Abstract
Background
Diabetic nephropathy (DN) is the major cause of end-stage renal disease. Recent studies suggest that it is probable that uric acid is involved in the pathogenesis of diabetic nephropathy. This study aim was to investigate the association between serum uric acid and kidney function in Iranian patients with type 2 diabetes mellitus.
Methods
In this case-control study, a total of 201 diabetic patients with or without impaired kidney function (glomerular filtration rate/GFR < 60 and GFR ≥ 60) were selected. In both groups, serum fasting glucose (FBS), HbA1c, urea, creatinine, uric acid and lipid profile, urine albumin and GFR were measured and results were compared between the two groups. The results also categorized into three groups based on uric acid tertiles.
Results
Serum levels of uric acid, urea and creatinine as well as urine albumin/creatinine ratio (ACR) were significantly different between the two groups. GFR, creatinine and also urea were significantly different between uric acid tertiles (p < 0.05).
Conclusions
Serum uric acid is associated with decreased GFR as well as albuminuria and can be used as an indicator of DN.
Keywords: Uric acid, Diabetic nephropathy, Albuminuria, GFR
Background
Diabetes Mellitus (DM) is one of the most common endocrine diseases and a big health challenges in the world, especially in developing countries which increasing prevalence in conjunction with poor control result in severe complications which are the most common causes of morbidity and mortality of diabetes [1, 2]. Diabetic nephropathy (DN) is the major cause of end-stage renal disease and 50% of end stage renal diseases (ESRD) are related to DM [3, 4]. It is predicted that about 50% of diabetic patients to develop DN after more than 20 years of diabetes. DN is characterized by pathological excretion of albumin in urine, glomerular lesions, and decreased glomerular filtration rate (GFR). DN is a multifactorial disease and different factors such as hyperglycemia, accumulation of glycated proteins, inflammation and also genetic factors influence the development of DN [5].
Although yet diagnosis of DN is based on detection of unusual albuminuria, but in some cases decreased GFR is present while urine albumin excretion is normal. In addition, diabetes may be accompanied by other non-diabetic renal disease in the absence or presence of DN which cannot be identified from DN [6]. Therefore, it seems that current methods for identification of DN are not ideal. Many studies have focused on the finding appropriate ways for early diagnosis of diabetes complication to prevent their occurrence or progression in many countries including Iran [7–13].
Recent studies suggest probable role of uric acid in the pathogenesis of DN [14]. Uric acid is the end product of purine metabolism, which is produced fromendogenous (purine nucleic acids metabolism) and exogenous (foods) sources and is excreted in urine.
The association of uric acid with different disorders including hypertension, metabolic syndrome, and cardiovascular disease has been confirmed, previously [2, 15–18].
Some studies in patients with diabetes (type 1 and 2) have shown that uric acid is an independent risk factor for DN development [19–23].
Regarding to availability and inexpensive measurement of uric acid compared to urine albumin, this parameter can be measured easily in different laboratories and may help in better and faster diagnosis of DN.
The aim of this study was to investigate the association between serum uric acid and renal function in patients with type 2 DM in order to find its value for DN diagnosis.
Methods
Subjects
In this case-control study, a total of 201 diabetic patients aged 30–75 years with or without diabetic nephropathy(GFR < 60 and GFR ≥ 60) and a history of more than 5 years of diabetes, referred to Diabetes Clinic affiliated to Tehran University of Medical Sciences, were included. Patients with heart failure, urinary tract infection, uncontrolled hypertension and diabetes (HbA1c > 9%), pregnancy, acute infections, hematuria, antihyperuricemic medication and recent heavy exercise were excluded.
The study protocol was approved by the Ethics Committee of the Endocrinology and Metabolism Research Institute (EMRI) affiliated to Tehran University of Medical Sciences and all participants signed written informed consent.
Clinical and laboratory measurements
Blood pressure was measured after 20-min rest in a sitting position and body mass index (BMI) was calculated after measurement of height and weight.
