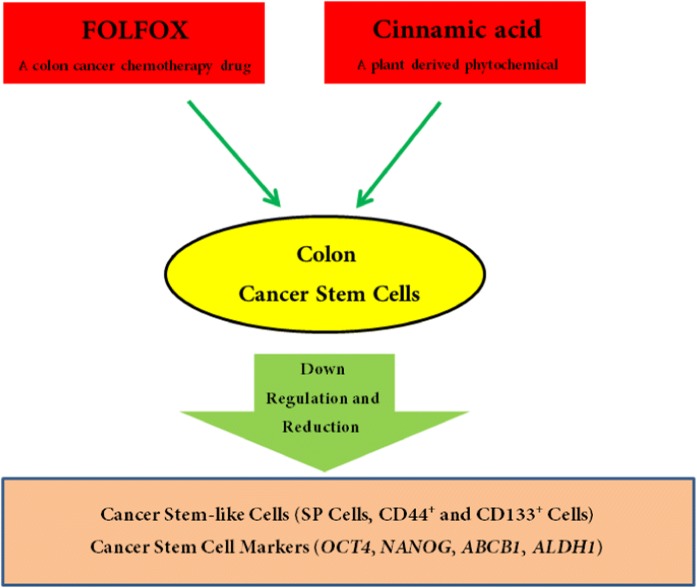
Abstract
Purpose
There is a lot of evidence suggesting that a small subset of cancer cells resistant to conventional chemotherapy and radiotherapy and known as cancer stem cells (CSCs) is responsible for promoting metastasis and cancer relapse. Therefore, targeting and eliminating the CSCs could lead to higher survival rates and a better quality of life. In comparison with conventional chemical drugs that may not be effective against CSCs, phytochemicals are strong anti-CSCs agents. The current study examines the effect of 5-fluorouracil plus oxaliplatin (FOLFOX) as a common chemotherapy drug on colorectal cancer as well as the influence of Cinnamic acid (CINN) as a plant-derived phytochemical on colon cancer stem-like cells in HT-29 adenocarcinoma cell line.
Methods
The anti-proliferative effect of FOLFOX and CINN was determined using MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Flow cytometry analysis was used for the identification of side population (SP), CD44, and CD133 positive cells. The expression of OCT4, NANOG, ABCB1, and ALDH1A was assessed by RT-PCR.
Results
The FOLFOX and CINN decreased cell viability in certain drug concentrations: IC50 = 5,40 μM oxaliplatin +220 μM 5-fluorouracil, and 13,50 mM for CINN. The CSC-associated markers (OCT4, NANOG, ABCB1, and ALDH1A) and the proportion of cancer stem-like cells (SP cells, CD44, and CD133 positive cells) were downregulated following the treatment of HT-29 adenocarcinoma cell line with IC50 concentrations of FOLFOX and CINN.
Conclusion
Our data suggests that CINN, a naturally occurring component, could be more effective than FOLFOX treatment in reducing the cancer stem-like cells and expression of CSC markers from HT-29 colon cancer cells.
Graphical abstract.
ᅟ
Keywords: Colon cancer stem cells, Side population, Cinnamic acid, FOLFOX, Cancer stem cell markers
Introduction
Cancer stem cells (CSCs) persist in tumors as a distinct population of pluripotent cancer cells with tumorigenic ability. Such cells result in the growth of a primary tumor and generate new tumors through the stem cell processes of self-renewal and differentiation into multiple cell types. In addition, it is found that CSCs are resistant to drug treatment due to having several mechanisms that inhibit cell death such as increased activity of detoxifying aldehyde dehydrogenases (ALDH) enzymes, enhanced DNA repair abilities, a slower cell-cycle, and an impressive drug efflux by upregulation of ATP-binding cassette (ABC) transporters [1–7]. The transport activity of ABC can be measured by the ability of side population (SP) cells to efflux fluorescent dyes such as Hoechst 33,342 and Rhodamine 123 (Rh123) [8–13]. There are a number of signaling pathways that play a significant role in maintaining self-renewal, ability of differentiation, and chemoresistance of CSCs, and these include the Wnt, Notch, and Hedgehog signaling pathways [14–21]. According to CSCs characteristics, this subpopulation of cancer cells is considered to be the main cause of treatment failure, relapse, and metastasis, therefore targeting the CSCs could be an effective and promising therapy for preventing recurrence and eliminating cancer [22–24].
Colorectal cancer is the third-most common cancer in men and women and a leading cause of death. It has been reported that cancer recurrence is prevalent in 50% of patients having colon cancer, this indicates that the current strategies of cancer therapy are ineffective [25, 26]. In colorectal cancer, CSCs are resistant to therapy and are also responsible for tumor recurrence. Although the combination chemotherapy of 5-fluorouracil (5-FU) with oxaliplatin (FOLFOX) is one of the most common treatments for colorectal cancer, providing regression in many cases, the relapse of cancer is also associated with the resistance of colorectal cancer cells to FOLFOX [27]. Furthermore, given the side effects of chemotherapy drugs like FOLFOX, researchers have been encouraged to find nontoxic active agents which can target and eliminate CSCs.
Several natural drugs have shown fewer side effects than synthetic chemotherapy drugs, hence many researchers tend to study plant-derived phytochemicals — evaluating their effects alone or together with conventional chemotherapy in eliminating CSCs, and thereby effectively treating cancer [28–32]. Cinnamic acid (CINN) as an organic compound is a phenylalanine deamination product and classified as an unsaturated carboxylic acid [33, 34]. This component and its derivative act as antimicrobial, anti-atherosclerotic, antioxidant, hypocholesterolemic, hepatoprotective, and antidiabetic agents [35–46]. Furthermore, many studies have shown the cytotoxic effects of CINN and its derivatives on different cancer cells, their anticancer potential in the treatment of various cancers has also been presented [47–51].
Until now, to our knowledge, the inhibitory effect of CINN on CSCs has been proven only in the lung adenocarcinoma cell line. Our study compared the effect of CINN (as a plant-derived phytochemical) with FOLFOX (as a common chemotherapy drug) on human colon cancer stem-like cells. For this purpose, the expression of OCT4, NANOG, ABCB1, and ALDH1A1 as CSC markers were quantified after treatment of human colorectal adenocarcinoma cell line HT-29 with FOLFOX and CINN. We also examined the effect of CINN and FOLFOX on the percentage of SP cells, and CD44+ and CD133+ (colon CSC markers) cells in HT-29 cell line.
Material and methods
Cell culture and reagents
The human colorectal cancer cell line HT-29 was obtained from Pasteur Institute of Iran, Tehran. This cell line was cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Biowest, France) which was supplemented with 10% fetal bovine serum (FBS; Biowest, France) and 1% penicillin-streptomycin (Biowest, France). The cell line was incubated at 37 °C in 5% CO2 atmosphere.
