Abstract
Background
The toxic metals and/or bacterial contaminants in illicit drugs are the main health problems in drug users worldwide. Hence, the potential risks of these contaminants were evaluated in some of the illicit drugs during 2015 and 2016.
Methods
The metals analysis were performed using graphite furnace atomic absorption spectrophotometry. In addition, all microbiological analysis stages, including handling procedures, dilution, and culture media, were conducted in accordance with the US Pharmacopeia (USP) which are harmonized with the European Pharmacopoeia (EP).
Results
In the present study, the highest lead (Pb; 138.10 ± 75.01 μg/g) and chromium (Cr; 447.38 ± 20.27 μg/g) levels were detected in opium samples. In addition, the highest prevalence of microbial contamination was observed in opium samples, and the lowest was recorded in heroin samples. Clostridium tetani, with about 50% of contaminant, was the most common bacteria in the analyzed samples.
Conclusions
Our results indicate that Pb exposure as well as bacterial contamination could be the major threats for drug users.
Graphical Abstract.
ᅟ
Keywords: Drug user, Opium, Heroin, Heavy metal toxicity
Introduction
Opioids are a class of drugs chemically related to alkaloids found in Papaver somniferum L [1]. Abuse of opioids is one the most significant public health problems, which have been identified in many developing countries [2, 3]. There has been a remarkable increase in the production and use of illicit drugs throughout the world over the last decades [4]. For instance, an increase in the opium poppy cultivation from 154,000 ha in 2012 to 209,000 ha in 2013 has been seem in Afghanistan, which has the world’s largest opium poppy cultivation [5]. On the other hand, Iran shares about 900 km border with Afghanistan, thus it has been a main transit route for drug-trafficking gangs [6]. These conditions lead to developing the use of illegal opioids, such as opium, heroin and crack, in Iran. It should be noted that crack is a street illicit drug in Iran which heroin is the main constituent and may also contain papaverine, caffeine, morphine, codeine, acetyl codeine, noscapine, and dextromethorphan, or corticosteroids with different amounts that make it even more harmful than pure heroin [7, 8]. Additionally, it has been reported that there is no difference between crack and crystal in term of ingredient in eastern of Iran [8].
According to the limited information available, about 16.5 million people which is almost 0.4% of the world’s adult population, use opiates such as opium and heroin [9]. In a meta-analysis study, the pooled prevalence of opium abuse in Iranian men and women was estimated 6.0 and 2.0%, respectively [10].
The illicit drug market is constantly changing and heroin and/or other opioid-like compounds are almost always mixed with other substances [11]. “Diluents” and “adulterants” are terms that refer to exogenous materials (either active or inactive) added to a specimen after its chemical synthesis or refinement before its retail sale [12]. These exogenous agents include harmless “diluting agents” such as different sugars and/or starch and pharmacologically active “adulterants” which may be co-responsible for increase the adverse side effects of the drugs, particularly in injectable drugs [11, 13].
Heavy metals are certainly one of the most toxicants known to humans [14]. The reports showed that salesmen and smugglers may add any kind of heavy metals such as lead (Pb), to opium for increasing its weight and more profit [15–18]. For instance, Aghaee-Afshar et al. [16] have been reported the presence of Pb in ten opium samples collected in South-East Iran. In addition, in a study carried out in Birjand city of Iran, it was reported that the mean blood-Pb in opium addicts was higher than healthy individuals [19]. In a case–control study, it has been reported some symptoms of thallium poisoning in opium addicts [15]. Therefore, one of the aspects to be considered in treating drug addicts is determining drug purity and identify the type of exogenous substances that might be added to drugs.
Infections have long been recognized as one of the most serious complications of drug abuse [20]. Drug users are susceptible to skin and soft tissue infections [21], endocarditis [22], and sexually transmitted infections [23, 24] caused by a wide range of microorganisms such as bacterial and viral pathogens. Jain et al. have been reported that tricuspid valve endocarditis occurs more frequently in heroin users than in other injection drug users (IDUs) [25]. Moreover, infective endocarditis in 33 drug abusers has been observed in intensive care unit (ICU) by Saydain et al. Accordingly, S. aureus was the more frequent pathogen, as it was observed in 94% patients, with 52% being methicillin resistant [26]. In the northern border of Mexico found that 96% of IDUs tested positive for hepatitis C virus (HCV) antibodies, while 2.8% were human immunodeficiency virus (HIV) positive [27].
In recent years, the risk of exposure to heavy metals and microbial contamination through abuse drugs has been limited to clinical studies and chemical analyzes on biological samples collected from abusers [28, 29]. However, there is little information about the level of these contaminants in drugs and consequently their effects on the human health profile. Therefore, knowledge about the presence of adulterants and/or bacterial contaminants in drugs is important for understanding of unexpected side effects in drug users. In the present study, the levels of several toxic metals and bacterial contaminants were determined in illegal opioid-like compounds in Iran.
Materials and methods
Sampling
During 2015 and 2016, a total of 30 samples of opium, heroin and crack, were collected from seized packages by security police in Iran. According to following equation, the number of sample for each illegal drugs was determined to be 10 (n = 10), and sampling method was defined in randomized manner [16].
