SUMMARY
Conditioned place preference (CPP) is a widely used model of addiction-related behavior whose underlying mechanisms are not understood. In this study, we used dual site silicon probe recordings in freely moving mice to examine interactions between the hippocampus and nucleus accumbens in cocaine CPP. We found that CPP was associated with recruitment of D2-positive nucleus accumbens medium spiny neurons to fire in the cocaine-paired location, and this recruitment was driven predominantly by selective strengthening of coupling with hippocampal place cells that encode the cocaine-paired location. These findings provide in vivo evidence suggesting that the synaptic potentiation in the accumbens caused by repeated cocaine administration preferentially affects inputs that were active at the time of drug exposure. This provides a potential physiological mechanism by which drug use becomes associated with specific environmental contexts.
INTRODUCTION
A key insight into addiction is that drug use becomes associated with the environmental context in which the drug was administered, and re-exposure to the associated context leads to cravings or drug-seeking behavior. A simple model of this association is cocaine conditioned place preference (CPP), in which cocaine is repeatedly paired with a specific spatial location causing the animal to spend more time in that location during subsequent exploration. Despite its simplicity and relevance, the neural mechanisms of cocaine CPP are still not understood.
The hippocampus (HPC) is a brain structure essential for spatial navigation, and it contains “place cells” that fire selectively in specific spatial locations (Kim et al., 2012; O’Keefe, 1976) or contexts (Komorowski et al., 2013). The CA1 and subiculum regions of the HPC send a strong projection to the nucleus accumbens (NAc) (Phillipson and Griffiths, 1985), and simultaneous HPC-NAc recordings in rats suggest that these HPC inputs carry spatial information to the NAc (Lansink et al., 2008; Lansink et al., 2009; Tabuchi et al., 2000; van der Meer and Redish, 2011a). Anatomical disconnection experiments indicate that HPC-NAc interactions are necessary for CPP (Ito et al., 2008), and NAc MSNs increase their firing rate near reward sites (Lansink et al., 2008; Lavoie and Mizumori, 1994; Miyazaki et al., 1998; van der Meer et al., 2010), possibly representing the readout of a stored location-reward association. Cocaine conditioning potentiates HPC synapses onto NAc MSNs, as tested in ex vivo slices (Britt et al., 2012; MacAskill et al., 2014; Pascoli et al., 2014), providing a potential substrate for storing this association. However, the neuronal mechanisms are unclear.
One possibility is that cocaine does not potentiate all HPC synapses in the NAc uniformly, as is often tacitly assumed. Plasticity at corticostriatal synapses generally requires presynaptic activity (Calabresi et al., 1999), raising the possibility that cocaine may selectively strengthen the synapses that were most active at the time of drug exposure. We thus hypothesized that cocaine would preferentially strengthen NAc coupling with HPC place cells that encode the cocaine-paired location. To test this hypothesis, we performed simultaneous dual site silicon probe recordings in the HPC and NAc of mice in a cocaine CPP paradigm. Our findings indicate that cocaine conditioning recruits location-specific MSN firing and suggest that this activity is driven predominantly by preferential strengthening of hippocampal inputs that encode the cocaine-paired location.
RESULTS
Dual site silicon probe recording during cocaine conditioned place preference
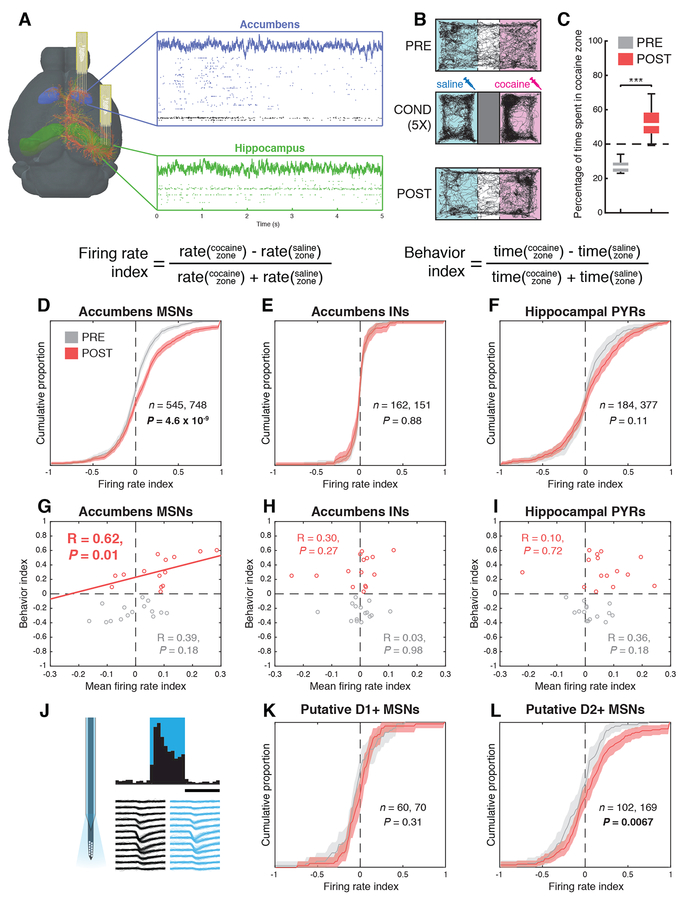
We measured interactions between posterior CA1 and the NAc in hippocampus-dependent cocaine CPP (Ito et al., 2008; Meyers et al., 2003) (Fig. S2A) using simultaneous silicon probe recordings in both structures (n = 10 mice) (Fig. 1A). We used established criteria to identify putative pyramidal cells (PYRs) (Bartho et al., 2004; McCormick et al., 1985) in the hippocampus (n = 561 PYRs) and medium spiny neurons (MSNs) and interneurons (INs) in the NAc (n = 1293 MSNs, 313 INs; Fig. S1) (Schmitzer-Torbert and Redish, 2008; Yamin et al., 2013). Recordings were performed in drug-free sessions either before (PRE) and after (POST) animals underwent cocaine place conditioning in a rectangular arena with a removable barrier (Fig. 1B, Fig. S2A). This conditioning paradigm induced CPP in POST sessions (PRE = 27% in cocaine zone, POST = 51%, rank sum test, n = 12 PRE, 15 POST sessions, P = 1.6 × 10−6; Fig. 1C, Table S1), which was attributable to an increase in the proportion of time spent immobile in the cocaine zone (Fig. S2).
Figure 1: Cocaine place conditioning increases activity of D2-positive MSNs in the cocaine zone.