A venous blood sample was obtained following an overnight fasting and early morning urine was collected. Serum samples were separated and stored in −70 C° until assay. In both groups, biochemical parameters including serum levels of glucose, urea, uric acid, triglyceride, cholesterol, High-density lipoprotein cholesterol (HDL-C), Low-density lipoprotein cholesterol (LDL-C), creatinine and urine albumin and creatinine were measured by commercial kits (Pars Azmun kit, Iran) and urine albumin to creatinine ratio (ACR) was calculated. HbA1c level was measured by High Performance Liquid Chromatography (TosohG8, Tosoh Bioscience, Japan) and Modification of Diet in Renal Disease (MDRD) formula was used for GFR calculation.
Statistical analysis
The results were classified based on GFR values and compared by independent sample t-test. The results were also categorized into three groups based on uric acid tertile levels (1sttertile: uric acid≤4.3 mg/dL, 2ndtertile: 4.4–5.6 mg/dL and 3rdtertile: >5.6 mg/dL). As distribution of ACR data was not normal, natural logarithm (Ln) of ACR and its tertiles were calculated and used for analysis. The results compared between groups of uric acid tertiles by one-way analysis of variance (ANOVA)- post hoc method. Patients were also categorized in three groups based on ACR tertiles (1sttertile:ACR ≤ 25 mg/g, 2ndtertile: 26–48 mg/g and 3rdtertile: >48 mg/g). Joint effects of uric acid and ACR tertiles were investigated.
Data analyzed using SPSS software version 21.00 for Windows by application of proper tests and p_ value equal or less than 0.05 was set as significance level.
Data availability
Data is available and will be sent on editor request.
Results
Patient’s demographic and biochemical parameters are given in Table 1. Patients with GFR less than 60 were older and had significantly higher systolic blood pressure, serum creatinine, urea, uric acid and urine ACR. Table 2 shows the result of comparison between uric acid tertile groups. Post hoc analysis demonstrated significant differences in serum creatinine, urea and GFR values between three groups.
Table 1.
Demographic characteristics and biochemical factors among diabetic patients with GFR ≥ 60 and with GFR < 60
| GFR ≥ 60 (n = 95) mean ± SD | GFR < 60 (n = 106) mean ± SD | |
|---|---|---|
| Female/Male (n) | 62/44 | 47/48 |
| Age (year) | 56.6 ± 7.8 | 62.5 ± 8.1† |
| Systolic blood pressure (mmHg) | 121 ± 12 | 125 ± 13† |
| Diastolic blood pressure (mmHg) | 78 ± 8.9 | 77 ± 7.0 |
| BMI(kg/m2) | 28.2 ± 3.8 | 29.4 ± 5.5 |
| GFR(mL/min/1.73m2) | 84.0 ± 17.1 | 58.7 ± 16.8† |
| FBS(mg/dL) | 142.3 ± 49.7 | 142.6 ± 41.4 |
| HbA1c (%) | 7.1 ± 0.8 | 7.2 ± 0.7 |
| Creatinine(mg/dL) | 0.98 ± 0.14 | 1.38 ± 0.38† |
| Urea(mg/dL) | 31.9 ± 9.3 | 46.7 ± 16.8† |
| Uric Acid(mg/dL) | 4.6 ± 1.2 | 5.5 ± 1.5† |
| Cholesterol(mg/dL) | 154.1 ± 31.6 | 153.3 ± 34.6 |
| Triglyceride(mg/dL) | 143.1 ± 69.1 | 149.1 ± 86.8 |
| HDL-c (mg/dL) | 47.6 ± 10.4 | 46.0 ± 11.6 |
| LDL-c(mg/dL) | 74.7 ± 19.0 | 75.5 ± 19.5 |
| ACR (mg/g) | 40.7 ± 27.7 | 64.2 ± 68.8† |
| Ln ACR | 3.5 ± 0.5 | 3.8 ± 0.8† |
†Mann-Whitney U P-value<0.05
BMI, body mass index; GFR, glomerular filtration rate; FBS, fasting blood sugar; ACR, albumin to creatinine ratio
Table 2.
