Abstract
Neurodegenerative diseases are a class of age-associated proteopathies characterized by the accumulation of misfolded and/or aggregation-prone proteins. This imbalance has been attributed, in part, to an age-dependent decay in the capacity of protein turnover. Most proteins are degraded by the ubiquitin-proteasome system (UPS), which is composed of ubiquitin ligases and regulatory particles, such as the 19S, that deliver cargo to the proteolytically active 20S proteasome core. However, a subset of clients, especially intrinsically disordered proteins (IDPs), are also removed by the action of the ubiquitin-independent proteasome system (UIPS). What are the specific contributions of the UPS and UIPS in the context of neurodegeneration? Here, we explore how age-associated changes in the relative contribution of the UPS and UIPS, combined with the IDP-like structure of many neurodegenerative disease-associated proteins, might contribute. Strikingly, the core 20S proteasome (20S) has been shown to predominate in older neurons and to preferentially act on relevant substrates, such as synuclein and tau. Moreover, pharmacological activation of the 20S has been shown to accelerate removal of aggregation-prone proteins in some models. Together, these recent studies are turning attention to the 20S proteasome and the UIPS as potential therapeutic targets in neurodegeneration.
INTRODUCTION TO THE PROTEASOME
The proteasome is a central protein degradation machine in eukaryotes1. Through hydrolysis activities, it removes damaged proteins and ensures the delivery of amino acids to support ongoing biosynthesis. In addition, the proteasome has been co-opted for more specialized tasks in regulating the cell cycle, differentiation, the inflammatory response, antigen presentation and apoptosis2,3. To enable these functions, the proteasome makes up a staggering 1 to 2% of the entire proteome in healthy cells. However, a decline in proteasome activity has been broadly implicated in ageing and age-associated diseases, including neurodegeneration. Presumably, this decline contributes to a catastrophic imbalance in proteostasis and accumulation of damaged and/or misfolded proteins. In this review, we explore the structure-function of the proteasome and its implications in the onset and progression of neurodegenerative disease. In addition, we focus on emerging therapeutic opportunities through pharmacological activation of this degradation machine.
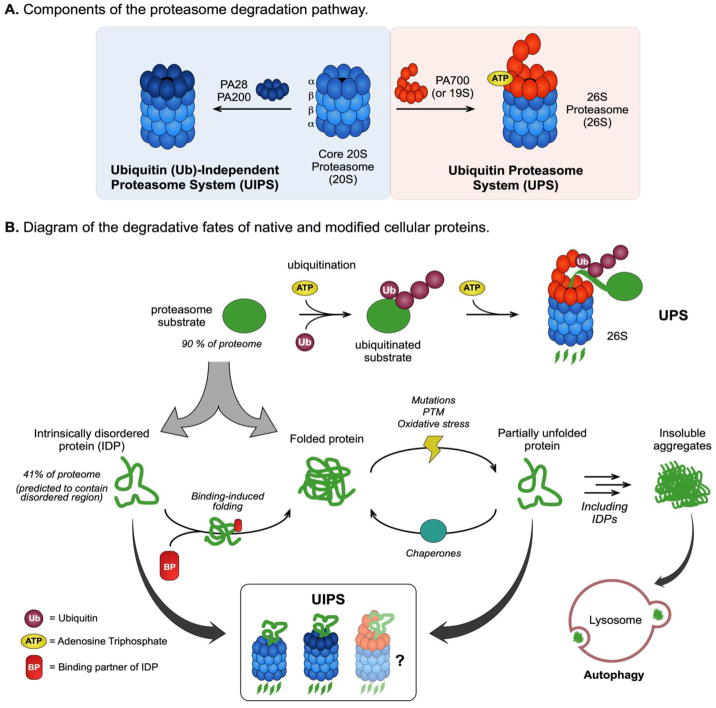
The 20S proteasome (20S) is a barrel-shaped complex comprised of four heptameric rings: two stacked β-rings that are sandwiched by two α-rings (Fig 1A). Three of the seven subunits (β1, β2, & β5) that make up the β-ring are proteases that hydrolyze peptide bonds of substrates. These active sites are sequestered in the interior of the 20S chamber, such that substrates must first traverse through the exterior α-rings. In its closed state, the α-rings have a narrow pore that occludes the entry of most proteins4. Thus, one key to understanding proteasome regulation is to learn how substrates are granted access to the proteolytic chamber. Substrates are targeted to the proteasome through two major pathways, the ubiquitin-proteasome system (UPS) and the ubiquitin-independent proteasome system (UIPS). Proteasome activators (PA), which are predominantly multi-protein complexes, help facilitate degradation by the 20S. There are many types of PAs and the specific one that is bound determines whether that 20S proteasome is coupled to the UPS or UIPS (Fig 1A). However, most of the PAs share a conserved tripeptide sequence, the HbYX (Hydrophobic-TYRosine-unspecified residue ‘X’), at their C-termini that interacts with pockets in the α-rings of the 20S to allosterically open the pore5.
Figure 1. Structure, Function, and Substrate Profile of the Proteasome Degradation Pathway.
(A) Components of the ubiquitin proteasome system (UPS) and the ubiquitin (Ub)-independent proteasome system (UIPS). (B) Diagram of substrate turnover by the different proteasome pathways. The 26S proteasome degrades over 90% of the proteome, including intrinsically disordered proteins (IDPs) and folded proteins, by the Ub- and ATP-dependent UPS. The UIPS targets substrates independent of Ub-conjugation and can effectively degrade IDPs, which constitute up to 41% of the proteome, but not folded proteins due to their three-dimensional structure. Cellular stresses, including mutations, post-translational modifications (PTMs) and oxidative damage, can partially unfold structured proteins making them susceptibility to turnover by the UIPS. Partially unfolded proteins (and IDPs) can self-associate to form insoluble aggregates, which cannot be processed by the proteasome, and are predominantly cleared from the cell through the lysosome-autophagy pathway.