A) Silicon probes were implanted in the hippocampus (green) and accumbens (blue), yielding LFP and single unit recordings (n = 1606 accumbens neurons, 725 hippocampal neurons). B) Mice (n = 10) were conditioned for five days with saline and cocaine 15 mg/kg IP. C) In POST sessions, animals exhibited a preference for the cocaine-paired zone (***P < 10−5, rank sum test). D) Accumbens MSNs fire preferentially in the cocaine zone after cocaine conditioning (t-test, P = 4.6 × 10−9). Shaded area represents 99% confidence intervals. Accumbens putative interneurons (INs, E) and hippocampal pyramidal cells (PYRs, F) exhibit no shift after cocaine conditioning. G) Strength of behavioral CPP expression in each recording session is correlated with firing rate index in MSNs in POST (R = 0.62, P = 0.01, permutation test) but not PRE (P = 0.18) conditions. Behavioral CPP expression is not correlated with firing rate index in INs (H) or hippocampal PYRs (I). J) Tapered optical fibers attached to the silicon probe enabled optogenetic tagging of putative D1+ and D2+ cells (scale bar = 50 ms). K) Putative D1+ MSNs showed no additional firing in the cocaine zone (N = 60, 70; t-test, P = 0.31), but putative D2+ MSNs (L) fired preferentially in the cocaine zone after conditioning (N = 102, 169 units; t-test, P = 0.0067).
Cocaine place conditioning recruits D2-positive MSNs
In POST sessions, NAc MSNs fire more when the animal is in the cocaine zone than the saline zone (firing rate index = −0.009 ± 0.011 PRE, 0.094 ± 0.013 POST, n = 545, 748 MSNs, t-test, P = 4.6 × 10−9; Fig. 1D). In contrast, firing rates were not different for NAc INs (n = 162, 151 INs, t-test, P = 0.88, Fig. 1E) or hippocampal PYRs (n = 184, 377 PYRs, P = 0.11, Fig. 1F). However, the density of PYR place fields was higher in the cocaine zone in POST sessions (P = 2.6 × 10−8, Fig. S4A). Further, we found that strength of behavioral CPP expression in a given POST session is correlated with the extent of increased MSN firing in the cocaine zone during that session (n = 15 POST sessions, R = 0.62, P = 0.01, Fig. 1G). This correlation was observed only in POST sessions and was not observed in NAc INs (R = 0.3, P = 0.27; Fig. 1H) or PYRs (R = 0.1, P = 0.72; Fig. 1I). Using established optotagging techniques (Stark et al., 2012), we found that D2-positive MSNs were selectively recruited to the cocaine zone (Fig. 1J-L).
MSNs entrained by hippocampal theta encode more spatial information and show greater recruitment by cocaine conditioning
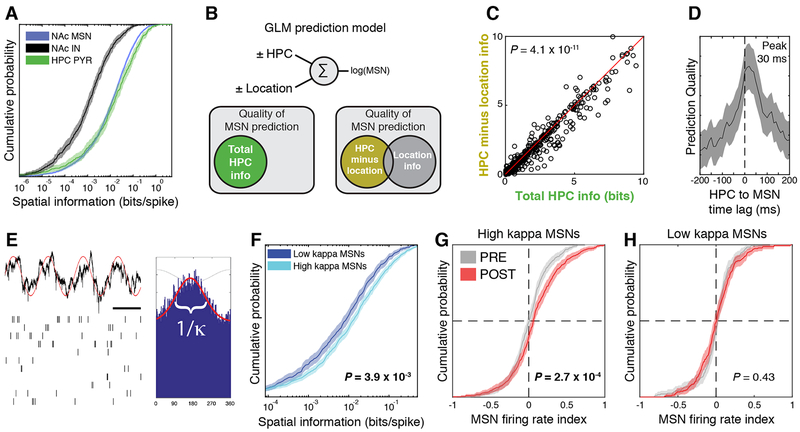
Because MSN firing is strongly modulated by running speed (not shown), and CPP results in correlations between location and running speed, (Fig. S2), we used generalized linear models (GLMs) with model cross-validation to separate the different sources of variance in our observations. We found that the best model fit included separate terms for location and speed modulation (Table S2). This enabled us to dissociate speed and location and determine that MSNs exhibit running speed modulation that is independent of location and stronger than PYRs (n = 1293 MSNs, 561 PYRs, rank sum test, P = 3.9 × 10−18) or NAc INs (Fig. S3A, n = 561 PYRs, 313 INs, rank sum test, P = 3.0 × 10−5). After speed correction, MSNs encoded approximately as much spatial information (Skaggs et al., 1993) as hippocampal PYRs (Fig. 2A, n = 1293 MSNs, 561 PYRs, rank sum test, P = 0.20) and significantly more than INs (n = 1293 MSNs, 313 INs, P = 1.1 × 10−38). To test whether MSNs received spatial information from the hippocampus, we used GLMs to predict MSN activity from combinations of location and spiking activity of hippocampal neurons (Fig. 2B). We found that the correlated component of HPC and MSN activity carries spatial information (see Methods, Fig. 2C, n = 1203 MSNs, signed rank test, P = 4.1 × 10−11). Prediction quality was highest when HPC led NAc by a time lag of ~30 ms (Fig. 2D), suggesting directional information transfer from HPC to NAc. A similar analysis found that MSNs also decode information about running speed independent of location (Fig. S3B-D). To test whether MSNs strongly modulated by HPC have different firing properties, we quantified phase locking of MSN firing to the HPC theta rhythm (Jones and Wilson, 2005; Lansink et al., 2009; Tabuchi et al., 2000; van der Meer and Redish, 2011a), using the parameter kappa of the Von Mises distribution (see Methods; Fig. 2E). We found that high kappa MSNs (strong phase locking) encode more spatial information (Fig. 2F; 0.014 ± 0.005 vs. 0.008 ± 0.003 bits/spike, rank sum test, P = 3.9 × 10−3) and exhibit larger recruitment to the cocaine zone than low kappa MSNs (Fig. 2G-H; high kappa: PRE 0.0082 ± 0.013, POST 0.087 ± 0.017, n = 261, 351 MSNs; t-test, P = 2.7 × 10−4; low kappa: PRE 0.0027 ± 0.016, POST 0.014 ± 0.016, n = 261, 351 MSNs; t-test, P = 0.43).
Figure 2: MSNs receive spatial information from hippocampus, and MSNs phase-locked to the hippocampal theta oscillation encode more spatial information and show greater recruitment by cocaine conditioning.
A) After correction for running speed, MSN and PYR activity carry similar amounts of spatial information (P = 0.20 for MSN vs. PYR), but both contain more spatial information than IN activity (rank sum test, P = 1.0 × 10−38). B) GLMs enable prediction of individual MSN spike trains from the combination of PYR activity and location as predictors. C) Adding hippocampal activity as a predictor to a model already containing explicit location information results in a smaller improvement in prediction quality (signed rank test, P = 4.1 × 10−11) than for a model without explicit location information (note that most points are right of the diagonal). This suggests that predicting MSN spike trains from PYR inputs implicitly decodes information about spatial location. D) MSN spike train prediction quality is highest when PYR activity leads MSN activity by ~30 ms, supporting the hypothesis that information is transferred from PYRs to MSNs. E) The parameter K (kappa) quantifies phase locking of MSN spikes (bottom) to the hippocampal theta rhythm (top). Scale bar = 100 ms. F) High kappa MSNs (stronger phase locking) carry more spatial information than low kappa MSNs (rank sum test, P = 3.9 × 10−3). G) High kappa MSNs exhibit increased activity in the cocaine zone after conditioning (t-test, P = 2.7 × 10−4), but low kappa MSNS (H) do not (t-test, P = 0.43). All shaded areas represent 99% confidence intervals.
