Abstract
The heterogeneity of tumor is considered as a major difficulty to victorious personalized cancer medicine. There is an extremeneed of consistent response evaluation for in vivo tumor heterogeneity anditscoupledconflict mechanisms. In this occasion researchers will be able to keep pace withpredictive, preventive, personalized, and Participatory (P4) medicine for cancer managements. In fact tumor heterogeneity is a central part of cancer evolution,soin order to progress in understanding of the dynamics within a tumor some diagnostic apparatus should be improved. Latest molecular techniques like Next generation Sequencing (NGS) and ultra-deep sequencing could disclose some clones within a liquid tumor biopsy which mainly responsible of treatment resistance. Circulating tumor DNA (ctDNA) as a main component of liquid biopsy is agifted biomarker for cancer mutation tracking as well as profiling. Personalized medicine facilitate learning regarding to genetic pools of tumor and their possible respond to treatment which could be much easier by using of ctDNA.With this information, cliniciansarelooking forward to find the best strategies for prevention, screening, and treatment in the way of precision medicine. Currently, numerous clinical efficacy of such informative improved treatment are in hand. Here we represent the review of plasma-derived ctDNA studies use in personalized cancer managements.
Keywords: Circulating tumor DNA (ctDNA), Personalized medicine, Cancer
Introduction
Cancer is one of the problematic issue of human health and the second main reason of death all over the word [1, 2]. Circulating tumor DNAs (ctDNA) are short tumor-derived fragments of DNA (≅166 base pairs) which are not associated with cells and freely are circulating in serum and plasma [3]. The precise mechanism of ctDNA release has not been cleared yet, but they are some suggesting role for tissue necrosis and apoptosis as well as dynamic secretion from tumor cells [4–8]. In the honor of ctDNA it can be said easily that it is a real time representative of tumor, so it can be checked for genetic and epigenetic changes of tumor in order to define the accurate treatment plan as well as monitoring the tumor progression during the therapy [9–11]. In reality using of ctDNA as a diagnostic or prognostic tool outweighs the other common biopsy methods like tissue biopsy [12, 13]. The ctDNA collection characteristics as a non-invasive biopsy method in addition to several sampling at different time after treatment will be possible and consequently keeping an eye on tumor progression and response to treatment will be much feasible [14].
One of the problematic issues of cancer therapy is drug-resistant tumors due to intra- and inter-tumor heterogeneity [15, 16]. Unfortunately even a minor genetic clone within the tumor if carries a drug-resistant mutation can be developed after treatment [17]. ctDNA is a repeatable non-invasive biopsy method and contrary to tissue biopsy as a ‘snapshot’, ctDNA is a ‘screenshot’ of the primary and metastatic tumor [18]. At the cutting-edge of targeted treatment approach, sequencing of ctDNA can be really informative for finding genetic hotspots of targeted tumor [19]. This is mainly significant for informing treatment specially when mutations are critical as drug targets [20, 21]. Consequently in each patient, personalizing targeted analysis of ctDNA can be promising by incorporating the liquid biopsies and common tissue biopsies [22].
Targeted approaches have the benefit of amplifying ctDNA in the course of polymerase chain reactions (PCR) or digital PCR (dPCR) [23]. It is above all essential because there are quite small amount of ctDNA circulating in the blood [23]. For that reason, amplification of interested region can considerably recover the weak points of ctDNA detection methods [24]. Unfortunately, PCR a an amplification tool can launch some known errors which will pass to the sequencing step [25].
The latest advances in whole genome and targeted next generation sequencing (NGS) techniques are breakthroughs for detection of genetic abnormalities of a patient’s tumor [26]. Moreover the Cancer Personalized Profiling by deep Sequencing (CAPP-Seq) is an insightful and sensitive method in order to quantify DNA in cancer because It measures ctDNA which is originated from tumor cells into the bloodstream [27]. This method can be widespread for any cancer type and is able to identify one molecule of mutant DNA in ten thousands molecules of normal DNA [28].