Ubiquitin-proteasome system (UPS)
Proteasomal degradation by the UPS first requires the conjugation of multiple ubiquitin (Ub) proteins onto the substrate, generating the polyUb signal that designates it as a substrate of the proteasome. Recent work has shown that conjugation of two or more polyUb chains is needed on the tagged substrate to efficiently interact with the UPS machine6. Thus, regulation of this pathway by the activity of the E1, E2 and E3 Ub ligases is a critical component of its function7, but will not be described in detail here. The canonical regulatory particle of the UPS is PA700 (or 19S), which is a 700 kDa proteasome activator complex that associates with the 20S to create the 26S proteasome (26S)8. PA700 is comprised of a ‘base’ and a ‘lid’. The lid contains subunits that bind to polyUb chains, as well as deubiquitinating enzymes (DUBs) that regulate association with the particle. The base contains the HbYX motifs that interact with the α-rings, and ATPases that unfold the substrate so that it can access the proteolytic chamber9. Recent reviews provide additional information about the structure of the 26S and its biological fuction6.
Ub-independent-proteasome system (UIPS)
Ub-independent degradation is coordinated by the 20S and may be amplified with UIPS-specific PAs, including PA200 and the heptameric PA2810. PA200 is a monomeric protein that uses a C-terminal HbYX motif to bind to and activate the 20S. PA28 is composed of multiple, different subunits (alpha, beta, and gamma) and it relies on an alternative (e.g. non-HbYX) motif for association with the 20S11,12. The UIPS-specific PAs typically lack the unfolding activity of PA700; rather, they open the α-ring gate through a binding-induced conformational change and increase the flux of suitable substrates into the proteolytic chamber13. As discussed below, this mechanism restricts UIPS substrates to unfolded proteins that can fit into the channel without an active unfoldase. However, PA700-bound 26S has an open α-ring gate too and is thus capable of facilitating ubiquitin-independent substrate turnover14. The relative contributions of the 26S in the UPS and UIPS pathways remain unclear (Fig 1B); and, for simplicity, we will only mention the contributions of the 26S to the UIPS in passing in this review. Finally, the free 20S (no PA) is likely to be a contributor to the UIPS. Although the 20S has relatively low enzymatic activity in the absence of PAs (see below), some small or unfolded substrates may be able to traverse the closed gates and be degraded by the minimal machine.
Substrate-targeting by the UPS and UIPS
Over 90% of the human proteome is regulated by the UPS15. These substrates include a vast array of structured (or folded) proteins, intrinsically disordered proteins (IDPs) and proteins containing intrinsically disordered regions (IDRs). Structured proteins must be unfolded prior to their degradation and can therefore only be cleared by the UPS16,17. Essentially, folded proteins cannot fit through the narrow axial pore of the 20S, making them inaccessible to degradation by the UIPS particles18. However, IDPs and IDR-containing proteins, which lack this three-dimensional structure, are thought to readily traverse the α-ring gate19. Twenty percent of cellular proteins are classified as IDPs and as many as 41% of the eukaryotic proteome is predicted to contain IDRs20,21, suggesting that the substrate pool of the UIPS may be considerably large. These substrates are particularly relevant for this discussion because they include the proteins that accumulate in neurodegenerative disorders, such as amyloid beta, tau, TDP-43 and α-synuclein (Table I)22,23.
Table I.
IDPs and associated neurodegenerative diseases. Adapted from Uversky40.
| Protein | Disorder by prediction (%)a | Function | Disease(s) |
|---|---|---|---|
| Amyloid-β | 16.9 | Peptidic fragment of APP, which regulates synapse formation, and neuronal plasticity | Alzheimer’s disease Amyloidosis |
|
| |||
| Tau | 77.6 | Promote the assembly of and stabilizes neuronal microtubules | Tauopathies Alzheimer’s disease Corticobasal degeneration Pick’s disease Progressive supranuclear palsy |
|
| |||
| TDP-43 | 57.3 | Transcriptional repression, pre-mRNA splicing, and translational regulation | Amyotrophic lateral sclerosis and frontotemporal lobar degeneration |
|
| |||
| α-Synuclein | 90.7 | Regulate synaptic vesicles | Alzheimer’s disease (α) Multiple system atrophy(α) Parkinson’s disease (α, β, γ) Diffuse Lewy body disease (α, β γ) |
| β-Synuclein | 87.3 | ||
| γ-Synculein | 100 | ||
|
| |||
| FUS | 90.7 | Transcriptional regulation (initiation & repression), and RNA-binding | Amyotrophic lateral sclerosis |
Disorder was predicted by PONDR® VSL2.
In cells, IDPs typically have shorter half-lives relative to structured proteins24. The UPS and UIPS have both been shown to facilitate the rapid proteasomal degradation of IDPs, such as p53 and p7325. Recognition of the IDPs by these pathways is mediated, in part, by disordered regions that act as signals (or degrons)24. Evidence for the UIPS in this process comes from experiments in which removal of the ubiquitinated lysine has been found to have little effect on turnover26,27. Thus, it seems that both the UPS and UIPS can contribute to the turnover of IDPs.
ROLE OF THE PROTEASOME IN AGEING AND NEURODEGENERATIVE DISEASES
Neurodegenerative disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS), are characterized by the progressive structural and functional impairment of neurons, resulting in neuronal death28. Although these diseases have different clinical symptoms, they are all associated with the accumulation of aggregated proteins29,30. This observation implicates the age-dependent decline in proteostasis and the contributing role of proteasomal dysfunction31. Consistent with this idea, species with increased longevity and long-lived individuals within a given species exhibit higher proteasome activity and are less susceptible to diseases, including neurodegeneration32,33.
