Abstract
Objectives:
1. Evaluate changes in subjective symptoms in patients following transmastoid canal plugging for superior semicircular canal dehiscence (SSCD) syndrome.
2. Quantify changes in hearing in patients who have undergone transmastoid canal plugging for SSCD syndrome.
Study Design:
Case series with chart review
Setting:
Single tertiary care institution
Subjects and Methods:
We retrospectively reviewed patients with SSCD who underwent repair with canal plugging via transmastoid approach between January 2012 and January 2017. Symptom severity was assessed prospectively (autophony, sound-/pressure-induced vertigo, disequilibrium, aural fullness, and pulsatile tinnitus) and after surgery. Pure tone and speech audiometry were measured before and after surgery. Two-sided Wilcoxon rank-sum tests were used to evaluate changes in subjective symptoms and audiometric outcomes.
Results:
Seventeen patients (19 ears) met inclusion criteria. The superior canal was successfully plugged via transmastoid approach in all cases. Patients reported a statistically significant improvement in autophony, vertigo, aural fullness, and pulsatile tinnitus (p<<0.01), without significant improvement in disequilibrium rating (p=0.06). There were no changes noted in PTA or WRS; however, there was a statistically significant improvement in air-bone gap (ABG) at 250 Hz of 10.9 dB (p=0.04) with 12.9 dB improvement in air-conduction thresholds (p=0.02) and no difference (0.9 dB, p=0.9) in bone-conduction thresholds.
Conclusion:
In our study, patients with SSCD demonstrated excellent hearing outcomes and resolution of most otologic symptoms after surgical repair. Transmastoid canal plugging, which has been described to date in only in smaller case series, is a safe and effective alternative to the traditional middle cranial fossa approach.
Introduction:
Superior semicircular canal dehiscence (SSCD) syndrome was first described only recently1, and has been called a “great otologic mimicker2“ with its clinical picture in some cases imitating a variety of other otologic disease, including Meniere’s disease, patulous Eustachian tube, and otosclerosis. Patients who experience SSCD syndrome typically experience sound- and/or pressure-induced vertigo, disequilibrium, autophony, hyperacusis, and aural fullness1–7. Clinically, patients often present with a low-frequency air-bone gap (ABG), decreased cervical vestibular evoked myogenic potential (VEMP) thresholds, and enhanced ocular VEMP amplitudes2,7. Computed tomography (CT) imaging of the temporal bone identifies absent or thinned bone overlying the superior canal, and this area of decreased cochlear input impedance provides the basis for the “third-window hypothesis,” in which clinical findings are explained by abnormal shunting of acoustic energy through the hyper-compliant area of bony dehiscence8–10.
Multiple clinical studies have demonstrated that surgical intervention can provide relief of clinical symptoms1,6,7,11–23, though no technique or approach has yet shown superior outcomes across the literature3–5,24,25. Studies continue to be limited based on the small sample size reported, making conclusions regarding operative technique difficult to assess. Here, we sought to investigate outcomes for a larger group of patients treated for SSCD syndrome. The purpose of this study was to quantify changes in both objective hearing outcomes and subjective symptom report in patients undergoing canal plugging via a transmastoid surgical approach.
Methods:
A retrospective chart review was conducted for all adult patients who treated for SSCD syndrome by the senior author between January 2012 and January 2017. Adult patients who underwent alternative surgery (either middle fossa approach or canal resurfacing) and those that declined surgical intervention were not included. No other exclusion criteria were used. The University of Colorado Anschutz Medical Campus Institutional Biosafety Committee and Review Board approved this study (COMIRB Exempt # 17–0210).