In the current review we are presenting the importance and value of ctDNA in the place of precision cancer medicine in both era of cancer diagnosis and cancer prognosis. The study was based on searching the PUBMED, Scopus, Web of Science, and EMBASE from 1990 to 2017.The search syntax were “cancer” or “neoplasm” or “tumor” and “cfDNA” or "circulationg tumor DNA" or “ctDNA” or "cell free DNA" or “CTC” or" circulating tumor DNA" and “personalized medicine” or “Precision medicine” or “P4 medicine” and “treatments” or “therapy” or “Diagnosis”. All final selected articles should be written in English (19 articles).
ctDNA as a liquid biopsy component
In spite of the fact that the cfDNA presence in the plasma was first accounted in 1948 by Mandel and Metais [29], it was just recent years that tumor-derived cfDNA was revealed that cancer patients had greater levels of plasma cfDNA than normal controls [30–32]. Actually the exact biological mechanism by which DNA is releasing into the peripheral blood has not been well understood; nonetheless, it is thinking to come about through multiple mechanisms, including extracellular vesicle secretion, tumor cell apoptosis, and necrosis [6, 33, 34]. The fact that dissimilar to genomic DNA, ctDNA is extremely fragmented around nucleosomes (approximately 150 base pairs in length) (Fig. 1), supports the hypothesis that ctDNA originates through cell necrosis or apoptosis [35, 36]. The DNA of eukaryotic cells is coiling around histone protein complexes, shaping nucleosomes as the basic form of chromatin [37]. Judge against to the naked DNA, DNA of nucleosome is fewer reachable to the transcription factors and regulatory elements [38, 39]. The precise physical situations of nucleosomes can affect vital process of cells including replication, DNA repair, and transcription [40]. Actually, depending on the cell type, nucleosome positioning is completely different so ctDNA deep sequencing, isolated from circulating blood plasma haven path of transcription factors [41]. It could be said that ctDNA nucleosome positioning is directly connected to the nuclear architecture and gene expression profile so it could be the exact representative of the origin of tumor [41–43].
Fig. 1.
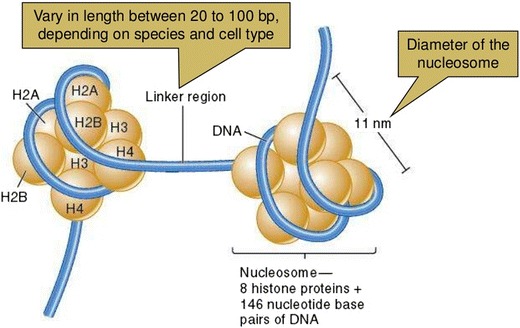
The “beads-on-a-string” structure taken from slide share (http://slideplayer.com/slide/5675348/). It is composed of eight specific histones come together (octamer). The linker DNA length is about 150 base pairs which are next to the ctDNA length
More than the origin of tumor the concentration of ctDNA can be an informative substances. Peripheral ctDNA isasmall proportion of DNA in bloodsteam, fewer than 100 ng/mL [44] and no more than a fraction of this whole ctDNA (< 1% of total ctDNA) is in certainty tumor-derived [45]. The quantityof released DNA into the peripheral blood is in coincide with the concentration of evident ctDNA sincectDNA shedding to the bloodis associatedwith the cell death,cell division rate, and tumor vascularization [30]. As a result the degree of metastatic tumor is joined to thevolume of ctDNA [33, 46–48]. By way of illustration, the direct existence of metastasis to the liver or bone has been straightattachedto thegreater levels of ctDNA [49].
Long beforepersonalized medicine, patients had the identical treatment, but next off it became clear that dependent to genetic profile of patients, certain treatments are much better for some patients than for others. This explained the dissimilar responses to cancer managementapproaches. Nowadays, personalized cancer treatment is an active branch of the treatment plan or even an essential part of a clinical trial. A few, but not all, of the cancers where targeted treatments are used consist of; breast cancer (BC), Lung cancer (LC), Gastrointestinal (GI) Cancer, and endocrine related tumors.