Assembly prediction analysis reveals increased hippocampus-accumbens coupling after cocaine conditioning
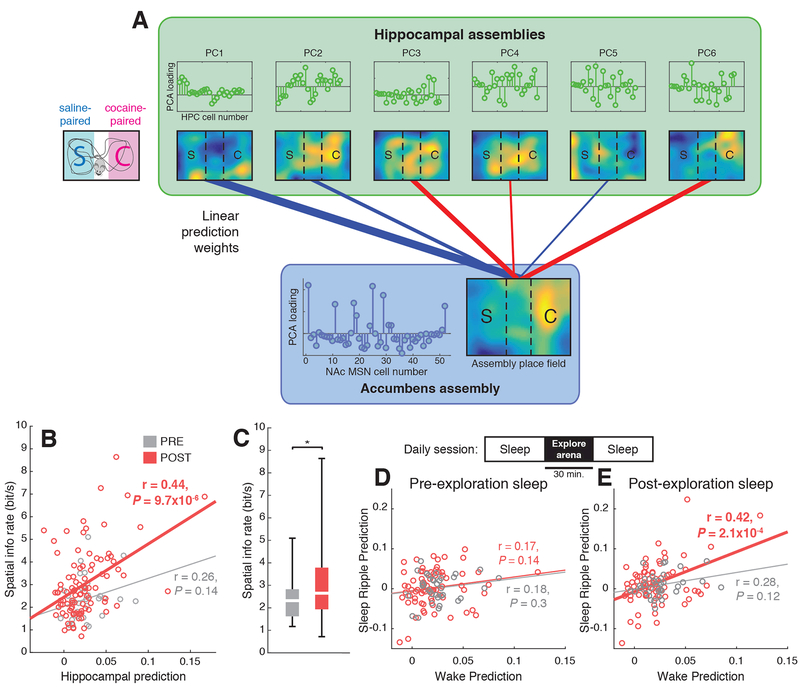
We next addressed the question of whether cocaine conditioning strengthens coupling between hippocampus and accumbens. We performed an assembly prediction analysis using PYR assemblies to predict the activity of a single MSN assembly (Fig. 3A, Methods). Consistent with our hypothesis that MSNs decode spatial information from hippocampal inputs, we found that the quality of the assembly prediction was correlated with the rate of spatial information encoded by the MSN assembly in POST, but not PRE, sessions (Fig. 3B). The overall spatial information rate was also higher in POST sessions (Fig. 3C, n = 34, 95 assemblies, rank sum test, P < 0.05), suggesting that cocaine conditioning strengthens hippocampus-accumbens coupling.
Figure 3: Cocaine conditioning increases the strength of functional coupling between hippocampal PYRs and accumbens MSNs.
A) Activity of PYR assemblies predicts activity of MSN assemblies. B) The spatial information rate of an MSN assembly is correlated with the accuracy by which its activity can be predicted from PYR assemblies, in POST (R = 0.44, P = 9.7 × 10−6), but not PRE (R = 0.26, P = 0.14) sessions. C) Spatial information rate of MSN assemblies increases after cocaine conditioning (rank sum test, *P = 0.02). D) Model predictions for MSN assemblies during awake locomotion are uncorrelated with model predictions during sharp wave ripples in pre-exploration sleep. E) Model predictions during awake locomotion are correlated with predictions during sharp wave ripples in post-exploration sleep in POST (P = 2.1 × 10−4), but not PRE (P = 0.12), sessions. This suggests that cocaine conditioning strengthens coordinated hippocampus-accumbens replay.
To ensure that our assembly prediction findings were not due to environmental cues or common inputs driving the HPC and NAc, we performed an analysis of sleep replay events. Replay events, which occur during HPC sharp-wave ripple oscillations, consist of temporally compressed firing of place cells sequences that encode recently visited spatial locations (Lee and Wilson, 2002; Nadasdy et al., 1999; Skaggs and McNaughton, 1996) and are generated locally in the hippocampus (Buzsaki et al., 1983). We used data from awake exploration of the CPP arena to fit the prediction weights of the GLM, then tested model predictions both on awake data withheld from the training set and on data collected during sleep ripples occurring before and after the animal explored the CPP arena. We found that during pre-exploration sleep, when replay events encoding locations in the CPP arena do not occur, the sleep ripple prediction quality was uncorrelated with wake prediction quality (Fig. 3D). In contrast, during post-exploration sleep the sleep ripple prediction quality was significantly correlated with wake prediction quality in POST sessions (Fig. 3E), indicating strengthened hippocampus-NAc coupling.
Location-specific strengthening of hippocampal coupling underlies MSN recruitment to the cocaine zone
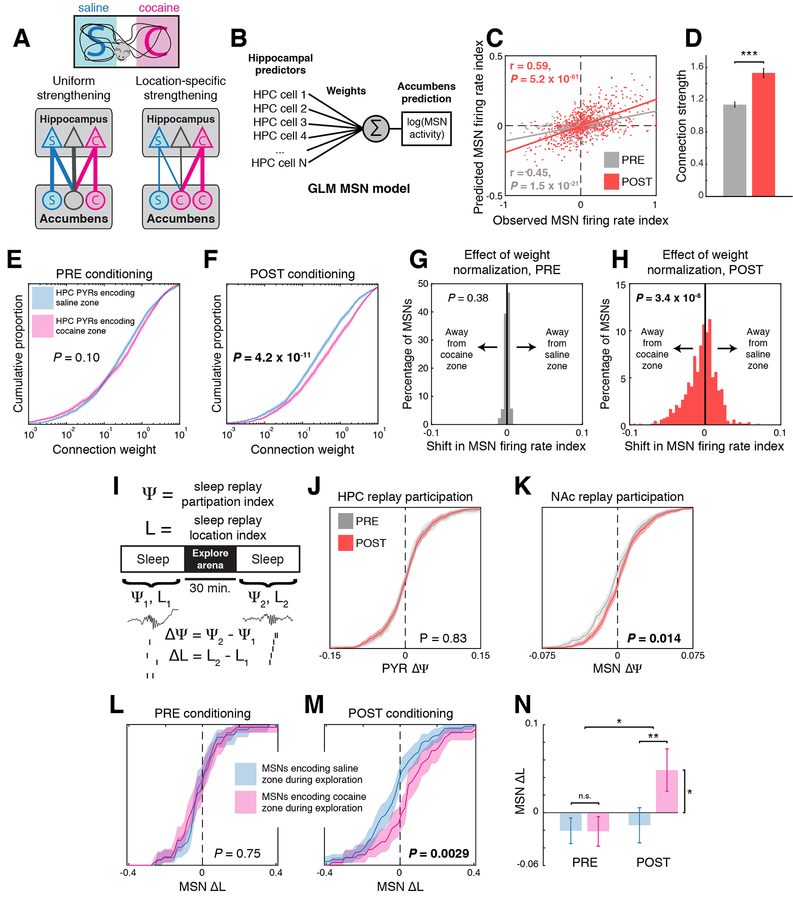
To distinguish whether strengthening of hippocampal coupling is uniform or location-specific (Fig. 4A), we fit a GLM to find connection weights that optimally predict MSN activity based solely on HPC activity (Fig. 4B). We then used this model to generate predictions of each MSN spike train, from which we calculated a firing rate index (see Methods). Although the model contains no explicit location information, the firing rate indices predicted from HPC activity were significantly correlated with the observed firing rate indices (n = 531, 713 MSNs; PRE R = 0.45, P = 1.2 × 10−7; POST R = 0.59, P = 6.8 × 10−68; Fig. 4C).