Demographic characteristics and biochemical factors among diabetic patients in different uric acid tertile groups
| Variable | Uric acid | ||
|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |
| Age (year) | 58.8 ± 7.6 | 58.7 ± 9.0 | 60.9 ± 8.4 |
| Systolic blood pressure (mmHg) | 120.1 ± 12.9 | 125.7 ± 13.5 | 124.4 ± 11.6 |
| Diastolic blood pressure (mmHg) | 77.4 ± 8.0 | 78.8 ± 8.6 | 78.4 ± 7.3 |
| BMI(kg/m2) | 28.2 ± 4.7 | 28.7 ± 4.7 | 29.4 ± 5.0 |
| GFR(mL/min/1.73m2) | 75.7 ± 22.4 | 72.6 ± 18.8 | 64.6 ± 21.0‡ |
| FBS(mg/dL) | 146.8 ± 53.8 | 142.9 ± 42.9 | 138.25 ± 39.7 |
| HbA1c (%) | 7.2 ± 0.70 | 7.1 ± 0.77 | 7.2 ± 0.70 |
| Creatinine(mg/dL) | 1.02 ± 0.22 | 1.14 ± 0.27 | 1.41 ± 0.41‡ * |
| Urea(mg/dL) | 33.9 ± 11.1 | 38.1 ± 14.3 | 46.4 ± 17.8‡ * |
| Uric Acid(mg/dL) | 3.5 ± 0.55 | 4.8 ± 3.9 | 6.7 ± 1.0 ‡ * |
| Cholesterol(mg/dL) | 155.3 ± 33.7 | 159.2 ± 31.3 | 146.9 ± 33.8 |
| Triglyceride(mg/dL) | 127.6 ± 65.6 | 155.3 ± 78.3 | 153.5 ± 87.4 |
| HDL-c (mg/dL) | 51.1 ± 12.4 | 47.5 ± 10.0 | 42.3 ± 9.2‡ |
| LDL-c(mg/dL) | 75.1 ± 20.5 | 79.0 ± 18.1 | 71.3 ± 18.7 |
| ACR (mg/g) | 45.4 ± 31.6 | 45.9 ± 39.8 | 66.7 ± 74.4 |
| Ln ACR | 3.6 ± 0.55 | 3.6 ± 0.64 | 3.8 ± 0.85 |
Mann-Whitney U P-value<0.05 Between tertile 1,2 †, Between tertile 1,3 ‡, Between tertile 2,3*
BMI, body mass index; GFR, glomerular filtration rate; FBS, fasting blood sugar; ACR, albumin to creatinine ratio
Linear regression analysis found a positive association between uric acid level and Ln ACR (β: 3.57 95% CI (3.21–3.93), P value: <0.001) in patients with ACR > 30 mg/g (r2: 0.13).
In a same analysis, we found a significant association between increasing of GFR and decreasing of systolic blood pressure and also uric acid level (r2: 0.19) (Table 3).
Table 3.
Linear regression analysis between GFR value, uric acid value and systolic blood pressure
| Variable | β | 95% CI | P value |
|---|---|---|---|
| Constant | 83.88 | 53.71–114.16 | <0.001 |
| Uric acid | −3.74 | (−5.65)-(−1.83) | <0.001 |
| Systolic Blood Pressure | −0.30 | (−0.51)-(−0.09) | 0.01 |
Multiple logistic regression analysis for evaluation of GFR values in different uric acid tertiles after adjusting for age and sex showed, second tertile of uric acid was associated with a decreased risk for GFR < 60 compared with the first tertile. Also third tertile of uric acid was associated with an increased risk for GFR < 60 compared with the first tertile (Table 4).
Table 4.