In this equation, standard deviation (σ), type І error (Z1-α) = 0.05, number of comparisons to be made (τ) = 3, power (Z1-β) = 0.8 and difference (μA-μB) < 0.3. It should be noted that all of the samples were placed into clean glass bottles, labeled and kept under suitable conditions until analysis.
Determination of metals
The samples were dried in an oven at 25 °C for 24 h. 20 mg of dried samples was weighed and put into Teflon reaction vessels. Concentrated HNO3 (2 mL, 35%) were added and, after closing the vessels with a lid, the samples were digested at 160 °C for 30 min. Then, the digested samples were cooled to room temperature. The resulting clear digest solutions were diluted with deionized water and filtered quantitatively into a 50 ml volumetric flask. The concentrations of lead (Pb), cadmium (Cd), chromium (Cr) in these digests were measured using graphite furnace atomic absorption spectrophotometry (Model AAnalyst 300, Perkin Elmer, USA). For measurements, argon, as the inert gas, and pyrolyticcoated graphite tubes with a platform were used. The atomic absorption signal was measured in peak area mode against a calibration curve.
In this study, calibration curves for metals were constructed using external standards in a range from 2.5 to 50 μg/L, prepared by dilution of a stock solution in deionized water. Then, the limits of detection (LOD) and limits of quantification (LOQ) were determined as (SD/S) × 3.3 and (SD/S) × 10, respectively, where SD/S is the ratio of standard deviation/slope of calibration curve. In addition to using validated methods, the reliability of the data was assessed by conducting internal quality control experiments. In this regard, recoveries of metals were recorded by analyzing a sample spiked with certain concentrations (10, 30 and 50 μg/g) of each metal.
Health risk assessment
Daily intake of metals (DIM) was calculated using the following equation: DIM = CM x DC/BW [30]. In this equation, CM, DC, and BW represent the metal level in drug samples (μg/g), daily consumption of drug (g), and average body weight (kg), respectively. It should be noted that the body weight for a adult was assumed to be 60 kg.
Microbiological analysis
One gram sample was suspended in 9 ml of sterilized distilled water and 1 ml was removed and inoculated directly on Sab agar plates at 37 °C for 24 h. Selective and nonselective culture media were used for isolation of the microbial contaminants, ie, nutrient agar (Oxoid, England), Sabouraud’s dextrose agar, thiosulfate-citrate-bile-sucrose agar, and MacConkey agar. For instance, Clostridium tetani was isolated by thioglycolate broth medium [31]. Briefly, samples were inoculated directly into thioglycolate broth medium and incubated at 37 °C for 24 h. The isolates were purified in cooked meat medium and used for further analysis. Finally, important microbiological testes include gram stain, urease, indole, citrate utilization, arginine dihydrolase, lysine decarboxylase, ornithine decarboxylase, gelatin hydrolysis, coagulase, amylase and inositol were performed in lab to identify the genus and species of unknown bacterial. All analyses stages, including handling procedures, dilution, and culture media, were conducted in accordance with the US Pharmacopeia (USP) which are harmonized with the European Pharmacopoeia (EP).
Statistical analysis
The results were presented as mean ± standard deviation (SD) and analyzed using Prism 6 software (Graph Pad Software, Inc., La Jolla, CA, USA) through one-way analysis of variance (ANOVA). Post hoc testing was done using the Tukey–Kramer HSD test. The P < 0.05 was considered statistically significant.
Results
Validation of method
The standard curve data for heavy metals detection using a graphite furnace atomic absorption spectrophotometry system are given in Table 1. The r2 indexes showed an acceptable calibration curves, which was linear in the defined ranges. In addition, the calculated LOD and LOQ values indicated a good performance at low statutory limits. As presented in Table 2, the relative standard deviation (RSD) values of the recovery experiment were within the acceptable ranges, indicating the good precision and accuracy of this analytical method.
Table 1.
Linearity range and detection limits for heavy metals analyses
| Heavy metals | Range (μg/L) | Linear equation | r 2 | LOD (μg/L) | LOQ (μg/L) |
|---|---|---|---|---|---|
| Lead (Pb) | 2.5–50 | Y = 0.0075X + 0.0044 | 0.999 | 1.01 | 3.03 |
| Cadmium (Cd) | 2.5–50 | Y = 0.0386X + 0.0051 | 0.999 | 0.66 | 2.20 |
| Chromium (Cr) | 2.5–50 | Y = 0.0207X-0.0185 | 0.999 | 0.57 | 1.89 |
LOD: limits of detection and LOQ: limit of quantitation
Table 2.
