cholesterol as a helper lipid followed by hydration in aqueous media [21, 22]. In architectural point of view, niosomes are similar to liposomes but more efficient in physicochemical properties, less toxicity due to their non-ionic nature [23, 24] and a promising carrier for the delivery of active compounds [25–27]. Vesicle formulations can be variable and controllable in characteristics. Change in vesicle composition, size, lamellarity, release rate, trapped volume and surface charge can control vesicle behaviors [28]. Thanks to the special structure of niosomes, hydrophilic and hydrophobic drugs can be encapsulating simultaneously so that the hydrophilic drug is encapsulated in the vesicular aqueous core or on the surface of noisome, and the lipophilic drug is encapsulated in the lipophilic region of the vesicle. Niosomes are osmotically active, stable and can increase the solubility of hydrophobic drugs [26]. Niosomes have numerous applications in immunological adjuvants [29]. They are also anticancer and anti-infective [30], carriers of anti-inflammatory drugs [31], imaging agents for diagnostic purposes [32] and also administered in different ways [33]. Innovative nanocarriers such as niosomes are able to tune some unfavorable chemical and physical features of phytochemicals and also promote antioxidant/radical scavenging activity and bioavailability in biological fluids [34–36]. Improving in antioxidant/radical scavenging activity of the active compounds arise from increasing the resistance of phytochemicals against degradative phenomena due to their encapsulation in the niosomes [37–39].
There have been no related study about encapsulating Lawsone in niosome for more efficient delivery. The purpose of this study is to develop a new approach for formulating Law-entrapped niosomes by thin film hydration method. Niosomes were formulated with Henna extract (HLaw) and standard Lawsone (SLaw), and their properties were compared in terms of size, morphology, stability, release profile and anticancer activity.
Materials and methods
Materials
Lawsone (2- hydroxy-1, 4-naphthoquinone), Span 60 (Sorbitan monostearate), Tween 60 (Polyoxyethylene sorbitan monostearate, 3-(4, 5-dimethylthiazol-2 yl) -2, 5-diphenyltetrazolium bromide (MTT reagent), cholesterol and dialysis bag (MWCO = 12,000 Da) were purchased from Sigma (St. Louis, MO, USA). Chloroform was obtained from Merck (Germany). All the reagents and chemicals were of analytical grade and used without further treatment.
Henna sample extraction and characterization
Fresh plant materials of the Henna were collected from Kerman district of Iran and were authenticated by a pharmacognosist. The leaves of Henna were air-dried at the room temperature. Then, 50 g of the dried and crushed leaves was extracted using alcoholic solution by soxhlet technique up to 24 h. The plant extract was filtered by membrane filters and concentrated in the rotatory evaporator at 40 °C [40].
The GC-MS analysis was performed by Varian 5300 Gas Chromatograph with a mass detector and HP-5 ms column (60 m × 0.25 mm × 025 μm DF). The instrument was set to an initial temperature of 60 °C and maintained at this temperature for 5 min. At the end of this period, the oven temperature was increased up to 270 °C, at the rate of an increase of 5 °C/min, and maintained for 5 min. Helium flow rate was 1 ml/min and injection port was set at 260 °C. The ionization voltage was 70 eV. The samples were injected in the split mode as 80:1. The fragmentation patterns earned from mass spectra were evaluated with those stored in the spectrometer database using National Institute of Standards and Technology Mass Spectral database (NIST-MS). The percentage of the components was compared with relative peak area in the chromatogram.
Preparation and optimization of Lawsone encapsulated niosomes
For niosome preparation, thin film hydration method was used as reported previously [41]. Specific molar ratios of Span 60, Tween 60 and cholesterol were dissolved in 10 mL of a chloroform solution containing 1 mg/ml of Lawsone and added to a 50 mL round bottom flask. Then, the solvent was evaporated at 60 °C under vacuum in a rotary evaporator (Eppendorf, Germany). The resulted thin lipid was hydrated with 10 ml of deionized water at 60 °C. The resulting solution was further sonicated in an ultrasonic bath for 30 min at 50 °C. Niosome purification was performed by a 0.22 um membrane filter (Sartorius AG, Göttingen, Germany).