Figure 4: Cocaine place conditioning preferentially strengthens coupling between MSNs and hippocampal pyramidal cells encoding the cocaine-paired location.
A) Two models: cocaine could either uniformly strengthen all hippocampal inputs equally or preferentially strengthen the inputs that encode the cocaine-paired location. B) A GLM that predicts MSN spiking activity from PYR spiking activity by fitting connection weights can estimate the effects of modifying the connections. C) Model-predicted MSN firing rate indices based solely on hippocampal activity are significantly correlated with observed MSN firing rate indices (R = 0.45, P = 1.2 × 10−7 PRE; R = 0.59, P = 6.8 × 10−68 POST). D) Estimated connection weights are increased after cocaine conditioning (rank sum test, ***P = 1.2 × 10−9). Graph represents mean ± SEM. E) In PRE sessions, connection weights from cocaine zone-encoding PYRs and saline zone-encoding PYRs have equal distributions. F) In POST sessions, connections from cocaine zone-encoding PYRs have stronger weights than connections from saline zone-encoding PYRs (rank sum test, P = 4.2 × 10−11). G) In PRE sessions, adjusting connection weights from cocaine zone-encoding PYRs to match the distribution of weights from saline zone-encoding does not change predicted MSN firing rate indices. H) In POST sessions, normalizing connection weights from cocaine zone-encoding PYRs significantly shifts predicted MSN firing rate indices away from the cocaine zone (t-test, P = 3.4 × 10−8), suggesting that the asymmetric connection weight distribution underlies increased MSN activity in the cocaine zone. I) Sleep replay analysis. During post-exploration sleep, the hippocampus replays spatial trajectories from inside the arena, but replay during pre-exploration sleep encodes locations outside the arena. The participation index Ψ represents the relative likelihood of a cell firing during replays encoding the cocaine zone vs. saline zone. A location index L is also calculated for each MSN, representing the average spatial location encoded by replay events that drove that MSN to fire, with −1 representing the saline zone and +1 representing the cocaine zone. ΔΨ and ΔL are the differences in Ψ and L, respectively, between post- and pre-exploration sleep. J) HPC PYR participation index Ψ does not change from PRE to POST, indicating that the PYR firing rate is the same in ripples encoding either location. K) For NAc MSNs, the Ψ index increases from PRE to POST (rank sum test, P = 0.014), indicating that in POST sessions, MSNs fire more spikes during sleep replays encoding the cocaine zone. L) In PRE sessions, MSNs that fired preferentially in either the cocaine or saline zones during exploration showed no difference in ΔL. M) In POST sessions, cocaine zone MSNs exhibited a larger ΔL than saline zone MSNs (rank sum test, **P = 0.0029), indicating that the MSNs that fire preferentially during sleep replay events encoding the cocaine zone are the same MSNs that fire preferentially during awake exploration of the cocaine zone. All shaded areas represent 99% confidence intervals. N) This effect is significantly different between PRE and POST sessions (2-way ANOVA, *P = 0.0197), and only the cocaine zone MSNs in POST sessions showed a ΔL that was significantly different from zero (signed rank test, *P = 0.014). Graph represents mean ± SEM.
We next examined the PYR to MSN connection weights and found that they increased in magnitude after cocaine conditioning (n = 4047, 3979 weights, rank sum test, P = 1.2 × 10−9; Fig. 4D). In PRE sessions there was no difference between connection strengths from PYRs encoding the cocaine and saline zones (n = 2011, 2036 weights, rank sum test, P = 0.10; Fig. 4E), but in POST sessions connection weights arising from cocaine zone PYRs were significantly larger (n = 2014, 1981 weights, rank sum test, P = 1.4 × 10−6; Fig. 4F), consistent with connection weights being strengthened in location-specific fashion. To test whether the location-specific distribution of connection weights was responsible for the recruitment of MSN activity in our model, we adjusted connection weights to equalize the distributions for PYRs encoding the cocaine and saline zones. In models fit to PRE sessions, this adjustment caused no change in MSN firing rate index (n = 531 MSNs, signed rank test, P = 0.39, Fig. 4G). However, in models fit to POST sessions the MSN firing rate indices shifted away from the cocaine zone (n = 713 MSNs, signed rank test, P = 3.4 × 10−8, Fig. 4H), indicating that connection weight asymmetry contributes to MSN recruitment to the cocaine zone.
Since cocaine conditioning also PYR place field density in the cocaine zone (Fig. S4A-B), we performed a similar analysis to determine the relative contribution of place field density to location-specific MSN activity. In PRE sessions there was no difference in distribution of PYR firing rate index magnitudes (Fig. S4C), but in POST sessions the magnitude of the indices was larger for cocaine zone PYRs (n = 164, 205 PYRs, median 0.15 vs. 0.24; rank sum test, P = 2.6 × 10−4, Fig. S4D), confirming that place cells over-represent the cocaine zone. Adjusting PYR spike trains to remove the asymmetry in PYR firing rate indices caused no change in MSN firing rate indices in PRE session models (n = 531 MSNs, signed rank test, P = 0.83, Fig. S4E), but in POST session models the MSN firing rate indices shifted away from the cocaine zone (n = 713 MSNs, signed rank test, P = 5.4 × 10−4, Fig. S4F), indicating that changes in PYR place field density contribute to MSN recruitment to the cocaine zone. Since place field changes and connection weight changes both contributed to increased MSN activity in the cocaine zone, we compared them directly to determine which was a larger contributor. The two effects contributed equally to the location tuning properties of MSNs encoding the saline zone (n = 355 MSNs, signed rank test, P = 0.29, Fig. S4G), but for MSNs encoding the cocaine zone, changes in connection weights were a larger contributor than changes in PYR place fields (n = 340 MSNs, signed rank test, P = 5.5 × 10−9, Fig. S4H).