Many of the proteins implicated in neurodegeneration are IDPs34. Due to their conformational flexibility, IDPs are prone to aggregation35 and present a particular risk in aged neurons, where the protein quality control network has deteriorated36. Against this backdrop, the proteasome likely plays a critical role because it rids neurons of damaged and misfolded proteins that may cause neuronal dysfunction. The role of the UPS in this process has received substantial attention due, in part, to the early observation that ubiquitinated proteins are found within disease-associated aggregates in postmortem brains31. Indeed, many recent reviews have focused on the connection between the UPS and neurodegeneration37. Similarly, the immunoproteasome, composed of alternative subunits38, likely plays a role, especially in the immune regulation of the diseases. The structure-function of the immunoproteasome has also been reviewed39 and will not be discussed here. Instead, we focus on the relatively under-studied position of the UIPS.
Cells employ the UIPS to cope with proteotoxicity
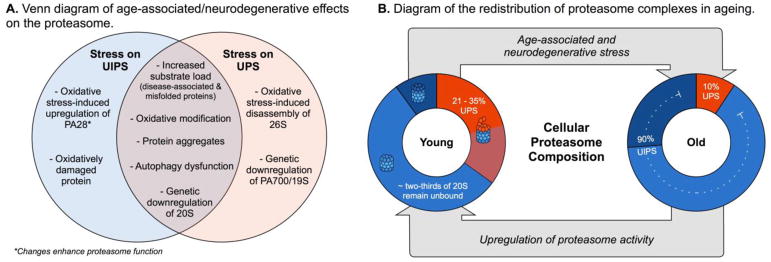
During neurodegeneration, neurons are challenged with a higher substrate load, potentially causing an imbalance in proteostasis and creating a feed-forward loop that compromises proteasome function41. Specifically, increased levels of misfolded proteins are though to “clog” the proteasome and impair its function42. There are several proposed mechanisms for this inhibition, including stabilization of the proteasome in its closed-gate conformation43,44. The feed-forward aspect of the relationship then comes about when the resulting proteotoxicity triggers mitochondrial dysfunction and increases reactive oxygen species (ROS) and DNA damage45. These processes contribute to further protein misfolding and inactivation of the proteasome through direct oxidation of its subunits46. Cells have adopted approaches to counteract this problem; for example, a rise in ROS induces expression of PA28α, which promotes UIPS activity47. Additionally, oxidative stress induces expression of 20S subunits through the oxidative stress sensor, Nrf2 (SKN-1)48. Interestingly, the levels of the UPS-associated PA700 remain constant under those conditions49, suggesting that oxidatively damaged proteins may be preferentially cleared by the UIPS. Consistent with this idea, oxidative stress also mediates the Ecm29-dependent disassembly of 26S (Fig 2A)50. Collectively, these findings suggest that challenged cells rely on the UIPS to degrade oxidatively damaged and unfolded proteins.
Figure 2. Proteasomal Regulation in Ageing and Neurodegenerative Disease Models.
(A) Partial list of stresses that effect UPS (orange) and UIPS (blues) function. (B) Summary of the cellular composition of proteasome complexes (20S, 26S, and UIPS-specific) in young vs. aged fibroblasts. See the text for citations. Briefly, proteasome complexes from young or old fibroblasts were placed into categories based on whether they were free 20S or whether they contain PA700 (26S/UPS) or PA28 (UIPS)51,55. Note that this simplification does not account for the contribution of the UPS to ubiquitin-independent turnover. (C) Schematic representation of the general decline in proteasome activity during ageing. In theory, small molecules might be used to partially restore this activity to boost function. 51,55
Aged cells contain a latent pool of free (e.g. unbound) 20S proteasome
What are the relative contributions of the free 20S, the UPS and the UIPS to proteotoxicity and neurodegeneration? The answers are not yet clear, but some clues come from reports that the relative levels of 20S and PA-bound 20S change with ageing and disease. Using label-free proteomics in nine different human cell lines, Fabre et al estimates that 21 – 35% of the total proteasome pool is PA700-bound (26S) and less than 10% is bound to UIPS-specific PAs, while the remaining ~66% is unbound 20S51 (Fig 2B). This is an interesting result because it suggests that cells may contain a latent pool of 20S proteasome that is not bound to any PA. Because PAs enhance the rate of proteolysis by as much as 20-fold52, these findings suggest that some cells have a pool of 20S that is poised to be activated. How does this ratio change during ageing and disease? It is known that this downregulation is mediated, in part, by diminished expression of proteasome genes themselves54. To date, the distribution of PA-bound and unbound 20S has not been surveyed in a neurodegeneration model, but the ratio of unbound (e.g. free 20S) proteasome to UPS-specific 26S has been reported to increase with age. For example, in cultured human fibroblasts from old individuals, the amount of 20S proteasome is decreased by 2.5-fold whereas the level of PA700 is lowered by 6.5-fold, compared to fibroblasts from young individuals55. Conversely, the levels of PA28 remained unchanged,55 suggesting a relative switch from UPS to UIPS pathways during ageing (Fig 2B). Although it is difficult to conclude a direct cause-and-effect, this increased availability of 20S roughly coincides with a decrease in total proteasome activity in many cells, tissues, and organisms during ageing53.