Recoveries of heavy metals in spiked drugs samples
| Metals | Metal spiked (μg/g) | Metal found (μg/g) | Recovery (%) | RSD (%)† |
|---|---|---|---|---|
| Lead (Pb) | 10 | 9.7 | 97 | 6.8 |
| 30 | 30.3 | 101 | 4.2 | |
| 50 | 46.3 | 92.6 | 3.6 | |
| Cadmium (Cd) | 10 | 8.9 | 89 | 5.5 |
| 30 | 28.4 | 94.6 | 3.6 | |
| 50 | 48.7 | 97.4 | 3.8 | |
| Chromium (Cr) | 10 | 9.3 | 93 | 6.2 |
| 30 | 26.2 | 87.3 | 4.4 | |
| 50 | 52.4 | 104.8 | 3.8 |
†Relative standard deviation (RSD) was obtained from triplicate tests
Determination of metals
The concentrations of three toxic metals in opium, heroin, and crack samples are shown in Table 3. The highest level of Pb contamination was observed in opium (138.10 ± 75.01 μg/g), and the lowest level was in crack samples (30.83 ± 16.26 μg/g). For assessing equality of variances, so called “homoscedasticity”, we used Levene’s test. Result of the test showed p-value more than 0.05, indicating equality of variances. In addition, statistical analyses (one-way ANOVA, post-hoc Tukey-Kramer test) showed a significant difference in Pb levels in opium samples when compared to heroin (P < 0.001) and crack (P < 0.001) samples. The highest level of Cd was detected in crack samples (0.60 ± 0.23 μg/g); however, no significant differences were observed among Cd levels in different samples. In the current study, the highest level of Cr was found in the opium samples (447.38 ± 20.27 μg/g), and the lowest level was detected in the heroin samples (413.70 ± 14.68 μg/g). However, no significant differences were observed among Cr levels in different samples.
Table 3.
Heavy metals contents in drug samples
| Metals | Lead (μg/g) | Cadmium (μg/g) | Chromium (μg/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Max | Min | Mean | SD | Max | Min | Mean | SD | Max | Min | |
| Opium | 138.10 | 75.01 | 243.30 | 52.80 | 0.45 | 0.29 | 1.03 | 0.14 | 447.38 | 20.27 | 479.00 | 419.50 |
| Heroin | 48.85* | 13.43 | 82.60 | 36.60 | 0.59 | 0.26 | 1.04 | 0.25 | 413.70 | 14.68 | 432.00 | 378.00 |
| Crack | 30.83* | 16.26 | 58.80 | 13.30 | 0.60 | 0.23 | 0.96 | 0.29 | 420.2 | 13.01 | 439.50 | 403.50 |
Data are expressed as mean, standard deviation (SD), minimum (Min), maximum (Max) values and n = 10 for each drug. * p < 0.001; statistically significant difference from opium samples (One-way ANOVA with Tukey–Kramer HSD test)
Microbiological analysis
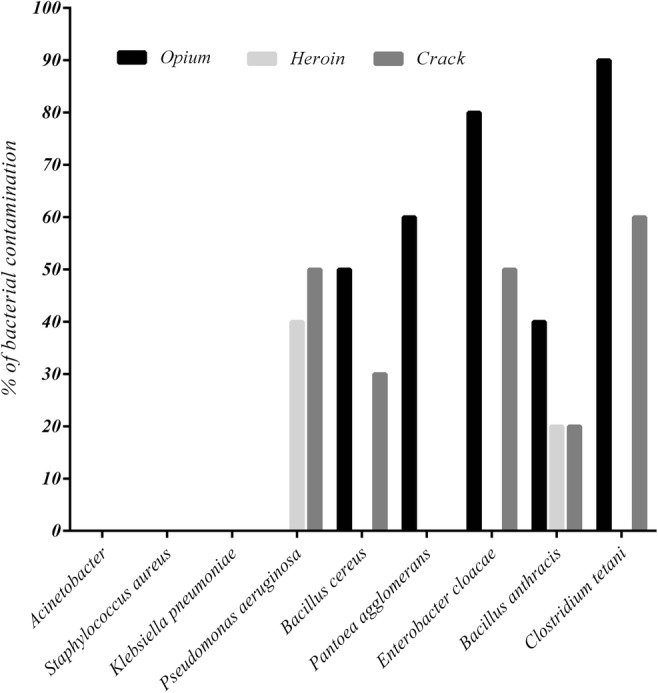
In microbiological analysis, all the samples tested were free from Klebsiella pneumoniae, Staphylococcus aureus, and Acinetobacter (Fig. 1). The highest prevalence of microbial contamination was observed in opium samples, and the lowest was recorded in heroin samples. Clostridium tetani, about 50% of contaminant, was the most common bacteria in the analyzed samples.
Fig. 1.