To address the possibility that correlations between activity in HPC and NAc could be brought about by environmental cues, behavior, or common inputs, we analyzed the effect of hippocampal replay events during sleep on MSN firing (Fig. 4I, Methods). By training a Bayesian decoder on HPC spike trains during awake exploration (Brown et al., 1998; Zhang et al., 1998), we were able to decode the locations encoded by replay events occurring during sleep. We found that HPC PYRs participated equally in replay events encoding the cocaine or saline zones (rank sum test, participation index 0.001 ± 0.003 PRE vs. 0.004 ± 0.002 POST, n = 231, 341 PYRs, P = 0.83, Fig. 4J), but in POST sessions NAc MSNs showed increased participation in replays encoding the cocaine zone (rank sum test, −0.002 ± 0.001 PRE vs. 0.002 ± 0.001 POST, n = 344, 381 MSNs, P = 0.014, Fig. 4K), consistent with a location-specific distribution of connection strengthening. To verify that the strengthened inputs we measured during sleep replays were related to the additional MSN firing in the cocaine zone, we calculated a location index L for each MSN, which represents the mean location encoded by replay events in which that MSN participated (see Methods). We found that MSNs in POST sessions that encoded the cocaine zone during exploration had an elevated ΔL, indicating that they also fired preferentially during sleep replays encoding the cocaine zone (PRE ΔL −0.007 ± 0.015 saline, −0.013 ± 0.017 cocaine; POST ΔL −0.032 ± 0.020 saline, 0.056 ± 0.024 cocaine, n = 55, 52 PRE; n = 76, 54 POST, rank sum test, P = 0.0029, Fig. 4L-N). These results suggest that MSN firing in the cocaine zone depends on location-specific strengthening of HPC inputs to the NAc.
DISCUSSION
In this study, we found that cocaine place conditioning recruits mainly D2-positive NAc MSNs, and to a lesser extent hippocampal PYRs, to fire in the cocaine-paired zone. Additionally, MSNs receiving strong PYR inputs encoded more spatial information and were preferentially recruited to the cocaine zone, suggesting that PYR inputs were driving MSN cocaine zone activity. Finally, we found that cocaine conditioning strengthens coupling between HPC PYRs and NAc MSNs in a location-dependent fashion during both waking and sleep.
Although extracellular recording is not able to make direct measurements of synaptic strength, ex vivo slice experiments have shown that repeated cocaine exposure potentiates hippocampal inputs to NAc MSNs (Britt et al., 2012; MacAskill et al., 2014; Pascoli et al., 2014), and our findings are consistent with this plasticity underlying the location-specific increase in hippocampus-NAc coupling. The majority of our POST sessions were recorded in early abstinence (<2 weeks), and our finding of recruitment of D2+ MSNs to fire in the cocaine zone is consistent with the results of MacAskill et al. (MacAskill et al., 2014), who found that cocaine conditioning increased the D2/D1 ratio of HPC inputs to the NAc. On the other hand, Pascoli et al. (Pascoli et al., 2014) found selective potentiation of HPC inputs onto D1-positive MSNs, but only in late cocaine abstinence (>2 weeks). A limitation of our study is that we primarily examined early abstinence, and understanding the longer-term effects of cocaine conditioning will be the focus of future work. Our results also suggest that cocaine may preferentially strengthen the inputs that are most active at the time of cocaine exposure, which could differ depending on the subregion of accumbens and environmental factors during conditioning.
It is worth emphasizing that our place conditioning paradigm (Fig. S2A) pairs cocaine with a spatial location defined relative to distal navigational cues, and proximal sensory cues were minimized and counterbalanced across cocaine/saline conditions. Under similar conditions, CPP has been shown to be dependent on dorsal hippocampus and not ventral hippocampus or amygdala (Ferbinteanu and McDonald, 2001; Meyers et al., 2003; Trouche et al., 2016). In CPP paradigms incorporating proximal sensory cues that differ between the cocaine and saline zones, NAc inputs from other structures such as the amygdala would likely be affected as well.
Our results may appear different from those of German et al. (German and Fields, 2007), who recorded neurons in the NAc during CPP POST sessions in rats and found that NAc MSNs exhibit decreased firing rates in the drug-paired zone. The most likely explanation for this difference is the fact that their study used morphine, and ours used cocaine. Cocaine and morphine act through different molecular mechanisms and are known to induce opposite changes in NAc dendritic spine density (Robinson and Kolb, 1999a, b). Our results suggest they may induce CPP through different physiological mechanisms at the level of the NAc as well. A recent study by Calipari et al. (Calipari et al., 2016) using fiber photometry appears to contradict our findings directly, as they used cocaine CPP and reported increased population activity in D1 cells and decreased activity in D2 cells in the cocaine zone in early abstinence. There are several possible explanations for this discrepancy, including differences in the conditioning protocol, nonlinear relationships between spike rate and calcium signal, inability to distinguish MSN and interneuron calcium signals, and the fact that we used GLMs to regress out movement-related MSN activity.
Location-dependent plasticity supports a plausible mechanistic model of cocaine CPP in which the NAc acts as an action-location-outcome associator (Berke and Hyman, 2000; van der Meer and Redish, 2011b). We hypothesize that the NAc integrates hippocampal inputs encoding a spatial location and prefrontal cortical inputs encoding an action plan to generate actions appropriate for a given spatial context. Under physiological conditions, performing an action in a specific location that generates a positive reward prediction error causes dopamine release in the NAc (Hart et al., 2014). This would strengthen hippocampal synapses encoding the rewarded location (Reynolds and Wickens, 2002), increasing the expected reward outcome associated with that action/location pairing. During cocaine place conditioning, NAc dopamine levels are artificially elevated, effectively rewarding all actions performed in the cocaine zone and increasing the strength of hippocampal inputs encoding that location. Upon exposure to the cocaine zone in POST sessions, the strengthened hippocampal inputs would drive increased firing in NAc MSNs, leading the animal to engage in activity in the cocaine zone rather than run to the saline zone. Determining whether this model is correct will require further studies involving multisite unit recording and manipulation of prefrontal cortex in CPP.
It is believed that drugs of abuse exert differential effects at various synaptic pathways in the brain – our results suggest that synaptic changes may be specific even at the level of functionally defined subsets of synapses within a single synaptic pathway. One important consequence of non-uniform changes in synaptic strength is that behavior could be driven by changes in a subset of synapses in a projection, while the average strength of the entire projection could be unchanged or even change in the opposite direction. This reveals an additional layer of complexity that is not recognized in many circuit models of drug addiction and suggests that some of these models may require revision. Selective plasticity of specific corticostriatal synapses based on presynaptic firing properties has been observed in other striatal regions (Xiong et al., 2015) and may represent a canonical mechanism by which sensory and contextual information can bias action selection based on reward history.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, György Buzsáki (Gyorgy.Buzsaki@nyumc.org).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animal models
Procedures were conducted using two BAC transgenic lines from GENSAT: Drd1a-Cre, (B6.FVB(Cg)-Tg(Drd1a-cre)EY217Gsat/Mmucd; RRID:MMRRC_034258-UCD) and A2a-Cre, (B6.FVB(Cg)-Tg(Adora2a-cre)KG139Gsat/Mmucd; RRID:MMRRC_036158-UCD) and one knock-in line from the Allen Institute: Ai32 Cre-dependent channelrhodopsin line (B6.Cg-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J; RRID:IMSR_JAX:024109). Experiments described in this study were conducted using 16 male mice in total (25–40 g, 12–30 weeks of age). 6 mice were wild-type C57BL/6J x FVB hybrids, 7 mice were Drd1a-Cre; Ai32 mice on a C57BL/6J background, and 3 mice were A2a-Cre; Ai32 mice on a C57BL/6J background. After implantation, animals were housed individually on a 12/12 h day/night schedule and were given ad libitum access to food and water. All experiments were conducted in accordance with the Institutional Animal Care and Use Committee of New York University Medical Center.
