Abstract
PURPOSE:
Preclinical studies performed in our laboratory have shown that high-dose selenium inhibits the development of carboplatin drug resistance in an ovarian cancer mouse xenograft model. Based on these data, as well as the potential serious toxicities of supranutritional doses of selenium, a phase I trial of a combination of selenium/carboplatin/paclitaxel was designed to determine the maximum tolerated dose, safety, and effects of selenium on carboplatin pharmacokinetics in the treatment of chemo-naive women with gynecologic cancers. Correlative studies were performed to identify gene targets of selenium..
METHODS:
Chemo-naïve patients with gynecologic malignancy received selenious acid IV on day 1 followed by carboplatin IV and paclitaxel IV on day 3. A standard 3 + 3 dose-escalating design was used for addition of selenium to standard dose chemotherapy. Concentrations of selenium in plasma and carboplatin in plasma ultrafiltrate were analyzed.
RESULTS:
Forty-five patients were enrolled and 291 treatment cycles were administered. Selenium was administered as selenious acid to 9 cohorts of patients with selenium doses ranging from 50 μg to 5000 μg. Grade 3/4 toxicities included neutropenia (66.6%), febrile neutropenia (2.2%), pain (20.0%), infection (13.3%), neurologic (11.1%), and pulmonary adverse effects (11.1%). The maximum tolerated dose of selenium was not reached. Selenium had no effect on carboplatin pharmacokinetics. Correlative studies showed post-treatment downregulation of RAD51AP1, a protein involved in DNA repair in both cancer cell lines and patient tumors.
CONCLUSION:
Overall, the addition of selenium to carboplatin/paclitaxel chemotherapy is safe and well tolerated, and does not alter carboplatin pharmacokinetics. A 5000 μg dose of elemental selenium as selenious acid is suggested as the dose to be evaluated in a phase II trial.
Keywords: Chemotherapy resistance, gynecologic cancer, selenium, chemotherapy, carboplatin
Introduction
Effective chemotherapy is essential in the treatment of advanced gynecological malignancies. Nevertheless, acquired resistance to platinum-based chemotherapy regimens, the standard-of-care in the treatment of many of these diseases, ultimately occurs in most patients [1–3]. New approaches are, therefore, urgently needed to overcome resistance to cytotoxic therapies [4].
Both platinum agents and taxanes are believed to exert anticancer effects through multiple mechanisms [5–7]. Some of the most well described modes of action of these 2 classes of drugs involve cell cycle arrest resulting in apoptotic cell death [6, 7]. These events are triggered by either the generation of lesions/crosslinks preferentially involving the purine bases of double-stranded DNA in the case of platinum agents, or taxane-induced stabilization of microtubules.
The mechanisms of resistance to these anticancer agents are also believed to be multifactorial in nature. In the case of platinum-based therapy, it has been proposed that these resistance mechanisms may be classified as “pre-target” (eg, reduced intracellular levels of drug mediated by transporter proteins; increased levels of glutathione which can reduce ROS), “on-target” (eg, increased proficiency of homologous recombination and other DNA repair mechanisms), “post-target” (eg, interference in components of apoptotic mechanisms), and “off-target” (eg, increase in cytoprotective autophagic processes) [6]. Many of these processes are also likely to interfere with the clinical activity of taxanes [7].
Selenium is a nutritionally essential trace element that forms a variety of biologically active organic (eg, selenomethionine, selenocysteine) and inorganic (eg, selenite, selenate) compounds, and is cotranslationally incorporated as selenocysteine into various selenoproteins, including glutathione peroxidases [8]. There have been many studies on the use of selenium for the prevention of cancer, but as shown in a recent meta-analysis, a significant effect has not been demonstrated [9, 10]. In contrast, the use of selenium compounds in the treatment of patients with cancer has not received extensive investigation. Nevertheless, a number of rationales exist for the inclusion of selenium in chemotherapy regimens.
Synergistic interactions between high-dose selenium and various cytotoxic drugs, including docetaxel, irinotecan, cisplatin, carboplatin, doxorubicin, and fluorouracil have been reported in a number of preclinical investigations involving in vivo studies of tumor xenografts [11–13]. These findings could be attributed to selenium-related enhancement of therapeutic effect or interference in processes of drug resistance. Regarding the latter possibility, our studies performed in nude mouse xenografts of ovarian cancer show that development of resistance to carboplatin chemotherapy is prevented when high doses of sodium selenite are administered prior to cytotoxic therapy. Furthermore, tumors treated with sodium selenite prior to carboplatin that were reimplanted into new animals maintain chemosensitivity to carboplatin [12]. In addition, proapoptotic effects of high-dose sodium selenite have been reported in studies of a number of different cancers [14]. It has also been proposed that the prooxidant characteristics of high-dose sodium selenite, while unlikely to directly cause DNA damage, can potentiate the action of other DNA damaging agents through induction of oxidative stress [15]. Interestingly, treatment of a xenograft mouse model of ovarian cancer with high-dose sodium selenite alone had no effect on tumor growth [4].
Several clinical studies have shown that addition of selenium-containing compounds to particular cytotoxic drug regimens may decrease toxicity and improve treatment tolerability, although the evidence with respect to this finding is mixed [16–19]. In addition, results from a randomized study of standard chemotherapy with or without high-dose sodium selenite in adult patients with non-Hodgkin’s lymphoma showed improved outcomes in the group receiving selenium [20]. However, clinical evidence supporting the safety of administering inorganic selenium compounds at relatively high dosages is limited [9, 13, 20–22], and these studies are critically important given the serious toxicities that have been reported when large quantities of selenium are accidentally ingested [23]. The primary objective of this phase I study is to investigate the safety of selenium as part of a therapeutic regimen for the treatment of women with gynecologic cancers.
Materials and Methods
Patient eligibility
Eligible patients had histologically or cytologically proven gynecologic malignancy. They were chemonaive and a regimen of carboplatin and paclitaxel chemotherapy was considered to be a standard option for their treatment. Other inclusion criteria included age greater than 18 years, estimated life expectancy of at least 6 months, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, and adequate hematologic, renal, and hepatic function.