Oxaliplatin and 5-FU were purchased from Mylan S.A.S, France and Ebewe Pharma, Austria, respectively. CINN (Cat. No. C80857) and Rh123 (Cat. No. R8004) were obtained from Sigma Aldrich, Munich, Germany. FITC Mouse Anti-Human CD44 Clone G44–26 (also known as C26) antibody (Cat. No. 560977) from BD Biosciences, San Jose, CA, U.S.A and Human CD133/2 (clone: 293C3) antibody from Miltenyi Biotec, Bergisch Gladbach, Germany were provided. FITC Mouse IgG1, isotype control clone MOPC-21 (Cat. No. 555748; BD Biosciences, San Jose, CA, USA) and PE Mouse IgG1, isotype control clone MOPC-21 (Cat. No. 554680, BD Biosciences, San Jose, CA, USA) served as the control in flow cytometry analysis. Moreover, staining with a non-vital DNA dye such as 7-aminoactinomycin D (7-AAD) (Cat. No. 559763; BD Biosciences, San Jose, CA, U.S.A) and propidium iodide (PI) (Cat. No. P4170; Sigma Aldrich, Munich, Germany) allows for discrimination of dead cells in flow cytometry
Growth inhibition assay
Inhibition of cell growth in response to FOLFOX or CINN was assessed by 3-(4,5-dimethylthiazol-2yl)-2, 5-diphenyltetrazoliumbromide (MTT) assay. Briefly, 9 × 103 cells were seeded into 96-well culture plates with three replicates. After 24 h of plating, the incubation was continued for another 48 h in the absence (control) or presence of different concentration of 5-FU plus oxaliplatin or CINN as shown in Fig. 1. At the end of the 48 h, the reaction was terminated by adding 20 μl of 5-mg/ml stock of MTT (Atocel, Austria) to each well. The reaction was allowed to proceed for 3 h at 37 °C. The culture medium was then removed. The formazan crystals were then dissolved by adding 0,10 ml of dimethyl sulfoxide. The intensity of the color, indicating the number of live cells, was measured using a microplate reader (BioTek-ELx800, USA) at a wavelength of 490 nm. The percentage of living cells was calculated by dividing the mean absorbance of treated cells in each well to the mean absorbance of control cells multiplied by 100. All the assays were performed in triplicates. The IC50 values of agents were calculated using Prism 6.0 (GraphPad Software, Inc., San Diego, California, USA).
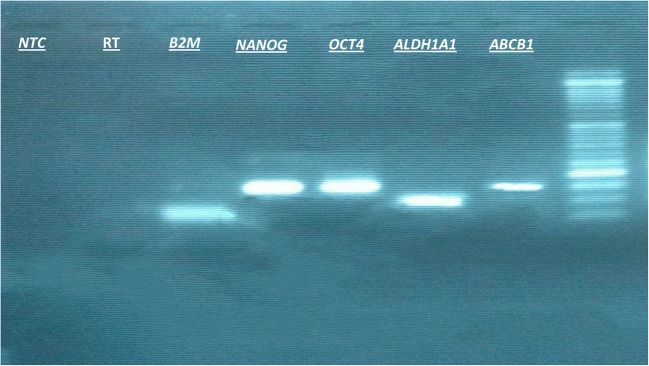
Fig. 1.
The accuracy of RT- PCR was further validated by gel electrophoresis. RT- PCR products were run in 2% agarose gel and bands were seen at the desired size (ABCB1: 151 bp; ALDH1A1: 98 bp; OCT4: 145 bp; NANOG: 149 bp; and β2M: 69 bp). The negative control lanes are indicated by RT minus (no reverse transcriptase for the reverse transcription reaction) and NTC (no-template control for the PCR reaction). A molecular weight marker (50 bp ladder) is used
Flow cytometry analysis
The treatment of HT-29 cell line with FOLFOX was carried out with 220 μM of 5-FU and 5,40 μM of oxaliplatin for 48 h. In order to study the effect of CINN, the cells were incubated with a medium containing 13,50 mM of CINN for 48 h. The flow cytometry analysis was carried out on the untreated control and FOFOLX or CINN-treated cells.
Rh123 staining
In order to analyze the SP cells, the untreated control and treated cells were suspended at 106 cell/ml in PBS containing 2% FBS and then incubated with 0,10 μg/ml Rh123 for 30 min at 37 °C. After washing with ice-cold PBS, the cells were incubated at 37 °C for 40 min in order to allow the cells to efflux the Rh123 dye. Finally, to exclude dead cells, the cells were suspended in PBS/FBS 2% with 1 μg/ml PI. The flow cytometric analysis of Rhodamine fluorescence was carried out with a Partec particle and cell sorting instrument (Munster, Germany). To inhibit the ABC transporters, 100 μm verapamil (Cat. No. V4629; Sigma-Aldrich, Munich, Germany) was added along with Rh123 to each cell suspension.
Antibodies
The untreated control and treated HT-29 colon cancer cells were subjected to direct immunofluorescence staining followed by flow cytometric analysis. Briefly, cells were treated with trypsin and washed with PBS containing 2% FBS. One million cells were suspended in 100 μl of PBS/ 2% FBS followed by the addition of 20 μl of FITC Mouse Anti-Human CD44 or 10 μl of PE Mouse Anti- Human CD CD133/2 and incubated for 30 min in the dark at room temperature. The samples were then washed and analyzed using flow cytometry. To detect dead cells, PI and 7-AAD was used with Mouse Anti-Human CD44 and Mouse Anti- Human CD133/2 antibody, respectively. FITC Mouse IgG1 isotype control and PE Mouse IgG1 isotype control served as control in flow cytometry.
Quantitative real-time PCR analysis
The total RNA was isolated from cells using Total RNA isolation kit (DENAzist Asia, Mashhad, Iran). The quantity and quality of RNA were assessed using a Nano drop and agarose gel electrophoresis. To avoid DNA contamination, the extracted RNAs were treated with RNase-free DNase I (Cat. No. EN0521; Thermo Scientific, Wilmington, USA). The cDNA was synthesized using M-MuLV reverse transcriptase (Cat. No. EP0441; Thermo Scientific, Wilmington, USA), according to protocol.
The real-time PCR (RT-PCR) was performed using SYBR Green master mix (Cat. No. PB20.11–01; Biosystems, Barcelona, Spain) in RT PCR System (Analytik Jena, Jena, Germany). To study the expression of OCT4, NANOG, ABCB1, ALDH1A1, and β2M, specific primers were designed using Oligo7 primer analysis software. The sequence of primers and product length are described in Table 1. The thermal cycling conditions involved initial denaturation at 95 °C for 4 min followed by amplification of OCT4, NANOG, and β2M cDNA for 40 cycles (95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s), and ALDH1A1 and ABCB1 cDNA for 40 cycles (95 °C for 30 s, 62 °C for 30 s, and 72 °C for 30 s). To derive the melting curves, the temperature was increased by 1 °C for 10 s from 60 to 95 °C. The analysis of melting curves clearly indicated that each of the primer pairs, described in Table 1, amplified only the expected product and the accuracy of amplification reaction was validated by gel electrophoresis (Fig. 1). The PCR efficiencies (E) were calculated for all used primers from the given slopes of standard curves, generated from serial dilutions of positive controls. The qPCR readings were performed in triplicate for each sample and the mean value of each triplicate was used for the calculation of the mRNA expression levels. To compare the level of gene expression in FOLFOX or CINN treated cells with the untreated control cells, β2M transcripts were used as an internal control and the fold-change in the target gene of treated cell relative to the untreated control sample was calculated according to the following equation: Fold change = 2(-ΔΔCT).
