Graphical abstract
Keywords: Fertility, Spermatotoxic, Sperm parameters, Telfairia occidentalis leaf extract, Testis
Highlights
-
•
Hormonal analysis showed that the reduction in testosterone levels highlighted TO caused untold damages to the testis at high dose.
-
•
Significant differences in sperm parameters showed an increase demonstrating the efficacy of T. occidentalis over a long period of time.
-
•
Significant differences (P < 0.05) in Histomorphometric analysis observed was as a result of the compensatory mechanism of TO leave extract.
-
•
Histological analysis showed improvement in the cytoarchitecture of the tubule at low doses but at high doses, distorted cytoarhitecture.
-
•
The study showed the spermatogenic properties of T. occidentalis at low doses while at high doses, its spermatotoxic properties were observed.
Abstract
Telfairia occidentalis (TO), commonly called Pumpkin is a plant with numerous medicinal values. Here we investigated the effects of Telfairia occidentalis aqueous leaves extract on the testis of Adult Male Wistar Rats (Rattus novergicus). Thirty-Five (35) adult male rats were grouped into seven (7) containing five (n = 5) rats in each group. Group A served as control, group B and C received 100 mg/kg body weight of Telfairia occidentalis for two and four weeks respectively, group D and E received 200 mg/kg body weight of Telfairia occidentalis for two and four weeks respectively, while group F and G received 300 mg/kg body weight of Telfairia occidentalis for two and four weeks respectively. Serum testosterone levels, testicular histomorphometry, Semen and histological analysis were observed. A dose dependent significant (P < 0.05) decrease in testosterone levels was observed in groups F and G when compared to control. Significant differences (P < 0.05) in sperm parameters and histomorphometric analysis were observed, while histological analysis showed an massive improvement in the cytoarchitecture of the seminiferous tubule at low doses but at high doses, it distorted seminiferous tubules cytoarhitecture when compared to the control group. In conclusion, the study showed that low doses of T. occidentalis leaf extract over a period of time had spermatogenic and testiculoprotective properties while at high doses, its spermatotoxic properties were observed.
1. Introduction
The affordability of herbs over expensive pharmaceutical drugs to treat diseases among non-industrialized societies is fast becoming revolutionized. In some countries, it has been integrated into the health scheme despite advances in orthodox medicine. It is believed that the natural products if utilized in the correct form and dosage are less harmful than synthetic products, which most often elicit some side effects [1].
Telfaira occidentalis is a vegetable consumed for its medicinal values possesses powerful in-vitro antioxidant activity that might be attributed to their phenolic and vitamin contents [2]. The presence of pharmacological active compounds was responsible for the analgesic activity in T. occidentalis [3]. TO possesses pro-fertility properties that can be exploited in fish fingerling production by hatchery operators [4] while Kuku et al. [5] reported that T. occidentalis (TO) seeds had a positive effect on nutrient metabolism and the performance of growth in rats.
The medicinal values of TO have been reported on hematological indices of starter broilers Onu [6], concentration of hemoglobin and packed cell volume [7], lipid peroxidation amelioration and cardiovascular disease reduction [8]. Its hepatoprotective effects on the liver have been reported [9], while Ajani and Akinyemi [10] highlighted its therapeutic efficacy on induced benign prostatic hyperplasia. Finally [11], reported that its seeds and leaves supplement had positive effects on biochemical enzymes on lipid profiles in the liver at varying dosages.
Therefore, it was on this basis that we decided to study the fertility indices by investigating the effects of Telfairia occidentalis aqueous leaves extract on the testis of adult male Wistar rats (Rattus novergicus).
1.1. Ethical approval
The experimental protocol was by the University ethical review committee, University of Ilorin, Ilorin, Nigeria. The research was approved to be in compliance with the institutional animal care and Use committee (IACUC).
2. Materials and methods
2.1. Animal grouping and treatments
Adult male rats (35, with average weight of 220 ± 3 g) were randomly assigned into 7 groups (A–G), each consisting of 5 rats (n = 5). The groups were treated orally as follows: Control group (received 2 ml daily for 28 days), group B (100 mg/kg body weight of T. occidentalis for 2 weeks), group C (100 mg/kg body weight of T. occidentalis for 4 weeks), group D (200 mg/kg body weight of T. occidentalis for 2 weeks), group E (200 mg/kg body weight for 4 weeks), group F (300 mg/kg body weight of T. occidentalis for 2 weeks) while, group F (300 mg/kg body weight of T. occidentalis for 4 weeks). Treatment doses adopted in this study were administered using an orogastric cannula and the experiment was based on the fact that it was closest to the traditional method of consumption of T. occidentalis in humans.