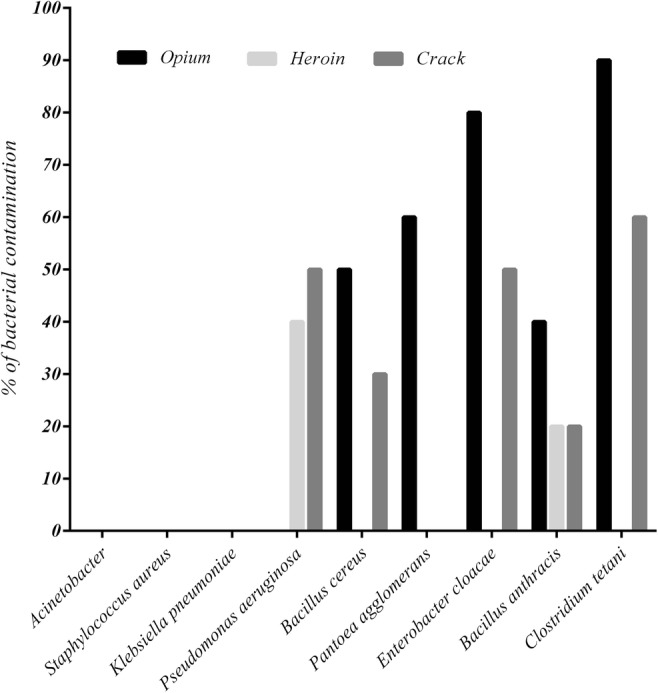
Percentage of bacterial contamination in drug samples
Discussion
In the current study, we evaluated several metal contaminations in illegal opioid-like compounds in Iran. The highest level of Pb contamination was observed in opium samples compared to heroin and crack samples (Table 3). In previous study, Aghaee-Afshar et al. reported the presence of Pb in ten opium samples collected from different sources in South-East Iran [16]. In addition, several studies have reported high blood Pb levels and their association with some adverse effects of Pb poisoning, such as anemia and abdominal pain, in patients addicted to opium [17, 28, 32, 33]. Therefore, we suggest that the Pb poisoning in these patients may be due to consumption of contaminated opium. To confirm this hypothesis, the daily intake of Pb was evaluated to estimate the degree of Pb exposure resulting from consumption of drugs in Iranian users. The FAO/WHO reported a provisional tolerable weekly intake (PTWI) of 25 μg Pb/kg body weight for humans [34], equaling 3.57 μg Pb/kg/daily. As shown in Table 4, our findings demonstrate that the Pb levels in these drugs pose a risks to opium addicts with a body weight of at least 60 kg, if the consumption of opium is assumed to be 3 g/daily. If the mean contents of Pb are considered in exposure assessment calculations, consuming more than 1.55 g/day of opium, 4.39 g/day of heroin, or 6.95 g/day of crack could put users at high risk of Pb poisoning.
Table 4.
Daily intake of heavy metals through consumption of drugs
| Metals | Lead (μg/kg/day) | Cadmium (μg/kg/day) | Chromium (μg/kg/day) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Max | Min | Mean | Max | Min | Mean | Max | Min | |
| Opium | 6.90 | 12.16 | 2.64 | 0.02 | 0.05 | 0.007 | 22.37 | 23.95 | 20.97 |
| Heroin | 2.44 | 4.13 | 1.83 | 0.03 | 0.05 | 0.01 | 20.68 | 21.60 | 18.90 |
| Crack | 1.54 | 2.94 | 0.66 | 0.03 | 0.05 | 0.01 | 21.01 | 21.97 | 20.17 |
The average consumption value for the mentioned drugs assumed to be 3 g/day
Lead contamination of opium may have several causes, the most important of which include water and soil pollution in farms, use of unsuitable methods and tools in opium production [18], and the addition of Pb to opium to increase its weight and therefore increase profits for opium salesmen and smugglers [15–17, 35]. Although these reasons suggest possible sources of contamination, the exact source of Pb in opium samples is still unknown and need futher studies.
Cadmium (Cd) is a nephrotoxic metal that is especially toxic to the proximal tubular cells of the kidneys, where it accumulates over time and can cause renal dysfunction [30]. In this study, the Cd content of the drug samples was determined (Table 3). Our results are in agreement with the published data about Cd content in different varieties of poppy. For instance, Knapek et al. described the average content of Cd as 0.64 mg/kg for poppy seeds [36], and Lachman et al. described the average Cd content as 0.56 mg/kg in different varieties of poppy [37]. Therefore, the Cd content in collected samples may be associated with its natural presence in poppies. The mean estimated daily intake for Cd was calculated to be 0.02 μg/kg for opium users and 0.03 μg/kg for heroin and crack users, respectively, which is much lower than the PTWI (420 μg of body weight, corresponding to 60 μg/day for a 60-kg adult) [38].
Chromium (Cr), especially hexavalent chromium compounds, has been classified as known human carcinogens based on data from animal studies and human epidemiological studies [39–41]. In the present study, the mean estimated daily intakes for this metal were calculated to be 22.37 μg/kg, 20.68 μg/kg, and 21.01 μg/kg for opium, heroin, and crack users, respectively (Table 4), which is much lower than Reference Doses (RfDs) for Cr (III) (1500 μg/kg/day), and higher than RfD for Cr (VI) (3 μg/kg/day) [42].
Clostridium tetani, with about 50% of contaminant, was the most common bacteria in the analyzed samples. Tetanus is an acute disease caused by Clostridium tetani and characterized by generalized rigidity and convulsive spasming of the skeletal muscles [43]. Tetanus in IDUs appears to be a case of the reemergence of an old disease in a new setting. Tetanus occurrences among IDUs have been reported worldwide and are often associated with poor outcomes and high mortality [44]. In the current study, the highest Clostridium tetani contamination was found in opium samples, and the lowest was observed in heroin. Despite the high prevalence of Clostridium tetani infection in heroin users worldwide [45], the prevalence of this bacterium in heroin samples is reported to be very low. Thus, in addition to the bacterial contamination rate in samples, other factors, such as bacteria spore levels and administration route, may influence the prevalence of this infection in drug users [46]. For instance, heroin is not water soluble and is often dissolved in weak acids followed by heating prior to use. The weak acids kill the non-spore-bearing bacteria, which serve as a source of competition for the surviving spore-bearers, and the heat treatment stimulates spore growth [47]. Sometimes, simultaneous injection of cocaine and heroin may induce soft tissue ischemia, making conditions favorable for this anaerobic bacterial growth [48].