Clinical Management:
The diagnosis of SSCD syndrome was confirmed in all patients by a correlation of clinical symptoms with positive audiometric, VEMP, and CT imaging studies. Audiometric testing consisted of a standard battery, including pure tone thresholds, speech recognition testing, tympanometry, and acoustic reflexes. Pure tone audiometric thresholds were obtained via both air conduction (250–800 Hz) and bone conduction (500–4000 Hz) with masking as required. Audiometers are calibrated to assess negative bone conduction thresholds and audiologists routinely assess bone conduction thresholds to the limit of the audiometer and transducer (−10 dB HL). A standard three-frequency (500, 1000, and 2000 Hz) air-conduction pure tone average (PTA) was calculated for each audiogram. All patients included in the study had both preoperative and postoperative audiometric results. Preoperative cervical VEMP measurements showed decreased thresholds on the affected side(s) for all patients, and high-resolution CT scans of the temporal bone obtained with 0.6 – 1.0 mm thick slices and reformatted in planes of Stenver and Pöschl confirmed the presence of bony dehiscence overlying the superior semicircular canal.
Subjective symptoms for five common complaints are regularly assessed in all patients as part of the work-up for SSCD syndrome. Patients are asked to rate the severity of autophony, ear fullness, pulsatile tinnitus, sound- and/or pressure-induced vertigo, and disequilibrium on a scale from 0 to 5, with 5 being the most severe and 0 representing an absence of the symptom. The patients are then asked to rate the symptoms as part of the postoperative evaluation at each follow-up appointment.
Surgical Technique:
Transmastoid canal plugging is performed by the senior author under general anesthesia in an outpatient surgical setting. The procedure begins with a standard mastoidectomy via a postauricular incision. After subperiosteal flaps are raised, a cutting burr is used to identify and skeletonize the sigmoid sinus, middle cranial fossa tegmen, and vestibular labyrinth. The superior semicircular canal is then blue-lined to visualize the area where the superior canal and middle cranial fossa dura intersect. The site of the fistula is approximated, but the dura is not elevated to explore the fistula. Two fenestrations are created in the bony labyrinth on either side of the fistula, maintaining the fenestrated canal under fluid to prevent air entry into the labyrinth (see Figure 1). Autologous bone dust graft (“pate”)26 is formed from bone dust collected during cortical drilling mixed with fibrin glue; this is used to plug the two fenestration sites, taking care not to overpack the areas. Finally, thinned or dehiscent middle cranial fossa dura can be repaired with a cartilage graft and/or autologous bone dust.
Figure 1:
Intraoperative photographs during transmastoid canal plugging. The semicircular canal is opened (A). Bone pate is packed into the fenestrations (B). After plugging, a right-angled hook placed through the dehiscence can be seen through the fenestration (C).
Statistical Analysis
Postoperative changes in hearing thresholds were evaluated in accordance with the American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) minimal reporting standard for audiometric data in clinical research. Statistical analyses for changes in both audiometric data and symptom severity were completed using functions in the Statistics and Machine Learning toolbox in Matlab. Audiometric data was examined for differences in air conduction thresholds, bone conduction thresholds, PTA, speech reception thresholds (SRT), and air-bone gap levels at 250 and 500 Hz, given that changes in low frequency hearing are a frequent clinical finding of SSCD syndrome. Symptom severity rating was also examined for significant changes following surgery. Audiometric and symptom severity were compared between the pre- and post-operative setting utilizing a Wilcoxon rank-sum test (ranksum function). This measure was chosen over a t-test as it is nonparametric and does not require an assumption of normal distributions. Statistical comparisons are assessed at the α = 0.05 level unless otherwise specified.
Results:
Thirty-six patients were diagnosed with SSCD syndrome during the reviewed period. Canal dehiscence was supported in all patients by correlation of symptoms with positive audiometric data, VEMP testing, and findings on CT imaging. Of all reviewed patients, 22 underwent surgical repair following positive diagnosis. Five patients were excluded based on surgical technique: 2 underwent canal plugging via a middle fossa approach and 3 were treated with transmastoid canal resurfacing. Seventeen patients (with 19 treated ears) who underwent transmastoid canal plugging remained and were included in the review.