Study Design
A standard 3 + 3 dose-escalating phase I trial evaluating administration of selenious acid followed by chemotherapy in cohorts of eligible patients was followed. Dose escalation was preceded in cohorts of three patients until a dose-limiting toxicity (DLT) was reported during the first cycle of therapy. If one patient out of three experienced a DLT, three additional patients were enrolled at that dose level. The maximum tolerated dose (MTD) was defined as the dose level at which ≥2 of 6 patients experienced a DLT.
The study protocol and amendments were approved by an institutional review board (IRB)-approved investigational trial conducted at the Rutgers Cancer Institute of New Jersey in accordance with the Belmont Report. Patients enrolled in this study provided written informed consent prior to study treatment.
Study Endpoints
The primary aim of this study was to determine the safety of selenium, administered intravenously (IV) as selenious acid, with carboplatin/paclitaxel in patients with gynecologic malignancies for whom standard therapy with carboplatin/paclitaxel was planned. This includes determination of the DLT and MTD of selenious acid in combination with carboplatin/paclitaxel. A secondary aim was to describe whether co-administration of selenious acid alters carboplatin pharmacokinetics.
An exploratory outcome measure included assessment of clinical response and progression-free survival (PFS) in the subgroup of patients with advanced ovarian cancer. In addition, correlative studies evaluating the effects of administration of selenious acid plus chemotherapy on gene expression in tumor specimens compared with ovarian and breast cancer cell lines, were also performed.
Treatment Protocol and Dose Cohorts
Selenium Injection (selenious acid) was purchased from American Regent, Inc. (Shirley, NY). Selenious acid-containing solutions were administered in a total volume of 500 mL, and were prepared by diluting specific volumes of aqueous selenious acid (65.5 μg/mL selenious acid corresponding to 40 μg/mL elemental selenium [Se]) with 5% dextrose in water.
Given the two pKas of selenious acid (2.7, 8.3) and the pH of blood (7.4), this compound in blood results in a mixture of partially and fully ionized forms of the compound. Treatment consisted of IV administration of these solutions over 5 h on day 1, followed by paclitaxel 175 mg/m2 IV and carboplatin (area under concentration [AUC] 5 for first cycle; AUC 6 for subsequent cycles) on day 3. A time delay of two days between administration of selenious acid and chemotherapy was chosen to approximate the delay between administration of selenium and carboplatin found to be most effective in the mouse xenograft studies [12]. Patients were assigned to 1 of 9 Se escalation dose cohorts ranging from 50 μg/dose to 5000 μg/dose (Table 2). (For reference, the recommended daily allowance of oral selenium for adults is 55 μg/day [24].)
Table 2:
Dose Escalation SchemaA
| Dose level | Selenious acid IV (μg) |
Carboplatin IV (AUC) | Paclitaxel IV (mg/m2) |
No. of ptsB | Total cycles | |
|---|---|---|---|---|---|---|
| Cycle 1 | Subsequent cycles |
|||||
| 1 | 50 | 5 | 6 | 175 | 3 | 14 |
| 2 | 100 | 5 | 6 | 175 | 6 | 35 |
| 3 | 200 | 5 | 6 | 175 | 4 | 25 |
| 4 | 400 | 5 | 6 | 175 | 3 | 24 |
| 5 | 800 | 5 | 6 | 175 | 7 | 42 |
| 6 | 1000 | 5 | 6 | 175 | 3 | 30 |
| 7 | 1200 | 5 | 6 | 175 | 7 | 52 |
| 8 | 2000 | 5 | 6 | 175 | 3 | 18 |
| 9 | 5000 | 5 | 6 | 175 | 9 | 51 |
IV- intravenous; AUC- area under the curve
There were three exceptions to dose escalation rules (dose levels 3, 5, and 7) that were approved by the primary investigator prior to treatment.
Clinical Toxicity Evaluation
All patients who received 1 cycle of protocol therapy were evaluated for toxicity. Adverse events were assessed weekly according to the National Cancer Institute (NCI) Terminology Criteria for Adverse Events (CTCAE) version 3.0. Dose limiting toxicity (DLT) was defined as an adverse event occurring in cycle 1 that met 1 of the following criteria: 1) treatment-related grade 3 or higher non-hematologic toxicity, excluding alopecia, hypersensitivity reactions, injection-site reactions, and dyspepsia, or 2) grade 4 neutropenia for at least 7 days, febrile neutropenia, thrombocytopenia accompanied by bleeding, or grade 3 or higher hematologic toxicity, excluding anemia and lymphocytopenia. The MTD was defined as the dose below the dose at which at least 2 patients out of 6 experienced DLT.
Clinical Response Evaluation
Patients were evaluated for response of measurable disease using CT of the abdomen/pelvis at baseline and after 3 cycles of protocol therapy and every 3 cycles thereafter according to RECIST version 1.1 criteria. Progression-free survival (PFS) was defined as the date of registration until disease progression or death, whichever came first (censored by the date of last contact prior to data analysis).
Statistical analyses
Pharmacokinetic findings were analyzed and parameters were summarized with mean ± SD, and compared with 95% confidence intervals (CIs) between cycle 1 and cycle 2 pharmacokinetic parameters. Confidence intervals at 95% of the mean were determined using OriginPro statistical software (Northampton, MA).
Supplementary Materials and Methods
See Supplementary Materials and Methods section for additional information related to patient eligibility, rationale for use of selenious acid/sodium selenite, treatment protocol and dose cohorts, determination of BRCA1/2 status, clinical toxicity evaluation, clinical response evaluation, selenium and carboplatin pharmacokinetics, cell lines, cell culture, cell viability and tumor specimens, microarray analysis and immunoblotting experiments, and determination of plasma selenoprotein P levels and plasma glutathione peroxidase activity.
Results
Patient Characteristics
Forty-five patients were enrolled in the study; 38 patients had a diagnosis of epithelial ovarian cancer, or cancer of the fallopian tubes or peritoneum, with 28 patients in that group diagnosed with stage III or IV disease. Patient baseline characteristics are represented in Table 1.