Bacillus anthracis, the etiologic agent of anthrax, is a facultatively anaerobic, gram-positive, spore-forming bacterium [49]. As shown in Fig. 1, Bacillus anthracis was the only bacterium found in all three types of drugs (26.6% of total samples). In several studies, anthrax infection has been reported in drug users in different areas of the world. For instance, since 2009, 31 cases of anthrax have been confirmed among drug users in Scotland [50]. In addition, Russell et al. (2013) have reported three cases of anthrax with soft tissue infection, sepsis and severe oedema among Danish heroin users [29]. The most recent outbreak started in 2012, when at least 13 cases occurred in Denmark, France, the United Kingdom, and Germany, five of whom died. Because all of these cases involved heroin use, contaminated heroin appears to be the most likely source of these infections [51]. Thus, contaminated drugs are a potential vehicle of infection for anthrax.
Enterobacter is a genus of a common gram-negative, rod-shaped, facultative, anaerobic, and non-spore-forming bacteria belonging to the family Enterobacteriaceae [52]. Among these, Enterobacter cloacae infections have the highest mortality rate among Enterobacter infections [53]. The well-known Enterobacter cloacae contributes to the occurrence of endocarditis, septic arthritis, osteomyelitis, and also skin and soft tissue infections, especially in drug users [52]. In the current study, Enterobacter cloacae and Pantoea agglomerans were identified in about 43.3 and 20% of the total samples, respectively; therefore, these pathogens could pose a potential risk to drug users. Interestingly, the highest Enterobacter cloacae and Pantoea agglomerans as well as Clostridium tetani contamination were observed in opium samples. The observations of security police indicate that salesmen and smugglers may add human and/or animal feces as an adulterant to opium to increase profits. In addition, several cases have been reported of opium being transferred by body packers in Iran [54, 55]. Due to presence of Enterobacter and Clostridium tetani in the human gut [56], contamination of opium samples with these bacteria may be associated with the presence of human feces in such samples.
Bacillus cereus is a spore-forming, gram-positive bacillus that is widely distributed environmentally [57], and Pseudomonas aeruginosa is a genus of gram-negative bacillus belonging to the Pseudomonadaceae family [58]. In the present study, Bacillus cereus and Pseudomonas aeruginosa were identified in about 26.6 and 30% of all drug samples, respectively. In agreement with our findings, only one previous study has positively linked a Bacillus cereus infection with a contaminated source of heroin [59]. Although there is little evidence concerning the presence of these two bacteria in drugs, their roles in the occurrence of various infectious diseases have been demonstrated in drug users. For instance, incidences of endocarditis [60] and septic arthritis by Pseudomonas aeruginosa [61], as well as crepitant cellulitis [59], have been reported in drug users.
A total microbial analysis showed that the microbial contamination level in heroin samples is much lower than other drug samples. Interestingly, Tuazon et al. were reported that most samples of street heroin have antibacterial effects against some of the bacterial species such as Staphylococcus aureus and Bacillus cereus [62]. This phenomenon may be due to the quinine content that used as an adulterant agent in heroin.
Conclusions
In summary, the presence of toxic metals, such as Pb, Cd and Cr, has been confirmed in different drugs. Our findings indicate that Pb contamination in opium samples could pose a serious threat to drug users. On the other hand, Clostridium tetani, Bacillus anthracis, Enterobacter cloacae, Pantoea agglomerans, Bacillus cereus and Pseudomonas aeruginosa were identified in 50%, 26.6%, 43.3%, 20%, 26.6 and 30% of total samples, respectively. These bacterial contaminants may be related to some of the bacterial infections in drug users. The presence of human and animal feces and drug preparation in non-sterile conditions may be contributed to the bacterial contamination of various drugs. Due to limitation of sample size in this study, further studies need with more samples and different drugs to evaluate more precise information.
Acknowledgements
This study was supported by grant No. 19710127901001 of Islamic Azad University, Shahreza Branch, Shahreza, Iran.
Compliance with ethical standards
Conflicts of interests
The authors declare that there is no conflict of interest.
Contributor Information
Rassoul Aghababaei, Email: ra.ag2772@yahoo.com.
Iraj Javadi, Email: irjava@yahoo.com.
Amir Nili-Ahmadabadi, Phone: +988138380031, Email: amirnili54@gmail.com.
Somayeh Parsafar, Email: Somayeh_pa@yahoo.com.
Davoud Ahmadimoghaddam, Email: d.ahmadimoghadam@umsha.ac.ir.