Of the 17 patients included in this review, 11 were female and 6 were male. The average age at presentation was 53 years, with a range of 38–77 years. Six patients presented with unilateral SSCD and 11 were determined to have bilateral disease (based on imaging); two patients with bilateral SSCD underwent sequential bilateral repair. Noted comorbidities included migraine in 9 patients and a history of prior concussion in 3 individuals. Three patients had previously underwent PE tube placement for suspected Eustachian tube dysfunction, one of whom developed a chronic perforation. Thirteen of the procedures were performed as outpatient surgery and 6 were completed with a 23-hour postoperative observation admission. Three of the procedures were revision of previous MCF resurfacing surgery. Patient characteristics are summarized in Table 1. Surgical complications are shown in Table 2, and included prolonged disequilibrium (n=2), new-onset sensorineural hearing loss (n=1), new-onset tinnitus (n=1), and wound infection (n=1). No patients were found to have change in lateral semicircular canal function as verified by postoperative head thrust testing, which is routinely assessed by the senior author as a part of the otologic physical exam in all patients.
Table 1:
Characteristics of study participants and surgical interventions
| Characteristics | Total n= 17 patients (19 ears) |
|---|---|
| Age, years (range) | 53 (38-77) |
| Sex, n (%) | |
| Male | 6 (35.3%) |
| Female | 11 (64.7%) |
| Unilateral/Bilateral Presentation, n (%) | |
| Unilateral | 6 (37.5%) |
| Bilateral | 10 (62.5%) |
| Comorbidities, n (%) | |
| Migraine | 9 (52.9%) |
| Concussion | 3 (17.6%) |
| History of pressure equalization tube | 3 (17.6%) |
| Tympanic membrane perforation | 1 (5.9%) |
| Admission, n (%) | |
| Outpatient | 11 (64.7%) |
| 23-hr Observation | 6 (35.3%) |
| Inpatient | 0 (0%) |
| Unilateral/Bilateral Procedures, n (%) | |
| Unilateral | 15 (88.2%) |
| Bilateral | 2 (11.8%) |
| Revision procedures, n (%) | 3 (17.6%) |
| Follow-up, months (range) | 9 (2-24) |
Table 2:
Surgical complications following transmastoid canal plugging for superior semicircular canal dehiscence
| Complication | Number of ears, n (%) |
|---|---|
| Prolonged disequilibrium | 2 (10.5%) |
| New-onset sensorineural hearing loss | 1 (5.3%) |
| New-onset tinnitus | 1 (5.3%) |
| Wound infection | 1 (5.3%) |
| Change in lateral canal functiona | 0 (0%) |
| Postoperative benign positional vertigo | 0 (0%) |
Change in lateral semicircular canal function by abnormal postoperative head thrust testing.
Patients are routinely seen after surgery at 1 week, 6 week, and 3 month intervals, and additionally as needed. All audiometric and subjective patient data is reported from the most recent patient follow-up, allowing for the greatest stabilization of postoperative changes. The mean patient follow-up at which time data was collected was 9 months (range: 2–24 months).