Morphology, size distribution and zeta potential of formulations
The morphological analysis of niosomes was carried out by scanning electron spectroscopy (SEM) (SBC-12, KYKY, China). For SEM analysis, a drop of niosome suspension added to a lam and air dried for 24 h. DLS (dynamic light scattering) (Malvern Instruments Ltd., Worcestershire, UK) was used to evaluate size, size distribution and zeta potential of the formulations. Also, the effective formation of the vesicles was investigated by transmission electron microscopy (TEM) analysis (EM10C, Zeiss, Germany). A drop of the dispersion was directly placed onto a formvar membrane-coated grid and stained by adding a drop of 2% (w/w) sodium phosphotungstate solution, removing the excess solution using a filter paper, followed by through air-drying.
Entrapment efficiency (EE) of lawsone into niosomes
Lawsone was spectrophotometrically determined using a UV–Vis spectrophotometer (Agilent Technologies, Cary 50, USA) at 673 nm, at which lawsone has maximum adsorption. To obtain calibration curve absorbance versus concentration of lawsone, different concentrations of lawsone dissolved in chloroform (1, 4, 8, 10, 15, 20, 60, 80, 100 and 150 ppm) were prepared. The limit of detection for UV-Vis spectroscopy was calculated and it was 0.399 ppm. The EE (%) was evaluated by quantifying free Lawsone in the supernatant after centrifugation procedure of niosome formulations. The samples were centrifuged at 15100 rpm for 60 min at 4 °C (5415D, Eppendorf, Germany). The free Lawsone concentration in the supernatant was evaluated by UV spectrophotometry at 673 nm. The difference between the total Lawsone content in the formulations and free Lawsone was EE (%).
Release behavior of Lawsone from loaded formulations
First, 1 ml of each formulation was added to a dialysis bag and, then, placed in a 50 ml beaker filled with PBS (pH = 7.4). The beaker was stirred by a magnetic stirrer (150 rpm) at 37 °C for 15 h. At a specific period of time, 1 mL of the released content was withdrawn and immediately replaced with the similar content of fresh PBS solution. Then, the concentration of Lawsone was determined by a spectrophotometer at 673 nm. The percentages of the released content of Lawsone in PBS were presented as % accumulative release [42].
Cytotoxicity assay
Cytotoxic effects of niosome formulations on MCF-7 cell line were performed by MTT assay. 7 × 103 MCF-7 cells were plated in 100 μL of DMEM solution and incubated for 24 h. On the next day, the incubated cells were treated with the concentration of 1.5 μM to 10 μM of niosome formulations suspended in PBS with pH = 7.4 and diluted with DMEM solution) for 24 h. After the incubation, 0.5 mg/ml of MTT was applied to the wells and incubation was carried out at 37 °C for 4 h in a dark place. Then, 100 μL of DMSO solvent was added. Finally, the absorbance was taken at 560 nm, and 630 nm was used as the reference wavelength [43].
Statistical analysis
One-way ANOVA was employed for the statistical analysis of various experiments [44]. A p-value <0.05 was considered statistically significant. Values are reported as mean ± SD.
Results and discussion
GC-MS analysis
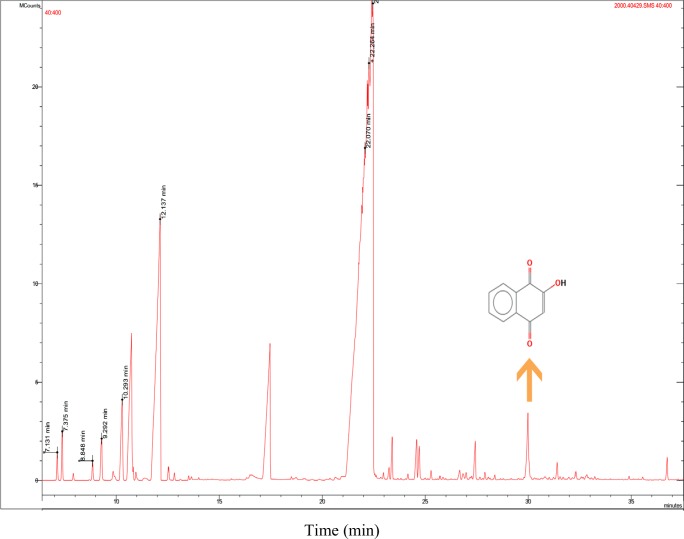
The phytochemical compounds in the alcoholic extracts of Henna leaves were identified by the GC-MS analysis. Retention time (RT), molecular formula (MF), area and percentage of the obtained peak in the extract were also evaluated. Totally, sixteen compounds were identified from the ethanol extract of the Henna leaves (data not shown). Presence of Lawsone in the extract was 1.03%, the RT of which was in the 30th minute (Fig. 1). As expected the amount of Lawsone in Henna extract was good enough for anti-cancer study. In Henna extract, there are some active ingredients that are responsible for antioxidant and antitumor activity.