Multiple linear regression analysis of relationships between uric acid tertile groups and GFR < 60
| Uric acid tertile groups | OR (95% CI) | P value |
|---|---|---|
| Uric acid < 4.3 mg/dL | 1.00 (ref) | 0.049 |
| Uric acid 4.3–5.6 mg/dL | 0.71 (0.29–1.74) | |
| Uric acid > 5.6 mg/dL | 1.97 (0.83–4.68) |
Finally Joint effects of uric acid and ACR tertiles were investigated and the result was shown in Fig. 1. Patients who were in both 3rdtertile of uric acid and ACR had lowest mean of GFR. In each tertile of uric acid, GFR showed a marked decline, with increasing ACR tertile. The same results were obtained when rise of uric acid was evaluated in each tertile of ACR.
Fig. 1.
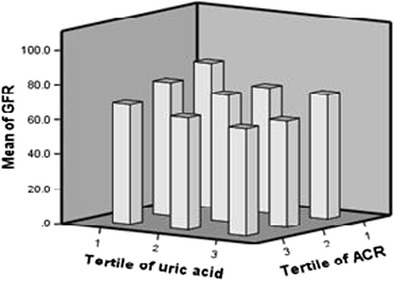
Joint effects of tertiles of albumin excretion rate and serum uric acid on GFR value
Discussion
The results of present study showed that uric acid in high normal levels is associated with decreasing GFR and increasing of urea and creatinine even after adjusting for confounding factors, including age and sex. In patients with ACR > 30 mg/g, there was a significant association between LnACR and uric acid values.
Influence of uric acid on renal function is not fully understood. It is not clear whether increased uric acid is a result of renal insufficiency, or vice viscera a risk factor for renal insufficiency [24]. Several studies signify the role of uric acid as an independent factor in development of albuminuria and chronic kidney diseases (CKD) in non diabetic population [25–28]. Also, studies have shown that uric acid is a useful tool for prediction of contrast-induced nephropathy [23].
There are also evidence about association of high-normal level of uric acid and CKD in patients with DM [12]. Some of previous studies in diabetic patients have reported uric acid as an independent risk factor for DN [19–23]. A recent study in Taiwan showed that increased serum level of uric acid is significantly associated with severity of albuminuria and retinopathy in diabetic patients [29].
In a cross sectional study Rosolowsky showed the association of high serum level of uric acid with low GFR and increased urinary albumin excretion [30]. In two different studies on patients with type1 DM, follow-up of patients showed strong association between high serum levels of uric acid and DN [21, 31].
In a prospective observational study on Japanese patients with DM type 2, high serum uric acid concentration was associated with reduced GFR in 12 months [32]. In a 5-year follow-up of Italian patients with DM type 2, hyperuricaemia was recognized as an independent risk factor for the development of DN [19]. A cross-sectional study on 343 Japanese men with type 2 DM, positive correlation was found between serum uric acid concentration and albuminuria [33]. Another cross- sectional study in china demonstrated that serum uric acid level was positively correlated with albuminuria and creatinine levels but negatively with GFR [34].
Our results are in line with the above studies. We found that high-normal uric acid level is associated positively with urea and creatinine and negatively with GFR. The difference is that we found significant correlation between serum uric acid level and urinary albumin excretion only in patients with ACR more than 30 mg/g. The reason might be difference in study design. Patients enrolled in present study had HbA1c less than 9% and had controlled blood pressure with urinary albumin excretion between 30 and 300 mg/g cr or mg/24 h.
The effects of uric acid on development of renal disease are still not entirely clear. Animal models studies have shown that uric acid can affect renal function via a variety of mechanisms. Uric acid is a potent antioxidant in the extracellular environment; on the other hand, it can act as a pro-oxidant inside the cell. Furthermore uric acid can be associated with endothelial dysfunction, inflammation, proliferation of vascular smooth muscle cells, increasing systemic and glomerular blood pressure and also decreasing renal blood flow [4, 24, 35].