Breast Cancer
There is a long-standing hope of precision medicine to find a genetic markers to guess reaction of a solid tumor to treatment, approximate patient prognosis and early prediction of tumor relapse [50]. Primary research were mainly paying attention to circulating tumor protein biomarkers in the glycosylated form, while it is now speedily altered to novel prospect like circulating tumor cells (CTCs), extracellular vesicles (exosomes), micro-RNAs and circulating tumor DNA (ctDNA) [50, 51]. In early-stagebreast cancer there are some appreciated indications for ctDNA quantifications [52]. In fact raised plasma ctDNA levels using specific digital droplet PCR (dd-PCR) assays in plasma samplesheaded clinical detection of tumor recurrence in patients [52]. Severalpreceding studies had focused on metastatic disease, in order to state the ctDNA amount and response to surgery, treatments or as a measurement tool of overall survival [53], such as colorectal [54], breast [55], ovarian and lung cancer [56]. The justification of this is that released tumor cells are typically phagocytosed by macrophages which engulf necrotic cells that release digested DNA fragments into the cell environment with a half-life in the circulation ranging from some minutes to several hours [57, 58]. Through tumor growth and turnover both wild-type and tumor-derived ctDNA can be shed into the blood, soaccording to the state and size of the tumor, the percentage of ctDNA that originates from tumor cells fluctuates [59]. It was shown by Sarah-Jane Dawson et al. that circulating tumor DNA was a permanent time-dependent inconsistent in the way that its levels were a signature of substandard overall survival of breast cancer patients [55]. The quantity of ctDNA was predictive of poor survival and ctDNA evaluating has worth as a observing component for early metastasis detection, therapy adjustment, and to support in overtreatment avoidance in the way of precision medicine [52].
More than ctDNA quantity the genetic and epigenetic alterations of ctDNA can be used for breast cancer personalized therapy. The level of plasma samples mutations imitate the clonal hierarchy concluded from sequencing of tumor biopsies [18]. The evaluation of biopsy and plasma samples in one metastatic breast cancer patient displays that ctDNA form a concurrent sampling of multifocal clonal evolution [18, 60]. Hopefully a study confirmed that ctDNA analysis via eTAm-Seq and digital PCR have high clinical validity in mutation detection [61]. Detection of Estrogen receptor alpha (ESR1) D538G mutation in circulating tumor cells (CTCs) and ctDNA can be used in for assessing response to endocrine therapies in breast cancer [62]. For resistance to subsequent aromatase inhibitor therapy ESR1 mutations can be strongly recognized with ctDNA analysis, and predict [63, 64]. ESR1 mutations are infrequently developed during adjuvant aromatase inhibitor (AI) therapy, but are frequently designated by therapy for metastatic disease, supporting that the mechanisms of resistance to targeted therapy possibly will be considerably dissimilar between the treatment of micro-metastatic and overt metastatic cancer [63]. Monitoring of ctDNA is extremely essential for preliminary security and efficacy checking of HER2-negative metastatic breast cancer treatment withPhosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) inhibitor Taselisib (GDC-0032) together with Tamoxifen in hormone receptor (HR) positive [65]. During a phase III clinical trial in postmenopausal women with endocrine-resistant HR+/HER2– advanced breast cancer, it was shown that checking the Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha (PIK3C) of ctDNA can guesses efficacy of Buparlisib (BUP) plus fulvestrant (FULV) [66].
In estrogen receptor (ER)–positive breast cancer, mutations of Phosphatidylinositol-4, 5-Bisphosphate 3-Kinase Catalytic Subunit Alpha (PIK3CA) are common genomic alterations and a self-governing analytical feature in breast cancer patients [67, 68]. Analysis of ctDNA in plasma could be used for minimal residual disease (MRD) monitoring in breast cancer [69]. It was verified that mutation tracking of ctDNA through sequencing could outline the genetic events of MRD in order to projected the genetic background of the subsequent metastatic relapse extra precisely than sequencing of the primary tumor [69]. Following adjuvant therapeutic interventions possibly will be personalized with the genetic profile existing in the MRD, a therapeutic approach that could solve the problem of intra-tumor genetic heterogeneity [69, 70].