Audiometric Data:
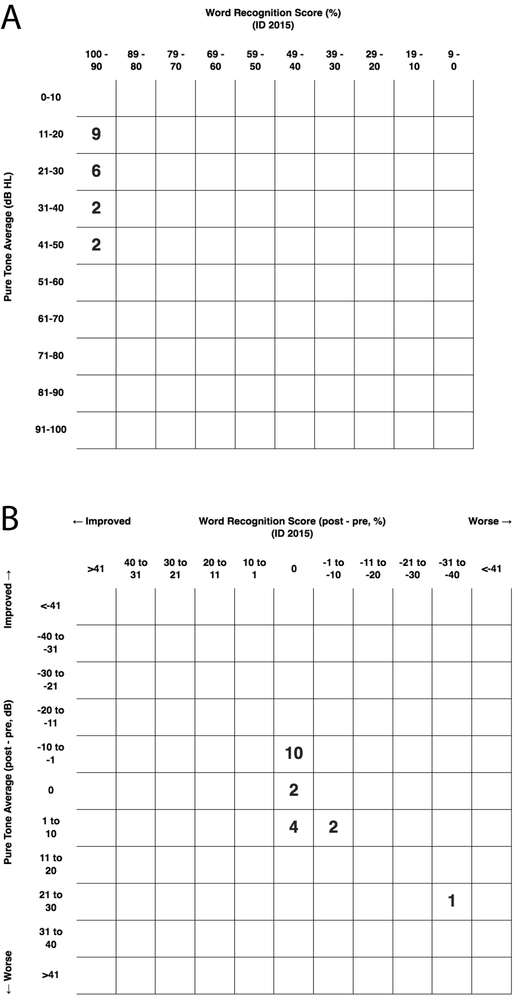
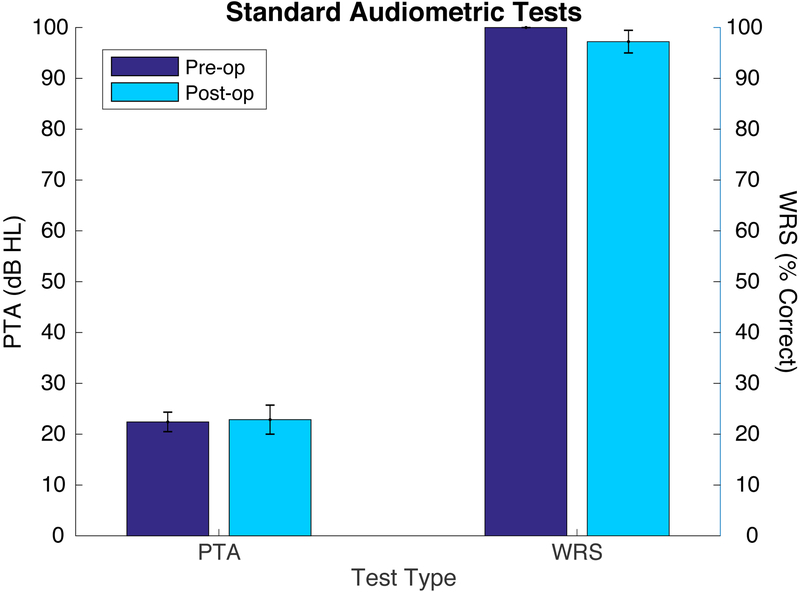
Preoperative and postoperative audiometric data for all patients are depicted as scattergrams of air-conduction pure tone average (PTA) and word recognition score (WRS), in accordance with the AAO-HNS minimal reporting standard (Figure 2). Mean PTA and WRS for all patients before and after surgery is shown in Figure 3. No statistically significant difference between pre- and post-operative results were seen.
Figure 2:
Preoperative and postoperative audiometric outcomes after transmastoid canal plugging by pure tone average (PTA) and word recognition score (WRS). The upper scattergram depicts preoperative hearing in study subjects (A), with postoperative results shown below (B).
Figure 3:
Mean preoperative (dark blue) and postoperative (light blue) results for three-frequency pure tone average (PTA) in dB HL and word recognition score (WRS) in percent correct. Error bars depict the standard error of the mean.
Given that the most frequent hearing change associated with SSCD syndrome is a low-frequency conductive hearing loss2,7, additional analysis was completed examining the 250 and 500 Hz audiometric data. Figure 4 illustrates changes in the air-conduction thresholds, bone-conduction thresholds, and ABG at 250 Hz and 500 Hz in the upper and lower panels, respectively. Though no significant change was seen in bone conduction thresholds at either frequency, a significant improvement in both air conduction thresholds and ABG was seen at 250 Hz.