Viral vectors
Adeno-associated viral vector (AAV) was used to express channelrhodopsin-2 in Cre-expressing neurons in the ventral striatum of D1-cre mice. The recombinant AAV vector was pseudotyped with AAV5 capsid protein and packaged by the University of North Carolina viral core.
METHOD DETAILS
Stereotactic surgery and data acquisition
All animals were implanted with either 32- or 64-site silicon probes (NeuroNexus) in posterior CA1 (AP −3.2 mm, ML 3 mm, DV ~1.5 mm) and in nucleus accumbens (AP 1.5 mm, ML 1.4 mm, DV 3.8 mm from brain surface) under isoflurane anesthesia, as described previously (Stark et al., 2013). Ground and reference wires were implanted in the skull above the cerebellum, and a grounded copper mesh hat was constructed shielding the probes. Probes were mounted on microdrives that were advanced to the CA1 pyramidal layer over the course of 3–5 days after surgery, and CA1 was identified by the presence of sharp wave ripple oscillations (Buzsaki et al., 1992). Animals were allowed to recover for at least one week prior to behavioral testing. After implantation, all animals were housed individually. Recordings were performed at 20 kHz using a 256-channel Amplipex system (Berenyi et al., 2014), and the animal’s position was determined by tracking the position of red and blue LEDs mounted on the recording headstage. Offline spike sorting was performed using KlustaKwik (Harris et al., 2000), followed by manual adjustment using Klusters. Isolated single units were assigned a putative cell type based on established criteria in the hippocampus (Bartho et al., 2004; McCormick et al., 1985) (Fig. S1B) and striatum (Schmitzer-Torbert and Redish, 2004; Yamin et al., 2013) (Fig. S1C). Recording location was confirmed by electrolytic lesioning and histology (Fig. S1F-G). Optotagging analysis was performed in D1-cre mice injected with AAV-FLEX-ChR2-EYFP or in Adora2A-cre; Ai32 mice that expressed ChR2 from a genomic locus. Units were defined as optically tagged based on published criteria (Stark et al., 2012), using a p-value cutoff of 10−4 and a modulation index cutoff of 0.6 (Fig. S1D). Putative D1 and D2 MSNs met the dual criteria of optogenetic response and classification as MSN based on waveform and bursting criteria. We found that >50% of tagged units responded in 5 ms or less (Fig. S1E), and restricting our analysis to the subset of neurons with <5 ms did not qualitatively change the results.
Recording sessions and behavioral conditioning
Animals were housed on a 12-hour light/dark cycle, and all recording sessions occurred 1–2 hours prior to the onset of the dark phase. For all recording sessions, animals were recorded while in the home cage for 30–60 minutes both prior to and after exploration of the CPP arena. During the first 1–3 days, animals were recorded in PRE sessions, in which they explored the CPP arena for 30 minutes (Fig. S2A). During five subsequent daily conditioning sessions, animals were injected with IP saline and confined to one side of the arena for 30 minutes, then returned to the home cage for 5 minutes. During this time, the CPP arena was cleaned and rotated 180o, and the animal was then injected with cocaine (15 mg/kg IP) and placed in the cocaine zone for 30 minutes. The cocaine zone was defined as the side of the arena the animal intrinsically preferred less during PRE sessions. The CPP arena was a featureless, symmetrical Plexiglas box designed to minimize local sensory cues. However, the side within the CPP arena in which the animal was conditioned changed daily, but the animal’s absolute room location (‘place’) during saline and cocaine pairing was constant from one day to the next. After five daily conditioning sessions, the animals were recorded in daily POST conditioning sessions in which conditions were identical to the PRE sessions. The strength of behavioral place preference tended to decrease with each POST session, and analysis of POST sessions was restricted to sessions in which the animal showed a place preference for the cocaine zone.
QUANTIFICATION AND STATISTICAL ANALYSIS
Firing rate and behavior indices
To quantify the relative firing rate of a cell in the saline and cocaine zones, we created a firing rate index φ defined as
where C is the firing rate of the cell in the cocaine zone, and S is the firing rate of the cell in the saline zone. A cell with φ=0 has an equal firing rate in both zones, while a cell with φ=−1 fires only in the saline zone, and a cell with φ=1 fires only in the cocaine zone. For analysis of behavior (Fig. 1G-I), we created a behavior index η defined as
where tC is the amount of time the animal spends in the cocaine zone, and tS is the amount of time the animal spends in the saline zone. Analogously, in a recording session with η = 0, the animal spends an equal amount of time in both zones. If η = −1, the animal spends the entire session in the saline zone, and if η = 1, the animal spends the entire session in the cocaine zone.
Generalized linear models (GLMs)
Because running speed and spatial location are correlated in CPP (Fig. S2B-E), we used GLMs to separate out the effects of these two variables. We tested several GLMs using 10-fold cross-validation (Harris et al., 2003; Peyrache et al., 2015; Truccolo et al., 2005). In this procedure, data is split into ten segments, and the GLM is trained on nine segments and tested on the last segment. This is repeated ten times so that each segment is tested once. The purpose of this procedure is to penalize model overfitting and allow direct comparison of GLMs containing different numbers of predictors. We found that the best predictions (Table S2) were with a GLM of the form
where X(t) is a location indicator vector that is 1 when the animal is in the cocaine zone and 0 in the saline zone, Z(t) is the linear speed of the animal in cm/s, and ϵ is the residual. This produces a measurement of speed modulation that is independent of position (ß2) and enables us to calculate the corrected cocaine index φcorr with running speed regressed out:
To include hippocampal activity as a predictor of MSN spike trains (Fig. 3), we extended the GLM to include the first n significant PC scores scPCx (see PCA section below):
Information measures
To compare the accuracy of different GLMs in predicting MSN spike trains, we used log-likelihood measures. Each GLM produces a predicted intensity function f, and the log-likelihood Lf of that intensity function producing the observed spike train (Stark et al.) is given by
which is in units of bits. The difference in GLM prediction quality is measured using the difference between the log-likelihoods Rx. When comparing GLMs with different numbers of predictors, this quantity represents the information about the predicted spike train carried by extra predictors in one GLM. We calculated the following R’s:
RPYR is the difference in L between a GLM containing hippocampal PYRs as predictors and a GLM containing no predictors (i.e. a constant baseline firing rate), and it represents the total information about MSN spiking carried by simultaneously recorded hippocampal pyramidal cells. RPYR-loc is the difference in L between a GLM containing PYRs and location and a GLM containing only location. RPYR-loc represents the additional information that PYRs carry about MSN spiking that is not location-related. The quantity RPYR – RPYR-loc therefore represents the location-related information that PYRs carry about MSN spiking, and RPYR – RPYR-spd represents the analogous information measure for running speed.