Lung cancer
The breathtaking advances in lung cancer therapy is the application of personalized chemotherapy planning according to the individual’s genetic profile [71]. It has been suggested that ctDNA “spill over” into an immediate outflow tract pulmonary venous blood (Pul.V) and peripheral blood (Peri.B), and after scattering to the whole body [72]. Thus, it can be inferred that ctDNA reflects the cancer progression and could function as a prognostic marker. It has been accepted that epidermal growth factor receptor EGFR mutation status is a delicate biomarker for the epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) therapy [73, 74]. In fact, patients with the L858R point mutation in exon 21 or deletion mutation in exon 19 display respectable response to EGFR-TKIs [74]. The problematic issue is that after chemotherapy EGFR mutation status might exchange from positive to negative [75]. For that reason, tracking theEGFR mutations is important to control an applicable treatment approach, mainly designed for the supervision of EGFR-TKIs to identify acquired resistance at early time [9, 76–78[. Indeed, ctDNA can be a potential source of tumor DNA alteration pursuing for the documentation of tumor-associated genetic changes in order to real-time tumor monitoring [4, 53, 78, 79]. For Non-Small Cell Lung Cancer Research (NSCLC), numerous clinical centers have investigated the diagnostic precision of ctDNA for EGFR mutation detection [80–83]. In 2016 the U.S. Food and Drug Administration (FDA) agreed to the EGFR Mutation Test v2, a blood-based companion diagnostic for the cancer drug Tarceva (Erlotinib) [84].
More than EGFR some other genetic and epigenetic changes has been considered for personalized lung cancer target therapy. By way of illustration, the existence of Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS) mutations in plasma might be an indicator of deprived prognosis and may also embrace predictive value [85, 86]. The clinical trial phase I, combination study of a kinase inhibitors in patients with RAS mutated cancers, indicated that increasing dose levels resulted in more consistent decreases in KRAS mutation in ctDNA, so the potential value of serial plasma ddPCR as a pharmacodynamic (PD) biomarker in early phase clinical trials was marked [87]. A promising step on the way to precision medicine is that genomic analysis of lung-tumor growth has been practiced to make personalized blood tests that allow successful clinical observing for early signs of cancer relapse [88]. Multiplex ctDNA Gene analysis in lung cancer revealed promising treatment options to guide clinicians to choice the accurate therapy plan for the right person [89].
Additional analysis of ctDNA through CAPP-Seqand resistance mechanisms in NSCLC patients cured with Rociletinib highlighted frequent intra-patient heterogeneity [90]. In fact, Met Proto-Oncogene copy number increasing involves in resistance recurrently [90]. Those results emphasized the position of tumor heterogeneity in NSCLC and the utility of ctDNA-based resistance mechanism calculation [90, 91]. Apart from genetic mutation some epigenetic changes of ctDNA can be recruited for prognosis and diagnosis [92]. By far the most important epigenetic alteration is DNA methylation that occurs by adding the methyl (CH3) group to DNA, in that way often modifying the function of the genes and affecting gene expression without changing the DNA sequences. Very recently the improvement of a highly sensitive blood-based non-invasive diagnostic assay for documentation of primary lung cancer stages, which can aid clinical decisions for patients with a CT scan positive for lung nodules, has been suggested [93]. This method can similarly be stretched to non-invasive early screening for various cancer types [93].