Fig. 1.
Chromatogram obtained from the GC-MS with the extract of Henna leaves
Mean size and physicochemical characterization of niosome formulations
The compositions and characteristics of the niosome formulations are presented in Table 1. The mean particle size of the niosomes ranged from 198 nm to 377 nm. A narrow peak distribution was obtained from DLS, which approved that the formulations had the uniform size. The average size of bare niosomes (198 ± 4.25 nm) compared to Nio/SLaw (316 ± 3.14 nm) and Nio/HLaw (377 ± 5.63) was changed significantly. Loading of HLaw and SLaw led to an increase in vesicle dimensions values (Table 1). The dimension increased when Law molecules were loaded in the vesicles, which was because of partial insertion of the guest drug in the bilayer, with a consequent decrease of the surface tension of the particles [45].
Table 1.
Composition and characteristics of niosome formulations (n = 3)
| Name | Size (nm) | PDI | Zeta potential (mV) | EE % (Law) |
|---|---|---|---|---|
| Bare Niosome | 198 ± 4.25 | 0.33 ± 0.07 | −19.8 ± 0.65 | – |
| Nio/SLaw | 316 ± 3.14 | 0.27 ± 0.01 | −27.6 ± 0.74 | 73 ± 1.45 |
| Nio/HLaw | 377 ± 5.63 | 0.22 ± 0.03 | −32.5 ± 0.68 | 69 ± 1.02 |
Zeta potential values were affected by the presence of Law. It has been reported that a stable nanosuspension mainly stabilized by electrostatic repulsion exhibits zeta potential values close to −30 mV [46]. In the current study, as soon as the law was loaded into niosomes, the zeta potential of these two formulations (Nio/SLaw and Nio/HLaw) moved to more negative values. Changing in zeta potential is maybe because of addition more negative charge density to the formulation bilayer that come from Lawsone. High and negative zeta potential values are an indication of stable preparations and prevent aggregation phenomena [47). PDI parameter approved homogeneity of our suspension that values of less than 0.3 corresponded to a single, rather than a sharp peak, in the DLS profile [48]. In the case of our samples, a single and homogeneous population was detected, except for bare niosome. As we reported above, bare niosome have a low zeta potential and because of this reason the stability of formulation is slight and they coagulate. The coagulation of formulation can increases the PDI value.
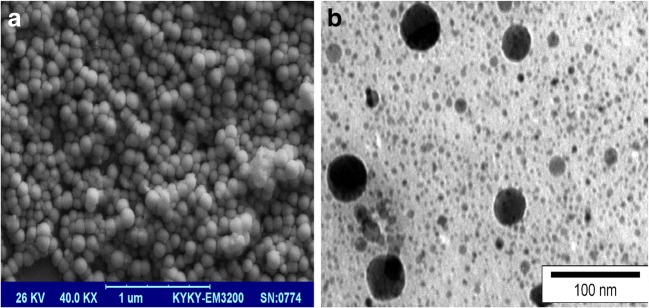
The morphology and size of niosome formulations were analyzed by SEM and TEM (Fig. 2A and B). The images showed nanometer sizes, semi-spherical and homogenies form for both Nio/HLaw and Nio/SLaw formulations. Moreover, the loading of niosomes did not cause any structural deformations, which confirmed high stability of the formulations. SEM and TEM analysis gave us lower sizes than DLS. Generally, TEM gives the size of nanoparticles in dried form while DLS tells the hydrodynamic diameter that includes core plus any molecule attached or adsorbed on surface, due to which the average size would be more.
Fig. 2.
SEM (a) and TEM (b) images of Nio/HLaw formulation, original magnification 40,000 X
EE %
EE measurement indicated that about 70 ± 1.02% of Law was entrapped within the niosomal bilayers. As shown in Table 1, SLaw formulation showed more entrapment efficiency that might be related to more structural affinity among SLaw (as hydrophobic moiety) and the non-polar palmitate moiety of Span 60 and Tween 60. High encapsulating efficiently of Lawsone in niosomes is because of hydrophobic nature of Lawsone that results in more affinity and tus more incorporation in niosomes bilayers. Law interacts with the acyl chains of niosome bilayers and can stabilize the formulation [49].