References
- 1.Martínez MA, Ballesteros S, Almarza E, Garijo J. Death in a legal poppy field in Spain. Forensic Sci Int. 2016;265:34–40. doi: 10.1016/j.forsciint.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Karbakhsh M, Salehian Zandi N. Acute opiate overdose in Tehran: the forgotten role of opium. Addict Behav. 2007;32(9):1835–1842. doi: 10.1016/j.addbeh.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Zaaijer ER, Bruijel J, Blanken P, Hendriks V, Koeter MWJ, Kreek MJ, Booij J, Goudriaan AE, van Ree JM, van den Brink W. Personality as a risk factor for illicit opioid use and a protective factor for illicit opioid dependence. Drug Alcohol Depend. 2014;145:101–105. doi: 10.1016/j.drugalcdep.2014.09.783. [DOI] [PubMed] [Google Scholar]
- 4.Van Nuijs AL, Castiglioni S, Tarcomnicu I, et al. Illicit drug consumption estimations derived from wastewater analysis: a critical review. Sci Total Environ. 2011;409(19):3564–3577. doi: 10.1016/j.scitotenv.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Rosen L, Katzman K. Afghanistan: Drug Trafficking and the 2014 Transition Washington: The Congressional Research Service, 2014. Available from: http://goodtimesweb.org/overseas-war/2014/R43540.pdf
- 6.Oskarsson K. The role of Iran in Afghanistan’s reconstruction & development. Civil-military fusion center, August, 2013.
- 7.Kazemifar AM, Solhi H, Badakhshan D. Crack in Iran: is it really cocaine. J Addict Res Ther. 2011;2(1):1–3. [Google Scholar]
- 8.Karrari P, Mehrpour O, Balali-Mood M. Iranian crystal: a misunderstanding of the crystal-meth. J Res Med Sci. 2012;17(2):203–204. [PMC free article] [PubMed] [Google Scholar]
- 9.Fallahzadeh MA, Salehi A, Naghshvarian M, Fallahzadeh MH, Poustchi H, Sepanlou SG, Gandomkar A, Malekzadeh R. Epidemiologic study of opium use in pars cohort study: a study of 9000 adults in a rural southern area of Iran. Arch Iran Med. 2017;20(4):205–210. [PubMed] [Google Scholar]
- 10.Menati W, Valizadeh R, Menati R, Niazi M, Nazarzadeh M, Bidel Z. Determination of opium abuse prevalence in Iranian young people: a systematic review and meta-analysis. J Subst Use. 2017;22(1):3–10. doi: 10.3109/14659891.2015.1130181. [DOI] [Google Scholar]
- 11.Cole C, Jones L, McVeigh J, Kicman A, Syed Q, Bellis M. Adulterants in illicit drugs: a review of empirical evidence. Drug Test Anal. 2011;3(2):89–96. doi: 10.1002/dta.220. [DOI] [PubMed] [Google Scholar]
- 12.Shesser R, Jotte R, Olshaker J. The contribution of impurities to the acute morbidity of illegal drug use. Am J Emerg Med. 1991;9(4):336–342. doi: 10.1016/0735-6757(91)90053-M. [DOI] [PubMed] [Google Scholar]
- 13.Schneider S, Meys F. Analysis of illicit cocaine and heroin samples seized in Luxembourg from 2005–2010. Forensic Sci Int. 2011;212(1):242–246. doi: 10.1016/j.forsciint.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Tchounwou PB, Yedjou CG, Patlolla AK, et al. Heavy metal toxicity and the environment, in Molecular, clinical and environmental toxicology Springer 2012; 133–64. [DOI] [PMC free article] [PubMed]
- 15.Ghaderi A, Vahdati-Mashhadian N, Oghabian Z, et al. Thallium exists in opioid poisoned patients. Daru. 2015;23(1):1–4. doi: 10.1186/s40199-015-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aghaee-Afshar M, Khazaeli P, Behnam B, Rezazadehkermani M, Ashraf-Ganjooei N. Presence of lead in opium. Arch Iran Med. 2008;11(5):553–554. [PubMed] [Google Scholar]
- 17.Khatibi-Moghadam H, Khadem-Rezaiyan M, Afshari R. Comparison of serum and urine lead levels in opium addicts with healthy control group. Hum Exp Toxicol. 2016;5:861–865. doi: 10.1177/0960327115607947. [DOI] [PubMed] [Google Scholar]
- 18.Karrari P, Mehrpour O, Abdollahi M. A systematic review on status of lead pollution and toxicity in Iran; guidance for preventive measures. Daru. 2012;20(1):2–17. doi: 10.1186/1560-8115-20-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghaemi K, Ghoreishi A, Rabiee N, et al. Blood lead levels in asymptomatic opium addict patients; a case control study. Emerg. 2017;5(1):e69. [PMC free article] [PubMed] [Google Scholar]
- 20.Lavender TW, McCarron B. Acute infections in intravenous drug users. Clin Med. 2013;13(5):511–513. doi: 10.7861/clinmedicine.13-5-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brett MM, Hood J, Brazier JS, et al. Soft tissue infections caused by spore-forming bacteria in injecting drug users in the United Kingdom. Epidemiol Infect. 2005;133(4):575–582. doi: 10.1017/S0950268805003845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farjo P, Jain A, Patel K. Bacterial endocarditis in an intravenous drug user with a ventricular septal aneurysm. W V med J. OA, 2017.