Table 1.
List of different PCR primers used in the study
| Gene name | Sequence (5′ to 3′) | Product size (bp) |
|---|---|---|
| Octamer-binding transcription factor 4 (OCT4) | F:CCGAAAGAGAAAGCGAACCAGTAT | 145 |
| R: CCACACTCGGACCACATCCTTC | ||
| Accession number: NM_002701.5 (Variant 1) | ||
| Accession number: NM_203289.5 (Variant 2) | ||
| Accession number: NM_001173531.2 (Variant 3) | ||
| Accession number: NM_001285986.1 (Variant 4) | ||
| Accession number: NM_001285987.1 (Variant 5) | ||
| Nanog homeobox (NANOG) | F: AATACCTCAGCCTCCAGCAGATG | 149 |
| R: CTGCGTCACACCATTGCTATTCT | ||
| Accession number: NM_024865.3 (Variant 1) | ||
| Accession number: NM_001297698.1 (Variant 2) | ||
| ATP Binding Cassette Subfamily B Member 1 (ABCB1) | F: CACCACTGGAGCATTGACTR | 151 |
| R: CAGTGTTAGTTGCCAACCAT | ||
| Accession number: NM_001348945.1 (Variant 1) | ||
| Accession number: NM_001348944.1 (Variant 2) | ||
| Accession number: NM_000927.4 (Variant 3) | ||
| Accession number: NM_001348946.1 (Variant 4) | ||
| Aldehyde dehydrogenase 1 family, member A1 (ALDH1A1) | F: TCAGCAGGAGTGTTTACCAA | 98 |
| R: CTTACCACGCCATAGCAA | ||
| Accession number: NM_000689.4 | ||
| Beta-2-Microglobulin (β2M) | F: CTCCGTGGCCTTAGCTGTG | 69 |
| Accession number: NM_004048.2 | R: TTTGGAGTACGCTGGATAGCCT |
Statistical analysis
All data was compiled from a minimum of three replicate experiments. Data for statistical analysis is expressed as the mean ± standard error. The comparison of results from treated versus control cells was done using the student’s t-test with SPSS Version 16 Software. A P-value less than 0.05 was considered statistically significant.
Results
Proliferative inhibition of FOFOX and CINN on HT-29 cell line
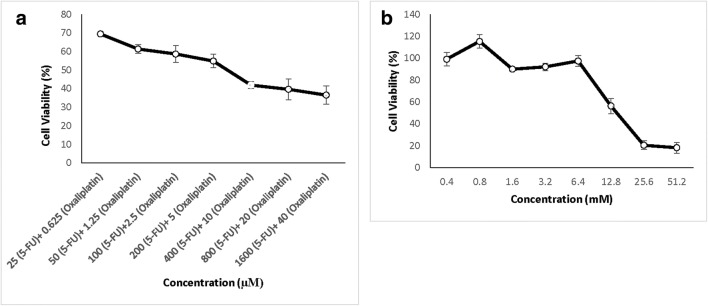
The cytotoxic effect of different concentrations of FOLFOX on HT-29 cells for 48 h was assessed by MTT assay (Fig. 2-a). The cell survival analysis showed that the FOLFOX has an inhibitory effect on the growth of HT-29 cells. The half maximal inhibitory concentration (IC50) values of FOLFOX was oxaliplatin: 5,40 μM and 5-FU: 220 μM after 48 h of treatment. In order to determine the IC50 value of CINN as a plant-derived phytochemical in HT-29 cells, they were treated with varying concentrations of CINN (0,40–51,20 mM) for 48 h. The results showed that CINN inhibited the HT-29 cells with IC50: 13,50 mM using MTT (Fig. 2-b).
Fig. 2.
Anti-proliferative activity of FOLFOX (a) and Cinnamic acid (b) on HT-29 cell line. HT-29 cells were treated with increasing concentrations of FOLFOX and Cinnamic acid for 48 h. All points represent results of cell viability percentage from three independent experiments performed in triplicate. Data is expressed as mean ± SD
FOLFOX and CINN decreased the percentage of SP cells, and CD44+ and CD133+ cells in human colon cancer cell line HT-29
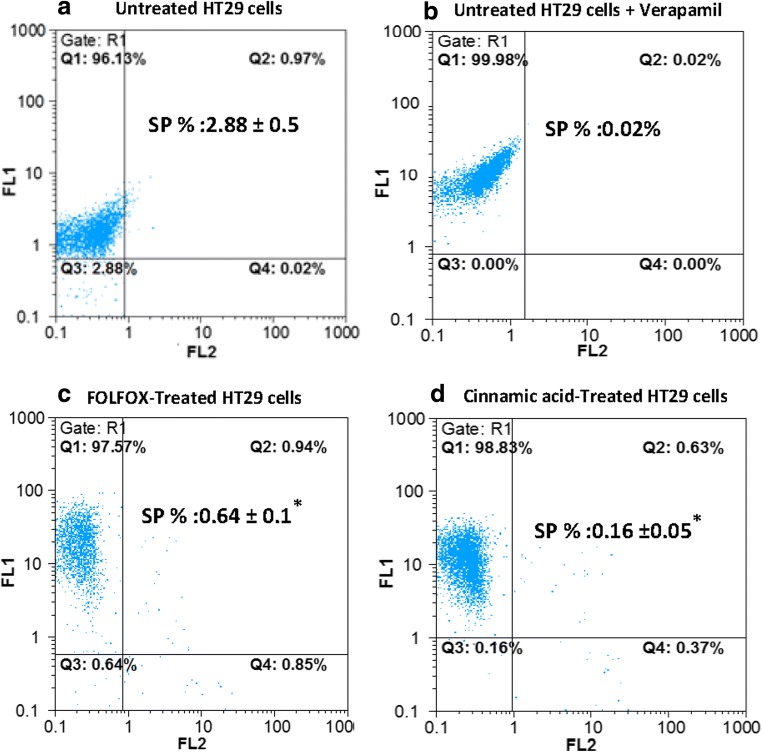
The flow cytometry analysis showed that the percentage of SP cells in the HT-29 cell line was about 2,88% of the total cells when stained with Rh123 alone and reduced significantly in the presence of verapamil (0,02%) (Figs. 3-a, b). This is because P-gp /ABCB1 is responsible for the efflux of Rh123 out of the cells. Therefore, the reduced Rh123 staining with verapamil as a P-gp inhibitor showed that the presence of SP cells is not due to the decreased initial dye uptake but is because of the role of P-gp /ABCB1 in the rapid efflux of dye out of cells [52–55]. We found that the proportion of SP cells in FOLFOX-treated cells and CINN-treated cells was about 4,50 and 18 folds lower than the control group, respectively (Figs. 3c, d).
Fig. 3.