Figure 4:
Mean preoperative (dark blue) and postoperative (light blue) air-conduction thresholds, bone-conduction thresholds, and air-bone gap at 250 Hz (A) and 500 Hz (B), with error bars depicting the standard error of the mean.
Subjective Report:
According to our standard clinical practice, patients reported the severity of clinical symptoms before and after surgery. Prevalence of preoperative symptoms for all study subjects is shown in Table 3, with autophony being the most common. Changes in symptom rating are shown in Figure 5. Preoperatively, autophony was rated as most severe and disequilibrium was noted to be least severe, according to patient ratings. A statistically significant decrease was noted in all symptoms, with the exception of disequilibrium (p=0.06).
Table 3:
Preoperative otologic findings in patients who underwent transmastoid canal plugging for superior semicircular canal dehiscence
| Symptom | Number of patients, n (%) |
|---|---|
| Autophony | 17 (100%) |
| Aural fullness | 14 (82.4%) |
| Pulsatile tinnitus | 15 (88.2%) |
| Sound- and/or pressure-induced vertigo | 15 (88.2%) |
| Disequilibrium | 11 (64.7%) |
| Air-bone gap >10 dB @250 Hz | 13 (76.5%) |
| Air-bone gap >10 dB @500 Hz | 9 (52.9%) |
Figure 5:
Preoperative (dark blue) and postoperative (light blue) mean results for symptom severity scores, with error bars depicting the standard error of the mean. Wilcoxon rank-sum showed statistically significant improvement in all symptoms, except for disequilibrium (p=0.06).
Discussion:
Results from this retrospective review reinforce the safety and efficacy of transmastoid canal plugging for treatment of SSCD syndrome. Postoperative complications in our data set were uncommon, with only one patient experiencing a significant worsening in hearing function. Most otologic symptoms improved following surgery, but no significant change was noted in disequilibrium. Given the significant improvement in other assessed symptoms, it is possible that this item was not specific to SSCD alone. The high prevalence of coexisting migraine in this data set as well as previously reported in the literature27 suggests that there may be additional confounding diagnoses contributing to lack of resolution of disequilibrium. Though 2 patients reported prolonged disequilibrium that required intervention with postoperative vestibular physical therapy, there did not appear to be evidence of lateral canal hypofunction on postoperative clinical exam. On average, we saw a significant improvement in air-conduction thresholds with ABG closure at low frequencies, and subjects demonstrated a significant improvement in severity of most typical SSCD syndrome symptoms. In our study, we used improvement in subjective symptom rating as a metric to determine procedure success, and all patients in this review met this criteria.
In the initial description of SSCD syndrome and its treatment, a middle cranial fossa approach was used1 and continues to be used for the benefit of direct access to the arcuate eminence12,13,17,21,23. Subsequently, the transmastoid approach was developed to avoid the risks associated with craniotomy6,7,11,14–16,18–20,22, though the resulting reduced morbidity and hospital stay are gained at the cost of requiring canal plugging prior to visualization of the fistula. No planned transmastoid plugging was avoided due to mastoid anatomy and all transmastoid pluggings were completed as planned. A single meta-analysis of surgical intervention for SSCD syndrome has been published to date, and the small number of published cases of transmastoid approach at that time precluded robust statistical conclusion regarding approach25.
Three methods for surgical repair have been described. Procedures in which the dehiscence is covered with hydroxyapatite cement (with or without fascia) are referred to as canal resurfacing1,11,16,23,28 while those in which a cartilage or bone graft alone is used are referred to as canal capping25. Canal plugging procedures involve occlusion of the lumen of the canal1,6,7,12–15,17–22,28. Most investigations suggest that canal plugging has a higher success rate than canal resurfacing25; however, lack of consensus regarding a metric for operative success and inconsistent reporting of surgical complications preclude a strong recommendation regarding a single surgical technique at this time3,4,25.
