Abstract
Background
There is a global perception that psychotropic utilization in children and adolescents is increasing in the US.
Methods
We present prevalent estimates for all psychotropics prescribed in the US (using commercial claims from Medicare and Medicaid) to children and adolescents in 2004 (total population N = 6,808,453) and in 2014 (total population N = 11,082.260). Further we evaluated if there has been a statistically significant change in prevalence during this time period. Analyses were stratified for the 6 major drug classes, all individuals’ psychotropics (87 drugs), age and sex.
Results
The prevalence of psychotropic drug prescription was 8.55% in 2004 and 9.00% in 2014 (age stratified in 2004 and 2014 toddlers: 3.08 and 2.63%, children: 8.74 and 8.73%, adolescents: 10.89% and 12.11). The prevalence for each drug class in 2004 and 2014 was: stimulants/other ADHD drugs 5.0 and 5.8%; antidepressants 2.8 and 2.7%; anxiolytic-hypnotic-sedative 2.2 and 2.3%; mood stabilizers 0.1 and 0.1%; antipsychotics 1.3 and 1.1%; and for drugs treating drug dependence 0.02 and 0.02%.
Conclusions
The perception that psychotropic utilization in children and adolescents is increasing in the US, derived from the 2 to 3 fold increase seen from the mid 80’s to the mid 90’s is not valid anymore. There has been a slowdown in the increase of prescribing psychotropics. In the last 10 years, in toddlers there was a decrease in the prescription; in children there was no change; and in adolescents there was a slight increase. The prescription of antidepressants, antipsychotics and mood stabilizers has decreased overall.
Graphical abstract.
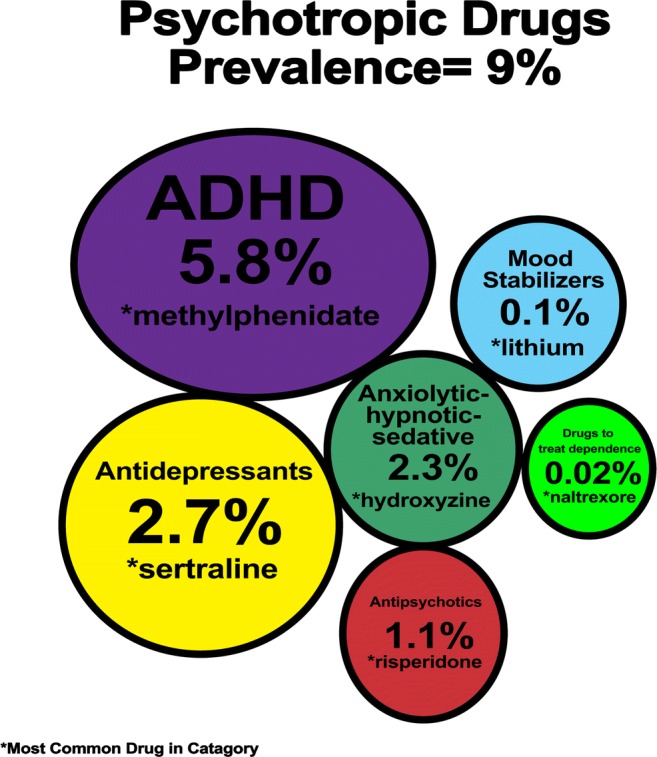
In the last 10 years there has been a slowdown in the increase of prescribing psychotropics. In toddlers there was a decrease in the prescription (3.08 and 2.63%); in children (8.74 and 8.73%) there was no change; and in adolescents there was a slight increase (10.89% and 12.11). The prescription of antidepressants, antipsychotics and mood stabilizers has decreased overall.
Electronic supplementary material
The online version of this article (10.1007/s40199-018-0204-6) contains supplementary material, which is available to authorized users.
Keywords: Psychotropic drugs, Antidepressive agents, Tranquilizing agents, Antipsychotic agents, Hypnotics and sedatives
Introduction
During the last decades, studies have reported an increase in the use of psychotropics in the United States (US) [1, 2]. The increase has been attributed to a surge in the number of children diagnosed with Attention Deficit Hyperactivity Disorder (ADHD) and autism [2, 3], and to the expanded labeling of several psychotropics to include children [4]. In addition, changes have been made in child and adolescent mental health care, due to clinical and regulatory changes in the US; multiple initiatives such as authorization requirement prior to the prescription of psychotropics; [5] and to the approval of new products and indications. Studies assessing the utilization of psychotropics in the US have focused on psychotropic use in general or by dividing them into different classes: stimulants/other drugs to treat ADHD, anxiolytics-hypnotics-sedatives, antidepressants, antipsychotics, mood stabilizers and drugs to treat drug dependence.