2.2. Preparation of treatment solution
The leaves of the plant T. occidentalis were procured from the Herbarium of the Department of Agriculture, University of Ilorin, Nigeria where it was identified an authenticated. The leaves were then washed and blended; the ground pastes were filtered (Whatmann filter paper No 2) and the extracted sediments were air dried for 7 days. TO was prepared in distilled water (30 mg/ml) and adjusted to pH 7.0 with 0.1 M PBS.
2.3. Sample collection and processing
On completion of treatments, rats for histological analysis were euthanized using 20 mg/kg body weight of ketamine intraperitoneally. Blood samples for hormonal assay were obtained from the orbital vein with the aid of a 2 ml syringe from all rats employed in the study. The serum was separated by centrifugation at 3000 rpm for 15 min. Histological staining was carried out in paraffin wax embedded sections according to [12] and stained with Haematoxylin and Eosin using the methods described by [13].
2.4. Determination of serum testosterone levels
Blood testosterone levels were measured by Enzyme-linked immunosorbant assay (ELISA) by (Monobind Inc. Lake Forest, CA 92630, USA) according to [14].
2.5. Semen analysis
Rat epididymis was placed in normal saline and used for evaluation of sperm quality (i.e. sperm count [15], sperm motility [16] and sperm morphology [17]. The concentration of spermatozoa was determined using the improved Neubauer Chamber Haemocytometer (Deep 1/10 mm, LABART, Germany).
2.6. Testicular histomorphometry
Testicular tissues sectioned at 3 μm and stained with Haematoxylin and Eosin stain was used for stereological studies. Histomorphometric data were collected with the aid of an Amscope digital microscope (AmScope Microsystem, USA) connected to a computer to evaluate the cross sectional area, lumen diameter and germinal epithelium diameter of the seminiferous tubules.
2.7. Light microscopy
For light microscopic studies, the testis sections on glass slides were captured using Olympus binocular research microscope (Olympus, New Jersey, USA) which was connected to a 5.0 MP Amscope Camera (Amscope Inc, USA.)
2.8. Statistical analysis
Data collected were analyzed using two-way analysis of variance (ANOVA) followed by Tukey’s (HSD) multiple comparison test with the aid of SPSS (V20; USA). Data were presented as means ± SEM (standard error of mean). P value (p < 0.05) was considered statistically significant. All graphs were drawn using the GraphPad Prism v.6 (GraphPad Software Inc., USA).
3. Results
3.1. Semen analysis
Significant increase (P < 0.05) in sperm count was observed in group C and E alone at 4 weeks compared to control while a non-significant increase (P > 0.05) in sperm motility and morphology were observed in the treatment groups at 2 and 4 weeks when compared to control (Table 1).
Table 1.
Effects of T. occidentalis aqueous leaves extract on semen analysis.
| Groups | Sperm count (X106/ml) | %Sperm motility | %Sperm morphology |
|---|---|---|---|
| A | 78.7 ± 2.03 | 84.0 ± 0.58 | 82.3 ± 1.45 |
| B | 85.7 ± 2.03 | 85.7 ± 1.76 | 83.3 ± 1.45 |
| C | 88.0 ± 1.16* | 82.5 ± 1.76 | 85.0 ± 1.56 |
| D | 80.3 ± 0.88 | 78.3 ± 1.20 | 80.7 ± 2.17 |
| E | 88.7 ± 1.76* | 85.3 ± 3.76 | 85.0 ± 2.03 |
| F | 82.3 ± 2.03 | 80.0 ± 1.56 | 82.0 ± 1.16 |
| G | 76.3 ± 2.19 | 79.7 ± 0.33 | 80.3 ± 0.88 |
Values are expressed as mean ± SEM showing the level of significance in *P < 0.05 compared to the control group.
3.2. Testicular histomorphometry
A significant decrease (P < 0.05) in seminiferous tubule diameter (STD) was observed in the groups B and F at 2 weeks and also groups C and at 4 weeks when compared to the control. A significant decrease (P < 0.05) in Lumen diameter (LD) was observed in groups B and D at 2 weeks and group E at 4 weeks respectively while a significant increase (P < 0.05) in group G alone at 4 weeks was observed when compared to the control. A significant increase (P < 0.05) in germinal epithelium diameter (GED) was observed in groups B and D at 2 weeks and also in groups E and G at 4 weeks when compared to the control group (Table 2).