Regarding the present study, our results seem at least comparable to middle fossa canal plugging and superior to resurfacing or capping techniques for symptom control and low-frequency hearing improvement. Three patients in our review underwent MCF revision procedures. Though one patient experienced initial prolonged disequilibrium requiring vestibular physical therapy, all three had complete air-bone gap closure and described resolution of subjective symptoms at their most recent follow-up. Based on revision procedures in our dataset, transmastoid canal plugging appears well suited for revision cases where mid-fossa repair may have increased risk of sensorineural hearing loss23.
Postoperative Changes in Air-Bone Gap
The presence of an air-bone gap in patients with otherwise normal middle ear function has been attributed to a cochlear conductive impairment, in which the presence of a pressure relief window leads to a decrease in cochlear impedance9,10. In SSCD syndrome, the lack of bony covering overlying the superior canal is postulated to be the third window, and it has been shown that the cochlear response will return to baseline following plugging of the canal defect in cadaveric models29.
In our review, we saw that twelve of thirteen cases with a preoperative ABG of greater than 10 dB improved by 10 dB or more following surgery, with an average improvement in the ABG of 22 dB and a range of 10–30 dB. Some studies have shown lower rates of improvement in the ABG with a higher rate of symptom recurrence23. Taken together, these data suggest that closure of the ABG may be a good clinical marker for “solid” plugging that returns cochlear fluid compliance to normal.
Postoperative Changes in Vestibular Symptoms
Several clinical studies have shown that canal plugging procedures can alter semicircular canal function28,30. Thorough vestibular testing can bring postoperative alterations in function to light, but the functional implication of these alterations remains somewhat unclear. Subjectively, some patients in our study and in the literature report persistent disequilibrium, though true vertigo is rare. In animals, it has been demonstrated that occlusion changes the phase and gain dynamics of the superior canal but does not deactivate it completely. Low frequency response amplitude is attenuated and phase shifted 90 degrees and high frequency responses are preserved31. Profound vestibular hypofunction can occur but is uncommon and was not present in our dataset.
Limitations:
Data presented in this study represent results from a single surgeon at a single institution, and thus may not be widely generalizable to all patient populations. Additionally, we did not examine outcomes for patients who underwent repair via a middle fossa craniotomy approach. Screening for postoperative changes in lateral canal function was assessed with head thrust alone. The head thrust vestibulo-oculo reflex test is subjective and dependent on interpreter expertise but has been shown to have high sensitivity for detecting large canal weaknesses and thus able to screen for large postoperative changes in canal function32,33. Standardized test batteries that include caloric and video head-impulse testing could provide a more objective assessment of postoperative vestibular function. Finally, though prospective collection of symptom scores is part of standard practice in our clinic and is preferable to a retrospective patient report, the symptom score remains subjective and is thus susceptible to bias.
Conclusions:
In our study, patients with superior semicircular canal dehiscence syndrome who underwent transmastoid canal plugging procedures demonstrated excellent hearing outcomes and resolution of most otologic symptoms. Transmastoid canal plugging has been described to date primarily in smaller case series. Here, we present a relatively larger review that supports the transmastoid approach as a safe and effective alternative to the traditional middle cranial fossa approach.
Acknowledgments
Funding:
RMBH was funded by NIH NIDCD T32 DC-012280.
Footnotes
Conflict of Interest Statement:
No potential conflict of interest was reported by the authors.