In vitro release study
Drug release rate is an important parameter in the design of drug delivery systems and must be carefully analyzed. In vitro results will give an insight from in vivo performance of niosomes [50, 51]. Fig. 3 illustrates the in vitro release of free Law, Nio/HLaw, and Nio/SLaw formulations. Niosomes like other vesicular drug delivery systems showed a biphasic release, so that rapid drug release happened and, then, a steady state of release was obtained. Nio/HLaw had a less release rate than Nio/Slaw due to a complex matrix of Henna extract. Free Law releases were very fast and almost all of the Law was released within 4 h because there is not any shell for sustained release. The Law release rate in our formulation was significantly slower than the free Law. These findings proved that niosomes have the capability of the sustainable and controlled release of Law. In fact, support studies working on other nanocarriers [51] have concluded that niosome formulations can be applicable for delivering phytochemicals in a sustained and controllable manner.
Fig. 3.
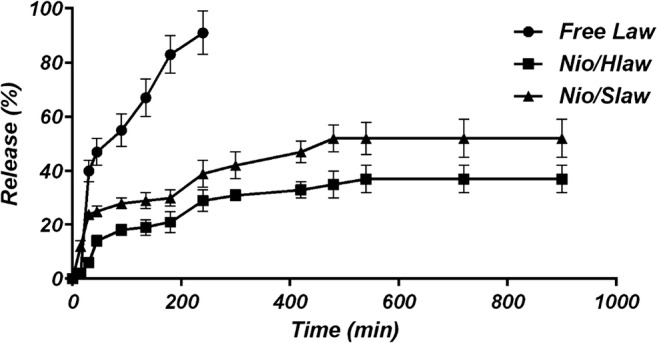
Sustained release (%) of free Law and Law entrapped in niosomes in PBS (pH 7.4) at 37°C. Data are expressed as the mean ± SD (n=3)
Stability of the system
Stability study showed that Nio/Hlaw have high stability. The stability of the encapsulated Law is a critical factor for the effective delivery of the formulation as reported by other authors [52]. The stability of the niosome formulations was assessed for over 60 days at 4 °C by DLS. Generally, size variation in the formulations by the lapse of time indicated that aggregation or fusion occurred and the system was destabilized [53]. The zeta potential is strongly related to the stability of the niosomes. In high zeta potential values, there is a high repulsive force between the particles that can stabilize the formulations [54]. On the other hand, close to zero or even low values lead to unstable systems with resultant aggregation or fusion processes. Our data showed that mean ± SD particle size of vesicles increased very slightly and change from 316 ± 8 nm to 374 ± 12 for Nio/Slaw and from 377 ± 10 to 402 ± 15 for Nio/Hlaw formulations (no statistically significant) which indicating good stability of both carriers. However, change in the size of Nio/HLaw was lower than Nio/SLaw formulation because of more zeta potential of Nio/HLaw formulation (Fig. 4).
Fig. 4.
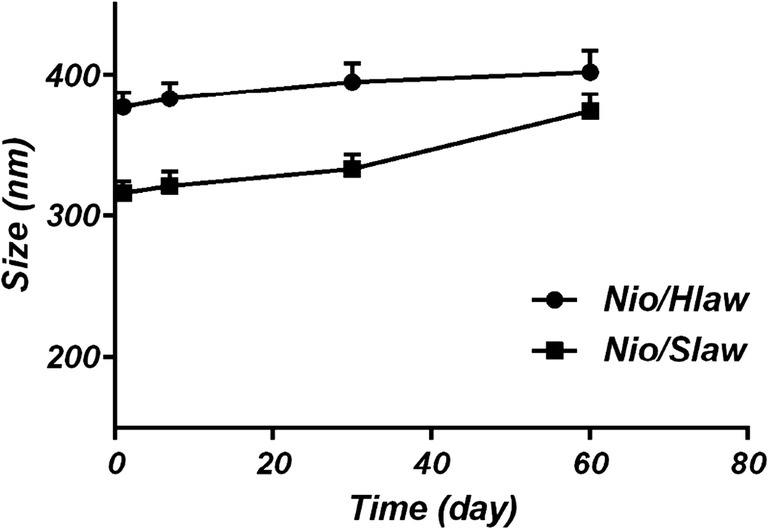
Stability measurements of the stored and freshly prepared Law-loaded niosomes for over 60 days at 4 °C by DLS. Nio/SLaw (■) and Nio/HLaw (●) prepared loaded niosomes. Data are expressed as the mean ± SD (n = 3)
Antitumor activity of loaded niosomes
Antitumor activity of free Law, bare niosomes (without Law) and Law-encapsulated niosomes were studied on MCF-7 cells at the concentrations 1.5 μM (based on our previous works) for 24 h. The cytotoxicity effect of Law-encapsulated formulations was significantly greater than that of the free Law. The enhanced cytotoxicity of the loaded niosomes is due to a synergistic effect of better internalizations of nanocarrier and a sustained release of Law. As expected, bare formulations did not show any cytotoxicity. Taken together, the present data indicate that loaded-niosomes have excellent cytotoxicity, thus rendering this formulation suitable for therapeutic purposes (Fig. 5).