- 23.Andrade AP, Pacheco SD, Silva FQ, et al. Characterization of hepatitis B virus infection in illicit drug users in the Marajó archipelago, northern Brazil. Arch Virol. 2017;162(1):227–233. doi: 10.1007/s00705-016-3060-z. [DOI] [PubMed] [Google Scholar]
- 24.Page K, Morris MD, Hahn JA, Maher L, Prins M. Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin Infect Dis. 2013;57(suppl_2):S32–S38. doi: 10.1093/cid/cit300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain V, Yang MH, Kovacicova-Lezcano G, et al. Infective endocarditis in an urban medical center: association of individual drugs with valvular involvement. J Inf Secur. 2008;57(2):132–138. doi: 10.1016/j.jinf.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Saydain G, Singh J, Dalal B, Yoo W, Levine DP. Outcome of patients with injection drug use–associated endocarditis admitted to an intensive care unit. J Crit Care. 2010;25(2):248–253. doi: 10.1016/j.jcrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Marín-Navarrete R, Magis-Rodríguez C, Strathdee SA. Sexually transmitted infections and substance use disorders: evidence and challenges in Mexico. Salud Ment. 2017;40(1):1–4. doi: 10.17711/SM.0185-3325.2017.001. [DOI] [Google Scholar]
- 28.Domeneh BH, Tavakoli N, Jafari N. Blood lead level in opium dependents and its association with anemia: a cross-sectional study from the capital of Iran. J Res Med Sci. 2014;19(10):939–943. [PMC free article] [PubMed] [Google Scholar]
- 29.Russell L, Pedersen M, Jensen AV, Søes LM, Hansen ABE. Two anthrax cases with soft tissue infection, severe oedema and sepsis in Danish heroin users. BMC Infect Dis. 2013;13(1):408. doi: 10.1186/1471-2334-13-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heshmati A, Karami-Momtaz J, Nili-Ahmadabadi A, Ghadimi S. Dietary exposure to toxic and essential trace elements by consumption of wild and farmed carp (Cyprinus carpio) and Caspian kutum (Rutilus frisii kutum) in Iran. Chemosphere. 2017;173:207–215. doi: 10.1016/j.chemosphere.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Hanif H, Anjum A, Ali N, et al. Isolation and antibiogram of clostridium tetani from clinically diagnosed tetanus patients. Am J Trop Med Hyg. 2015;93(4):752–756. doi: 10.4269/ajtmh.15-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masoodi M, Zali MR, Ehsani-Ardakani MJ, Mohammad-Alizadeh AH, Aiassofi K, Aghazadeh R, Shavakhi A, Somi MH, Antikchi MH, Yazdani S. Abdominal pain due to lead-contaminated opium: a new source of inorganic lead poisoning in Iran. Arch Iran Med. 2006;9(1):72–75. [PubMed] [Google Scholar]
- 33.Jalili M, Azizkhani R. Lead toxicity resulting from chronic ingestion of opium. West J Emerg Med. 2009;10(4):244–246. [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JA, Lee SH, Choi SH, Jung KK, Park MS, Jeong JY, Hwang MS, Yoon HJ, Choi DW. Heavy metal risk management: case analysis. Toxicol Res. 2012;28(3):143–149. doi: 10.5487/TR.2012.28.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehrpour O, Karrari P, Abdollahi M. Chronic lead poisoning in Iran; a silent disease. Daru. 2012;20(1):8. doi: 10.1186/2008-2231-20-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knapek J, Buchtova R. Vosmerova D. Determination of cadmium in poppy seeds and in poppy seeds containing products. Book of abstract. 4th international symposium on recent advances in food analysis. Prag 2009; 226–26.
- 37.Lachman J, Hejtmankova A, Miholova D, et al. Relations among alkaloids, cadmium and zinc contents in opium poppy (Papaver somniferum L.) Plant Soil Environ. 2006;52(6):282–288. doi: 10.17221/3442-PSE. [DOI] [Google Scholar]
- 38.Joint FA, World Health Organization, WHO Expert Committee on Food Additives. Evaluation of certain Food Addit Contam: seventy-seventh report of the Joint FA. 2013: World Health Organization.