Table 1:
Baseline Patient and Disease Characteristics
| Age, years | |
| Median | 54 |
| Range | 36–74 |
| Age groups, years (n,%) | |
| 30–49 | 14 (31%) |
| 50–69 | 28 (62%) |
| 70–79 | 3 (7%) |
| Race (n,%) | |
| Asian | 2 (4%) |
| Black or African American | 4 (9%) |
| White | 39 (87%) |
| ECOG performance status (n,%) | |
| 0 | 29 (64%) |
| 1 | 14 (31%) |
| 2 | 2 (4%) |
| Ovarian, Fallopian tube, or peritoneal cancer | |
| No. of patients | 38 |
| Stage (n,%) | |
| Stage I | 2 (5.3%) |
| Stage II | 6 (15.8%) |
| Stage III | 18a (47.4%) |
| Stage IV | 10 (26.3%) |
| Stage unavailable | 2 (5.3%) |
| Uterine cancer | |
| No. of patients | 6 |
| Stage/classification (n,%) | |
| Stage IV | 1 (16.6%) |
| Recurrent | 5 (83.3%) |
| Cervical cancer | |
| No. of patients | 1 |
| Stage, (n,%) | |
| Stage IV | 1 (100%) |
One patient classified as having cancer of both the ovary and the uterus.
Patients received treatment either in the neoadjuvant setting or following surgery (See Supplementary Table 4). For the group of patients with ovarian, fallopian tube, or peritoneal cancer, 12 received neoadjuvant therapy, and 15 and 11 received adjuvant treatment following optimal or suboptimal cytoreductive surgery, respectively. Hence, 23 patients in this group had measurable disease at initiation of treatment.
Maximum Tolerated Dose
A total of 291 treatment cycles were administered. A median of 6 cycles were given, with a range of one to 13 cycles per patient. Thirty-three patients (73%) received 6 or more cycles. There were no treatment-related deaths. A summary of the number of cycles in which specific grade 3/4 adverse events were experienced is presented in Table 3. Grade 3 and 4 toxicities, regardless of attribution, from all 291 cycles are shown. Only three cycle 1-related DLTs occurred. Worst grade hematologic toxicities per patient summarized in Supplementary Table 1 show that grade 3/4 neutropenia and thrombocytopenia occurred in 66.6% and 0% of patients, respectively. Rates of grade 3/4 anemia and leukopenia were very low (Supplementary Table 1).
Table 3:
Numbers of Cycles Per Se Dose Level in Which Grade 3/4 (Worst Grade) Adverse EventsA Occurred
| Selenious acid dose (μg) | 50 | 100 | 200 | 400 | 800 | 1000 | 1200 | 2000 | 5000B | |||||||||
| Total number of cycles per selenious acid dose | 14 | 35 | 25 | 24 | 42 | 30 | 52 | 18 | 51 | |||||||||
| Hematologic Toxicities | ||||||||||||||||||
| Grade | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 |
| Neutropenia | 1 | 1 | 4 | 1 | 2 | 3 | 3 | 3 | 4 | 5 | 2 | 7 | 3 | |||||
| Febrile neutropenia | 1 | |||||||||||||||||
| Leukopenia | 2C,F | |||||||||||||||||
| Anemia | 1 | |||||||||||||||||
| Thrombocytopenia | ||||||||||||||||||
| Non-hematologic Toxicities | ||||||||||||||||||
| Grade | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 4 |
| Cardiovascular | 2 | |||||||||||||||||
| ConstitutionalD | 1 | 1 | 1 | 1 | ||||||||||||||
| Dermatology | 2 | |||||||||||||||||
| Endocrine | 1 | |||||||||||||||||
| Gastrointestinal | 1 | 1 | 3 | |||||||||||||||
| Hepatobiliary | 2 | 2 | ||||||||||||||||
| Infection | 1 | 1 | 1C | 3 | ||||||||||||||
| Metabolic | 1 | |||||||||||||||||
| Musculoskeletal | 1C | |||||||||||||||||
| Neurologic | 1 | 1 | 1 | 2E | ||||||||||||||
| Pain | 1 | 1 | 3 | 1 | 4 | |||||||||||||
| Pulmonary | 1 | 2 | 1 | 1 | ||||||||||||||
Regardless of attribution. More than 1 of the same adverse events occurring during a cycle is reported only once at highest grade level
Expansion cohort
One cycle 1 DLT
Constitutional adverse events include fatigue, fever, anxiety
Restlessness is a neurologic adverse event
Occurred in expansion cohort.
Relatively few patients experienced grade 3 or 4 non-hematologic adverse events. Injection-site reactions are reported as dermatological adverse events, and were noted to occur at a higher rate at the 1200 μg dose of selenious acid. As a consequence, higher doses of selenious acid were subsequently administered through a central venous catheter.
Dose reductions were required in four patients: two patients had a 25% dose reduction of paclitaxel; one patient had a 25% dose reduction of carboplatin; and one patient received AUC 5 for all cycles. Treatment was discontinued early in 6 patients due to treatment-related toxicity (3 bone marrowrelated events, 2 grade 2 neuropathy, 1 carboplatin hypersensitivity reaction). Supplementary Table 2 lists reasons for treatment discontinuation in all patients who terminated therapy. Interestingly, only one patient receiving the highest dose of selenium terminated treatment early (ie, after 4 cycles) and this was due to grade 3 neuropathy.
Only one of the first 6 patients receiving selenious acid at the 5000 μg Se dose experienced a cycle 1 DLT and an MTD was not reached in this study. In view of the favorable safety profile seen with selenious acid doses up to and including 5000 μg Se, the protocol was amended to explore a treatment regimen including the 5000 μg dose in a dose expansion cohort (n=9). Of the additional 3 patients enrolled in the expansion cohort, one patient experienced a cycle 1 DLT (grade 3 leukopenia). In light of these results, a selenious acid dose of 5000 μg Se is suggested as the dose to be evaluated in a phase II study.