Gastrointestinal Cancer
Regarding to the liquid biopsy components it can be said easily that in patients with cancer of the gastrointestinal cancer (GI), major advances have been completed in the use of circulating tumor cells (CTCs) and ctDNAs for monitoring tumor evolution [94]. This is principally right in the case that in the peripheral blood circulation of GI cancers patients, the mutant form of “driver” genes and “drug-resistant” alleles of tumor are represented in the circulating cell-free tumor DNA (cfDNA) [95–97]. The discriminative accuracy of ctDNA the amount for diagnosis of gastrointestinal cancer contrast to the benign inflammatory diseaseshas been distinguished [98–102].In order to prove the comprehensive diagnostic value of ctDNA through diverse gastrointestinal tumor types, ctDNA of 640 patients evaluated by Bettegowda et al. [53]. The NGS method used to find out target mutations of tumor tissue, and then by using RT-PCR quantified in ctDNA [53]. Moreover, complete cfDNA and tumor-specific ctDNA have been exposed in several researches to be higher in patients with colorectal cancers (CRC) compared with healthy controls [103–107]. Use of RAS mutations in cfDNA of patients with metastatic colorectal cancer brought a promising personalized dashboard for this cancer [108, 109]. Clinical utility of ctDNA sequencing in advanced CRC can provide appropriate information on potential mutations, in that way to ease clinical trial enrollment and enlightening the supposed value of care [110]. The quantitative relationship of cfDNA with tumor specific mutations in plasma from metastatic colorectal cancer (mCRC) patients was related to the efficacy of third line treatment with cetuximab and irinotecan [111]. Checking the quantity of ctDNA levels within a post-surgery surveillance study by Reinert and colleagues in and five no relapsing and six relapsing patients with colorectal cancer showed that relapses could be detected months in advance compared to conventional follow-up [112]. Moreover, ctDNA analysis can be used for tumor burden and standard chemotherapy reaction estimation in patients with early-stage colorectal cancer [113]. Gastric cancer is a leading cause of cancer deaths in the world with highly heterogeneous etiology and clinical characteristics [114]. The Cancer Genome Atlas (TCGA) network shed light on the heterogeneity and possible targeted therapeutics for various subtypes of gastric cancer according to comprehensive genomic platforms [95, 115]. The most usual mesenchymal tumors of the gastrointestinal tract are gastrointestinal stromal tumors (GISTs) [116, 117]. GISTs are described by mutations in a receptor tyrosine family (mainly KIT gene) which are linked to the mast cell growth factor receptor or in the platelet-derived growth factor receptor alpha(PDGFRA) coding gene [118–121]. The relationship between tumor genotype and positive effect of adjuvant imatinib stated that GIST with a KIT exon 11-deletion beneficially respond to treatment, with a considerably extended progression free survival (PFS) compared with placebo [122–124]. It was shown that mutation detection in cfDNA of GIST patients with metastatic disease can be recruited for personalized usage of imatinib and monitoring of early treatment adaptations [125, 126]. A panel called (‘SiRe’) with 568 mutations in six genes (EGFR, KRAS, NRAS, BRAF, cKIT and PDGFRα) evaluated in different cancers including GIST and can be optimized for its precision medicine in the near future [19]. A comparison of cfDNA levels after the six months after surgery and at the time of recurrence were considered in 18 gastric cancer patients who did not receive adjuvant chemotherapy, indicated to the fact that this patients had high pre- and postoperative cfDNA [127].
Thyroid tumors
The increasing prevalence of thyroid nodules and tumors had been resulted in a higher demand for the accurate diagnosis of thyroid nodules, and the best treatment strategies for this aggressive disease. A usual diagnostic tool is fine needle aspiration (FNA) samples from thyroid nodules with a mutations profiles that typically includes BRAF, RAS, RET/PTC, and PAX8/PPARg [128–132]. Combining the use of these molecular markers of ctDNA and new high-throughput molecular techniques will improve significantly the accuracy of cancer diagnosis in thyroid nodules [133]. By way of illustration, Anaplastic Thyroid Carcinoma (ATC) is an aggressive type of thyroid cancers that requires rapid diagnosis and multimodality management approaches. At the MD Anderson Cancer Center of University of Texas the NGS platforms over 70 genes of 23 patients ctDNA suggested that both tumor-based and ctDNA examination in the setting of clinical-trial application is beneficial for ATC patients [134]. Further innovative, realistically designed therapeutic strategies are under active expansion both for patients with Differentiated Thyroid Tumors (DTC) and for patients with ATC, within several phase II and phase III randomized clinical trials currently continuing [135] (Table 1).