Table 2.
Effects of Telfairia occidentalis aqueous leaves extract on testicular histomorphometry.
| Groups | STD (μm2 ×109) | LD (μm ×10) | GED (μm ×102) |
|---|---|---|---|
| A | 0.63 ± 0.10 | 0.81 ± 0.10 | 0.17 ± 0.02 |
| B | 0.37 ± 0.01* | 0.53 ± 0.03* | 0.28 ± 0.02* |
| C | 0.34 ± 0.03* | 0.80 ± 0.03 | 0.17 ± 0.02 |
| D | 0.62 ± 0.16 | 0.61 ± 0.06* | 0.26 ± 0.01* |
| E | 0.39 ± 0.05* | 0.58 ± 0.12* | 0.31 ± 0.04* |
| F | 0.51 ± 0.03* | 0.73 ± 0.10 | 0.18 ± 0.03 |
| G | 0.88 ± 0.17 | 1.07 ± 0.19* | 0.29 ± 0.02* |
Values are expressed as mean ± SEM showing the level of significance in *P < 0.05 compared to the control group.
Seminiferous tubule diameter (STD), Lumen diameter (LD), germinal epithelium diameter (GED).
3.3. Hormonal analysis
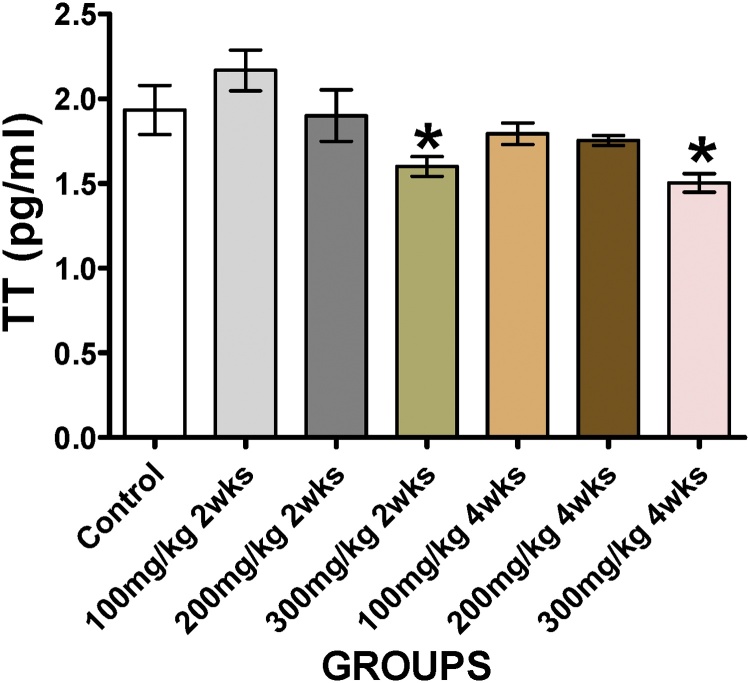
Significant reduction (P < 0.05) in testosterone levels was observed in groups F and G at 2 and 4 weeks respectively when compared to the control group (Fig. 1).
Fig. 1.
Showing the serum Testosterone concentration of animals treated with varying doses and duration of T. occidentalis. *(P ≤ 0.05) - significantly different compared to control group.