Proteasomal upregulation accelerates the clearance of pathogenic proteins
Together, these findings suggest that decreased proteasome activity, driven by a combination of reduced expression of 20S and an increase in free (i.e. non-PA bound) proteasome, may contribute to neurodegeneration. Accordingly, this model suggests that boosting proteasome activity may counteract the process. This goal could theoretically be achieved in a number of ways. For example, delivery of purified 20S to cells through direct injection has been shown to accelerate clearance of tau56. Similarly, cells expressing a 20S mutant, in which the gating N-termini of the α-subunits are deleted, are also partially protected from tau aggregation57. Pharmacologically, Finley et al identified small molecules that inactivate the deubiquitinating activity of USP14, the PA700-associated DUB, allosterically activate proteasomal degradation of polyUb-conjugated proteins and, consistent with the model, accelerate turnover of tau in vitro58,59. These findings have motivated others to search for molecules that directly bind the 20S to promote its activity (see below). Such a strategy might take advantage of the fact that only <10% of total proteasomes in a neurodegenerative disease model are intact 26S and a large pool is free 20S (Fig 2B). Consistent with this idea, over-expression of PA28 enhances protein clearance in ageing models60. The pharmacological equivalent of this approach would be to activate the 20S, in the absence of a PA. In the next sections, we discuss the opportunities and challenges of this possibility.
PHARMACOLOGICAL ACTIVATORS OF THE 20S PROTEASOME
The proteasome has long been the subject of studies to identify inhibitors. Early work identified natural products, such as lactacystin, that inactivate it by mimicking peptide substrates and covalently modifying the β-subunits61. These compounds became widely used chemical probes and, subsequently, a few were approved for the treatment of multiple myeloma patients53, where they seem to exploit the need of cancer cells for high protein turnover. More recently, other strategies have been developed to create inhibitors with different mechanisms62. Activators of the proteasome, in contrast, have received less attention; as mentioned above, such molecules would ideally bind to the unbound 20S and open its pore, acting as “artificial activators” to compensate for the loss of natural PAs and proteasome activity during ageing (Fig 3). However, the size and complexity of the 20S coupled with an incomplete understanding of the mechanisms of activation, has made the discovery of activators a difficult task. Furthermore, the field of 20S activators lacks the serendipitous discovery of natural product leads (such as lactacystin) as starting points. The next sections introduce the early chemical efforts to create proteasome activators and point out the substantial challenges that remain in the discovery, optimization and deployment of these molecules. For clarity, the sections are divided based on the chemical composition of the compounds.
Figure 3. Schematic representation of the general decline in proteasome activity during ageing.
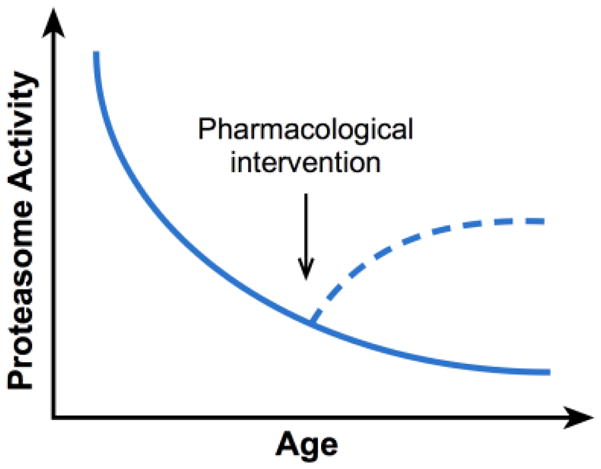
In theory, small molecules might be used to partially restore this activity to boost function.
Denaturants
The first reported proteasome activator was the detergent, sodium dodecyl sulfate (SDS), which was identified in vitro by measuring the chymotryptic activity of the 20S against the fluorogenic peptide substrate, succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (LLVY-amc). Addition of SDS increases the turnover of LLVY-amc by 20-fold, but at levels above its critical micellar concentration. This result suggests that SDS acts as a detergent, likely activating by partially denaturing the α-ring gate and allowing access of substrate into the pore. Another sign of this mechanism is that stimulation occurs within a narrow concentration range: lower concentrations activate turnover, while higher levels inhibit activity, likely by more extensive denaturation of the 20S63,64. Similar trends are seen for polycations (e.g. polylysine), polyanionic lipids (e.g. cardolipin & heparin), fatty acids (e.g. oleic, linoleic, & linolenic acids) and the natural product oleuropein65–69 (Table IIA and B). Molecules with this type of concentration-activity signature are likely to be rather non-specific activators, with mechanisms that make them difficult to optimize.
Table II.
Activators of the 20S proteasome.
Small molecules
Betulinic acid is a triterpene natural product that stimulates the 20S at low micromolar concentrations70. Unlike the detergents, betulinic acid preferentially stimulates the chymotryptic activity of the 20S (and not the other two activities). However, attempts to improve the potency of betulinic acid through medicinal chemistry have only yielded analogs that inhibit (rather than stimulate) proteasome activity, suggesting complex structure-activity relationships (SAR)71. More recent reports have turned to high-throughput chemical screens to identify alternative molecules. These assays often rely on measurement of proteasome activity through the hydrolysis of LLVY-amc, while secondary assays are used to measure degradation of biologically relevant substrates72,73. For example, Trader et al screened a subset of the NIH Clinical Collection (NCC) library to discover two compounds, AM-404 and MK-866, that stimulate the 20S in vitro and enhance turnover of α-synuclein by 3 to 4-fold in cells72. In a parallel screen of the Natural Product Library (NPL) of the NCC, Coleman & Trader identified three additional molecules (denoted NPL-1, 2, & 3) that activate the 20S at low micromolar concentrations74 (Table IIC). Beyond the specific molecules identified, one of the highlights of that work is the introduction of a rigorous suite of assays to triage non-specific mechanisms. However, additional medicinal chemistry and structural biology efforts will be needed to advance chemical probes with the desired selectivity and potency.