Table 1.
The summary of studies related to ctDNA and personalized cancer management
| Author | Country | Year | Type of Cancer | Result |
|---|---|---|---|---|
| Eleonor Olsson [52] | Sweden | 2015 | Breast Cancer | • ctDNA quantity was a prognostic tool of poor survival • ctDNA is a monitoring tool for early metastasis detection, therapy modification, and to aid in avoidance of overtreatment |
| Chetan Bettegowda [53] | USA | 2014 | Pancreatic Cancer Ovarian Cancer Colorectal Cancer Bladder Cancer Gastro esophageal Breast Cancer Melanoma Hepatocellular Carcinoma Head and neck Cancers |
• ctDNA KRAS gene mutations as a broadly applicable, sensitive, and specific biomarker that can be used for a variety of clinical and research purposes in patients with multiple different types of cancer. |
| Sarah-Jane Dawson [55] | United Kingdom | 2013 | Breast cancer | • Circulating tumor DNA is an informative, inherently specific, and highly sensitive biomarker of metastatic breast cancer. |
| Muhammed Murtaza [56] | United Kingdom | 2012 | Breast Cancer Ovarian Cancer Lung Cancer |
• Exome-wide analysis of ct DNA could complement current invasive biopsy approaches to identify mutations associated with acquired drug resistance in advanced cancers • Serial analysis of cancer genomes in plasma constitutes a new paradigm for the study of clonal evolution in human cancers. |
| Nicholas C. Turner [62] | United Kingdom | 2016 | Breast Cancer | • ESR1 mus, detected in plasma ctDNA, were identified in a high percentage of pts. with HR+ MBC confirming an important role in endocrine-resistance • P + F treatment provided significant benefit for MBC pts. with and without ESR1 mus |
| Richard D. Baird [65] | USA | 2016 | Breast cancer | • Taselisib in combination with tamoxifen is generally well tolerated. Preliminary evidence of anti-tumor activity was seen, in some patients preceded by a fall in plasma PIK3CA ctDNA levels. The recommended phase II dose of taselisib in combination with tamoxifen is 4 mg on a daily continuous schedule. Clinical trial information: NCT02285179. |
| Cristofanilli M [147] | United Kingdom | 2016 | Metastatic Breast cancer | • Fulvestrant plus palbociclib was associated with significant and consistent improvement in progression-free survival compared with fulvestrant plus placebo, the combination could be considered as a therapeutic option for patients with recurrent hormone-receptor-positive, HER2-negative metastatic breast cancer that has progressed on previous endocrine therapy. |
| A Bosch [67] | USA | 2015 | Breast Cancer | • Increased ER transcriptional activity may be a reactive mechanism that limits the activity of PI3K inhibitors and that combined PI3K and ER inhibition is a rational approach to target these tumors. |
| Cloud P. Paweletz [87] | USA | 2016 | Lung Cancer | • Increasing dose levels resulted in more consistent decreases in KRAS mutation in cfDNA, consistent with a dose-dependent pharmaco-dynamic effect. These results highlight the potential value of serial plasma ddPCR as a PD marker in early phase clinical trials. |
| Smadar Geva [89] | Israel | 2016 | Lung Cancer | • Liquid biopsy ctDNA testing revealed possible treatment options for more than two-thirds of patients analyzed, including FDA-approved drugs as well as eligibility for clinical trials and guide clinicians to select the right therapy for the right patient. |
| Jacob Chabon [90] | 2016 | USA. | Lung Cancer | • Underscore the importance of tumor heterogeneity in NSCLC and the utility of ctDNA-based resistance mechanism assessment |
| D.J. Merriott [91] | USA | 2017 | Lung cancer | • Analysis of ctDNA potentially allows early identification of NSCLC patients who will have DCB from ICIs • Response assessment by ctDNA may therefore be useful in clinical studies examining combinations of ICIs and radiotherapy |
| Spindler KL [111] | Denmark | 2012 | Metastatic Colorectal Cancer (mCRC) | • cfDNA alleles and KRAS and BRAF mutation alleles analysis in plasma is a viable alternative to tissue analysis • Quantitative levels of cfDNA and pmKRAS are strongly correlated and hold promise of clinical application. |