Fig. 5.
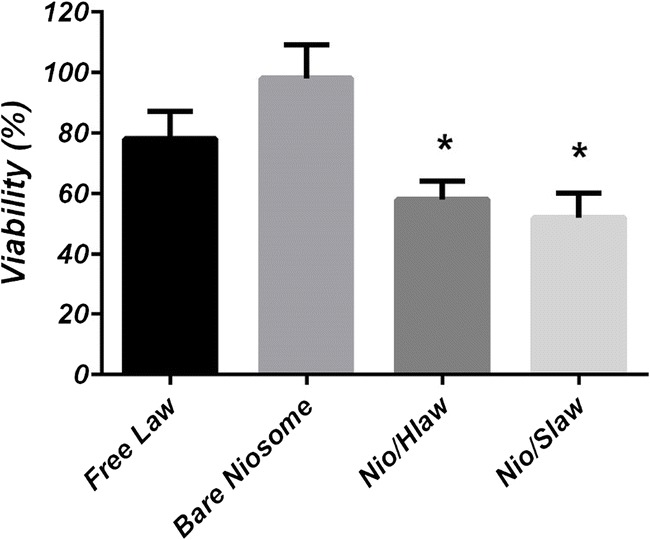
Antitumor activity of loaded niosomes compare to that of free Law and bare niosomes at the concentration of 1.5 μM. * means p < 0.05
Conclusions
In this study, proved that Lawsone-loaded niosomes can be used as a promising carrier for cancer therapy. The law-loaded formulation had bigger size than bare niosomes. The release profile showed similar release behaviors for both loaded formulations; however, Nio/SLaw had a faster release. Antitumor study on MCF-7 cell lines showed that, when Lawsone was encapsulated in niosome, the cytotoxicity significantly increased compared to the free Law. Finally, according to the results of this study, can conclude that niosomes can efficiently encapsulate phytochemical compounds and also improve the efficiency of nanocarrier for cancer therapy in a controlled and sustained manner.
Abbreviations
- Law
Lawsone
- SLaw
Standard Lawsone
- HLaw
Henna Extract containing Lawsone
- Bare niosomes
Niosomes without Law (Free niosomes)
- Nio/HLaw
Niosome that loaded by HLaw
- Nio/Slaw
Niosome that loaded by SLaw
Compliance with ethical standards
Conflict of interest
The authors of this study declare that they have no conflict of interest.
References
- 1.Demarque DP, Fitts SMF, Boaretto AG, da Silva JCL, Vieira MC, Franco VN, et al. Optimization and technological development strategies of an antimicrobial extract from Achyrocline alata assisted by statistical design. PLoS One. 2015;10(2):e0118574. doi: 10.1371/journal.pone.0118574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicolaou K, Yang Z, Liu J, Ueno H, Nantermet P, Guy R, et al. Total synthesis of taxol. Nature. 1994;367(6464):630–634. doi: 10.1038/367630a0. [DOI] [PubMed] [Google Scholar]
- 3.Castro FAV, Mariani D, Panek AD, Eleutherio ECA, Pereira MD. Cytotoxicity mechanism of two naphthoquinones (menadione and plumbagin) in Saccharomyces cerevisiae. PLoS One. 2008;3(12):e3999. doi: 10.1371/journal.pone.0003999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rates SMK. Plants as source of drugs. Toxicon. 2001;39(5):603–613. doi: 10.1016/S0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 5.Preeti P. Pharmacognostical and phytochemical evaluation of Grewia asiatica Linn Tiliaceae fruit pulp and seed. International Journal of Pharmaceutical & Biological Archive (IJPBA). 2013;4(2).