- 39.Thompson CM, Kirman CR, Proctor DM, Haws LC, Suh M, Hays SM, Hixon JG, Harris MA. A chronic oral reference dose for hexavalent chromium-induced intestinal cancer. J Appl Toxicol. 2014;34(5):525–536. doi: 10.1002/jat.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park RM, Bena JF, Stayner LT, Smith RJ, Gibb HJ, Lees PSJ. Hexavalent chromium and lung cancer in the chromate industry: a quantitative risk assessment. Risk Anal. 2004;24(5):1099–1108. doi: 10.1111/j.0272-4332.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 41.Proctor DM, Suh M, Campleman SL, Thompson CM. Assessment of the mode of action for hexavalent chromium-induced lung cancer following inhalation exposures. Toxicology. 2014;325:160–179. doi: 10.1016/j.tox.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Naz A, Mishra BK, Gupta SK. Human health risk assessment of chromium in drinking water: a case study of Sukinda chromite mine, Odisha, India. Exposure and Health. 2016;8(2):253–264. doi: 10.1007/s12403-016-0199-5. [DOI] [Google Scholar]
- 43.Derbie A, Amdu A, Alamneh A, Tadege A, Solomon A, Elfu B, Mekonnen D, Mezgebu Y, Worku S, Biadglegne F. Clinical profile of tetanus patients attended at Felege Hiwot referral hospital, Northwest Ethiopia: a retrospective cross sectional study. Springerplus. 2016;5(1):892. doi: 10.1186/s40064-016-2592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaushik KS, Kapila K, Praharaj AK. Shooting up: the interface of microbial infections and drug abuse. J Med Microbiol. 2011;60(4):408–422. doi: 10.1099/jmm.0.027540-0. [DOI] [PubMed] [Google Scholar]
- 45.Kimura AC, Higa JI, Levin RM, et al. Outbreak of necrotizing fasciitis due to Clostridium sordellii among black-tar heroin users. Clin Infect Dis. 2004;38(9):87–91. doi: 10.1086/383471. [DOI] [PubMed] [Google Scholar]
- 46.Gordon RJ, Lowy FD. Bacterial infections in drug users. N Engl J Med. 2005;353(18):1945–1954. doi: 10.1056/NEJMra042823. [DOI] [PubMed] [Google Scholar]
- 47.Brazier JS, Morris TE, Duerden BI. Heat and acid tolerance of Clostridium novyi type a spores and their survival prior to preparation of heroin for injection. Anaerobe. 2003;9(3):141–144. doi: 10.1016/S1075-9964(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 48.Murphy EL, DeVita D, Liu H, Vittinghoff E, Leung P, Ciccarone DH, Edlin BR. Risk factors for skin and soft-tissue abscesses among injection drug users: a case-control study. Clin Infect Dis. 2001;33(1):35–40. doi: 10.1086/320879. [DOI] [PubMed] [Google Scholar]
- 49.Ascough S, Altmann DM. Anthrax in injecting drug users: the need for increased vigilance in the clinic. Expert Rev Anti-Infect Ther. 2015;13(6):681–684. doi: 10.1586/14787210.2015.1032255. [DOI] [PubMed] [Google Scholar]
- 50.Booth MG, Hood J, Brooks TJ, et al. Anthrax infection in drug users. Lancet. 2010;375:1345–1346. doi: 10.1016/S0140-6736(10)60573-9. [DOI] [PubMed] [Google Scholar]
- 51.Grunow R, Klee SR, Beyer W, et al. Anthrax among heroin users in Europe possibly caused by same Bacillus anthracis strain since 2000. Euro Surveill. 2013;18(13):1–9. [PubMed] [Google Scholar]
- 52.Davin-Regli A, Pages JM. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front Microbiol. 2015;6:1–10. doi: 10.3389/fmicb.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J, Zeng T, Su G, et al. The dissemination mode of drug-resistant genes in Enterobacter cloacae. Indian J Med Microbiol. 2015;33:87–92. doi: 10.4103/0255-0857.150967. [DOI] [PubMed] [Google Scholar]
- 54.Shadnia S, Faiaz-Noori MR, Pajoumand A, Talaie H, Khoshkar A, Vosough-Ghanbari S, Abdollahi M. A case report of opium body packer; review of the treatment protocols and mechanisms of poisoning. Toxicol Mech Methods. 2007;17(4):205–214. doi: 10.1080/15376510600992574. [DOI] [PubMed] [Google Scholar]
- 55.Hassanian-Moghaddam H, Abolmasoumi Z. Consequence of body packing of illicit drugs. Arch Iran Med. 2007;10(1):20–23. [PubMed] [Google Scholar]
- 56.Van Schaik W. The human gut resistome. Phil Trans R Soc B. 2015;370(1670):20140087. doi: 10.1098/rstb.2014.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23(2):382–398. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pier Gerald B. Goldman's Cecil Medicine. 2012. Pseudomonas and Related Gram-Negative Bacillary Infections; pp. 1877–1881. [Google Scholar]
- 59.Dancer SJ, McNair D, Finn P, et al. Bacillus cereus cellulitis from contaminated heroin. J Med Microbiol. 2002;51(3):278–281. doi: 10.1099/0022-1317-51-3-278. [DOI] [PubMed] [Google Scholar]
- 60.Sousa C, Botelho C, Rodrigues D, Azeredo J, Oliveira R. Infective endocarditis in intravenous drug abusers: an update. Eur J Clin Microbiol Infect Dis. 2012;31(11):2905–2910. doi: 10.1007/s10096-012-1675-x. [DOI] [PubMed] [Google Scholar]
- 61.Peterson TC, Pearson C, Zekaj M, Hudson I, Fakhouri G, Vaidya R. Septic arthritis in intravenous drug abusers: a historical comparison of habits and pathogens. J Emerg Med. 2014;47(6):723–728. doi: 10.1016/j.jemermed.2014.06.059. [DOI] [PubMed] [Google Scholar]
- 62.Tuazon CU, Miller H, Shamsuddin D. Antimicrobial activity of street heroin. J Infect Dis. 1980;142(6):944. doi: 10.1093/infdis/142.6.944. [DOI] [PubMed] [Google Scholar]