In a parallel effort, Jones et al screened the NCC and Prestwick libraries, revealing chlorpromazine as a putative agonist of the 20S. This compound stimulated the activity of the 20S by 20-fold in vitro. Unbiased docking of chlorpromazine to the 20S predicted possible interactions with the intersubunit pocket of the α-ring, reminiscent of the natural HbYX motif. Thus, this chemical series may take advantage of intrinsic allosteric mechanisms to promote turnover. Chlorpromazine is a well-known dopamine D2 agonist, but chemical modifications suggested that the SAR for proteasome activation was distinct. However, the potency of these compounds remains limited (Table IIC)73.
Peptides
Another strategy for stimulating the proteasome is to directly create mimics of the HbYX motif. Indeed, peptides inspired by the HbYX-containing C-termini of PAs can stimulate the 20S in vitro at mid-micromolar concentrations5. Moreover, the potency of these HbYX peptides seems to depend on which subunits of PA700 they were taken from 75,76. Some of these HbYX peptides can enhance proteasomal degradation of a model substrate and rescue the 20S from inhibition by toxic amyloid-β oligomers43. While promising, peptide-based activators of the 20S have intrinsic challenges that will need to be overcome. For example, they have poor membrane permeability, uncertain selectivity and typically low metabolic stability. Despite these hurdles, the recently reported crystal structure of the HbYX peptide-bound eukaryotic 20S77 might provide a structure-guided way to advance this approach. It is important to note that any compounds that bind this site would expectedly displace the UPS and UIPS-specific PAs. Thus, even though they might stimulate the pool of free 20S, their pharmacology is expected to be complex.
CONCLUSION and PROSPECTUS
There is mounting evidence that decreased activity of the proteasome contributes to neurodegeneration. Although decreased 26S is assuredly a key component of this decline, we have focused here on the contributions of the UIPS and the free 20S. This focus is based on evidence that both the PA28-bound and free 20S seem to become more prominent during ageing. Moreover, many of the neurodegenerative disease-associated proteins, such as tau, are IDPs and thus particularly good substrates for these complexes.
The increased level of free 20S during ageing has interesting implications for drug discovery. Specifically, molecules that boost the function of this “low activity” 20S pool might partially compensate for diminished proteasomal function. While a compelling idea, there are major challenges to pursuing this concept and the current molecules are not yet up to this task. One challenge is that many compounds can have detergent-like activity that is potent, but ultimately untenable for the creation of activators. Thus, any screening effort is highly likely to produce denaturation-like artifacts that must be removed through subsequent secondary assays and careful analysis of their dose dependence. In addition, one cannot use the typical approach of adding Tween or Triton-X in screening buffers to minimize the discovery of aggregators or pan-assay interference (PAINS) molecules78. Thus, additional types of artifacts are likely to populate the list of apparent “hits”. These two challenges are expected to result in higher-than-normal failure rates during compound triage and special care will be needed to select high quality scaffolds.
Acknowledgments
The authors have reviewed the journal’s policy on potential conflicts of interest and have none to declare. Both authors have reviewed the journal’s authorship agreement and approved the document. Our work on the proteasome is funded by the NIH (AG053619).
Abbreviations
- 20S
core 20S proteasome
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- ATP
adenosine triphosphate
- BBB
blood-brain barrier
- DUB
deubiquitinating enzyme
- HbYX
Hydrophobic-Tyrosine-unspecified residue ‘X’
- IDP
intrinsically disordered protein
- IDR
intrinsically-disordered region
- LLVY-amc
succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin
- NCC
NIH (National Institute of Health) Clinical Collection
- NPL
Natural Product Library
- PA
proteasome activators
- PAINS
pan-assay interference compounds
- PD
Parkinson’s disease
- ROS
reactive oxygen species
- Rpt
regulatory particle of triple-ATPase
- SAR
structure-activity relationship
- tau
microtubule-associated protein tau (MAPT)
- TDP-43
trans-activation response element (TAR) DNA-binding protein 43
- Ub
ubiquitin
- UIPS
Ubiquitin-independent proteasome system
- UPS
ubiquitin-proteasome system
- USP-14
Ubiquitin-specific-processing protease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith DM, Benaroudj N, Goldberg A. Proteasomes and their associated ATPases: A destructive combination. J Struct Biol. 2006;156(1):72–83. doi: 10.1016/j.jsb.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg AL. Functions of the proteasome: from protein degradation and immune surveillance to cancer therapy. Biochem Soc Trans. 2007;35(1):12–17. doi: 10.1042/BST0350012. http://www.biochemsoctrans.org/content/35/1/12.abstract. [DOI] [PubMed] [Google Scholar]