- 6.Lauro M, De Simone F, Sansone F, Iannelli P, Aquino R. Preparations and release characteristics of naringin and naringenin gastro-resistant microparticles by spray-drying. J Drug Delivery Sci Technol. 2007;17(2):119–124. doi: 10.1016/S1773-2247(07)50018-3. [DOI] [Google Scholar]
- 7.Lauro M, Maggi L, Conte U, De Simone F, Aquino R. Rutin and quercetin gastro-resistant microparticles obtained by spray-drying technique. J Drug Delivery Sci Technol. 2005;15(5):363–369. doi: 10.1016/S1773-2247(05)50066-2. [DOI] [Google Scholar]
- 8.Sansone F, Picerno P, Mencherini T, Villecco F, D’ursi A, Aquino R, et al. Flavonoid microparticles by spray-drying: influence of enhancers of the dissolution rate on properties and stability. J Food Eng. 2011;103(2):188–196. doi: 10.1016/j.jfoodeng.2010.10.015. [DOI] [Google Scholar]
- 9.Sansone F, Rossi A, Del Gaudio P, De Simone F, Aquino RP, Lauro MR. Hesperidin gastroresistant microparticles by spray-drying: preparation, characterization, and dissolution profiles. AAPS PharmSciTech. 2009;10(2):391–401. doi: 10.1208/s12249-009-9219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebrahimi Ahmad Khajeh, Barani Mahmood, Sheikhshoaie Iran. Fabrication of a new superparamagnetic metal-organic framework with core-shell nanocomposite structures: Characterization, biocompatibility, and drug release study. Materials Science and Engineering: C. 2018;92:349–355. doi: 10.1016/j.msec.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Muhammad H, Muhammad S. The use of Lawsonia inermis Linn.(henna) in the management of burn wound infections. Afr J Biotechnol. 2005;4(9)
- 12.Hosein HKM, Zinab D. Phenolic compounds and antioxidant activity of henna leaves extracts (Lawsonia inermis) World J Dairy Food Sci. 2007;2(1):38–41. [Google Scholar]
- 13.McKelvey EM, Lomedico M, Lu K, Chadwick M, Loo TL. Dichloroallyl lawsone. Clin Pharmacol Ther. 1979;25(5part1):586–590. doi: 10.1002/cpt1979255part1586. [DOI] [PubMed] [Google Scholar]
- 14.Udapurkar P, Bhusnure O, Kamble S, Biyani K. Phyto-phospholipid complex vesicles for phytoconstituents and herbal extracts: a promising drug delivery system. Int J Herbal Med. 2016;4(5):14–20. [Google Scholar]
- 15.Singh RP, Narke R. Preparation and evaluation of phytosome of Lawsone. Int J Pharm Sci Res. 2015;6(12):5217. [Google Scholar]
- 16.Singh DK, Luqman S. Lawsonia inermis (L.): a perspective on anticancer potential of mehndi/henna. Biomedical Research and Therapy (BMRAT) 2014;1(04):112–120. [Google Scholar]
- 17.Knecht W, Henseling J, Löffler M. Kinetics of inhibition of human and rat dihydroorotate dehydrogenase by atovaquone, lawsone derivatives, brequinar sodium and polyporic acid. Chem Biol Interact. 2000;124(1):61–76. doi: 10.1016/S0009-2797(99)00144-1. [DOI] [PubMed] [Google Scholar]
- 18.El-Hag A, Al-Jabri A, Habbal O. Antimicrobial properties of Lawsonia inermis (henna): a review. Australian Journal of Medical Herbalism (AJMH) 2007;19(3):114. [Google Scholar]
- 19.Chaudhary G, Goyal S, Poonia P. Lawsonia inermis Linnaeus: a phytopharmacological review. International Journal of Pharmaceutical Sciences and Drug Research (IJPSDR) 2010;2(2):91–98. [Google Scholar]
- 20.LÓPEZ LÓPEZ LI, FLORES N, Daniel S, SILVA BELMARES SY, SÁENZ GALINDO A. Naphthoquinones: biological properties and synthesis of lawsone and derivatives-a structured review. Vitae. 2014;21(3):248–258. [Google Scholar]
- 21.Srinivas S, Kumar YA, Hemanth A, Anitha M. Preparation and evaluation of niosomes containing aceclofenac. Dig J Nanomater Bios. 2010;5(1):249–254. [Google Scholar]