FOLFOX and Cinnamic acid diminished SP cells in HT-29 cells. Untreated HT-29 cells in absence (a) and presence of verapamil (b), FOLFOX (220 μM 5-FU/ 5.4 μM Oxaliplatin) treated (c) and Cinnamic acid (13.5 Mm) treated HT-29 cells (d) were stained with Rh123 and propidium iodide dyes and analyzed using flow cytometry. The cell population that excludes Pi and Rh123 are representative of SP cells and were counted in the left low quadrants. The data represents the mean (± standard deviation, SD) of three independent experiments and the difference in fraction of SP cells between control and treated cells was significant according to student’s t-test (*P < 0.05)
CSCs are identified by specific surface epitopes. Some of the important surface markers in colon.
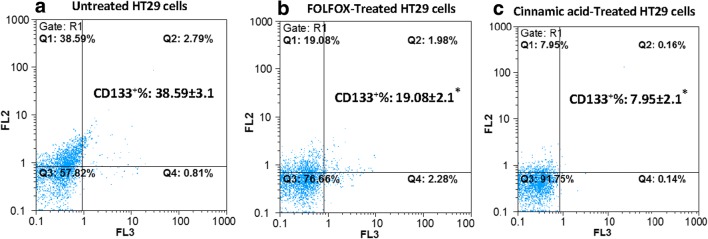
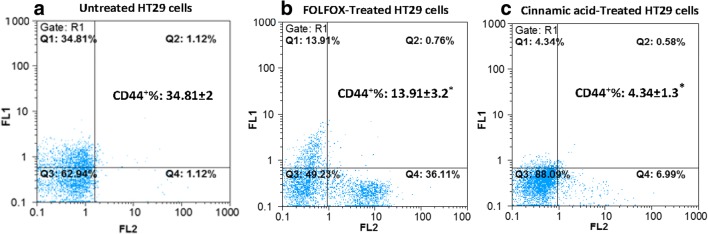
CSCs are CD44, CD166, and CD133 [56, 57]. Here, untreated control and FOLFOX or CINN- treated HT-29 cells were analyzed for colon cancer stem-like cells by tagging them separately with CD44 and CD133 antibodies, and then subsequently, they were tested by flow cytometry. The results revealed that the percentage of CD44+ and CD133+ cells in untreated control cells was about 34–38% of total cells. However, the FOLFOX treatment diminished the percentage of positive CD44 and CD133 cells to around 19 and 13%, respectively. After CINN treatment, the expression of CD133 and CD44 expression was reflected in 7,90% and 4,30% cells, respectively, suggesting that CINN might be more effective than FOLFOX in inhibiting colon CSCs in HT-29 cell line (Figs. 4 and 5).
Fig. 4.
FOLFOX and Cinnamic acid decreased the proportion of CD133+ cells in HT-29 cell line. Untreated HT-29 (a), HT-29 cells which were incubated with FOLFOX (220 μM 5-FU/ 5.4 μM Oxaliplatin) (b), and in medium containing 13.5 Mm Cinnamic acid (c) for 48 h were analyzed after incubation with PE anti-human CD133 Antibody+7-AAD with flow cytometry. The results obtained are from the mean ± SD of three independent experiments. Each CD133/7-AAD dot plot represents one of the three independent experiments. *P < 0.05 compared to the control as tested by the student’s t-test
Fig. 5.
FOLFOX and Cinnamic acid decreased the percentage of CD44+ cells in HT-29 cell line. Untreated HT-29 (a), HT-29 cells which were incubated with FOLFOX (220 μM 5-FU/ 5.4 μM Oxaliplatin) (b), and in medium containing 13.5 Mm Cinnamic acid (c) for 48 h were analyzed after incubation with FITC anti-human CD44 Antibody+ Pi with flow cytometry. The results are obtained from the mean ± SD of three independent experiments. Each CD44/PI dot plot represents one of the three independent experiments. *P < 0.05 compared to the control as tested by the Student’s t-test
Downregulation of stem cell-associated genes by FOLFOX and CIINN
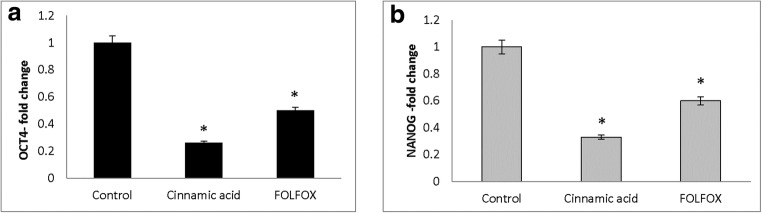
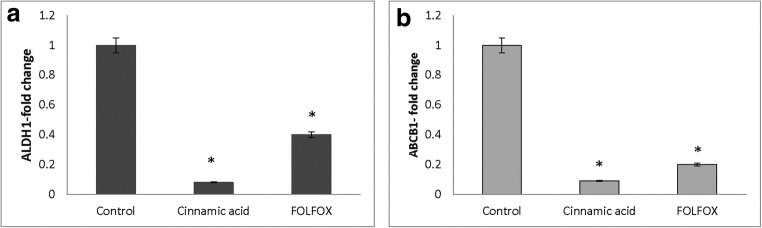
The expression of stem cell-associated genes including OCT4, NANOG, ABCB1, and ALDH1A1 were analyzed by RT after treating the HT-29 cell line with FOLFOX and CINN. The results indicated that after treatment, OCT4, NANOG, ABCB1, and ALDH1A1 were downregulated. FOLFOX decreased the fold-change of OCT4 and NANOG to 0,50 ± 0,01 and 0,60 ± 0,05 whereas CINN decreased the fold-change of OCT4 and NANOG to 0,26 ± 0,20 and 0,33 ± 0,10, respectively (Fig. 6). In addition, the expression of ABCB1 and ALDH1A1 also significantly decreased after CINN (fold-change of 0,09 ± 0,07 and 0,08 ± 0,03) and FOLFOX treatment (fold-change of 0,20 ± 0,05 and 0,40 ± 00) (Fig. 7).
Fig. 6.
Downregulation of OCT4 (a) and NANOG (b) in HT-29 by FOLFOX and Cinnamic acid. HT-29 cells were treated with Cinnamic acid (13.5 Mm) and FOLFOX (220 μM 5-FU/ 5.4 μM Oxaliplatin) for 48 h. The Y-axis represents the fold-change in transcript levels compared with untreated HT-29 cells (designated as 1.0). The graph represents the mean data ± SD of at least three independent experiments. Asterisk indicates significant (p < 0.05) difference in mRNA expression in comparison with untreated cells
Fig. 7.