3.4. Histological observation
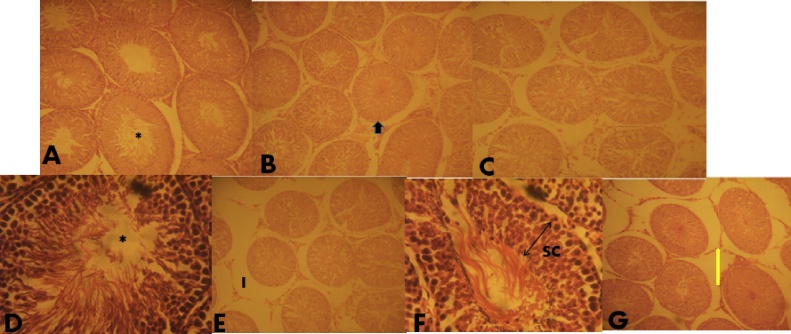
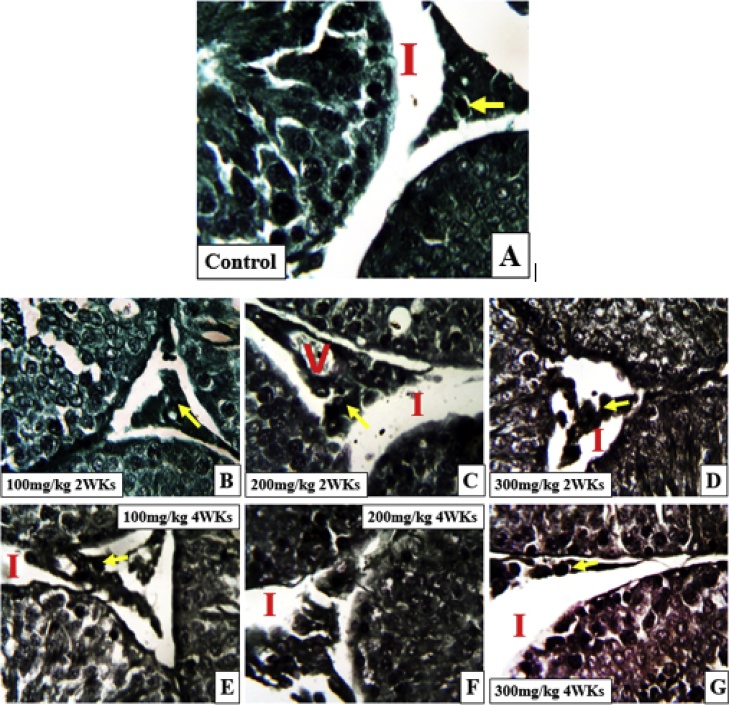
Histological observation using H & E in groups’ 2a–c showed normal testicular cytoarchitectural array depicting an oval shaped seminiferous tubule with abundant and progressively proliferating spermatogenic series, abundant spermatozoa in the lumen, intact basement membrane and presence of testosterone producing Leydig cells in the interstitial spaces. Figures D and E showed an degenerated seminiferous tubule and basement membrane Groups F and G showed an elongated seminiferous tubule with scanty spermatozoa in the lumen (*), very few Leydig cells in the interstitial spaces at 2 weeks while at 4 weeks, a rounded/oval seminiferous tubule and wide interstitial spaces with reduced Leydig cells was observed (Fig. 2) while histological observation using Mason Trichrome Stain in groups 3A–C showed a seminiferous tubule with progressively proliferating spermatogenic cells and an interstitial space with abundant testosterone producing Leydig cells at 2 and 4 weeks while groups 3D–G showed widened interstitial spaces, presence of vacuolation in the interstitial space and very few Leydig cells at 4 weeks of administration (Fig. 3).
Fig. 2.
(a–g) General structure of the testes of animals treated with varying doses and duration of Telfairia occidentalis. I- interstital space Stain, arrow head-basement membrane, spanned arrow- spermatogenic cells, Star- lumen. Yellow arrow- Leydig cells Haematoxylin and Eosin. Mag. ×100. Testicular cytoarchitecture of groups a, b and c were in normal arrays, d and e showed a degenerated seminiferous tubule and basement membrane. Groups f and g showed an elongated seminiferous tubule with scanty spermatozoa in the lumen (*), very few leydig cells in the interstitial spaces at 2 weeks while at 4 weeks, a rounded/oval seminiferous tubule and wide interstitial spaces with reduced Leydig cells was observed.
Fig. 3.
(a–g) General structure of the testes of animals treated with varying doses and duration of Telfairia occidentalis. I- interstital space Stain, Arrow head-basement membrane, V- vacuolation, Spanned arrow- spermatogenic cells, Star- lumen. Yellow arrow- Leydig cells Mason Trichrome stain Mag. ×100.
4. Discussion
Previous reports on the effects of T. occidentalis on the testis presented a plethora of apparently conflicting results, due to their largely limited scope. Ho wever, reports from this study hits the nail on the head by revealing the effects of T. occidentalis leaf extract on reproductive parameters. The investigation of sperm parameters proffers solutions to male fertility issues. Hence, factors which damage the testes could hamper fertility. The dose dependent significant increase in sperm count alone and non significant increase in sperm motility and morphology observed in this study demonstrated the efficacy of T. occidentalis over a long period of time at a substantial dose. However, it negated studies by [18] whose reports showed a significant decrease (P < 0.05) in sperm count and morphology. Another reason for the increase in sperm count may be due to the reparative effect of the phytochemicals present in the TO.