| Evan J Lipson [113] | USA | 2014 | Melanoma | • Levels of ctDNA correlated with clinical and radiologic outcomes, and, in one case, preceded eventual tumor regression. Further prospective analysis is required to assess the utility of ctDNA as an early biomarker of clinical outcomes in patients receiving immune checkpoint blocking drugs. |
| Weixin Yan [95] | USA | 2016 | Gastrointestinal stromal tumors (GISTs) | • Mutant tumor DNA derived “driver” and “drug-resistant” alleles that are present in the cfDNA could be widely applied for minimally invasive molecular testing in the therapeutic management of GISTs. |
| Wada N [124] | Japan | 2016 | Gastrointestinal stromal tumors (GISTs) | • Detection of secondary C-KIT mutations in ctDNA could be useful for the selection of targeted agents and prediction of antitumor effects. |
| PA Boonstra [125] | USA | 2017 | Gastrointestinal stromal tumors (GISTs) | • Mutation detection in cfDNA of GIST patients with metastatic disease is feasible, which may guide early treatment adaptations. |
| Marcia S Brose [137] | USA | 2016 | Thyroid Tumor | • Vemurafenib showed anti-tumor activity in patients with progressive, ctDNABRAFV600E-positive papillary thyroid cancer refractory to radioactive iodine that had never been treated with a multi-kinase inhibitor |
| Elena Pereira [141] | USA | 2015 | Gynecologic Cancers | • ctDNA was an independent predictor of survival in patients with ovarian and endometrial cancers. Earlier recognition of disease persistence and/or recurrence and the ability to stratify into better and worse outcome groups through ctDNA surveillance may open the window for improved survival and quality and life in these cancers. |
In advanced Medullary Thyroid Carcinoma (MTC), ctDNA RET M918 T mutations of circulating tumor DNA can be predictive for overall survival (OS) and could take part in a role in monitoring response to treatment [136]. Moreover, in thyroid tumors the published result related to a phase II clinical study in Philadelphia demonstrated treating metastatic thyroid cancer patients with the targeted therapy of Vemurafenib to launch the activity of Vemurafenib in the only patients with BRAFV600E-positive papillary thyroid [137]. In fact it was shown that Vemurafenib had antitumor activity in patients with progressive, BRAFV600E-positive papillary thyroid cancer refractory to radioactive iodine who had never been cured with a multi-kinase inhibitor [137, 138]. More than that it has been revealed that detectable levels of BRAF(V600E) ctDNA pre-operatively, thus BRAF(V600E) ctDNA can be a discriminative tool between benign and malignant thyroid nodules [139, 140].
In fact personalized ctDNA biomarkers dynamically can be a good predictor of treatment response and survival in a wide range of cancer types including gynecologic cancers [141, 142]. It was shown that ctDNA level increased in advanced stage of ovarian cancers compared to controls, so ctDNA quantity can be useful for noninvasive screening and this disease surveillance [143]. Although point mutations have been extensively studied, chromosomal rearrangements have confirmedsuperior tumor specificity [144, 145]. A panel of individualized junctions consequent from tumor DNA possibly will be an useful way to monitor cancer patients for relapse and therapeutic efficacy using ctDNA [144]. ctDNA as non-invasive biomarkers of gynecological cancers, ovarian, endometrial. For example ctDNA can detect more mutations than DNA extracted from solid tumor and when performing genetic profiling in order to precision medicine programs should consider cfDNA to optimize finding of the molecular diversity of ovarian cancer [146].
Conclusion
Both ctDNA quantity and genetic hallmarks of ctDNA can be taken into consideration for personalized cancer managements. There is a big hope that by utilizing of ctDNA mutation the problem of resistance to drug in some patients will be overcome specially in breast, lung and colorectal cancers.
Acknowledgements
Special thanks to Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author.