- 3.Chen LH, CH Proteasome Regulators: Activators and Inhibitors. Curr Med Chem. 2009;16(8):931–939. doi: 10.2174/092986709787581860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kish-Trier E, Hill CP. Structural Biology of the Proteasome. Annu Rev Biophys. 2013;42(1):29–49. doi: 10.1146/annurev-biophys-083012-130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith DM, Chang SC, Park S, Finley D, Cheng Y, Goldberg AL. Docking of the proteasomal ATPases’ carboxyl termini in the 20S proteasome’s α Ring Opens the Gate for Substrate Entry. Mol Cell. 2007;27(5):731–744. doi: 10.1016/j.molcel.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finley D. Recognition and Processing of Ubiquitin-Protein Conjugates by the Proteasome. Annu Rev Biochem. 2009;78(1):477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finley D, Ulrich HD, Sommer T, Kaiser P. The ubiquitin-proteasome system of Saccharomyces cerevisiae. Genetics. 2012;192(2):319–360. doi: 10.1534/genetics.112.140467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeMartino GN, Moomaw CR, Zagnitko OP, et al. PA700, an ATP-dependent activator of the 20 S proteasome, is an ATPase containing multiple members of a nucleotide-binding protein family. J Biol Chem. 1994;269(33):20878–20884. [PubMed] [Google Scholar]
- 9.Glickman MH, Rubin DM, Fried Va, Finley D. The regulatory particle of the Saccharomyces cerevisiae proteasome. Mol Cell Biol. 1998;18(6):3149–3162. doi: 10.1128/mcb.18.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt M, Hanna J, Elsasser S, Finley D. Proteasome-associated proteins: Regulation of a proteolytic machine. Biol Chem. 2005;386(8):725–737. doi: 10.1515/BC.2005.085. [DOI] [PubMed] [Google Scholar]
- 11.Sadre-Bazzaz K, Whitby FG, Robinson H, Formosa T, Hill CP. Structure of a Blm10 Complex Reveals Common Mechanisms for Proteasome Binding and Gate Opening. Mol Cell. 2010;37(5):728–735. doi: 10.1016/j.molcel.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Rechsteiner M. Molecular dissection of the 11S REG (PA28) proteasome activators. Biochimie. 2001;83(3–4):373–383. doi: 10.1016/S0300-9084(01)01236-6. [DOI] [PubMed] [Google Scholar]
- 13.Förster A, Masters EI, Whitby FG, Robinson H, Hill CP. The 1.9 Å structure of a proteasome-11S activator complex and implications for proteasome-PAN/PA700 interactions. Mol Cell. 2005;18(5):589–599. doi: 10.1016/j.molcel.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Strickland E, Hakala K, Thomas PJ, DeMartino GN. Recognition of misfolded proteins by PA700, the regulatory subcomplex of the 26S proteasome. J Biol Chem. 2000;275(8):5565–5572. doi: 10.1074/jbc.275.8.5565. [DOI] [PubMed] [Google Scholar]
- 15.Kwon YT, Ciechanover A. The Ubiquitin Code in the Ubiquitin-Proteasome System and Autophagy. Trends Biochem Sci. 2017;42(11):873–886. doi: 10.1016/j.tibs.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Rock KL, Gramm C, Rothstein L, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78(5):761–771. doi: 10.1016/S0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 17.Craiu a, Gaczynska M, Akopian T, et al. Lactacystin and clasto-lactacystin beta-lactone modify multiple proteasome beta-subunits and inhibit intracellular protein degradation and major histocompatibility complex class I antigen presentation. J Biol Chem. 1997;272(20):13437–13445. doi: 10.1074/jbc.272.20.13437. [DOI] [PubMed] [Google Scholar]
- 18.Hagai T, Levy Y. Ubiquitin not only serves as a tag but also assists degradation by inducing protein unfolding. Proc Natl Acad Sci. 2010;107(5):2001–2006. doi: 10.1073/pnas.0912335107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlowski M, Wilk S. Ubiquitin-independent proteolytic functions of the proteasome. Arch Biochem Biophys. 2003;415(1):1–5. doi: 10.1016/S0003-9861(03)00197-8. [DOI] [PubMed] [Google Scholar]
- 20.Baugh JM, Viktorova EG, Pilipenko EV. Proteasomes can degrade a significant proportion of cellular proteins independent of ubiquitination. J Mol Biol. 2009;386(3):814–827. doi: 10.1016/j.jmb.2008.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunker AK, Obradovic Z, Romero P, Garner EC, Brown CJ. Intrinsic protein disorder in complete genomes. Genome informatics. 2000;11:161–171. doi: 10.11234/gi1990.11.161. [DOI] [PubMed] [Google Scholar]
- 22.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35(43):13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 23.Uversky VN, Oldfield CJ, Dunker AK. Intrinsically disordered proteins in human diseases: introducing the D2 concept. Annu Rev Biophys. 2008;37(1):215–246. doi: 10.1146/annurev.biophys.37.032807.125924. [DOI] [PubMed] [Google Scholar]
- 24.van der Lee R, Lang B, Kruse K, et al. Intrinsically disordered segments affect protein half-life in the cell and during evolution. Cell Rep. 2014;8(6):1832–1844. doi: 10.1016/j.celrep.2014.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asher G, Tsvetkov P, Kahana C. A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73 service A mechanism of proteasomal degradation of the tumor suppressors p53 and p73. Genes Dev. 2005;19:316–321. doi: 10.1101/gad.319905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peña MMO, Xing YY, Koli S, Berger FG. Role of N-terminal residues in the ubiquitin-independent degradation of human thymidylate synthase. Biochem J. 2006;394(1):355–363. doi: 10.1042/BJ20051479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiggins CM, Tsvetkov P, Johnson M, et al. BIMEL, an intrinsically disordered protein, is degraded by 20S proteasomes in the absence of poly-ubiquitylation. J Cell Sci. 2011;124(6):969–977. doi: 10.1242/jcs.058438. [DOI] [PubMed] [Google Scholar]
- 28.Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20(11):1242–1253. doi: 10.1038/nm.3739. [DOI] [PubMed] [Google Scholar]
- 29.Walker LC, LeVine H. The cerebral proteopathies: neurodegenerative disorders of protein conformation and assembly. Mol Neurobiol. 2000;21(1–2):83–95. doi: 10.1385/MN:21:1-2:083. [DOI] [PubMed] [Google Scholar]