Downregulation of ALDH1A1 (a) and ABCB1 (b) in HT-29 by FOLFOX and Cinnamic acid. HT-29 cells were treated with Cinnamic acid (13.5 Mm) and FOLFOX (220 μM 5-FU/ 5.4 μM Oxaliplatin) for 48 h. Y-axis represents the fold-change in transcript levels compared with untreated HT-29 cells (designated as 1.0). The graph represents the mean data ± SD of at least three independent experiments. Asterisk indicates significant (p < 0.05) difference in mRNA expression in comparison with untreated cells
Discussion
According to the CSC theory, CSCs constitute a small proportion of tumor cells responsible for cancer initiation, invasion, metastasis, and recurrence [58, 59]. Therefore, identifying therapeutic agents that can target CSCs is considered more effective for tumor destruction as well as for reducing the risk of recurrence. The CSCs resistance to various chemotherapy drugs is attributed to the increased expression of ABC transporters and elevated activity of ALDH, which are a superfamily of enzymes with detoxification capabilities [22, 23, 60–67]. The increased expression of ABC transporters such as ABCB1 (multidrug resistance protein 1 [MDR1] or P-gp), ABCC1 (multidrug resistance-associated protein 1 [MRP1]), and ABCG2 (breast cancer resistance protein [BRCP]) in CSCs can be detected by their ability to efflux fluorescent dyes, such as Hoechst 33,342 and Rh123, which is then measured by flow cytometry [66–68]. This population of negatively stained cells is known as SP cells [8–13, 68, 69]. The SP cells isolated from various cancer cell lines and tumors possess CSC properties such as self-renewal capabilities, ability to differentiate into heterogeneous cells, high proliferation, and high colony forming potential [8–13, 70–72]. Therefore, in this study we used SP cell analysis as a tool to evaluate the effect of CINN and FOLFOX on elimination of CSCs. The fact that CSCs constitute a small proportion of cancer cells has been demonstrated in our study which showed that only 2–3% of the total colon cancer HT-29 cells are SP cells. Furthermore, the present study indicated that CINN reduced the proportion of SP cells more effectively than FOLFOX. In addition to the SP phenotype, the CSCs carry lineage-specific surface markers. Several cell surface biomarkers have been detected to identify and isolate CSCs in various types of cancers [73–80]. In colon CSCs, multiple cell surface markers including CD133, CD166, CD44, CD24, beta1 integrin-CD29, Lgr5, EpCAM (ESA), ALDH-1, Msi-1, DCAMLK1, or EphB receptors have been identified. Among these markers, CD133, CD166, and CD44 are the three main markers [25, 56, 57, 81–85]. The detection of colorectal CSC markers —CD44 and CD133 — in this study showed that after incubation of the HT-29 cell line with CINN and FOLFOX, these CSC markers reduced significantly in the CINN-treated cells compared to the FOLFOX-treated cells. Consequently, the flow cytometry results showed that CINN has a better inhibitory effect on size of the cancer stem-like cells including SP cells, and CD 44 and CD133 positive cells. The CSCs and normal stem cells share some markers such as OCT4, NANOG, and SOX2 which are key factors in maintaining pluripotency and self-renewal of stem cells [68, 86–88]. Therefore, added support for the effect of FOLFOX and CINN on colon CSCs can be derived from the expression analysis of two stem cell-associated factors including OCT4 and NANOG as well as ALDH1A1 and ABCB1 as CSC markers using RT-PCR. The results showed that CINN is effective in the downregulation of OCT4, NANOG, ABCB1, and ALDH1A1 compared to FOLFOX.
CINN is a plant-derived component displaying a wide range of biological activities including cytotoxic effects on cancer cells [47–51]. The anti-cancer activity of CINN has been demonstrated only in the lung adenocarcinoma cell line, wherein the CSC-like abilities were diminished by decreasing the proliferation, invasive abilities, and in vivo tumorigenicity of sphere-derived stem cells. Furthermore, the CINN improved the sensitivity of CSCs to chemotherapeutic drugs via apoptosis induction [89]. In order to support the antitumor effect of CINN, we investigated the effect of CINN on colon CSCs and showed that the incubation of colon cancer cell line with CINN led to the reduction of colon cancer stem-like cells and the downregulation of CSC markers. Accordingly, CINN was demonstrated to be a more effective chemotherapeutic agent than FOLFOX to eliminate HT-29 CSCs.
Similar to our results, the comparison of chemotherapy drugs and plant-derived phytochemicals in other studies showed that chemotherapy drugs are less effective in CSCs eradication than plant-derived phytochemicals. For instance, while the exposure of HCT-116 colon cancer cell line to FOLFOX led to the enrichment of CSCs phenotype, treatment with curcumin alone or together with FOLFOX or dasatinib could target the CSC subpopulation [90–94]. Several studies have shown that breast cancer cell treatment with Berberine as an isoquinoline alkaloid, isolated from medicinal herbs, leads to decreased expression of ABCG2, stem cell-associated genes, and SP fraction [31, 95, 96]. However, no statistically significant decrease was detected at the percentage of SP cells and expression level of ABCG2 after exposure of MCF-7 breast cancer cells to chemotherapy drugs such as doxorubicin and docetaxel, or mitoxantrone [31]. Likewise, oxymatrine is a plant alkaloid that reduced the percentage of SP cells and downregulated the activity of Wnt/b-catenin signaling in MCF-7, whereas cisplatin treatment was not able to do so [30]. Resveratrol and quercetin are naturally occurring polyphenolic compounds affecting CSCs by inducing apoptosis, inhibiting self-renewal capacity, reducing ALDH1 activity, and downregulating pluripotency [97–99]. In addition, it was proven that apigenin and baicalein, which are plant-derived flavones, and sulforaphane, a major glucosinolate in broccoli/broccoli sprouts, suppresses self-renewal capacity, cell growth, clonogenicity, and migration of CSCs in various cancers [32, 63, 96, 100–106]. To sum up, many reports indicate a low efficacy of common anti-cancer drugs compared to plant-derived compounds in combating CSCs. In addition, the adverse effects of synthetic drugs and the lower toxicity of plant-derived components make the latter a better option for cancer treatment [107–111]. Similarly, we have demonstrated that CINN as a natural agent not only inhibits the growth of HT-29 cells in cell-culture, but also leads to a dramatic decrease in colon cancer stem-like cell population. Furthermore, CINN has stronger anti-colon CSCs properties, which indicates that CINN either alone or together with FOLFOX could be more effective in eliminating colorectal cancer.
Acknowledgements
The authors thank Saeed Esmaeili Mahani at Department of Biology, Faculty of science, Shahid Bahonar University of Kerman for providing experimental equipment, Vice Chancellor for Research, Kerman University of Medical Science, Kerman, Iran for financial support.