Principal component analysis (PCA)
PCA was applied on binned spike trains (25 ms bins). The number of significant principal components (PCs) was determined by the upper limit λmax of the Marchenko-Pastur distribution (Peyrache et al., 2010; Peyrache et al., 2009)
where B is the number of time bins and N the number of neurons. Binned spike trains were projected onto the significant PCs, resulting in a time series of activation of each individual PC, referred to as PC scores scPCx.
Assembly prediction analysis
We first performed PCA separately on binned MSN and PYR spike trains to obtain correlated MSN and PYR assemblies, retaining only significant PCs. To quantify the functional relationship between these assemblies, we used a cross-validated procedure. During each training epoch, a single PC score of MSN data was linearly regressed against all significant PYR PC scores. The linear model was then used to construct a prediction of the MSN PC score during the test epoch. The likelihood of the prediction was measured by the correlation coefficient between the predicted and observed PC scores.
The same method was used to quantify the prediction during sleep sharp wave ripples. Each significant MSN PC score was linearly regressed against all hippocampal PC scores. The resulting linear model was used to build predictors of individual MSN PC scores during hippocampal ripples. The likelihood of the prediction was defined as the correlation coefficient between the prediction and the observed PC score during sharp wave ripples.
Connection weight modeling
To model spiking activity of MSNs using hippocampal PYR activity as predictors, we performed singular value decomposition (SVD) on the binned PYR spike trains. SVD was used instead of PCA because PCA effectively normalizes each PYR spike train, discarding differences in firing rate of PYRs that could vary systematically with place field location. All connection weight analyses (Fig. 4) were also performed with PCA and produced qualitatively similar results (not shown). SVD factorizes QPYR, which is the B x NPYR matrix of binned PYR spike trains (B time bins, NPYR PYRs):
U and V are orthonormal matrices, and S is a diagonal matrix of eigenvalues that denote how much variance is accounted for by each column of U and V. Since the null distribution of eigenvalues can not be calculated analytically as in PCA, we performed parallel analysis to determine the number n of how many significant columns of U and V to retain. We then predicted a single binned MSN spike train QMSN (t) using a GLM of the form:
In matrix form, this becomes
where , , and denote U, S, and V with only the first n columns retained, and is the n x 1 vector of GLM coefficients. Because V is orthonormal, V −1 = V T, therefore
we substitute for in the GLM equation yielding
Here, is an NPYR x 1 vector containing estimates of the connection weights between all NPYR of the PYRs and a single MSN.
Place field shifting and connection weight scaling
We found that the distribution of PYR place field locations and connection weights was asymmetrical with respect to the cocaine/saline zones, meaning that the cocaine zone had a higher place field density and stronger connection weights (Fig. 4, S4). To determine the effects of this asymmetry, we adjusted the distribution of place field locations and connection weights in our model so that the distribution for PYRs encoding the cocaine zone matched PYRs encoding the saline zone. We then examined the changes in MSN spatial tuning predicted by the model to determine how much the asymmetries contributed to the additional MSN activity in the cocaine zone. To accomplish this, we first calculated the cumulative distribution functions (CDFs) of PYR cocaine index and connection weights for PYRs that encode the saline and cocaine zones (i.e,. with negative or positive cocaine indices). For the cocaine zone PYRs, we assigned each cocaine index or connection weight a percentile score within the cocaine zone PYRs, then adjusted each value to match the value from a corresponding saline zone PYR at the same percentile within saline zone PYRs. This had the effect of making the CDFs of PYR cocaine index or connection weight identical for PYRs encoding the saline and cocaine zones.
To adjust PYR place field location, we calculated location-dependent scaling factors so that each spike would contribute a non-integer value slightly more or less than 1.0 to the binned spike train, depending on which zone the animal was in when the spike occurred. To obtain unique solutions, we also enforced a constraint that the sum of the binned spike train (i.e., the “firing rate”) over the entire trial would be unchanged. We started with the adjusted cocaine index
with the firing rate constraint: CtC + StS = CadjtC + SadjtS where C and S are PYR firing rates in the cocaine and saline zones, and tC and tS are the amount of time spent in the cocaine and saline zones, respectively. We then determined the location-dependent scaling factors to multiply with each element of the binned spike train to adjust the cocaine index to equal φadj
All spike bins in which the animal was in the saline zone were multiplied by Sadj/S, and spike bins where the animal was in the cocaine zone were multiplied by Cadj/C. This had the effect of changing the PYR cocaine index without affecting the firing rate averaged over the entire exploration session.
Sleep replay analysis
For the sleep replay analysis (Fig. 4I-N), we used a Bayesian decoder to estimate the probability of PYR spiking encoding the cocaine or saline zones. To calculate the participation index Ψ, we separated replays into the third of events most likely to encode the saline zone, the third most likely to encode the center zone, and the third most likely to encode the cocaine zone. If ΨS is the probability of a neuron firing during a saline zone replay, and ΨC is the probability of the neuron firing during a cocaine zone replay, then the participation index Ψ = ΨC – ΨS. ΔΨ represents the difference in Ψ between pre-exploration sleep and post-exploration sleep.
The location index L represents the mean location encoded by a replay event in which a given cell fires (saline zone = −1, cocaine zone = 1). ΔL thus represents the difference in L between pre-exploration sleep and post-exploration sleep. The comparison (Fig. 4L-N) between MSNs encoding the saline and cocaine zones is based on MSNs with negative firing rate indices during exploration (saline) vs positive firing rate indices (cocaine). For all replay analyses, cells that fired in fewer than 1% of all sharp wave ripples were omitted from analysis.
Statistical analysis
All analyses and statistical tests were performed using MATLAB (The MathWorks). Most comparisons employed nonparametric Wilcoxon rank sum tests or sign rank tests (for paired statistics). Comparisons of cocaine index were performed using two-tailed t-tests for two reasons: 1) cocaine indices are approximately normally distributed, and 2) changes in cocaine index represent changes in a relatively sparse subpopulation of cells that are better captured by the mean of a distribution than the median. Two-way comparisons were performed with either standard two-way ANOVA or mixed model between/within subjects ANOVA when appropriate. Confidence intervals plotted on CDFs represent α = 0.05 unless stated otherwise and were derived from bootstrap resampling of the sample distribution with replacement. Data points were excluded if the MSN firing rate was below 0.01 Hz and/or if the GLM failed to converge. When hippocampal activity is a predictor, ~7% of MSNs were excluded by these criteria.
DATA AND SOFTWARE AVAILABILITY
The data and code that support the findings of this study will be made available from the corresponding author upon reasonable request.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Gord Fishell, Lisa Roux, Viola Woo, Sujit Thomas, and the members of the Buzsáki lab for support, technical assistance, and comments on the manuscript. This work was supported by the Leon Levy Foundation (L.S.), the Brain and Behavior Research Foundation (L.S.), and NIH grants K08 DA036657 (L.S.) and MH107396 (G.B.).
Footnotes
DECLARATION OF INTERESTS
The authors have no conflicts of interest to report.