The insignificant decrease in sperm motility and morphology observed at 300 mg/kg body weight of TO over a long period of time had reducing effects on semen quality by causing cellular testicular damage in a dose dependent fashion. These findings corroborated with the values obtained from results of serum testosterone levels which were in consonance with [1] who reported that T. occidentalis leaves extract reduced sperm motility further highlighting its anti-spermatogenic properties. However, it negated reports by [19] who reported a significant increase in sperm quality after studies on the effects of T. occidentalis on the testes of male rats.
The reduction in serum testosterone levels observed in the group that received 300 mg/kg body weight of TO at 2 and 4 weeks respectively further highlighted that at high doses TO may cause untold damages to the testis. These results corroborated reports from the histological analysis which depicted clearly, the widely spaced interstitium, reduced leydig cells and presence of vacuolation in the leydig cells. Thus, bringing to the fore that production of quality spermatozoa is highly dependent on the histological integrity of the testis.
The significant increase (P < 0.05) in germinal epithelium diameter (GED) observed in groups B and D at 2 weeks and also in groups E and G at 4 weeks when compared to the control group was as a result of the compensatory mechanism which was triggered by the significant reductions in Lumen diameter (LD) and seminiferous tubule diameter (STD) observed in this study. This result corroborated with [2] who reported that different doses of Telfairia occidentalis leave extract, preserved histomorphometric profiles of the testes. They reported that the antioxidant contents like phenolic and vitamin present in TO may be the reason for its testiculoprotective and spermatogenic properties. The significant decrease observed negated reports by [20] who observed that aqueous leaf extract of T. occidentalis maintained the histoachitecture of the testis by increasing spermatogonia proliferative activities, maintaining volume density of the interstitium and possessing pro-fertility potentials respectively.
Histological analysis from this study showed various degrees of depleted cytoarchitectural array of the seminiferous tubule for groups TO was mostly administered over a long period bringing to the fore the inhibition of the different stages of spermatogenesis in the testis. This was in concert with studies by [21] who reported that higher doses of TO coupled with a longer period of administration provoked varying degrees of severe testicular oxidative status, degenerated testis and deranged sperm parameters. These defects may be linked to the presence of phytochemical constituents in the extract like tanins, alkaloids and saponins. Saponins, alkaloid and tannins at high doses could become pro-oxidant, thereby increasing lipid peroxidation in the testis [22].
5. Conclusion
In conclusion, the link between the dosage and duration of the extract as a precursor to various degrees of spermatotoxic and antispermatogenic damages has been explained lucidly. Therefore, Telfairia occidentalis leaf extract have potential to reduce male sexual function considering the observed effects it had on semen quality (sperm morphology and motility), plasma testosterone level, histological and histomorphometric presentations of the testis. However, a moderate use and consumption of the extract should be encouraged.
Conflict of interest
None.
Acknowledgment
I will like to acknowledge the inputs of ObasiKosi and the Technologists at the Department of Anatomy, LAUTECH. Osun State, Nigeria.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2018.08.009.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Adisa W.A., Okhiai O., Bankole J.K., Iyamu O.A., Aigbe O. Testicular damage in Telfairia occidentalis extract treated Wistar rats. Am. J. Med. Biol. Res. 2014;2(2):37–45. [Google Scholar]
- 2.Mohammed A., Mada S.B., Olagunju A., Muhammad A., Bala S.M., Garba A., Hafsat A.M., Mustafa S., Nasir Nakakana H., Ahmadu H., Aliyu S. Comparative in vitro antioxidant studies of ethanolic extracts of Psidiumguajava stem bark and Telfairia occidentalis leaf. Int. J. Modern Biochem. 2012;1(1):18–26. [Google Scholar]