References:
- 1.Minor LB, Solomon D, Zinreich JS, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998;124(3):249–258. [DOI] [PubMed] [Google Scholar]
- 2.Zhou G, Gopen Q, Poe DS. Clinical and diagnostic characterization of canal dehiscence syndrome: a great otologic mimicker. Otol Neurotol. 2007;28(7):920–926. [PubMed] [Google Scholar]
- 3.Palma Diaz M, Cisneros Lesser JC, Vega Alarcón A. Superior Semicircular Canal Dehiscence Syndrome - Diagnosis and Surgical Management. Int Arch Otorhinolaryngol. 2017;21(2):195–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward BK, Carey JP, Minor LB. Superior Canal Dehiscence Syndrome: Lessons from the First 20 Years. Front Neurol. 2017;8:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandolini C, Modugno GC, Pirodda A. Dehiscence of the superior semicircular canal: a review of the literature on its possible pathogenic explanations. Eur Arch Otorhinolaryngol. 2014;271(3):435–437. [DOI] [PubMed] [Google Scholar]
- 6.Teixido MT, Artz GJ, Kung BC. Clinical experience with symptomatic superior canal dehiscence in a single neurotologic practice. Otolaryngol Head Neck Surg. 2008;139(3):405–413. [DOI] [PubMed] [Google Scholar]
- 7.Brantberg K, Bergenius J, Mendel L, Witt H, Tribukait A, Ygge J. Symptoms, findings and treatment in patients with dehiscence of the superior semicircular canal. Acta Otolaryngol. 2001;121(1):68–75. [DOI] [PubMed] [Google Scholar]
- 8.Luers JC, Pazen D, Meister H, et al. Acoustic effects of a superior semicircular canal dehiscence: a temporal bone study. Eur Arch Otorhinolaryngol. 2015;272(3):563–571. [DOI] [PubMed] [Google Scholar]
- 9.Merchant SN, Rosowski JJ. Conductive hearing loss caused by third-window lesions of the inner ear. Otol Neurotol. 2008;29(3):282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosowski JJ, Songer JE, Nakajima HH, Brinsko KM, Merchant SN. Clinical, experimental, and theoretical investigations of the effect of superior semicircular canal dehiscence on hearing mechanisms. Otol Neurotol. 2004;25(3):323–332. [DOI] [PubMed] [Google Scholar]
- 11.Powell HRF, Khalil SS, Saeed SR. Outcomes of Transmastoid Surgery for Superior Semicircular Canal Dehiscence Syndrome. Otol Neurotol. 2016;37(7):e228–e233. [DOI] [PubMed] [Google Scholar]
- 12.Thomeer H, Bonnard D, Castetbon V, Franco-Vidal V, Darrouzet P, Darrouzet V. Long-term results of middle fossa plugging of superior semicircular canal dehiscences: clinically and instrumentally demonstrated efficiency in a retrospective series of 16 ears. Eur Arch Otorhinolaryngol. 2016;273(7):1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward BK, Agrawal Y, Nguyen E, et al. Hearing outcomes after surgical plugging of the superior semicircular canal by a middle cranial fossa approach. Otol Neurotol. 2012;33(8):1386–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao YC, Somers T, van Dinther J, Vanspauwen R, Husseman J, Briggs R. Transmastoid repair of superior semicircular canal dehiscence. J Neurol Surg B Skull Base. 2012;73(4):225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beyea JA, Agrawal SK, Parnes LS. Transmastoid semicircular canal occlusion: a safe and highly effective treatment for benign paroxysmal positional vertigo and superior canal dehiscence. Laryngoscope. 2012;122(8):1862–1866. [DOI] [PubMed] [Google Scholar]
- 16.Amoodi HA, Makki FM, McNeil M, Bance M. Transmastoid resurfacing of superior semicircular canal dehiscence. Laryngoscope. 2011;121(5):1117–1123. [DOI] [PubMed] [Google Scholar]