Selenium pharmacokinetics
This study is the first to evaluate the pharmacokinetics of selenious acid in women with gynecologic cancer treated with standard chemotherapy of paclitaxel and carboplatin. In order to describe the pharmacokinetics, the baseline Se concentration at time zero was subtracted from the measured values and the resulting values were subjected to pharmacokinetic estimates. Use of the baseline value to estimate the plasma selenium level over the course of several days is supported by a study conducted in healthy women showing minimal variation in plasma selenium levels over several weeks in nonpregnant women, and over several months in pregnant women [25]. The estimated selenium pharmacokinetic parameters are listed in Table 4. The baseline plasma concentration of selenium ranged from 76141μg/L, (average±SD 116.6±21.2), which is similar to values previously reported in the literature for an American population [26]. Plasma Se levels at the initial cohorts 50, 100, 200 μg and 400 μg doses were ‘noisy’ and almost within the baseline fluctuations. Therefore, the pharmacokinetics of selenious acid was performed only in patients treated with Se doses of 800, 1000, 1200, 2000, and 5000 μg. The average plasma levels of Se in different selenious acid dose cohorts are presented in Supplementary Figure 1. Selenium concentration in plasma increased steadily until the end of infusion and thereafter declined gradually with an average plasma half-life of 25 h (range 8.2–74.4 h). This finding is similar to the median plasma half-life of 18.25 h reported from pharmacokinetic analyses of data from a phase I trial of IV sodium selenite administered to patients with a variety of advanced cancers [27]. The maximum Se concentration (Cmax) in plasma exhibited a dose-related increase (Supplementary Figure 1; Table 4). The maximal concentration of plasma selenium observed in patients receiving the 5000 μg dose of Se as selenious acid was 667 μg/L, although this concentration decreased by approximately half within 24 h. The time to maximum concentration (Tmax) corresponded to the time of end of infusion, which was 5–5.2 h. Area under concentration-time curves (AUCs) showed a dose-dependent linear increase during cycle 1 and cycle 2 (Table 4). The average clearance and the 95% confidence intervals (lower, upper) of selenium in cycle 1 and cycle 2 were 478 (279.7, 623.0) L/h and 692.4 (416.4, 898.2) L/h, respectively.
Table 4.
Plasma Selenium Pharmacokinetic ParametersA
| Dose(μg) | Cmax (μg/L) | AUC (μg/mL*h) | Half- life (T1/2 h) | Clearance (L/h) | ||||
|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C1 | C2 | C1 | C2 | C1 | C2 | |
| 800 | 101.3 ±43.9 |
72.2 ±18.6 |
2562.7 ±2191 |
1038.9 ±613.9 |
19.1 ±9.3 |
23.2 ±19.2 |
527.7 ±575.8 |
841.5 ±654.1 |
| 1000 | 101.6 ±11.5 |
90.3 ±5.5 |
2014.2 ±602.1 |
1396.3 ±1225.8 |
34.4 ±7.4 |
17.5 ±10.6 |
291.0 ±114.1 |
976.9 ±542.6 |
| 1200 | 162.8 ±24.9 |
154.4 ±47.4 |
3763.7 ±2319.9 |
3000.4 ±2133.5 |
35.1 ±26.4 |
38.1 ±25.8 |
432.8 ±370.8 |
552.9 ±450.3 |
| 2000 | 269.8 96.5± |
230.9 ±43.9 |
5090.7 ±1864.8 |
4578.5 ±450.2 |
28.5 ±0.1 |
18.8 ±1.9 |
305.6 ±115.1 |
390.8 ±111.3 |
| 5000 | 537.4 ±90.4 |
517.9 ±92.6 |
10950.2 ±3614.7 |
9552.0 ±1303.2 |
21.2 ±6.7 |
19.3 ±6.7 |
416.8 ±198.8 |
443.8 ±92.3 |
The pharmacokinetic parameters were estimated using Se concentrations derived after baseline value was subtracted from the measured concentration at each time point (n=5, 800 μg); (n=3, 1000 μg); (n=5, 1200 μg); (n=3, 2000 μg); (n=4, 5000 μg). Cmax- maximum selenium concentration; AUC- area under the curve; T1/2- Half-life; CL- average clearance; C- cycle.
Carboplatin pharmacokinetics
Carboplatin pharmacokinetic parameters were determined during the first two cycles of therapy. The AUC for the first dose was 5, and was increased to 6 for the second dose. The pharmacokinetics parameters associated with carboplatin were evaluated in 33 patients during the first cycle and 27 patients during the second cycle (Table 5). There was variation in AUCs between patients, the average observed AUC at cycle 1 was 4.5 (95% CI, 4.21, 4.80), and 5.74 at cycle 2 (95% CI, 5.31, 6.17) of the target AUC (Table 5). Between cycle 1 (AUC=5) and cycle 2 (AUC=6), the estimated pharmacokinetic parameters, average clearance and half-life, showed very little difference (<5%), and the 95% CIs for clearance and half-life were (123, 154 mL/min) vs (120, 147 mL/min) and (253, 371 min) vs (225, 384 min), respectively, thus suggesting that selenium does not affect carboplatin pharmacokinetics.
Table 5.