Currently, less information is known about other types of psychotropic use such as benzodiazepines and non-benzodiazepine sedatives and anxiolytics, and drugs to treat alcohol, nicotine or opioid dependence in children and adolescents in the US. To our knowledge, only one study has been published describing the utilization of anxiolytics in children, showing that between 1995 and 2010, the utilization of anxiolytics for children had remained the same while in adolescents they had increased [6].
Various studies assessing the utilization of psychotropics in the US have focused on psychotropic use in general or by drug class. However, less attention has been given to single medications. The objective of this study is to estimate the year prevalence of (i) total use of psychotropics, (ii) each of the 6 major drug classes and (iii) all individual psychotropic drugs prescribed in the US to children and adolescents in the years 2004 and 2014 and to evaluate if there has been a statistically significant change in prevalence during this time period.
Methods
Source of data
Data for the analyses were obtained from the MarketScan Commercial Claims and Medicare database and the MarketScan Medicaid database [7]. These are medical claims databases from the USA, maintained by Truven Health Analytics. These databases contain de-identified, person-specific health care data, including clinical utilization, expenditures, insurance enrollment, plan benefit, and outpatient prescribing information.
Study samples
The analytic sample included all individuals 2 to 18 years of age. Two cohorts were created, one which included patients enrolled for at least 12 months between 01 January 2004 and 31 December 2004 (N = 6,808,453) and one between 01 January 2014 and 31 December 2014 (N = 11,082,260). From the study population defined above, all individuals prescribed one or more psychotropic drugs at least once during the calendar year were identified (2004 N = 582,232; 2014 N = 997,594).
Exposure definition
MarketScan utilizes the Systematized Nomenclature of Medicine (SNOMED) to classify drugs. This system is based on a multiaxial, hierarchical, classification system. The level that selects all generic and commercial drugs and all formulations was selected.
Our analyses focused on studying psychotropic drugs as a whole, into 6 different drug classes and for the individual drugs. Patients were included in the psychotropic drug use category if they were prescribed with one or more individual psychotropic drugs during the calendar year. Further, individuals were allocated into six different drug classes (1.stimulants/other drugs to treat ADHD, 2. anxiolytics-hypnotics-sedatives 3. antidepressants, 4.antipsychotics, 5.mood stabilizers and 6.drugs to treat drug dependence) according to their prescribing information. If the individual was prescribed with different psychotropics, they contributed to several groups. Age groups were divided in three: preschool (age 2 to 4), children (age 5–12) and adolescents (age 13–18).
Statistical analysis
For ease of interpretation, year prevalence was presented and referred to as percentage. For each study year, percentage was calculated based on 3 separate levels i) Psychotropic Medication: calculated as the number of persons prescribed with one or more of the 87 drugs (numerator) divided by the total number of children in the database (denominator) multiplied by 100. ii) For each of the 6 drug classes, calculated as the number of persons in each drug class (numerator) divided by the total number of children in the database (denominator) multiplied by 100. iii) Finally for each of the 87 individual drugs: calculated as the number of persons prescribed with an individual drug (numerator) divided by the total number of children in the database (denominator) multiplied by 100. For the i and ii categories, the prevalence was further stratified by sex and by age. Confidence intervals for all estimated percentages were constructed using the exact Clopper-Pearson method [8]. Confidence intervals for the ratios of percentages were constructed using the standard asymptotic interval estimator [9].
Data availability
The data that support the findings of this study are available from Truven Health Analytics but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Truven Health Analytics.