- 17.Crane BT, Lin FR, Minor LB, Carey JP. Improvement in autophony symptoms after superior canal dehiscence repair. Otol Neurotol. 2010;31(1):140–146. [DOI] [PubMed] [Google Scholar]
- 18.Fiorino F, Barbieri F, Pizzini FB, Beltramello A. A dehiscent superior semicircular canal may be plugged and resurfaced via the transmastoid route. Otol Neurotol. 2010;31(1):136–139. [DOI] [PubMed] [Google Scholar]
- 19.Deschenes GR, Hsu DP, Megerian CA. Outpatient repair of superior semicircular canal dehiscence via the transmastoid approach. Laryngoscope. 2009;119(9):1765–1769. [DOI] [PubMed] [Google Scholar]
- 20.Kirtane MV, Sharma A, Satwalekar D. Transmastoid repair of superior semicircular canal dehiscence. J Laryngol Otol. 2009;123(3):356–358. [DOI] [PubMed] [Google Scholar]
- 21.Yuen H-W, Eikelboom RH, Atlas MD. Auditory manifestations of superior semicircular canal dehiscence. Otol Neurotol. 2009;30(3):280–285. [DOI] [PubMed] [Google Scholar]
- 22.Agrawal SK, Parnes LS. Transmastoid superior semicircular canal occlusion. Otol Neurotol. 2008;29(3):363–367. [DOI] [PubMed] [Google Scholar]
- 23.Limb CJ, Carey JP, Srireddy S, Minor LB. Auditory function in patients with surgically treated superior semicircular canal dehiscence. Otol Neurotol. 2006;27(7):969–980. [DOI] [PubMed] [Google Scholar]
- 24.Chilvers G, McKay-Davies I. Recent advances in superior semicircular canal dehiscence syndrome. J Laryngol Otol. 2015;129(3):217–225. [DOI] [PubMed] [Google Scholar]
- 25.Vlastarakos PV, Proikas K, Tavoulari E, Kikidis D, Maragoudakis P, Nikolopoulos TP. Efficacy assessment and complications of surgical management for superior semicircular canal dehiscence: a meta-analysis of published interventional studies. Eur Arch Otorhinolaryngol. 2009;266(2):177–186. [DOI] [PubMed] [Google Scholar]
- 26.Gersdorff MC, Robillard TA. A new procedure for bone reconstruction in oto-microsurgery: a mixture of bone dust and fibrinogen adhesive. Laryngoscope. 1985;95(10):1278–1280. [DOI] [PubMed] [Google Scholar]
- 27.Jung DH, Lookabaugh SA, Owoc MS, McKenna MJ, Lee DJ. Dizziness is more prevalent than autophony among patients who have undergone repair of superior canal dehiscence. Otol Neurotol. 2015;36:126–132. [DOI] [PubMed] [Google Scholar]
- 28.Carey JP, Migliaccio AA, Minor LB. Semicircular canal function before and after surgery for superior canal dehiscence. Otol Neurotol. 2007;28(3):356–364. [DOI] [PubMed] [Google Scholar]
- 29.Pisano DV, Niesten MEF, Merchant SN, Nakajima HH. The effect of superior semicircular canal dehiscence on intracochlear sound pressures. Audiol Neurootol. 2012;17(5):338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agrawal Y, Migliaccio AA, Minor LB, Carey JP. Vestibular hypofunction in the initial postoperative period after surgical treatment of superior semicircular canal dehiscence. Otol Neurotol. 2009;30(4):502–506. [DOI] [PubMed] [Google Scholar]
- 31.Rabbitt RD, Boyle R, Highstein SM. Influence of surgical plugging on horizontal semicircular canal mechanics and afferent response dynamics. J Neurophysiol. 1999;82(2):1033–1053. [DOI] [PubMed] [Google Scholar]
- 32.Perez N, Rama-Lopez J. Head-Impulse and Caloric Tests in Patients With Dizziness. Otol Neurotol. 2003;24:913–917. [DOI] [PubMed] [Google Scholar]
- 33.Helmchen C, Knauss J, Trillenberg P, Frendl A, Sprenger A. Role of the Patient’s History of Vestibular Symptoms in the Clinical Evaluation of the Bedside Head-Impulse Test. Front Neurol. 2017;8:737–738. [DOI] [PMC free article] [PubMed] [Google Scholar]