Plasma Carboplatin (Ultrafiltrate) Pharmacokinetic ParametersA
| Patient # | Se Dose (μg) | AUC (mg/ml*min) | Half-life (T½ min) | Clearance (mL/min) | |||
|---|---|---|---|---|---|---|---|
| C1 | C 2 | C1 | C2 | C1 | C2 | ||
| 1 | 50 | 3.93 | 6.24 | 288 | 245 | 187 | 149 |
| 2 | 50 | 5.23 | 4.92 | 263 | 257 | 120 | 128 |
| 4 | 100 | 5.65 | 5.95 | 383 | 259 | 99.8 | 140 |
| 5 | 100 | 5.73 | 4.9 | 660 | 344 | 99.4 | 107 |
| 6 | 100 | 6.89 | 5.51 | 823. | 1302 | 81.6 | 73 |
| 7 | 100 | 4.37 | 6.69 | 423 | 237 | 152.9 | 135 |
| 9 | 100 | 3.86 | 5.19 | 209 | 275 | 179.4 | 160 |
| 10 | 200 | 3.95 | 5.40 | 208 | 252 | 131.1 | 132 |
| 11 | 200 | 4.1 | 5.10 | 343 | 353 | 95.7 | 103 |
| 12 | 200 | 3.7 | 238 | 161 | |||
| 13 | 200 | 5.3 | 6.50 | 887 | 277 | 90 | 122 |
| 14 | 400 | 4.7 | 4.0 | 239 | 268 | 127 | 168 |
| 15 | 400 | 4.4 | 6.5 | 247 | 279 | 159 | 134 |
| 16 | 400 | 3.8 | 4.0 | 282 | 254 | 270 | 205 |
| 17 | 800 | 4.0 | 195 | 124 | |||
| 18 | 800 | 3.1 | 270 | 175 | |||
| 19 | 800 | 4.4 | 6.1 | 230 | 239 | 113 | 108 |
| 20 | 800 | 4.0 | 291 | 148 | |||
| 21 | 800 | 4.0 | 5.1 | 352 | 295 | 186 | 180 |
| 22 | 800 | 5.9 | 4.9 | 315 | 291 | 107 | 118 |
| 23 | 800 | 4.6 | 4.8 | 277 | 245 | 171 | 170 |
| 24 | 1000 | 5.4 | 5.7 | 290 | 266 | 182 | 158 |
| 25 | 1000 | 5.2 | 5.7 | 217 | 253 | 107 | 107 |
| 26 | 1000 | 5.1 | 8.99 | 266 | 274 | 92 | 82 |
| 27 | 1200 | 3.58 | 251 | 137 | |||
| 32 | 1200 | 4.47 | 250 | 104 | |||
| 33 | 1200 | 3.32 | 4.81 | 270 | 243 | 159 | 142 |
| 35 | 2000 | 4.2 | 4.8 | 280 | 257 | 241 | 211 |
| 36 | 2000 | 5.35 | 7.45 | 258 | 261 | 101 | 114 |
| 37 | 5000 | 4.36 | 6.9 | 172 | 242 | 109 | 98 |
| 38 | 5000 | 3.91 | 6.7 | 257 | 254 | 127 | 153 |
| 39 | 5000 | 4.11 | 6.23 | 173 | 247 | 129 | 113 |
| 40 | 5000 | 3.99 | 5.95 | 182 | 254 | 92 | 95 |
| Average ± (SD) | 4.5 (0.8) | 5.74 (1.08) |
312 (166) | 305 (201) | 138 (44) | 134 (35) | |
| Median | 4.4 | 5.7 | 266 | 257 | 127 | 132 | |
| 95% CI of Mean | (4.21–4.80) | (5.31–6.17) | (253–371) | (225–384) | (123–154) | (120–147) | |
AUC- area under the curve; C1- Cycle 1; C2 – Cycle 2. Carboplatin AUC=5 in Cycle 1 and AUC=6 in Cycle
2. SD- standard deviation, 95% CI- 95% confidence interval (lower, upper).
Selenoprotein P and glutathione peroxidase determination
Serum glutathione peroxidase levels did not change significantly after selenium treatment compared with pretreatment levels. Similarly, no changes in selenoprotein P levels were detected after each cycle of selenium compared with baseline (data not shown). These results, together with the measured baseline selenium levels (Supplementary Figure 1), suggest that patients were not selenium deficient prior to study enrollment.
Clinical response
A summary of the results of the clinical response evaluation is presented in Supplementary Table 3. The median PFS for 28 patients with stage III and IV malignancies was 15 months (95% CI, 10.9 – 34.5 months; Supplementary Figure 2). Thirty-three patients had elevated serum CA-125 at initiation of therapy; 21/33 of these patients had normalization of CA-125 (< 35 U/ml) after cycle 2 [n=14], and after cycle 6 [n= 7]).
Twelve patients enrolled in the study were tested for germline deleterious BRCA alterations. Of the 3 patients found to have a deleterious mutation in either BRCA1 or BRCA2, one patient experienced a PR with an overall survival (OS) of 79 months, while two patients receiving adjuvant therapy are alive with disease at 81 and 105 months. Interestingly, seven of the nine patients in this tested group without a deleterious germline BRCA1/2 mutation experienced prolonged OS ranging from 60–120 months. Of those seven patients, three patients remain with no evidence of disease at 62, 69, and 114 months, while one patient is alive with disease at 120 months. Only one patient enrolled in the study subsequently developed another cancer; this patient developed breast cancer in the setting of a deleterious germline BRCA mutation.
Correlative studies
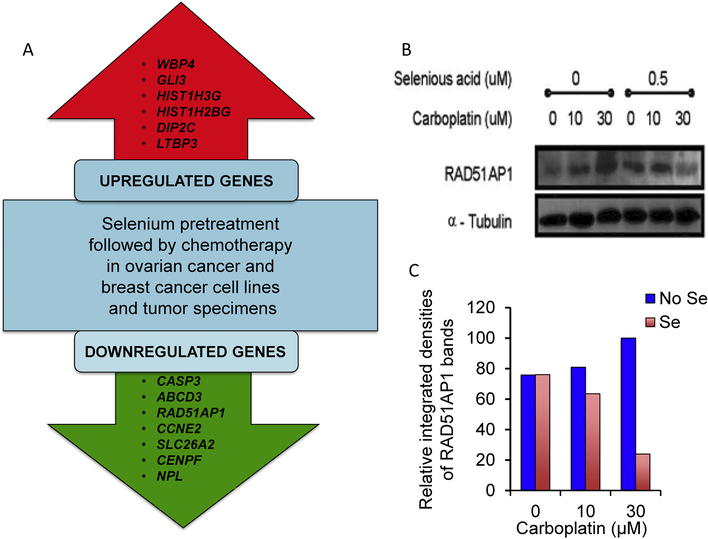
Differential RNA expression in breast and ovarian cancer cell lines, as well as two sets of pre- and posttreatment tumor specimens from patients, were evaluated. The doses of selenious acid and carboplatin used in the cell studies were selected on the basis of results of MTT assays (see Supplementary Materials and Methods; data not shown). The gene expression analysis was limited to those mRNAs that converged with either over- or under-expression after selenious acid plus chemotherapy exposure in both cell lines and patient tumors compared with the control specimens (Figure 1A). The downregulation of several genes was of particular interest within the context of chemosensitivity/chemoresistance.