- 22.Nematollahi MH, Pardakhty A, Torkzadeh-Mahanai M, Mehrabani M, Asadikaram G. Changes in physical and chemical properties of niosome membrane induced by cholesterol: a promising approach for niosome bilayer intervention. RSC Adv. 2017;7(78):49463–49472. doi: 10.1039/C7RA07834J. [DOI] [Google Scholar]
- 23.James N, Coker R, Tomlinson D, Harris J, Gompels M, Pinching A, et al. Liposomal doxorubicin (Doxil): an effective new treatment for Kaposi's sarcoma in AIDS. Clin Oncol. 1994;6(5):294–296. doi: 10.1016/S0936-6555(05)80269-9. [DOI] [PubMed] [Google Scholar]
- 24.Bartelds R, Nematollahi MH, Pols T, Stuart MC, Pardakhty A, Asadikaram G, et al. Niosomes, an alternative for liposomal delivery. PLoS One. 2018;13(4):e0194179. doi: 10.1371/journal.pone.0194179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172(1–2):33–70. doi: 10.1016/S0378-5173(98)00169-0. [DOI] [Google Scholar]
- 26.Baillie A, Florence A, Hume L, Muirhead G, Rogerson A. The preparation and properties of niosomes—non-ionic surfactant vesicles. J Pharm Pharmacol. 1985;37(12):863–868. doi: 10.1111/j.2042-7158.1985.tb04990.x. [DOI] [PubMed] [Google Scholar]
- 27.Nematollahi MH, Torkzadeh-Mahanai M, Pardakhty A, Ebrahimi Meimand HA, Asadikaram G. Ternary complex of plasmid DNA with NLS-mu-mu protein and cationic niosome for biocompatible and efficient gene delivery: a comparative study with protamine and lipofectamine. Artif Cell Nanomed B. 2017:1–11. 10.1080/21691401.2017.1392316. [DOI] [PubMed]
- 28.Sezgin-Bayindir Z, Yuksel N. Investigation of formulation variables and excipient interaction on the production of niosomes. AAPS PharmSciTech. 2012;13(3):826–835. doi: 10.1208/s12249-012-9805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brewer J, Alexander J. The adjuvant activity of non-ionic surfactant vesicles (niosomes) on the BALB/c humoral response to bovine serum albumin. Immunology. 1992;75(4):570–575. [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmermann GR, Lehar J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12(1):34–42. doi: 10.1016/j.drudis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Raja NR, Pillai G, Udupa N, Chandrashekar G. Anti-inflammatory activity of niosome encapsulated diclofenac sodium in arthritic rats. Indian J Pharm. 1994;26(1):46. [Google Scholar]
- 32.Luciani A, Olivier J-C, Clement O, Siauve N, Brillet P-Y, Bessoud B, Gazeau F, Uchegbu IF, Kahn E, Frija G, Cuenod CA. Glucose-receptor MR imaging of tumors: study in mice with PEGylated paramagnetic Niosomes 1. Radiology. 2004;231(1):135–142. doi: 10.1148/radiol.2311021559. [DOI] [PubMed] [Google Scholar]
- 33.Schreier H, Bouwstra J. Liposomes and niosomes as topical drug carriers: dermal and transdermal drug delivery. J Control Release. 1994;30(1):1–15. doi: 10.1016/0168-3659(94)90039-6. [DOI] [Google Scholar]
- 34.Aditya N, Aditya S, Yang H, Kim HW, Park SO, Ko S. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem. 2015;173:7–13. doi: 10.1016/j.foodchem.2014.09.131. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed K, Li Y, McClements DJ, Xiao H. Nanoemulsion-and emulsion-based delivery systems for curcumin: encapsulation and release properties. Food Chem. 2012;132(2):799–807. doi: 10.1016/j.foodchem.2011.11.039. [DOI] [Google Scholar]
- 36.Barras A, Mezzetti A, Richard A, Lazzaroni S, Roux S, Melnyk P, Betbeder D, Monfilliette-Dupont N. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int J Pharm. 2009;379(2):270–277. doi: 10.1016/j.ijpharm.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 37.Puglia C, Lauro MR, Tirendi GG, Fassari GE, Carbone C, Bonina F, et al. Modern drug delivery strategies applied to natural active compounds. Expert Opin Drug Deliv. 2016:1–14. [DOI] [PubMed]