Results
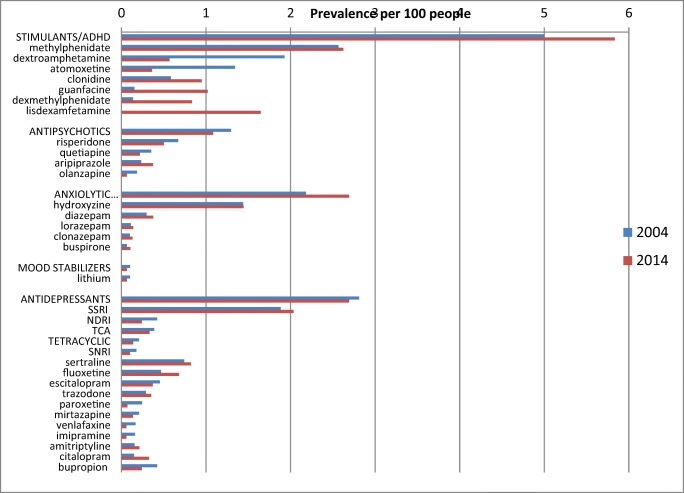
The percentage of children and adolescents who received psychotropic drugs increased slightly from 8.55% (95% CI 8.53–8.57) in 2004 to 9.00% (95% CI 8.98–9.02) in 2014 (Ratio 1.05, 95% CI 1.05–1.06 p-value 0.000). The percentage in each drug class in 2004 and in 2014 respectively was: stimulants/other drugs to treat ADHD 5.00 and 5.83% (p-value 0.000); antidepressants 2.81 and 2.69% (p-value 0.000); anxiolytic-hypnotic-sedative 2.18 and 2.26% (p-value 0.000); mood stabilizers 0.10 and 0.06% (p-value 0.000); antipsychotics 1.29 and 1.08% (p-value 0.000); and for the drug class to treat drug dependence 0.02 and 0.02% (p-value 0.000). (Fig. 1 and Supplement 1).
Fig. 1.
Percentage of children and adolescents prescribed with a psychotropic
In both time periods, the most frequently utilized drugs for the different classes was methylphenidate (stimulant/ADHD), risperidone (antipsychotic), hydroxyzine (sedative), sertraline (antidepressant), lithium (mood disorders) and naltrexone (drug dependence) (Fig. 2).
Fig. 2.
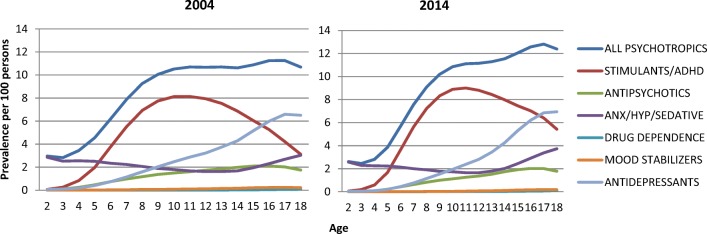
Prevalence of psychotropic drug class by age
In relation to age, prescriptions decreased for preschool children (3.08 in 2004, 2.63 in 2014) and children 5 to 12 years (8.74 in 2004, 8.73 in 2014), but slightly increased for adolescents (10.89 in 2004, 12.11 in 2014) (supplement Table 1). The most prescribed drug class in preschool children (age 2–4) was anxiolytics-hypnotics-sedatives (driven by hydroxyzine); for the ages 6 to 16, stimulants and drugs to treat ADHD; and for ages 17 and 18, antidepressants. In both 2004 and 2014, there were more males prescribed with an antipsychotic. When stratifying by sex, males were prescribed more often with stimulants/other drugs to treat ADHD, antipsychotics and mood stabilizers than females. Females were prescribed more often than males with anxiolytics-hypnotics-sedatives, antidepressants, and with drugs to treat drug dependence (Fig. 3).
Fig. 3.
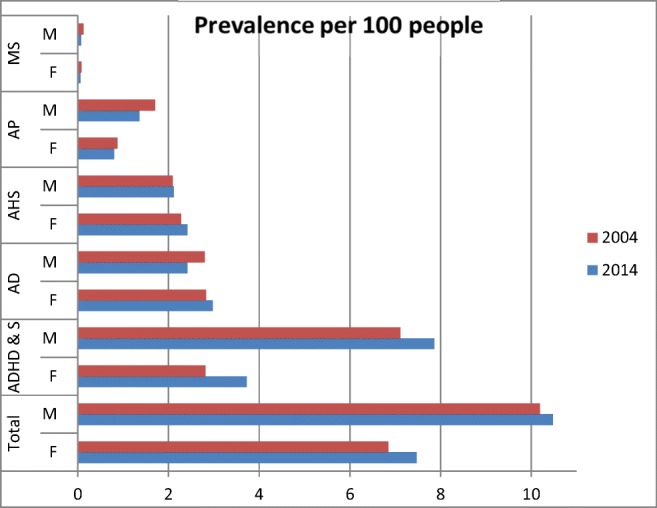
Prevalence of Psychotropic drug class by sex. ADHD & S = attention deficit hyperactivity disorder and stimulants, AHS = anxiolytics hypnotics or sedatives, AD = antidepressants, AP = antipsychotics, MS = mood stabilizers Drug Dependence less than 0.1 not included in figure
For the stimulants/drug to treat ADHD drug class, methylphenidate was the most prescribed in both 2004 and 2014 (2.57%, 2.62%). Lisdexamfetamine was not used in 2004, and in 2014 it became the second most prescribed in this drug class (0%, 1.65%). The prescribing of dextroamphetamine (1.93%, 0.57%) and atomoxetine (1.34%, 0.37%) decreased by more than half; while it increased for clonidine (0.58%, 0.95%), guanfacine (0.15%, 1.02%) and dexmethylphenidate (0.14%, 0.84%). For antipsychotics, the only drug that had an increase was aripiprazole (0.23%, 0.37%). The prescribing of risperidone (0.67%, 0.50%), quetiapine (0.35, 0.22%) and olanzapine (0.18%, 0.06%) decreased. In the anxiolytic, hypnotic and sedatives group, the estimate was driven by hydroxyzine (1.44%, 1.44%). The prescribing of diazepam (0.30%, 0.38%), lorazepam (0.11%, 0.14%), clonazepam (0.10%, 0.13%) and buspirone (0.07%, 0.11%) also increased. In relation to mood stabilizers, the prescribing of lithium decreased (0.10%, 0.06%). In relation to antidepressants, SSRIs (1.88%, 2.03%) were the most frequently prescribed. Of the SSRIs sertraline (0.74%, 0.82%), followed by fluoxetine (0.47%, 0.68%) were the most prescribed.. There was a decrease in the prescribing of tricyclics (TCAs) (0.39%, 0.33%) and tetracyclics (0.21%, 0.14%) antidepressants. The prescribing of amitriptyline increased (0.16%, 0.21%), whereas it decreased for all the other TCAs and tetracyclics. The prescribing of drugs to treat dependence was very low, the most prescribed was naltrexone (0.01, 0.01).