Figure 1:
Alterations in gene and protein expression following treatment with selenious acid plus chemotherapy. A. Genes up- and down-regulated following treatment with selenium plus chemotherapy. Shown are only those genes which exhibited an increase or decrease in expression in all samples tested by microarray analysis (ie, breast and ovarian cancer cell lines, as well as patient tumor specimens) following exposure to selenious acid plus chemotherapy compared with the control samples. CASP3: Caspase 3, apoptosis-related cysteine peptidase; ABCD3: ATP-binding cassette, sub-family D (ALD), member 3; RAD51AP1: RAD51 associated protein 1; CCNE2: Cyclin E2; SLC26A2: Solute carrier family 26 (sulfate transporter), member 2; CENPF: Centromere protein F, 350/400 KDa (mitosin); NPL: Nacetylneuraminate pyruvate lyase (dihydrodipicolinate synthase); WBP4: WW domain binding protein 4; GLI3: Glioma-associated oncogene family zinc finger 3 (Greig cephalopolysyndactyly syndrome); HIST1H3G: Histone Cluster 1 H3 family member G; HIST1H2BG: Histone cluster 1 H2B family member G; DIP2C: Disco interacting protein 2 homolog C; LTBP3: Latent transforming growth factor beta binding protein 3. B. Downregulation of RAD51AP1 protein expression in the presence of selenious acid plus carboplatin. Western blot of MCF-7/Adr cells showing changes in RAD51AP1 protein expression following treatment with selenious acid, carboplatin, and the combination of selenious acid and chemotherapy. C. Quantification of Western blot image shown in Figure 1B using ImageJ software (https://imagej.nih.gov/ij/) available from NIH. The corrected (eg, background subtracted) integrated densities of the RAD51AP1 bands are plotted at three carboplatin concentrations with (red bars) and without selenious acid (blue bars). Results are normalized with respect to the integrated density of the RAD51AP1 band without selenious acid (set at 100%).
Results of immunoblotting experiments evaluating RAD51AP1 protein expression in lysates from MCF7/Adr cells pretreated with selenious acid followed by chemotherapy compared with no treatment or carboplatin chemotherapy alone showed substantially lower expression of RAD51AP1 at higher concentrations of carboplatin when selenious acid was present vs not. Figure 1B shows that cells treated with increasing amounts of carboplatin responded with an increase in RAD51AP1 protein expression. However, when they were pretreated with selenious acid, the expression of RAD51AP1 decreased at higher concentrations of carboplatin. This result is consistent with the results of the gene expression profiling studies showing decreased expression of RAD51AP1 when breast and ovarian cancer cells or patient’s tumor were treated with the combination of selenium and chemotherapy compared with controls.
Discussion
The results of this phase I trial demonstrate that selenious acid can be safely administered to patients with advanced gynecologic malignancies receiving carboplatin and paclitaxel chemotherapy at doses up to 5000 μg Se. While none of the patients enrolled in this study had grade 3 or 4 thrombocytopenia, 66.6% experienced grade 3 or 4 neutropenia. For comparison, hematologic toxicities observed in several Gynecologic Oncology Group (GOG) trials of chemo-naive patients with advanced ovarian cancer receiving carboplatin/paclitaxel combination chemotherapy, rates of grade 3 or 4 neutropenia or granulocytopenia were 89% of patients with optimally resected stage III ovarian cancer receiving thrice weekly carboplatin/paclitaxel as reported by Ozols et al. (GOG 0158), and 72% and 83% for patients enrolled in the GOG 0262 as reported by Chan et al. for patients receiving weekly (dose-dense) vs every 3 week regimens, respectively [1, 3]. Burger et al. reported (GOG 0218) grade 4/5 neutropenia rates of 63%, irrespective of bevacizumab use for patients receiving carboplatin/paclitaxel on a once every 3week schedule [2]. Reported rates of grade 3/4 thrombocytopenia in these GOG studies varied between 16% and 39%, although they were not included in the GOG 0218 trial report [1–3]. Although it cannot be concluded from these data that selenious acid pretreatment ameliorated the hematologic toxicity of chemotherapy, the observed rates of chemotherapy-associated neutropenia and thrombocytopenia observed in this study are somewhat lower compared with historical controls from the large GOG randomized trials [1–3]. Interestingly, it has recently been reported that administration of relatively low daily doses of selenium glycine over a period of one month was associated with increased neutrophil counts in children with solid tumor cancers [28]. It has also been proposed that simultaneous seleniuminduced protection of normal cells from cytotoxic damage and selenium-induced enhancement of cytotoxic damage to TP53-mutant cancer cells may be related to p53-mediated upregulation of DNA repair [29]. Such a hypothesis may be reasonable in the setting of gynecologic cancers, many of which are p53 deficient due to inactivating TP53 mutations.
Some of the reported adverse effects of acute ingestion of very high quantities of selenium include hypotension, tachycardia, cardiac abnormalities, abdominal symptoms such as nausea, vomiting, and pain, pulmonary edema, and neurologic symptoms [23]. Long-term exposure to high dietary levels of selenium has also been associated with brittleness and loss of nails and hair, gastrointestinal disturbances, and neurologic symptoms [30]. In this study, rates of most grade 3/4 adverse events were similar to those reported in several trials evaluating patients with advanced gynecologic malignancies receiving carboplatin and paclitaxel combination chemotherapy [1, 3, 31, 32]. Nevertheless, we cannot exclude the possibility that some of the adverse events observed in this study were associated with administration of sodium selenite.
With regard to pharmacokinetic measurements, addition of selenious acid on day 1 did not affect the pharmacokinetics of carboplatin administered on day 3. Given the estimated half-life of plasma selenious acid/selenite, plasma levels of selenium on day 3 were substantially lower than the maximal concentrations observed during day 1 of its administration. Nevertheless, the administration of selenious acid on day 1 is also likely to influence tissue stores of this element [33]. Of note, a study in patients with aggressive non-Hodgkin’s lymphoma undergoing their first treatment with chemotherapy, radiotherapy or both showed that a higher serum Se concentration at presentation was a positive predictor for dose delivery, treatment response and long-term survival [34].
Patients with stage III or stage IV ovarian cancer receiving the combination of selenious acid, carboplatin and paclitaxel had a median PFS of 15 months which is similar to the median PFS times of 14.1 and 14.9 months observed for the bevacizumab-containing arms of the GOG 0218 and GOG-0262 (dose-dense) trials, respectively [1–3], although PFS times were shorter in the non-bevacizumab-containing arms of those studies (10.3 months in both studies). Nevertheless, while these data support the conclusion that pretreatment with selenious acid followed by administration of standard chemotherapy did not negatively impact clinical outcomes, it is not possible to conclude that seleniuminduced an increase in PFS, given that the study was not powered to answer this question. However, the few cases of patients with ovarian cancer exhibiting a long-term response in this trial are noteworthy. Although this finding should be considered anecdotal, it is consistent with a similar observation made in a phase I trial of selenomethionine administered in combination with irinotecan in patients with solid tumors [17], and a phase I trial of sodium selenite in patients with advanced cancers [27].