Funding
This study was funded by Kerman University of Medical Science, Kerman, Iran (grant number: 96000157).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Thomas M, Coyle K, Sultan M, Vaghar-Kashani A, Marcato P. Chemoresistance in cancer stem cells and strategies to overcome resistance. Chemotherapy. 2014;3(125):2. [Google Scholar]
- 2.Blagosklonny MV. Why therapeutic response may not prolong the life of a cancer patient: selection for oncogenic resistance. Cell Cycle. 2005;4(12):1693–1698. doi: 10.4161/cc.4.12.2259. [DOI] [PubMed] [Google Scholar]
- 3.Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7(3):330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hipkens J, Struck R, Gurtoo H. Role of aldehyde dehydrogenase in the metabolism-dependent biological activity of cyclophosphamide. Cancer Res. 1981;41(9 Part 1):3571–3583. [PubMed] [Google Scholar]
- 5.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 6.Mathews LA, Cabarcas SM, Hurt EM, Zhang X, Jaffee EM, Farrar WL. Increased expression of DNA repair genes in invasive human pancreatic cancer cells. Pancreas. 2011;40(5):730. doi: 10.1097/MPA.0b013e31821ae25b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS One. 2008;3(8):e3077. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo Z, Jiang JH, Zhang J, Yang HJ, Zhong YP, Su J, et al. Side population in hepatocellular carcinoma HCCLM3 cells is enriched with stem-like cancer cells. Oncol Lett. 2016;11(5):3145–3151. doi: 10.3892/ol.2016.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murase M, Kano M, Tsukahara T, Takahashi A, Torigoe T, Kawaguchi S, et al. Side population cells have the characteristics of cancer stem-like cells/cancer-initiating cells in bone sarcomas. Br J Cancer. 2009;101(8):1425–1432. doi: 10.1038/sj.bjc.6605330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnenberg VS, Landreneau RJ, Donnenberg AD. Tumorigenic stem and progenitor cells: implications for the therapeutic index of anti-cancer agents. J Control Release. 2007;122(3):385–391. doi: 10.1016/j.jconrel.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci U S A. 2004;101(3):781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 14.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 15.Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, et al. Loss of β-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12(6):528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flahaut M, Meier R, Coulon A, Nardou K, Niggli F, Martinet D, et al. The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/β-catenin pathway. Oncogene. 2009;28(23):2245. doi: 10.1038/onc.2009.80. [DOI] [PubMed] [Google Scholar]
- 17.Ulasov IV, Nandi S, Dey M, Sonabend AM, Lesniak MS. Inhibition of sonic hedgehog and notch pathways enhances sensitivity of CD133+ glioma stem cells to temozolomide therapy. Mol Med. 2011;17(1):103. doi: 10.2119/molmed.2010.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo S, Liu M, Gonzalez-Perez RR. Role of notch and its oncogenic signaling crosstalk in breast cancer. Biochimica et Biophysica Acta (BBA)-reviews on. Cancer. 2011;1815(2):197–213. doi: 10.1016/j.bbcan.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reguart N, He B, Taron M, You L, Jablons DM, Rosell R. The role of Wnt signaling in cancer and stem cells. Future Oncol. 2005;1(6):787–97. doi: 10.2217/14796694.1.6.787. [DOI] [PubMed] [Google Scholar]
- 20.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 21.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 22.Vasiliou V, Nebert DW. Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum Genomics. 2005;2(2):138. doi: 10.1186/1479-7364-2-2-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15(12):4234–4241. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 24.Ikawa M, Impraim CC, Wang G, Yoshida A. Isolation and characterization of aldehyde dehydrogenase isozymes from usual and atypical human livers. J Biol Chem. 1983;258(10):6282–6287. [PubMed] [Google Scholar]
- 25.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 27.Park SH, Sung JY, Han S-H, Baek JH, Oh JH, Bang S-M, et al. Oxaliplatin, folinic acid and 5-fluorouracil (FOLFOX-4) combination chemotherapy as second-line treatment in advanced colorectal cancer patients with irinotecan failure: a Korean single-center experience. Jpn J Clin Oncol. 2005;35(9):531–535. doi: 10.1093/jjco/hyi140. [DOI] [PubMed] [Google Scholar]
- 28.Chung SS, Vadgama JV. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3–NFκB signaling. Anticancer Res. 2015;35(1):39–46. [PMC free article] [PubMed] [Google Scholar]
- 29.Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK, et al. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122(2):267–273. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Piao B, Zhang Y, Hua B, Hou W, Xu W, et al. Oxymatrine diminishes the side population and inhibits the expression of β-catenin in MCF-7 breast cancer cells. Med Oncol. 2011;28(1):99–107. doi: 10.1007/s12032-010-9721-y. [DOI] [PubMed] [Google Scholar]
- 31.Kim JB, Ko E, Han W, Shin I, Park SY, Noh D-Y. Berberine diminishes the side population and ABCG2 transporter expression in MCF-7 breast cancer cells. Planta Med. 2008;74(14):1693–1700. doi: 10.1055/s-0028-1088313. [DOI] [PubMed] [Google Scholar]
- 32.Gu Y-Y, Liu L-P, Qin J, Zhang M, Chen Y, Wang D, et al. Baicalein decreases side population proportion via inhibition of ABCG2 in multiple myeloma cell line RPMI 8226 in vitro. Fitoterapia. 2014;94:21–28. doi: 10.1016/j.fitote.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Budavari S. An encyclopedia of chemicals, drugs, and biologicals. The Merck Index. 1989;246.
- 34.Garbe D. Cinnamic Acid. In: Wiley J, editor. Ullmann’s Encyclopedia of Industrial Chemistry. Hoboken, New York: Wiley-VCH Verlag GmbH & Co. KGaA; 2000. [Google Scholar]
- 35.Lafay S, Gil-Izquierdo A. Bioavailability of phenolic acids. Phytochem Rev. 2008;7(2):301. [Google Scholar]
- 36.Clifford MN. Chlorogenic acids and other cinnamates–nature, occurrence, dietary burden, absorption and metabolism. J Sci Food Agric. 2000;80(7):1033–1043. [Google Scholar]
- 37.Simonyan A. Activity of cinnamic acid derivatives and new methods for their synthesis. Pharm Chem J. 1993;27(2):92–100. [Google Scholar]
- 38.Sharma P. Cinnamic acid derivatives: a new chapter of various pharmacological activities. J Chem Pharm Res. 2011;3(2):403–423. [Google Scholar]
- 39.Narasimhan B, Belsare D, Pharande D, Mourya V, Dhake A. Esters, amides and substituted derivatives of cinnamic acid: synthesis, antimicrobial activity and QSAR investigations. Eur J Med Chem. 2004;39(10):827–834. doi: 10.1016/j.ejmech.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 40.Carvalho SA, da Silva EF, de Souza MV, Lourenço MC, Vicente FR. Synthesis and antimycobacterial evaluation of new trans-cinnamic acid hydrazide derivatives. Bioorg Med Chem Lett. 2008;18(2):538–541. doi: 10.1016/j.bmcl.2007.11.091. [DOI] [PubMed] [Google Scholar]
- 41.Tonari K, Mitsui K, Yonemoto K. Structure and antibacterial activity of cinnamic acid related compounds. J Oleo Sci. 2002;51(4):271–273. [Google Scholar]
- 42.Gupta A, Soni L, Hanumantharao P, Sambasivarao S, Babu MA, Kaskhedikar S. 3D-QSAR analysis of some cinnamic acid derivatives as antimalarial agents. Asian J Chem. 2004;16(1):67. [Google Scholar]