- 30.Sherman MY, Goldberg AL. Cellular defenses against unfolded proteins: A cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29(1):15–32. doi: 10.1016/S0896-6273(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 31.Dantuma NP, Bott LC. The ubiquitin-proteasome system in neurodegenerative diseases: precipitating factor, yet part of the solution. Front Mol Neurosci. 2014 Jul;7:1–18. doi: 10.3389/fnmol.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruby JG, Smith M, Buffenstein R. Naked mole-rat mortality rates defy gompertzian laws by not increasing with age. Elife. 2018;7:1–18. doi: 10.7554/eLife.31157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chondrogianni N, Petropoulos I, Franceschi C, Friguet B, Gonos ES. Fibroblast cultures from healthy centenarians have an active proteasome. Exp Gerontol. 2000;35(6–7):721–728. doi: 10.1016/S0531-5565(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 34.Deger JM, Gerson JE, Kayed R. The interrelationship of proteasome impairment and oligomeric intermediates in neurodegeneration. Aging Cell. 2015;14(5):715–724. doi: 10.1111/acel.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine ZA, Larini L, LaPointe NE, Feinstein SC, Shea J-E. Regulation and aggregation of intrinsically disordered peptides. Proc Natl Acad Sci. 2015;112(9):2758–2763. doi: 10.1073/pnas.1418155112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40(2):427–446. doi: 10.1016/s0896-6273(03)00606-8. The ubiquitin proteasome system in neurodeg. doi:S0896627303006068 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Dennissen FJA, Kholod N, van Leeuwen FW. The ubiquitin proteasome system in neurodegenerative diseases: Culprit, accomplice or victim? Prog Neurobiol. 2012;96(2):190–207. doi: 10.1016/j.pneurobio.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Kniepert A, Groettrup M. The unique functions of tissue-specific proteasomes. Trends Biochem Sci. 2014;39(1):17–24. doi: 10.1016/j.tibs.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Ferrington DA, Gregerson DS. Immunoproteasomes: Structure, Function, and Antigen Presentation. 2012 doi: 10.1016/B978-0-12-397863-9.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uversky VN. Alpha-synuclein misfolding and neurodegenerative diseases. Curr Protein Pept Sci. 2008;9(5):507–540. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 41.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.San Martín Á, Rodriguez-Aliaga P, Molina JA, Martin A, Bustamante C, Baez M. Knots can impair protein degradation by ATP-dependent proteases. Proc Natl Acad Sci. 2017;114(37):201705916. doi: 10.1073/pnas.1705916114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thibaudeau TA, Anderson RT, Smith DM. A common mechanism of proteasome impairment by neurodegenerative disease-associated oligomers. Nat Commun. 2018 doi: 10.1038/s41467-018-03509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett EJ, Bence NF, Jayakumar R, Kopito RR. Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol Cell. 2005;17(3):351–365. doi: 10.1016/j.molcel.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 45.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 46.Reichmann D, Voth W, Jakob U. Maintaining a Healthy Proteome during Oxidative Stress. Mol Cell. 2018;69(2):203–213. doi: 10.1016/j.molcel.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, Powell SR, Wang X. Enhancement of proteasome function by PA28 overexpression protects against oxidative stress. FASEB J. 2011;25(3):883–893. doi: 10.1096/fj.10-160895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Matilainen O, Jin C, Glover-Cutter KM, Holmberg CI, Blackwell TK. Specific SKN-1/NrF stress responses to perturbations in translation elongation and proteasome activity. PLoS Genet. 2011;7(6):9–11. doi: 10.1371/journal.pgen.1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pickering AM, Staab TA, Tower J, Sieburth D, Davies KJA. A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster. J Exp Biol. 2013;216(4):543–553. doi: 10.1242/jeb.074757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Chemmama IE, Yu C, et al. The proteasome-interacting Ecm29 protein disassembles the 26S proteasome in response to oxidative stress. J Biol Chem. 2017;292(39):16310–16320. doi: 10.1074/jbc.M117.803619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fabre B, Lambour T, Garrigues L, et al. Label-free quantitative proteomics reveals the dynamics of proteasome complexes composition and stoichiometry in a wide range of human cell lines. J Proteome Res. 2014;13(6):3027–3037. doi: 10.1021/pr500193k. [DOI] [PubMed] [Google Scholar]
- 52.Masson P, Lundin D, Söderbom F, Young P. Characterization of a REG/PA28 proteasome activator homolog in dictyostelium discoideum indicates that the ubiquitin- And ATP-independent REGγ proteasome Is an ancient nuclear protease. Eukaryot Cell. 2009;8(6):844–851. doi: 10.1128/EC.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt M, Finley D. Regulation of proteasome activity in health and disease. Biochim Biophys Acta. 2014;1843(1):13–25. doi: 10.1016/j.bbamcr.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee C, Klopp RG, Weindruch R, Prolla TA. Gene Expression Pro le of Aging and Its Retardation by Caloric Restriction. Science (80-) 1999 Aug;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 55.Hwang JS, Hwang JS, Chang I, Kim S. Age-associated decrease in proteasome content and activities in human dermal fibroblasts: restoration of normal level of proteasome subunits reduces aging markers in fibroblasts from elderly persons. J Gerontol. 2007;62(5):490–499. doi: 10.1093/gerona/62.5.490. 62/5/490 [pii] [DOI] [PubMed] [Google Scholar]