Discussion
The results of this study showed that in a 10 year period the overall use of psychotropic increased slightly (from 8.55 to 9.00%). The small increase was driven by the adolescent group aged 13 to 18. Prescribing decreased for pre-school children age 2–4 and remained the same for children ages 5 to 12. The prescribing increased for stimulants/other drugs to treat ADHD, and anxiolytics-hypnotics-sedatives. On the contrary, the prescribing of antidepressants, antipsychotics and mood stabilizers decreased. These results are in line with previous studies [1, 10–14], which show that the prescription of psychotropics is departing from the 2 to 3 fold increase seen in the mid 80’s to the beginning of the twenty-first century [15–17].
Several events in the last decade have impacted the pattern of utilization of psychotropics in the treatment of ADHD. One was the 2011 update of the Clinical Guidelines on the diagnosis and treatment of ADHD in children of the American Academy of Pediatrics, which now includes guideline for ages 4 to 18 (instead of ages 6 to 12) [18]. Another was the Food and Drug Administration’s (FDA) approval of new treatment options for ADHD such as lisdexamfetamine and non-stimulant drugs. And finally, several communications issued by FDA between 2005 and 2007 regarding cardiovascular safety and risk of suicidal ideation [19]. Concerning these safety topics, in 2009 the Committee for Medicinal Products for Human use concluded that overall the benefit of these medications outweigh the risks [20].
In relation to antidepressants, between 2003 and 2006 the FDA issued a Public Health Advisory about the risk of suicidality in patients taking antidepressants, proposed a boxed warning, and revised the product labeling and medication guide. These measures were controversial and concerns were expressed that these actions may have resulted in excessive declines in antidepressant prescribing, placing depressed youth at increased risks and increasing suicide attempts [21, 22]. Utilization studies of antidepressants performed at a later date showed that the use of antidepressants declined or stayed the same [1, 23], however; the changes to the individual antidepressants are not known.
Concerning antipsychotics, although use increased during the 90’s and early 2000s, there has been a reduction in their utilization among children and adolescents [10, 14]. The decline is most likely due to increasing awareness of the metabolic effects of second-generation antipsychotics [24–26], and to the campaign lead by the American Psychiatric Association targeting the overuse of antipsychotic medication, urging physicians to not routinely prescribe antipsychotic medications to treat behavioral disorders in children [27]. In addition, the pattern of utilization of psychotropics in general might have been affected given that in the last decade the FDA approved the use of atypical antipsychotics in pediatric patients for irritability associated with autism, schizophrenia and bipolar disorder [4].