- 3.Okokon J.E., Ashana D., Muhammad I.C. Chemical constituents and analgesic activity of Telfaria occidentalis. Phytopharm. 2012;3(2):359–366. [Google Scholar]
- 4.Dada A.A., Ejete-Iroh V.C. Dietary fluted pumpkin (Telfairia occidentalis) improves reproductive indices in male African catfish (Clariasgariepinus) broodstock. J. Agric. Sci. 2015;7(7):228–234. [Google Scholar]
- 5.Kuku A., Etti U.J., Ibironke I.S. Processing of fluted pumpkin seeds, Telfairia occidentalis (Hook F) as it affects growth performance and nutrient metabolism in rats. Afr. J. food. Agric. Dev. 2014;14(5):1992–2014. [Google Scholar]
- 6.Onu P.N. Effect of aqueous extract of Telfairia occidentalis leaf on the performance and haematological indices of starter broilers. Vet. Sci. 2012;3(2):25–31. doi: 10.5402/2012/726515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obeagu E.I., Chikelu I.M., Obarezi T.M., Ogbuabor B.N., Anaebo Q.B. Haematological effects of fluted pumpkin (Telfairia occidentalis) leaves in rats. Int. J. Life Sci. Biotechnol. Pharm. Res. 2014;3:172–182. [Google Scholar]
- 8.Daramola O.O., Wahab A., Oyeyemi W.A., Onyendilefu G. Effects of methanol extract of Telfairia occidentalis seed on serum lipid profile, biochemical and antioxidant activity in female Wistar rats. Eur. J. Med. Phys. 2016;15(2):1–8. [Google Scholar]
- 9.Ekpenyong C.E., Akpan E.E., Udoh N.S. Phytochemistry and Toxicity studies of Telfairia occidentalis aqueous leaf extract on liver biochemical indices in Wistar rats. Am. J. Med. Sci. 2012;2:103–110. [Google Scholar]
- 10.Ajani R.S., Akinyemi A.R. Telfairia occidentalis leaf and seed extract as possible preventive and therapeutic agents for induced benign prostatic hyperplasia. Eur. J. Med. Plants. 2016;12(1):1–11. [Google Scholar]
- 11.Onuegbu A.J., Olisekodiaka J.M., Ude T., Amah K.U., Okwara E.J., Ifeadike C. Effects of Telferia occidentalis seeds on the serum lipid profile and atherogenic indices of male albino Wistar rats. Pak. J. Nutr. 2015;14(9):557–562. [Google Scholar]
- 12.Canene-Adams K. Preparation of formalin-fixed paraffin-embedded tissue for immunohistochemistry. Methods Enzymol. 2013;533:225–233. doi: 10.1016/B978-0-12-420067-8.00015-5. [DOI] [PubMed] [Google Scholar]
- 13.Fischer A.H., Jacobson K.A., Rose J., Zeller R. Hematoxylin and Eosin (H & E) staining. CSH Protoc. 2005;2008 doi: 10.1101/pdb.prot4986. [DOI] [PubMed] [Google Scholar]
- 14.Tietz N.W. Saunders W.B. Co.; Philadephia: 1986. Textbook of Clinical Chemistry; pp. 509–512. [Google Scholar]
- 15.Keel B.A., Webster B.W. CRC Press Incorporation; Boca Raton: 1990. CRC Handbook of the Laboratory Diagnosis and Treatment of Infertility; p. 37. [Google Scholar]
- 16.Yan J., Agresti M., Bruce T., Yan Y., Granlund A., Matloub H. Effects of cellular phone emissions on sperm motility in rats. Fertil. Steril. 2007;88:957–964. doi: 10.1016/j.fertnstert.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Atessahin A.I., Karahan G., Turk S., Yilmaz S., Ceribasi A.O. Protective role of lycopene on cisplatin induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Reprod. Toxicol. 2006;21:42–47. doi: 10.1016/j.reprotox.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Akang E.N., Oremosu A.A., Osinubi A.A., Dosumu O.O., Kusemiju T.O., Adelakun S.S., Umaru M.L. Histomorphometric studies of the effects of Telfairia occidentalis on alcohol-induced gonado-toxicity in male rats. Toxicol. Rep. 2015;2:968–975. doi: 10.1016/j.toxrep.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christopher S.L., Onovughakpo‑Sakpa O.E., Okhimamhe A.F. Profertility effects of aqueous leaf extract of Telfairia occidentalis in adult male Wistar rats. JECA. 2017;14(2):88–94. [Google Scholar]
- 20.Saalu L.C., Kpela T., Benebo A.S., Oyewopo A.O., Anifowope E.O., Oguntola J.A. The dose-dependent testiculoprotective and testiculotoxic potentials of Telfairia occidentalis Hook F. leaves extract in rat. Int. J. Appl. Res. Nat. Prod. 2010;3(3):27–38. [Google Scholar]
- 21.Akube A.R. Chemical composition of Telfairia occidentalis plant. Plant Media. 1980;38:33–43. [Google Scholar]
- 22.Nworgu F.C., Ekemezie A.A., Ladele A.O., Akinrolabu B.M. Performance of broiler chickens served heat-treated fluted pumpkin (Telfaria occidentalis) leaves extract supplement. Afr. J. Biotechnol. 2007;6(6):818–825. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.