- 56.Han DH, Na H-K, Choi WH, et al. Direct cellular delivery of human proteasomes to delay tau aggregation. Nat Commun. 2014;5:5633. doi: 10.1038/ncomms6633. [DOI] [PubMed] [Google Scholar]
- 57.Choi WH, de Poot SAH, Lee JH, et al. Open-gate mutants of the mammalian proteasome show enhanced ubiquitin-conjugate degradation. Nat Commun. 2016;7:10963. doi: 10.1038/ncomms10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee B-H, Lee MJ, Park S, et al. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature. 2010;467(7312):179–184. doi: 10.1038/nature09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boselli M, Lee BH, Robert J, et al. An inhibitor of the proteasomal deubiquitinating enzyme USP14 induces tau elimination in cultured neurons. J Biol Chem. 2017;292(47):19209–19225. doi: 10.1074/jbc.M117.815126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vilchez D, Saez I, Dillin A. Organismal Ageing and Age-Related Diseases. Nat Commun. 2014;5:1–13. doi: 10.1038/ncomms6659. [DOI] [PubMed] [Google Scholar]
- 61.Kim KB, Myung J, Sin N, Crews CM. Proteasome inhibition by the natural products epoxomicin and dihydroeponemycin: Insights into specificity and potency. Bioorg Med Chem Lett. 1999;9(23):3335–3340. doi: 10.1016/S0960-894X(99)00612-5. [DOI] [PubMed] [Google Scholar]
- 62.McDaniel TJ, Lansdell TA, Dissanayake AA, et al. Substituted quinolines as noncovalent proteasome inhibitors. Bioorganic Med Chem. 2016;24(11):2441–2450. doi: 10.1016/j.bmc.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orlowski N, Wilk S. A multicatalytical protease complex from pituitary that forms enkephalin and enkephalin containing peptides. Biochem Biophys Res Commun. 1981;101(3):814–822. doi: 10.1016/0006-291X(81)91823-4. [DOI] [PubMed] [Google Scholar]
- 64.Wilk S, Orlowski M. Evidence that Pituitary Cation-Sensitive Neutral Endopeptidase Is a Multicatalytic Protease Complex. J Neurochem. 1983;40(3):842–849. doi: 10.1111/j.1471-4159.1983.tb08056.x. [DOI] [PubMed] [Google Scholar]
- 65.Ohkubo I, Gasa S, Namikawa C, Makita A, Sasaki M. Human Erythrocyte multicatalytic proteinase: Activation and binding to sulfated galacto- and lactosylceramides+ Biochem Biophys Res Commun. 1991;174(14):1133–1140. doi: 10.1016/0006-291x(91)91538-n. [DOI] [PubMed] [Google Scholar]
- 66.Ruiz de Mena I, Mahillo E, Arribas J, Castaño JG. Kinetic mechanism of activation by cardiolipin (diphosphatidylglycerol) of the rat liver multicatalytic proteinase. Biochem J. 1993;296(Pt 1):93–97. doi: 10.1042/bj2960093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe N, Yamada S. Activation of 20S proteasomes from spinach leaves by fatty acids. Plant Cell Physiol. 1996;37(2):147–151. doi: 10.1093/oxfordjournals.pcp.a028925. [DOI] [PubMed] [Google Scholar]
- 68.Tanakas K, Yoshimura T, Ichihara A, et al. A High Molecular Weight Protease in the Cytosol of Rat Liver. J Biol Chem. 1986;261(32):15204–15207. [PubMed] [Google Scholar]
- 69.Katsiki M, Chondrogianni N, Chinou I, Rivett AJ, Gonos ES. The olive constituent oleuropein exhibits proteasome stimulatory properties in vitro and confers life span extension of human embryonic fibroblasts. Rejuvenation Res. 2007;10(2):157–172. doi: 10.1089/rej.2006.0513. [DOI] [PubMed] [Google Scholar]
- 70.Kashiwada Y, Hashimoto F, Cosentino LM, Chen CH, Garrett PE, Lee KH. Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J Med Chem. 1996;39(5):1016–1017. doi: 10.1021/jm950922q. [DOI] [PubMed] [Google Scholar]
- 71.Huang L, Ho P, Chen CH. Activation and inhibition of the proteasome by betulinic acid and its derivatives. FEBS Lett. 2007;581(25):4955–4959. doi: 10.1016/j.febslet.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trader DJ, Simanski S, Dickson P, Kodadek T. Establishment of a suite of assays that support the discovery of proteasome stimulators. Biochim Biophys Acta. 2017;1861(4):892–899. doi: 10.1016/j.bbagen.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones CL, Njomen E, Sjögren B, Dexheimer TS, Tepe JJ. Small Molecule Enhancement of 20S Proteasome Activity Targets Intrinsically Disordered Proteins. ACS Chem Biol. 2017;12(9):2240–2247. doi: 10.1021/acschembio.7b00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coleman RA, Trader DJ. Development and Application of a Sensitive Peptide Reporter to Discover 20S Proteasome Stimulators. ACS Comb Sci. 2018 doi: 10.1021/acscombsci.7b00193. acscombsci.7b00193. [DOI] [PubMed] [Google Scholar]
- 75.Gillette TG, Kumar B, Thompson D, Slaughter Ca, DeMartino GN. Differential roles of the COOH termini of AAA subunits of PA700 (19 S regulator) in asymmetric assembly and Activation of the 26 S proteasome. J Biol Chem. 2008;283(46):31813–31822. doi: 10.1074/jbc.M805935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim Y-C, DeMartino GN. C Termini of Proteasomal ATPases Play Nonequivalent Roles in Cellular Assembly of Mammalian 26 S Proteasome. J Biol Chem. 2011;286(30):26652–26666. doi: 10.1074/jbc.M111.246793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Witkowska J, Gizyńska M, Grudnik P, et al. Crystal structure of a low molecular weight activator Blm-pep with yeast 20S proteasome - Insights into the enzyme activation mechanism. Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-05997-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baell JB, Nissink JWM. Seven Year Itch: Pan-Assay Interference Compounds (PAINS) in 2017 - Utility and Limitations. ACS Chem Biol. 2018;13(1):36–44. doi: 10.1021/acschembio.7b00903. [DOI] [PMC free article] [PubMed] [Google Scholar]