- 43.Lee S, Han J-M, Kim H, Kim E, Jeong T-S, Lee WS, et al. Synthesis of cinnamic acid derivatives and their inhibitory effects on LDL-oxidation, acyl-CoA: cholesterol acyltransferase-1 and-2 activity, and decrease of HDL-particle size. Bioorg Med Chem Lett. 2004;14(18):4677–4681. doi: 10.1016/j.bmcl.2004.06.101. [DOI] [PubMed] [Google Scholar]
- 44.Shahidi F, Chandrasekara A. Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochem Rev. 2010;9(1):147–170. [Google Scholar]
- 45.Neogi P, Lakner FJ, Medicherla S, Cheng J, Dey D, Gowri M, et al. Synthesis and structure–activity relationship studies of cinnamic acid-based novel thiazolidinedione antihyperglycemic agents. Bioorg Med Chem. 2003;11(18):4059–4067. doi: 10.1016/s0968-0896(03)00393-6. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, Hudgins WR, Shack S, Yin MQ, Samid D. Cinnamic acid: a natural product with potential use in cancer intervention. Int J Cancer. 1995;62(3):345–350. doi: 10.1002/ijc.2910620319. [DOI] [PubMed] [Google Scholar]
- 47.De P, Baltas M, Bedos-Belval F. Cinnamic acid derivatives as anticancer agents-a review. Curr Med Chem. 2011;18(11):1672–1703. doi: 10.2174/092986711795471347. [DOI] [PubMed] [Google Scholar]
- 48.Sova M, Zizak Z, Stankovic JAA, Prijatelj M, Turk S, Juranic ZD, et al. Cinnamic acid derivatives induce cell cycle arrest in carcinoma cell lines. Med Chem. 2013;9(5):633–641. doi: 10.2174/1573406411309050002. [DOI] [PubMed] [Google Scholar]
- 49.de Oliveira Niero EL, Machado-Santelli GM. Cinnamic acid induces apoptotic cell death and cytoskeleton disruption in human melanoma cells. J Exp Clin Cancer Res. 2013;32(1):31. doi: 10.1186/1756-9966-32-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu B, Shang B, Li Y, Zhen Y. Inhibition of histone deacetylases by trans-cinnamic acid and its antitumor effect against colon cancer xenografts in athymic mice. Mol Med Rep. 2016;13(5):4159–4166. doi: 10.3892/mmr.2016.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pontiki E, Hadjipavlou-Litina D, Litinas K, Geromichalos G. Novel cinnamic acid derivatives as antioxidant and anticancer agents: design, synthesis and modeling studies. Molecules. 2014;19(7):9655–9674. doi: 10.3390/molecules19079655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Germann UA, Ford PJ, Shlyakhter D, Mason VS, Harding MW. Chemosensitization and drug accumulation effects of VX-710, verapamil, cyclosporin a, MS-209 and GF120918 in multidrug resistant HL60/ADR cells expressing the multidrug resistance-associated protein MRP. Anti-Cancer Drugs. 1997;8(2):141–155. doi: 10.1097/00001813-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Dantzig AH, Shepard RL, Pratt SE, Tabas LB, Lander PA, Ma L, et al. Evaluation of the binding of the tricyclic isoxazole photoaffinity label LY475776 to multidrug resistance associated protein 1 (MRP1) orthologs and several ATP-binding cassette (ABC) drug transporters. Biochem Pharmacol. 2004;67(6):1111–1121. doi: 10.1016/j.bcp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Ford JM, Hait WN. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990;42(3):155–199. [PubMed] [Google Scholar]
- 55.Shudo N, Mizoguchi T, Kiyosue T, Arita M, Yoshimura A, Seto K, et al. Two pyridine analogues with more effective ability to reverse multidrug resistance and with lower calcium channel blocking activity than their dihydropyridine counterparts. Cancer Res. 1990;50(10):3055–3061. [PubMed] [Google Scholar]
- 56.Dalerba P, Dylla SJ, Park I-K, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci. 2007;104(24):10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 58.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15(9):494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Moharil RB, Dive A, Khandekar S, Bodhade A. Cancer stem cells: an insight. J Oral Maxillofac Pathol. 2017;21(3):463. doi: 10.4103/jomfp.JOMFP_132_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheung A, Wan T, Leung J, Chan L, Huang H, Kwong Y, et al. Aldehyde dehydrogenase activity in leukemic blasts defines a subgroup of acute myeloid leukemia with adverse prognosis and superior NOD/SCID engrafting potential. Leukemia. 2007;21(7):1423–1430. doi: 10.1038/sj.leu.2404721. [DOI] [PubMed] [Google Scholar]
- 62.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4):1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin M-G, Liu L-P, Li C-Y, Zhang M, Chen Y, Qin J, et al. Scutellaria extract decreases the proportion of side population cells in a myeloma cell line by down-regulating the expression of ABCG2 protein. Asian Pac J Cancer Prev. 2013;14(12):7179–7186. doi: 10.7314/apjcp.2013.14.12.7179. [DOI] [PubMed] [Google Scholar]
- 64.Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268(1):1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 65.Andrews TE, Wang D, Harki DA. Cell surface markers of cancer stem cells: diagnostic macromolecules and targets for drug delivery. Drug Deliv Transl Res. 2013;3(2):121–142. doi: 10.1007/s13346-012-0075-1. [DOI] [PubMed] [Google Scholar]
- 66.Choi YH, Yu AM. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm. 2014;20(5):793–807. doi: 10.2174/138161282005140214165212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Webb M, Raphael CL, Asbahr H, Erber WN, Meyer BF. The detection of rhodamine 123 efflux at low levels of drug resistance. Br J Haematol. 1996;93(3):650–655. doi: 10.1046/j.1365-2141.1996.d01-1680.x. [DOI] [PubMed] [Google Scholar]
- 68.Begicevic R-R, Falasca M. ABC transporters in Cancer stem cells: beyond Chemoresistance. Int J Mol Sci. 2017;18(11):2362. doi: 10.3390/ijms18112362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glavinas H, Krajcsi P, Cserepes J, Sarkadi B. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr Drug Deliv. 2004;1(1):27–42. doi: 10.2174/1567201043480036. [DOI] [PubMed] [Google Scholar]
- 70.Xiong B, Ma L, Hu X, Zhang C, Cheng Y. Characterization of side population cells isolated from the colon cancer cell line SW480. Int J Oncol. 2014;45(3):1175–1183. doi: 10.3892/ijo.2014.2498. [DOI] [PubMed] [Google Scholar]
- 71.Fukuda K, Saikawa Y, Ohashi M, Kumagai K, Kitajima M, Okano H, et al. Tumor initiating potential of side population cells in human gastric cancer. Int J Oncol. 2009;34(5):1201–1207. [PubMed] [Google Scholar]
- 72.Hadnagy A, Gaboury L, Beaulieu R, Balicki D. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res. 2006;312(19):3701–3710. doi: 10.1016/j.yexcr.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 73.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5(7):738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 74.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 76.Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103(6):2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prince M, Sivanandan R, Kaczorowski A, Wolf G, Kaplan M, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Plesa A, Elhamri M, Clapisson G, Mattei E, Gazzo S, Hequet O, et al. Higher percentage of CD34+ CD38− cells detected by multiparameter flow cytometry from leukapheresis products predicts unsustained complete remission in acute myeloid leukemia. Leuk Lymphoma. 2015;56(3):622–629. doi: 10.3109/10428194.2014.927453. [DOI] [PubMed] [Google Scholar]