- 38.MUSTAFA MAZ. DEVELOPMENT OF. NIOSOME CONTAINING ROASTED COFFEE RESIDUE EXTRACT FOR ANTIAGING PREPARATION: THAMMASAT UNIVERSITY; 2015. [Google Scholar]
- 39.Sebaaly C, Jraij A, Fessi H, Charcosset C, Greige-Gerges H. Preparation and characterization of clove essential oil-loaded liposomes. Food Chem. 2015;178:52–62. doi: 10.1016/j.foodchem.2015.01.067. [DOI] [PubMed] [Google Scholar]
- 40.Devendran G, Sivamani G. Phytochemical analysis of leaf extract of plant Costus spicatus by GCMS method. Journal of Drug Delivery and Therapeutics (JDDT) 2015;5(4):24–26. [Google Scholar]
- 41.Arunothayanun P, Uchegbu I, Craig D, Turton J, Florence A. In vitro/in vivo characterisation of polyhedral niosomes. Int J Pharm. 1999;183(1):57–61. doi: 10.1016/S0378-5173(99)00044-7. [DOI] [PubMed] [Google Scholar]
- 42.Hao Y-M, Ka L. Entrapment and release difference resulting from hydrogen bonding interactions in niosome. Int J Pharm. 2011;403(1):245–253. doi: 10.1016/j.ijpharm.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 43.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 44.Stoline MR. The status of multiple comparisons: simultaneous estimation of all pairwise comparisons in one-way ANOVA designs. Am Stat. 1981;35(3):134–141. [Google Scholar]
- 45.Marianecci C, Rinaldi F, Di Marzio L, Mastriota M, Pieretti S, Celia C, et al. Ammonium glycyrrhizinate-loaded niosomes as a potential nanotherapeutic system for anti-inflammatory activity in murine models. Int J Nanomedicine. 2014;9:635. doi: 10.2217/nnm.13.67. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Hans M, Lowman A. Biodegradable nanoparticles for drug delivery and targeting. Curr Opinion Solid State Mater Sci. 2002;6(4):319–327. doi: 10.1016/S1359-0286(02)00117-1. [DOI] [Google Scholar]
- 47.Müller R, Jacobs C, Kayser O. Nanosuspensions as particulate drug formulations in therapy: rationale for development and what we can expect for the future. Adv Drug Deliv Rev. 2001;47(1):3–19. doi: 10.1016/S0169-409X(00)00118-6. [DOI] [PubMed] [Google Scholar]
- 48.Galante R, Rediguieri CF, Kikuchi IS, Vasquez PA, Colaço R, Serro AP, et al. About the sterilization of chitosan hydrogel nanoparticles. PLoS One. 2016;11(12):e0168862. doi: 10.1371/journal.pone.0168862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harvey RD, Ara N, Heenan RK, Barlow DJ, Quinn PJ, Lawrence MJ. Stabilization of distearoylphosphatidylcholine lamellar phases in propylene glycol using cholesterol. Mol Pharm. 2013;10(12):4408–4417. doi: 10.1021/mp400140u. [DOI] [PubMed] [Google Scholar]
- 50.Mahjub R, Dorkoosh FA, Amini M, Khoshayand MR, Rafiee-Tehrani M. Preparation, statistical optimization, and in vitro characterization of insulin nanoparticles composed of quaternized aromatic derivatives of chitosan. AAPS PharmSciTech. 2011;12(4):1407–1419. doi: 10.1208/s12249-011-9716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hao Y, Zhao F, Li N, Yang Y. Li Ka. Studies on a high encapsulation of colchicine by a niosome system. Int J Pharm. 2002;244(1):73–80. doi: 10.1016/S0378-5173(02)00301-0. [DOI] [PubMed] [Google Scholar]
- 52.Owen SC, Chan DP, Shoichet MS. Polymeric micelle stability. Nano Today. 2012;7(1):53–65. doi: 10.1016/j.nantod.2012.01.002. [DOI] [Google Scholar]
- 53.Soussan E, Cassel S, Blanzat M, Rico-Lattes I. Drug delivery by soft matter: matrix and vesicular carriers. Angew Chem Int Ed. 2009;48(2):274–288. doi: 10.1002/anie.200802453. [DOI] [PubMed] [Google Scholar]
- 54.Freitas C, Müller RH. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN™) dispersions. Int J Pharm. 1998;168(2):221–229. doi: 10.1016/S0378-5173(98)00092-1. [DOI] [Google Scholar]