Due to the cross-sectional nature of this study, we were unable to prove a causal association between uric acid and DN and this is a limitation of our study. Confirmation of such association must be obtained in additional prospective studies. Since the DN is a multifactorial disease and many factors may influence the development of DN, the results of present study should be interpreted carefully.
Conclusion
In conclusion, serum uric acid is independently associated with decreased GFR and kidney function and can acts as an indicator of DN.
Acknowledgements
The authors wish to thank staffs at Diabetes Research Center for their assistance in the study.
Abbreviations
- ACR
Albumin to Creatinine Ratio
- ANOVA
One-way analysis of variance
- CKD
Chronic Kidney Diseases
- DN
Diabetic Nephropathy
- ESRD
End Stage Renal Diseases
- FBS
Fasting Blood Sugar
- GFR
Glomerular Filtration Rate
- MDRD
Modification of Diet in Renal Disease
Author contributions
FR: study design and concept, performing assay and approving final manuscript.
FB: Data collection, statistical analysis and approving final manuscript.
EN: data interpretation, writing first manuscript draft and approving final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the Endocrinology and Metabolism Research Institute (EMRI) affiliated to Tehran University of Medical Sciences and all participants signed written informed consent.
Competing interest
The authors have no conflict of interest regarding this study to report.
References
- 1.Nasli-Esfahani E, Farzadfar F, Kouhnavard M, Ghodssi-Ghassemabadi R, Khajavi A, Peimani M, Razmandeh R, Vala M, Shafiee G, Rambod C, Sanjari M, Aalaa M, Ghodsi M, Razi F, Bandarian F, Larijani B. Iran diabetes research roadmap (IDRR) study: a preliminary study on diabetes research in the world and Iran. J Diabetes Metab Disord. 2017;16:9. doi: 10.1186/s40200-017-0291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandarian F, Omidvar M, Farideh R, Nasli-Esfahani E, Saeedi S, Larijani B. Iran diabetes research roadmap (IDRR) study; knowledge gap in Ge-netic research on diabetes mellitus in Iran: a review article. Iran J Public Health. 2017;46:53. [Google Scholar]
- 3.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME. Diabetic kidney disease: a report from an ADA consensus conference. Diabetes Care. 2014;37:2864–2883. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raptis AE, Viberti G. Pathogenesis of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S424–S437. doi: 10.1055/s-2001-18600. [DOI] [PubMed] [Google Scholar]
- 5.Wolf G, Ziyadeh FN. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol. 2007;106:26–31. doi: 10.1159/000101797. [DOI] [PubMed] [Google Scholar]
- 6.Jayashankar C, Andrews HP, Vijayasarathi VBP, Shashidharan B, Kumar HN, Vemulapalli S. Serum uric acid and low-density lipoprotein cholesterol levels are independent predictors of coronary artery disease in Asian Indian patients with type 2 diabetes mellitus. J Nat Sci Biol Med. 2016;7:161–165. doi: 10.4103/0976-9668.184703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soleymanian T, Hamid G, Arefi M, Najafi I, Ganji MR, Amini M, Hakemi M, Tehrani MRM, Larijani B. Non-diabetic renal disease with or without diabetic nephropathy in type 2 diabetes: clinical predictors and outcome. Ren Fail. 2015;37:572–575. doi: 10.3109/0886022X.2015.1007804. [DOI] [PubMed] [Google Scholar]
- 8.Razi F, Esfahani EN, Farzami MR, Tootee A, Qorbani M, Ebrahimi SA, et al. Effect of the different assays of HbA1c on diabetic patients monitoring. J Diabetes Metab Disord. 2015;14:65. doi: 10.1186/s40200-015-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saeedi S, Bandarian F, Meshkani R, Nourbakhsh M, Nasli-Esfahani E, Larijani B. Iran diabetes research roadmap (IDRR) study; trends of basic sciences publication: a review article. Iran J Public Health. 2017;46:60. [Google Scholar]