- 79.Wang B-B, Li Z, Zhang F-F, Hou H-T, Yu J-K, Li F. Clinical significance of stem cell marker CD133 expression in colorectal cancer. Histol Histopathol. 2016;31:299–306. doi: 10.14670/HH-11-676. [DOI] [PubMed] [Google Scholar]
- 80.Liu D, Sun J, Zhu J, Zhou H, Zhang X, Zhang Y. Expression and clinical significance of colorectal cancer stem cell marker EpCAMhigh/CD44+ in colorectal cancer. Oncol Lett. 2014;7(5):1544–1548. doi: 10.3892/ol.2014.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Langan RC, Mullinax JE, Raiji MT, Upham T, Summers T, Stojadinovic A, et al. Colorectal cancer biomarkers and the potential role of cancer stem cells. J Cancer. 2013;4(3):241–250. doi: 10.7150/jca.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thenappan A, Li Y, Shetty K, Johnson L, Reddy E, Mishra L. New therapeutics targeting colon cancer stem cells. Curr Colorectal Cancer Rep. 2009;5(4):209–216. doi: 10.1007/s11888-009-0029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horst D, Kriegl L, Engel J, Kirchner T, Jung A. Prognostic significance of the cancer stem cell markers CD133, CD44, and CD166 in colorectal cancer. Cancer Investig. 2009;27(8):844–850. doi: 10.1080/07357900902744502. [DOI] [PubMed] [Google Scholar]
- 84.Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, et al. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90(12):5013–5021. [PubMed] [Google Scholar]
- 85.Wang C, Xie J, Guo J, Manning HC, Gore JC, Guo N. Evaluation of CD44 and CD133 as cancer stem cell markers for colorectal cancer. Oncol Rep. 2012;28(4):1301–1308. doi: 10.3892/or.2012.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rizzino A. Sox2 and Oct-3/4: a versatile pair of master regulators that orchestrate the self-renewal and pluripotency of embryonic stem cells. Wiley Interdiscip Rev Syst Biol Med. 2009;1(2):228–236. doi: 10.1002/wsbm.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takeda J, Seino S, Bell GI. Human Oct3 gene family: cDNA sequences, alternative splicing, gene organization, chromosomal location, and expression at low levels in adult tissues. Nucleic Acids Res. 1992;20(17):4613–4620. doi: 10.1093/nar/20.17.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113(5):643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 89.Huang Y, Zeng F, Xu L, Zhou J, Liu X, Le H. Anticancer effects of cinnamic acid in lung adenocarcinoma cell line h1299-derived stem-like cells. Oncol Res. 2012;20(11):499–507. doi: 10.3727/096504013X13685487925095. [DOI] [PubMed] [Google Scholar]
- 90.Yu Y, Kanwar SS, Patel BB, Nautiyal J, Sarkar FH, Majumdar AP. Elimination of colon cancer stem-like cells by the combination of curcumin and FOLFOX. Transl Oncol. 2009;2(4):321–328. doi: 10.1593/tlo.09193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nautiyal J, Kanwar SS, Yu Y, Majumdar AP. Combination of dasatinib and curcumin eliminates chemo-resistant colon cancer cells. J Mol Signal. 2011;6(1):7. doi: 10.1186/1750-2187-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patel BB, Gupta D, Elliott AA, Sengupta V, Yu Y, Majumdar AP. Curcumin targets FOLFOX-surviving colon cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Res. 2010;30(2):319–325. [PMC free article] [PubMed] [Google Scholar]
- 93.Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS One. 2013;8(2):e57218. doi: 10.1371/journal.pone.0057218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang D, Kong X, Li Y, Qian W, Ma J, Wang D, et al. Curcumin inhibits bladder cancer stem cells by suppressing sonic hedgehog pathway. Biochem Biophys Res Commun. 2017;493(1):521–527. doi: 10.1016/j.bbrc.2017.08.158. [DOI] [PubMed] [Google Scholar]
- 95.Park S, Sung J, Chung N. Berberine diminishes side population and down-regulates stem cell-associated genes in the pancreatic cancer cell lines PANC-1 and MIA PaCa-2. Mol Cell Biochem. 2014;394(1–2):209–215. doi: 10.1007/s11010-014-2096-1. [DOI] [PubMed] [Google Scholar]
- 96.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci. 1997;94(19):10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shankar S, Nall D, Tang S-N, Meeker D, Passarini J, Sharma J, et al. Resveratrol inhibits pancreatic cancer stem cell characteristics in human and Kras G12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PLoS One. 2011;6(1):e16530. doi: 10.1371/journal.pone.0016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007;12:4839–4854. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- 99.Zhou W, Kallifatidis G, Baumann B, Rausch V, Mattern J, Gladkich J, et al. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int J Oncol. 2010;37(3):551–561. doi: 10.3892/ijo_00000704. [DOI] [PubMed] [Google Scholar]
- 100.Kim B, Jung N, Lee S, Sohng JK, Jung HJ. Apigenin inhibits Cancer stem cell-like phenotypes in human glioblastoma cells via suppression of c-met signaling. Phytother Res. 2016;30(11):1833–1840. doi: 10.1002/ptr.5689. [DOI] [PubMed] [Google Scholar]
- 101.Erdogan S, Doganlar O, Doganlar ZB, Serttas R, Turkekul K, Dibirdik I, et al. The flavonoid apigenin reduces prostate cancer CD44+ stem cell survival and migration through PI3K/Akt/NF-κB signaling. Life Sci. 2016;162:77–86. doi: 10.1016/j.lfs.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 102.Erdogan S, Turkekul K, Serttas R, Erdogan Z. The natural flavonoid apigenin sensitizes human CD44+ prostate cancer stem cells to cisplatin therapy. Biomed Pharmacother. 2017;88:210–217. doi: 10.1016/j.biopha.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 103.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27(6):962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y, Zhang T, Korkaya H, Liu S, Lee H-F, Newman B, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16(9):2580–2590. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kallifatidis G, Rausch V, Baumann B, Apel A, Beckermann BM, Groth A, et al. Sulforaphane targets pancreatic tumour-initiating cells by NF-κB-induced antiapoptotic signalling. Gut. 2009;58(7):949–963. doi: 10.1136/gut.2008.149039. [DOI] [PubMed] [Google Scholar]
- 106.Rausch V, Liu L, Kallifatidis G, Baumann B, Mattern J, Gladkich J, et al. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Res. 2010;70(12):5004–5013. doi: 10.1158/0008-5472.CAN-10-0066. [DOI] [PubMed] [Google Scholar]
- 107.Pistollato F, Giampieri F, Battino M. The use of plant-derived bioactive compounds to target cancer stem cells and modulate tumor microenvironment. Food Chem Toxicol. 2015;75:58–70. doi: 10.1016/j.fct.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 108.Scarpa E-S, Ninfali P. Phytochemicals as innovative therapeutic tools against cancer stem cells. Int J Mol Sci. 2015;16(7):15727–15742. doi: 10.3390/ijms160715727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dandawate P, Padhye S, Ahmad A, Sarkar FH. Novel strategies targeting cancer stem cells through phytochemicals and their analogs. Drug Deliv Transl Res. 2013;3(2):165–182. doi: 10.1007/s13346-012-0079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Torquato FVH, Goettert IM, Justo ZG, Paredes-Gamero JE. Anti-Cancer Phytometabolites targeting Cancer stem cells. Curr Genomics. 2017;18(2):156–174. doi: 10.2174/1389202917666160803162309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taylor WF, Jabbarzadeh E. The use of natural products to target cancer stem cells. Am J Cancer Res. 2017;7(7):1588–1605. [PMC free article] [PubMed] [Google Scholar]