In this context it is also worth noting that, despite previous findings that patients with germline mutations in BRCA are more likely to be sensitive to platinum-based chemotherapy and to achieve better clinical outcomes due to pre-existing impairments in the process of homologous recombination [35], the three patients with deleterious germline mutations in either BRCA1 or BRCA2 did not appear to receive greater benefit from platinum-based chemotherapy plus selenium compared with the group without these mutations. It is tempting to suggest that selenium may interfere with DNA repair in a manner similar to BRCA deficiency, particularly in light of the observed changes in gene expression related to the RAD51AP1 gene, thereby eliminating the advantage of BRCA deficiency in the setting of carboplatin chemotherapy. However, the number of patients with BRCA1/2-related cancers enrolled in this study is too low to draw such a conclusion.
The putative underlying modes of action of selenium as a component of cancer treatment are likely to be multifactorial. Some of the changes observed in the expression of several genes after selenious acid exposure are consistent with a selenium-related enhancement of therapeutic effect or its interference in the development of chemoresistance. Genes shown in this study to be downregulated with selenium pretreatment that may enhance sensitivity to chemotherapy and/or decrease disease aggressiveness in ovarian cancer include RAD51AP1, ABCD3, and CCNE2. RAD51AP1, the protein that is encoded for by the gene RAD51AP1, interacts with RAD51 and has been shown to have a role in mitotic homologous recombination and double-stranded DNA repair [36]. RAD51AP1 has also been reported to be upregulated in ovarian cancer [37]. Furthermore, knockdown of RAD51 has been shown to increase sensitivity to anticancer agents that cause DNA damage and/or interfere in homologous recombination processes [38]. Another gene shown to be downregulated in this setting is ABCD3 which encodes for a transporter protein previously shown to be expressed at higher levels in high-grade serous ovarian cancer compared with other subtypes [39]. With respect to CCNE2, a known oncogene in many cancers which encodes for cyclin proteins that regulate cell cycle progression, its upregulation has been associated with poor prognosis in ovarian cancer [40].
In conclusion, the results of this study support the safety of adding high-dose selenious acid to the combination of carboplatin and paclitaxel in the treatment of patients with advanced gynecologic malignancies. A phase II trial using selenious acid or sodium selenite at a dose of 5000 μg Se is being planned.
Supplementary Material
Highlights:
Selenious acid (5000 μg Se) can be safely combined with carboplatin/paclitaxel
Pharmacokinetics of carboplatin on day 3 is not affected by selenious acid on day 1
Average plasma half-life of selenious acid/sodium selenite is 25 hours
Selenious acid administered with carboplatin may downregulate RAD51AP1
Acknowledgements
The authors would like to thank Eric Rubin, MD, formerly of the Rutgers Cancer Institute of New Jersey and currently of Merck Research Laboratories, Inc. in Kenilworth, NJ for helpful discussions and for reviewing an earlier draft of the manuscript, Raymond F. Burk, MD and Kristina E. Hill of Vanderbilt University for their help with selenoprotein P and glutathione peroxidase assays, and Gunter Schemmann, PhD for his assistance with the microarray analyses. In addition, the authors would like to acknowledge Susan Moench and Vaishali Kulkarni for their assistance with drafting and editing the manuscript. The authors would like to dedicate this work in memoriam of Merrill J. Egorin, MD who generously advised the PI during the conception of the study and early analyses of the data.
Funding
This trial was supported by New Jersey Commission on Cancer Research (03–1093-CCR-EO) and the following shared resources: Laboratory Support Services and Biometrics, Biospecimen Repository Service, and the Office of Human Research Services funded by NIH grant P30CA072720.
Footnotes
Conflicts of Interest
The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chan JK, Brady MF, Penson RT, Huang H, Birrer MJ, Walker JL, et al. Weekly vs. Every-3-Week Paclitaxel and Carboplatin for Ovarian Cancer. N Engl J Med. 2016;374:738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. [DOI] [PubMed] [Google Scholar]
- [3].Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. [DOI] [PubMed] [Google Scholar]
- [4].Frenkel GD, Caffrey PB. A prevention strategy for circumventing drug resistance in cancer chemotherapy. Curr Pharm Des. 2001;7:1595–614. [DOI] [PubMed] [Google Scholar]
- [5].Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–83. [DOI] [PubMed] [Google Scholar]
- [7].Fitzpatrick JM, de Wit R. Taxane mechanisms of action: potential implications for treatment sequencing in metastatic castration-resistant prostate cancer. Eur Urol. 2014;65:1198–204. [DOI] [PubMed] [Google Scholar]
- [8].Gromer S, Eubel JK, Lee BL, Jacob J. Human selenoproteins at a glance. Cell Mol Life Sci. 2005;62:2414–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vinceti M, Filippini T, Cilloni S, Crespi CM. The Epidemiology of Selenium and Human Cancer. Adv Cancer Res. 2017;136:1–48. [DOI] [PubMed] [Google Scholar]
- [10].Vinceti M, Filippini T, Del Giovane C, Dennert G, Zwahlen M, Brinkman M, et al. Selenium for preventing cancer. Cochrane Database Syst Rev. 2018;1:CD005195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Caffrey PB, Frenkel GD. Selenium compounds prevent the induction of drug resistance by cisplatin in human ovarian tumor xenografts in vivo. Cancer Chemother Pharmacol. 2000;46:74–8. [DOI] [PubMed] [Google Scholar]
- [12].Caffrey PB, Frenkel GD. Prevention of carboplatin-induced resistance in human ovarian tumor xenografts by selenite. Anticancer Res. 2013;33:4249–54. [PubMed] [Google Scholar]