- 10.Mehrabzadeh M, Pasalar P, Karimi M, Abdollahi M, Daneshpour M, Asadolahpour E, et al. Association between ELMO1 gene polymorphisms and diabetic nephropathy in an Iranian population. J Diabetes Metab Disord. 2016;15:43. doi: 10.1186/s40200-016-0265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asdadollahpour E, Daneshpour M, Khayat BS, Hashemiaghdam A, Amoli MM, Qorbani M, et al. Non-muscle myosin heavy chain 9 gene (MYH9) polymorphism (rs4821481) is associated with urinary albumin excretion in Iranian diabetic patients. Iran Red Cres Med J. 2017;e40076:19. [Google Scholar]
- 12.Karimoei M, Pasalar P, Mehrabzadeh M, Daneshpour M, Shojaee M, Forouzanfar K, Razi F. Association between apolipoprotein E polymorphism and nephropathy in Iranian diabetic patients. Saudi J Kidney Dis Transpl. 2017;28:997–1002. doi: 10.4103/1319-2442.215137. [DOI] [PubMed] [Google Scholar]
- 13.Razi F, Daneshpour MS, Karimoei M, Mehrabzadeh M, Bandarian F, Bahreini E, Qorbani M, Pasalar P. AGTR1 rs5186 variants in patients with type 2 diabetes mellitus and nephropathy. Meta Gene. 2018;15(Supplement C):50–54. doi: 10.1016/j.mgene.2017.11.001. [DOI] [Google Scholar]
- 14.Behradmanesh S, Horestani MK, Baradaran A, Nasri H. Association of serum uric acid with proteinuria in type 2 diabetic patients. J Res Med Sci. 2013;18:44–46. [PMC free article] [PubMed] [Google Scholar]
- 15.Bagheri B, Zargari M, MeShKInI F, DInarvanD K, Mokhberi V, Azizi S, et al. Uric acid and coronary artery disease, two sides of a single coin: A determinant of antioxidant system or a factor in metabolic syndrome. J Clin Diagn Res: JCDR. 2016;10:OC27. doi: 10.7860/JCDR/2016/16335.7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebrahimpour P, Fakhrzadeh H, Heshmat R, Bandarian F, Larijani B. Serum uric acid levels and risk of metabolic syndrome in healthy adults. Endocr Pract. 2008;14:298–304. doi: 10.4158/EP.14.3.298. [DOI] [PubMed] [Google Scholar]
- 17.Meshkani R, Zargari M, Larijani B. The relationship between uric acid and metabolic syndrome in normal glucose tolerance and normal fasting glucose subjects. Acta Diabetol. 2011;48:79–88. doi: 10.1007/s00592-010-0231-3. [DOI] [PubMed] [Google Scholar]
- 18.Ficociello LH, Rosolowsky ET, Niewczas MA, Maselli NJ, Weinberg JM, Aschengrau A, Eckfeldt JH, Stanton RC, Galecki AT, Doria A, Warram JH, Krolewski AS. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes. Diabetes Care. 2010;33:1337–1343. doi: 10.2337/dc10-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoppini G, Targher G, Chonchol M, Ortalda V, Abaterusso C, Pichiri I, Negri C, Bonora E. Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care. 2012;35:99–104. doi: 10.2337/dc11-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling Y, Li X, Gao X. Cross-sectional association of serum C-reactive protein and uric acid with albuminuria in Chinese type 2 diabetic patients. Chin Med J. 2013;126:4023–4029. [PubMed] [Google Scholar]
- 21.Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving H-H. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes an inception cohort study. Diabetes. 2009;58:1668–1671. doi: 10.2337/db09-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hovind P, Rossing P, Johnson RJ, Parving H-H. Serum uric acid as a new player in the development of diabetic nephropathy. J Ren Nutr. 2011;21:124–127. doi: 10.1053/j.jrn.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Mendi MA, Afsar B, Oksuz F, Turak O, Yayla C, Ozcan F, Johnson RJ, Kanbay M. Uric acid is a useful tool to predict contrast-induced nephropathy. Angiology. 2017;68:627–632. doi: 10.1177/0003319716639187. [DOI] [PubMed] [Google Scholar]