Several strengths and limitations of this study should be noted. The major strength was using a database which included approximately 11 million in 2014 and 7 million in 2004. The large number of individuals included provided the necessary precision and power to evaluate less commonly prescribed classes of psychotropic medications. To our knowledge, this study provides the first estimates of the annual prevalence of each individual psychotropic prescribing in the United States.
Concerning the limitations, first, the fact that a drug prescription was filled does not necessary mean that the child consumed the drug. Second, if the patient payed out of pocket or bought the medication over the counter it is not included in the database. Third, these data are aggregate across the country and do not describe regional or provider variation. Fourth, the data relies on a claims database which lacks chart data, therefore it is possible that some medications were used to treat non-psychiatric conditions such as pain relief (sedatives/hypnotics), allergies (antihistamines), or to help relax before and after surgery (sedatives). Even though in some cases the drug prescription did not imply that the child had a diagnosed psychiatric disorder, these drugs are psychotropics and have effects on psychological functions. The other limitation of this study was comparing only two years (2004 & 2014) rather than one-year time intervals between 2004 and 2014.
Conclusion
The global perception that psychotropic utilization in youth is increasing in the US is not valid anymore. In the last 10 years there has been a slowdown in the increase of prescribing psychotropics. In toddlers there was a decrease in the prescription (3.08 and 2.63%); in children (8.74 and 8.73%) there was no change; and in adolescents there was a slight increase (10.89 and 12.11%). The prescription of antidepressants, antipsychotics and mood stabilizers has decreased overall. These changes are likely a response to the risk minimization measures which include FDA communications, box warnings, guidelines, initiatives and campaigns issued in the last 10 years addressing the concerns that children and adolescents are being over treated with medication, especially in the US.
Electronic supplementary material
(DOCX 89 kb)
Acknowledgements
The authors would like to thank Emil Scosyrev, Ph.D. for quality control of the statistical analyses.
SLL and BW are employees of Novartis Pharmaceuticals Corporation.
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- FDA
food and drug administration’s
- SNOMED
systematized nomenclature of medicine
- US
United States
Authors’ contributions
Analyzed and interpreted the data. SL- analyzed and interpreted the data, study design, analyses, analyzed data, wrote manuscript. MIL study design, interpret data and contributor in writing the manuscript. BW- interpretation of data, contributor in writing manuscript. LRL study design, writing of sections of the manuscript, all figures of the study. All authors read and approved the final manuscript.
Funding
SLL, BW and ES are employees of Novartis Pharmaceuticals Corporation.
Ethics approval and consent to participate
An ethics committee approved the data collected. We received unidentified data and all data is presented in aggregated form.
Consent for publication
All authors give consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Olfson M, Druss BG, Marcus SC. Trends in mental health care among children and adolescents. N Engl J Med. 2015;373:1079. doi: 10.1056/NEJMc1504261. [DOI] [PubMed] [Google Scholar]
- 2.Garfield CF, Dorsey ER, Zhu S, Huskamp HA, Conti R, Dusetzina SB, Higashi A, Perrin JM, Kornfield R, Alexander GC. Trends in attention deficit hyperactivity disorder ambulatory diagnosis and medical treatment in the United States, 2000-2010. Acad Pediatr. 2012;12:110–116. doi: 10.1016/j.acap.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg RE, Daniels AM, Law JK, Law PA, Kaufmann WE. Trends in autism spectrum disorder diagnoses: 1994-2007. J Autism Dev Disord. 2009;39:1099–1111. doi: 10.1007/s10803-009-0723-6. [DOI] [PubMed] [Google Scholar]
- 4.Lorberg B, Robb A, Pavuluri M, Chen DT, Wilens T. Pediatric psychopharmacology: food and drug administration approval through the evidence lens. J Am Acad Child Adolesc Psychiatry. 2014;53:716–719. doi: 10.1016/j.jaac.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Schmid I, Burcu M, Zito JM. Medicaid prior authorization policies for pediatric use of antipsychotic medications. JAMA. 2015;313:966–968. doi: 10.1001/jama.2015.0763. [DOI] [PubMed] [Google Scholar]
- 6.Olfson M, Blanco C, Wang S, Laje G, Correll CU. National trends in the mental health care of children, adolescents, and adults by office-based physicians. JAMA Psychiat. 2014;71:81–90. doi: 10.1001/jamapsychiatry.2013.3074. [DOI] [PubMed] [Google Scholar]
- 7.Hansen L, Chang S. Health research data for the real world: the Marketscan databases. 1-12-0017. Ref Type: Internet Communication.