REFERENCES
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, and Buzsaki G (2004). Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol 92, 600–608. [DOI] [PubMed] [Google Scholar]
- Berenyi A, Somogyvari Z, Nagy AJ, Roux L, Long JD, Fujisawa S, Stark E, Leonardo A, Harris TD, and Buzsaki G (2014). Large-scale, high-density (up to 512 channels) recording of local circuits in behaving animals. J Neurophysiol 111, 1132–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, and Hyman SE (2000). Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25, 515–532. [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, and Bonci A (2012). Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76, 790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Frank LM, Tang D, Quirk MC, and Wilson MA (1998). A statistical paradigm for neural spike train decoding applied to position prediction from ensemble firing patterns of rat hippocampal place cells. The Journal of neuroscience : the official journal of the Society for Neuroscience 18, 7411–7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Leung LW, and Vanderwolf CH (1983). Cellular bases of hippocampal EEG in the behaving rat. Brain research 287, 139–171. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Horvath Z, Urioste R, Hetke J, and Wise K (1992). High-frequency network oscillation in the hippocampus. Science 256, 1025–1027. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Marfia GA, and Bernardi G (1999). Glutamate-triggered events inducing corticostriatal long-term depression. The Journal of neuroscience : the official journal of the Society for Neuroscience 19, 6102–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Pena CJ, Walker DM, Pirpinias ST, Guise KG, Ramakrishnan C, et al. (2016). In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci U S A 113, 2726–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, and McDonald RJ (2001). Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus 11, 187–200. [DOI] [PubMed] [Google Scholar]
- German PW, and Fields HL (2007). Rat nucleus accumbens neurons persistently encode locations associated with morphine reward. J Neurophysiol 97, 2094–2106. [DOI] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G, and Buzsaki G (2003). Organization of cell assemblies in the hippocampus. Nature 424, 552–556. [DOI] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, and Buzsaki G (2000). Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol 84, 401–414. [DOI] [PubMed] [Google Scholar]
- Hart AS, Rutledge RB, Glimcher PW, and Phillips PE (2014). Phasic dopamine release in the rat nucleus accumbens symmetrically encodes a reward prediction error term. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Pennartz CM, and Everitt BJ (2008). Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 6950–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, and Wilson MA (2005). Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS biology 3, e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Ganguli S, and Frank LM (2012). Spatial information outflow from the hippocampal circuit: distributed spatial coding and phase precession in the subiculum. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 11539–11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski RW, Garcia CG, Wilson A, Hattori S, Howard MW, and Eichenbaum H (2013). Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 8079–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, Joosten RN, McNaughton BL, and Pennartz CM (2008). Preferential reactivation of motivationally relevant information in the ventral striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 6372–6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, and Pennartz CM (2009). Hippocampus leads ventral striatum in replay of place-reward information. PLoS biology 7, e1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie AM, and Mizumori SJ (1994). Spatial, movement- and reward-sensitive discharge by medial ventral striatum neurons of rats. Brain research 638, 157–168. [DOI] [PubMed] [Google Scholar]
- Lee AK, and Wilson MA (2002). Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194. [DOI] [PubMed] [Google Scholar]
- MacAskill AF, Cassel JM, and Carter AG (2014). Cocaine exposure reorganizes cell type- and input-specific connectivity in the nucleus accumbens. Nature neuroscience 17, 1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, and Prince DA (1985). Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54, 782–806. [DOI] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, and Neisewander JL (2003). Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport 14, 2127–2131. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Mogi E, Araki N, and Matsumoto G (1998). Reward-quality dependent anticipation in rat nucleus accumbens. Neuroreport 9, 3943–3948. [DOI] [PubMed] [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, and Buzsaki G (1999). Replay and time compression of recurring spike sequences in the hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience 19, 9497–9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J (1976). Place units in the hippocampus of the freely moving rat. Experimental neurology 51, 78–109. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, and Luscher C (2014). Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509, 459–464. [DOI] [PubMed] [Google Scholar]
- Peyrache A, Benchenane K, Khamassi M, Wiener SI, and Battaglia FP (2010). Principal component analysis of ensemble recordings reveals cell assemblies at high temporal resolution. J Comput Neurosci 29, 309–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrache A, Khamassi M, Benchenane K, Wiener SI, and Battaglia FP (2009). Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat Neurosci 12, 919–926. [DOI] [PubMed] [Google Scholar]
- Peyrache A, Lacroix MM, Petersen PC, and Buzsaki G (2015). Internally organized mechanisms of the head direction sense. Nat Neurosci 18, 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson OT, and Griffiths AC (1985). The topographic order of inputs to nucleus accumbens in the rat. Neuroscience 16, 275–296. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, and Wickens JR (2002). Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw 15, 507–521. [DOI] [PubMed] [Google Scholar]
- Robinson TE, and Kolb B (1999a). Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci 11, 1598–1604. [DOI] [PubMed] [Google Scholar]
- Robinson TE, and Kolb B (1999b). Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse 33, 160–162. [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert NC, and Redish AD (2008). Task-dependent encoding of space and events by striatal neurons is dependent on neural subtype. Neuroscience 153, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, and McNaughton BL (1996). Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271, 1870–1873. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Gothard KM, and Markus EJ (1993). An information-theoretic approach to deciphering the hippocampal code.
- Stark E, Koos T, and Buzsaki G (2012). Diode probes for spatiotemporal optical control of multiple neurons in freely moving animals. J Neurophysiol 108, 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi ET, Mulder AB, and Wiener SI (2000). Position and behavioral modulation of synchronization of hippocampal and accumbens neuronal discharges in freely moving rats. Hippocampus 10, 717–728. [DOI] [PubMed] [Google Scholar]
- Trouche S, Perestenko PV, van de Ven GM, Bratley CT, McNamara CG, Campo-Urriza N, Black SL, Reijmers LG, and Dupret D (2016). Recoding a cocaine-place memory engram to a neutral engram in the hippocampus. Nat Neurosci 19, 564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truccolo W, Eden UT, Fellows MR, Donoghue JP, and Brown EN (2005). A point process framework for relating neural spiking activity to spiking history, neural ensemble, and extrinsic covariate effects. J Neurophysiol 93, 1074–1089. [DOI] [PubMed] [Google Scholar]
- van der Meer MA, Johnson A, Schmitzer-Torbert NC, and Redish AD (2010). Triple dissociation of information processing in dorsal striatum, ventral striatum, and hippocampus on a learned spatial decision task. Neuron 67, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer MA, and Redish AD (2011a). Theta phase precession in rat ventral striatum links place and reward information. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 2843–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer MA, and Redish AD (2011b). Ventral striatum: a critical look at models of learning and evaluation. Current opinion in neurobiology 21, 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q, Znamenskiy P, and Zador AM (2015). Selective corticostriatal plasticity during acquisition of an auditory discrimination task. Nature 521, 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamin HG, Stern EA, and Cohen D (2013). Parallel processing of environmental recognition and locomotion in the mouse striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Ginzburg I, McNaughton BL, and Sejnowski TJ (1998). Interpreting neuronal population activity by reconstruction: unified framework with application to hippocampal place cells. J Neurophysiol 79, 1017–1044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.