- 24.Johnson RJ, Nakagawa T, Jalal D, Sánchez-Lozada LG, Kang D-H, Ritz E. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant. 2013:gft029. [DOI] [PMC free article] [PubMed]
- 25.Fan X, Cai J, Gao B, Mou L, Li J, Liu X, Wu JX, Meng QY, Wang HY, Liu LL, Li H, Li XM, Li XW. The relationship between urinary albumin excretion and serum uric acid in general population. Zhonghua nei ke za zhi. 2011;50:550–554. [PubMed] [Google Scholar]
- 26.Chang H-Y, Lee P-H, Lei C-C, Tung C-W, Hsu Y-C, Huang T-J, Lu LC, Lin CL. Hyperuricemia is an independent risk factor for new onset micro-albuminuria in a middle-aged and elderly population: a prospective cohort study in Taiwan. PLoS One. 2013;8:e61450. doi: 10.1371/journal.pone.0061450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konta T, Hao Z, Abiko H, Ishikawa M, Takahashi T, Ikeda A, Ichikawa K, Takasaki S, Kubota I. Prevalence and risk factor analysis of microalbuminuria in Japanese general population: the Takahata study. Kidney Int. 2006;70:751–756. doi: 10.1038/sj.ki.5001504. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Dov IZ, Kark JD. Serum uric acid is a GFR-independent long-term predictor of acute and chronic renal insufficiency: the Jerusalem lipid research clinic cohort study. Nephrol Dial Transplant. 2011;26:2558–2566. doi: 10.1093/ndt/gfq740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang CC, Lin PC, Lee MY, Chen SC, Shin SJ, Hsiao PJ, et al. Association of Serum Uric Acid Concentration with Diabetic Retinopathy and Albuminuria in Taiwanese Patients with Type 2 Diabetes Mellitus. Int J Mol Sci. 2016;17:E1248. doi: 10.3390/ijms17081248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosolowsky ET, Ficociello LH, Maselli NJ, Niewczas MA, Binns AL, Roshan B, Warram JH, Krolewski AS. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2008;3:706–713. doi: 10.2215/CJN.04271007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jalal DI, Rivard CJ, Johnson RJ, Maahs DM, McFann K, Rewers M, Snell-Bergeon JK. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the coronary artery calcification in type 1 diabetes study. Nephrol Dial Transplant. 2010;25:1865–1869. doi: 10.1093/ndt/gfp740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito H, Abe M, Mifune M, Oshikiri K, Antoku S, Takeuchi Y, Togane M. Hyperuricemia is independently associated with coronary heart disease and renal dysfunction in patients with type 2 diabetes mellitus. PLoS One. 2011;6:e27817. doi: 10.1371/journal.pone.0027817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukui M, Tanaka M, Shiraishi E, Harusato I, Hosoda H, Asano M, Kadono M, Hasegawa G, Yoshikawa T, Nakamura N. Serum uric acid is associated with microalbuminuria and subclinical atherosclerosis in men with type 2 diabetes mellitus. Metabolism. 2008;57:625–629. doi: 10.1016/j.metabol.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Yan D, Tu Y, Jiang F, Wang J, Zhang R, Sun X, Wang T, Wang S, Bao Y, Hu C, Jia W. Uric acid is independently associated with diabetic kidney disease: a cross-sectional study in a Chinese population. PLoS One. 2015;10:e0129797. doi: 10.1371/journal.pone.0129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez-Lozada LG, Lanaspa MA, Cristóbal-García M, García-Arroyo F, Soto V, Cruz-Robles D, et al. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol. 2012;121:e71-e8. doi: 10.1159/000345509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available and will be sent on editor request.