- 8.Desu MMRD. Nonparametric statistical methods for complete and censored data. 2004. [Google Scholar]
- 9.Rothman KJ, Greenland S, Lash TL: Applications of stratified analysis methods. In Modern Epidemiology. 3rd edition. Edited by Lippincott W&W. Philadelphia, PA; 2008.
- 10.Constantine R, Tandon R. Changing trends in pediatric antipsychotic use in Florida's Medicaid program. Psychiatr Serv. 2008;59:1162–1168. doi: 10.1176/ps.2008.59.10.1162. [DOI] [PubMed] [Google Scholar]
- 11.Hartz I, Skurtveit S, Steffenak AK, Karlstad O, Handal M. Psychotropic drug use among 0-17 year olds during 2004-2014: a nationwide prescription database study. BMC Psychiatry. 2016;16:12. doi: 10.1186/s12888-016-0716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohr WD, Chowning RT, Stevenson MD, Williams PG. Trends in atypical antipsychotics prescribed to children six years of age or less on Medicaid in Kentucky. J Child Adolesc Psychopharmacol. 2015;25:440–443. doi: 10.1089/cap.2014.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mojtabai R, Olfson M, Han B. National Trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics. 2016;138:e20161878. doi: 10.1542/peds.2016-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olfson M, King M, Schoenbaum M. Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry. 2015;72:867–874. doi: 10.1001/jamapsychiatry.2015.0500. [DOI] [PubMed] [Google Scholar]
- 15.Cooper WO, Arbogast PG, Ding H, Hickson GB, Fuchs DC, Ray WA. Trends in prescribing of antipsychotic medications for US children. Ambul Pediatr. 2006;6:79–83. doi: 10.1016/j.ambp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Safer DJ. Changing patterns of psychotropic medications prescribed by child psychiatrists in the 1990s. J Child Adolesc Psychopharmacol. 1997;7:267–274. doi: 10.1089/cap.1997.7.267. [DOI] [PubMed] [Google Scholar]
- 17.Zito JM, Safer DJ, dosReis S, Gardner JF, Magder L, Soeken K, Boles M, Lynch F, Riddle MA. Psychotropic practice patterns for youth: a 10-year perspective. Arch Pediatr Adolesc Med. 2003;157:17–25. doi: 10.1001/archpedi.157.1.17. [DOI] [PubMed] [Google Scholar]
- 18.Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007–1022. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornfield R, Watson S, Higashi AS, Conti RM, Dusetzina SB, Garfield CF, Dorsey ER, Huskamp HA, Alexander GC. Effects of FDA advisories on the pharmacologic treatment of ADHD, 2004-2008. Psychiatr Serv. 2013;64:339–346. doi: 10.1176/appi.ps.201200147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Union. Referrals document: Questions and answers on the review of medicines containing methylphenidate. EMA. 2009. (Accessed January, 6, 2017 at http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Methylphenidate_31/WC500011125.pdf)
- 21.Lineberry TW, Bostwick JM, Beebe TJ, Decker PA. Impact of the FDA black box warning on physician antidepressant prescribing and practice patterns: opening Pandora's suicide box. Mayo Clin Proc. 2007;82:518–520. doi: 10.4065/82.4.518. [DOI] [PubMed] [Google Scholar]
- 22.Friedman RA. Antidepressants' black-box warning--10 years later. N Engl J Med. 2014;371:1666–1668. doi: 10.1056/NEJMp1408480. [DOI] [PubMed] [Google Scholar]
- 23.Lu CY, Zhang F, Lakoma MD, Madden JM, Rusinak D, Penfold RB, Simon G, Ahmedani BK, Clarke G, Hunkeler EM, Waitzfelder B, Owen-Smith A, Raebel MA, Rossom R, Coleman KJ, Copeland LA, Soumerai SB. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. doi: 10.1136/bmj.g3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Consensus development conference on antipsychotic drugs and obesity and diabetes Diabetes Care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 25.Tarricone I, Ferrari GB, Serretti A, Grieco D, Berardi D. Weight gain in antipsychotic-naive patients: a review and meta-analysis. Psychol Med. 2010;40:187–200. doi: 10.1017/S0033291709990407. [DOI] [PubMed] [Google Scholar]
- 26.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuehn BM. APA targets unnecessary antipsychotic use. JAMA. 2013;310:1909–1910. doi: 10.1001/jama.2013.281140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 89 kb)
Data Availability Statement
The data that support the findings of this study are available from Truven Health Analytics but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Truven Health Analytics.