- [13].Evans SO, Khairuddin PF, Jameson MB. Optimising Selenium for Modulation of Cancer Treatments. Anticancer Res. 2017;37:6497–509. [DOI] [PubMed] [Google Scholar]
- [14].Brozmanova J, Manikova D, Vlckova V, Chovanec M. Selenium: a double-edged sword for defense and offence in cancer. Arch Toxicol. 2010;84:919–38. [DOI] [PubMed] [Google Scholar]
- [15].Manikova D, Letavayova LM, Vlasakova D, Kosik P, Estevam EC, Nasim MJ, et al. Intracellular diagnostics: hunting for the mode of action of redox-modulating selenium compounds in selected model systems. Molecules. 2014;19:12258–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fakih MG, Pendyala L, Smith PF, Creaven PJ, Reid ME, Badmaev V, et al. A phase I and pharmacokinetic study of fixed-dose selenomethionine and irinotecan in solid tumors. Clin Cancer Res. 2006;12:1237–44. [DOI] [PubMed] [Google Scholar]
- [17].Fakih MG, Pendyala L, Brady W, Smith PF, Ross ME, Creaven PJ, et al. A Phase I and pharmacokinetic study of selenomethionine in combination with a fixed dose of irinotecan in solid tumors. Cancer Chemother Pharmacol. 2008;62:499–508. [DOI] [PubMed] [Google Scholar]
- [18].Sieja K, Talerczyk M. Selenium as an element in the treatment of ovarian cancer in women receiving chemotherapy. Gynecol Oncol. 2004;93:320–7. [DOI] [PubMed] [Google Scholar]
- [19].Hu YJ, Chen Y, Zhang YQ, Zhou MZ, Song XM, Zhang BZ, et al. The protective role of selenium on the toxicity of cisplatin-contained chemotherapy regimen in cancer patients. Biol Trace Elem Res. 1997;56:331–41. [DOI] [PubMed] [Google Scholar]
- [20].Asfour IA, Fayek M, Raouf S, Soliman M, Hegab HM, El-Desoky H, et al. The impact of high-dose sodium selenite therapy on Bcl-2 expression in adult non-Hodgkin’s lymphoma patients: correlation with response and survival. Biol Trace Elem Res. 2007;120:1–10. [DOI] [PubMed] [Google Scholar]
- [21].Corcoran NM, Hovens CM, Michael M, Rosenthal MA, Costello AJ. Open-label, phase I doseescalation study of sodium selenate, a novel activator of PP2A, in patients with castration-resistant prostate cancer. Br J Cancer. 2010;103:462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Forceville X, Laviolle B, Annane D, Vitoux D, Bleichner G, Korach JM, et al. Effects of high doses of selenium, as sodium selenite, in septic shock: a placebo-controlled, randomized, double-blind, phase II study. Crit Care. 2007;11:R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nuttall KL. Evaluating selenium poisoning. Ann Clin Lab Sci. 2006;36:409–20. [PubMed] [Google Scholar]
- [24].Selenium. https://ods.od.nih.gov/factsheets/Selenium-Consumer/. Accessed on June 30
- [25].Karita K, Takano T, Satoh K, Suzuki T. Variations in plasma selenium levels as a result of the menstrual cycle and pregnancy in healthy Japanese women. Biol Trace Elem Res. 2004;99:83–91. [DOI] [PubMed] [Google Scholar]
- [26].Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168:404–10. [DOI] [PubMed] [Google Scholar]
- [27].Brodin O, Eksborg S, Wallenberg M, Asker-Hagelberg C, Larsen EH, Mohlkert D, et al. Pharmacokinetics and Toxicity of Sodium Selenite in the Treatment of Patients with Carcinoma in a Phase I Clinical Trial: The SECAR Study. Nutrients. 2015;7:4978–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rocha KC, Vieira ML, Beltrame RL, Cartum J, Alves SI, Azzalis LA, et al. Impact of Selenium Supplementation in Neutropenia and Immunoglobulin Production in Childhood Cancer Patients. J Med Food. 2016;19:560–8. [DOI] [PubMed] [Google Scholar]
- [29].Fischer JL, Mihelc EM, Pollok KE, Smith ML. Chemotherapeutic selectivity conferred by selenium: a role for p53-dependent DNA repair. Mol Cancer Ther. 2007;6:355–61. [DOI] [PubMed] [Google Scholar]
- [30].Morris JS, Crane SB. Selenium toxicity from a misformulated dietary supplement, adverse health effects, and the temporal response in the nail biologic monitor. Nutrients. 2013;5:1024–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pignata S, Scambia G, Katsaros D, Gallo C, Pujade-Lauraine E, De Placido S, et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014;15:396–405. [DOI] [PubMed] [Google Scholar]
- [32].Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–8. [DOI] [PubMed] [Google Scholar]
- [33].Blodgett DJ, Bevill RF. Acute selenium toxicosis in sheep. Vet Hum Toxicol. 1987;29:233–6. [PubMed] [Google Scholar]
- [34].Last KW, Cornelius V, Delves T, Sieniawska C, Fitzgibbon J, Norton A, et al. Presentation serum selenium predicts for overall survival, dose delivery, and first treatment response in aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2003;21:2335–41. [DOI] [PubMed] [Google Scholar]
- [35].Bolton KL, Chenevix-Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA. 2012;307:382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dunlop MH, Dray E, Zhao W, San Filippo J, Tsai MS, Leung SG, et al. Mechanistic insights into RAD51-associated protein 1 (RAD51AP1) action in homologous DNA repair. J Biol Chem. 2012;287:12343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Miles GD, Seiler M, Rodriguez L, Rajagopal G, Bhanot G. Identifying microRNA/mRNA dysregulations in ovarian cancer. BMC Res Notes. 2012;5:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yang Z, Waldman AS, Wyatt MD. Expression and regulation of RAD51 mediate cellular responses to chemotherapeutics. Biochem Pharmacol. 2012;83:741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Elsnerova K, Mohelnikova-Duchonova B, Cerovska E, Ehrlichova M, Gut I, Rob L, et al. Gene expression of membrane transporters: Importance for prognosis and progression of ovarian carcinoma. Oncol Rep. 2016;35:2159–70. [DOI] [PubMed] [Google Scholar]
- [40].Xie L, Li T, Yang LH. E2F2 induces MCM4, CCNE2 and WHSC1 upregulation in ovarian cancer and predicts poor overall survival. Eur Rev Med Pharmacol Sci. 2017;21:2150–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.