Abbreviations
- ATC
Anaplastic Thyroid Cancer
- AI
Aromatase inhibitor
- BC
Breast Cancer
- CAPP-Seq
Cancer Personalized Profiling by deep Sequencing
- CRC
Colorectal cancers
- ctDNA
Circulating Tumor DNA
- CTCs
Circulating Tumor Cells
- ddPCR
Droplet Digital PCR
- EGFR-TKIs
Epidermal growth factor receptor tyrosine kinase inhibitors
- EGFR
Epidermal growth factor receptor
- ESR1
Estrogen receptor alpha
- FDA
U.S. Food and Drug Administration
- HER2/neu
Human epidermal growth factor receptor 2
- KRAS
Kirsten Rat Sarcoma Viral Oncogene Homolog
- mCRC
Metastatic colorectal cancer
- MRD
Minimal residual disease
- MTC
Medularlly Thyroid Cancer
- NSCLC
Non-Small Cell Lung Cancer Research
- NGS
Next-generation sequencing
- PFS
Progression free survival
- PIK3C
Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- PDGFRA
Platelet-derived growth factor receptor alpha
Authors’ contributions
Professor Seyed Mohammad Tavangar made substantial contributions to conception and design, supervision, acquisition of data, and interpretation of data. Mrs. Fatemeh Khatami had been involved in drafting the manuscript or revising it critically for important intellectual content.
Funding
This article was a part of a larger project which was granted by the National Institute for Medical Research Development (NIMAD, Grant number: 957222).
Ethics approval and consent to participate
This manuscript does not report on or involve the use of any animal or human data or tissue, so ethical approval is not applicable in this section.
Consent for publication
This review article does not contain data from any individual person; consequently the consent for publication is “Not applicable” in this section.
Competing interests
All authors declare that they have no competing interests” in this section.
Contributor Information
Fatemeh Khatami, Email: f-Khatami@farabi.tums.ac.ir.
Seyed Mohammad Tavangar, Phone: +98 21 84902187, Email: Tavangar@ams.ac.ir.
References
- 1.Larijani B, Shirzad M, Mohagheghi M, Haghpanah V, Mosavi-Jarrahi A, Tavangar S, et al. Epidemiologic analysis of the Tehran cancer institute data system registry (TCIDSR) Asian Pac J Cancer Prev. 2004;5(1):36–39. [PubMed] [Google Scholar]
- 2.Haghpanah V, Soliemanpour B, Heshmat R, Mosavi-Jarrahi A, Tavangar S, Malekzadeh R, Larijani B. Endocrine cancer in Iran: based on cancer registry system. Indian J Cancer. 2006;43(2):80–85. doi: 10.4103/0019-509x.25889. [DOI] [PubMed] [Google Scholar]
- 3.Underhill HR, Kitzman JO, Hellwig S, Welker NC, Daza R, Baker DN, et al. Fragment length of circulating tumor DNA. PLoS Genet. 2016;12(7):e1006162. doi: 10.1371/journal.pgen.1006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11(6):426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 5.Stroun M, Anker P. Nucleic acids spontaneously released by living frog auricles. Biochem J. 1972;128(3):100P. doi: 10.1042/bj1280100pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stroun M, Lyautey J, Lederrey C, Olson-Sand A, Anker P. About the possible origin and mechanism of circulating DNA: apoptosis and active DNA release. Clin Chim Acta. 2001;313(1):139–142. doi: 10.1016/s0009-8981(01)00665-9. [DOI] [PubMed] [Google Scholar]
- 7.Anker P, Stroun M, Maurice PA. Spontaneous release of DNA by human blood lymphocytes as shown in an in vitro system. Cancer Res. 1975;35(9):2375–2382. [PubMed] [Google Scholar]
- 8.Rogers JC, Boldt D, Kornfeld S, Skinner SA, Valeri CR. Excretion of deoxyribonucleic acid by lymphocytes stimulated with phytohemagglutinin or antigen. Proc Natl Acad Sci. 1972;69(7):1685–1689. doi: 10.1073/pnas.69.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz Jr LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamakawa T, Kukita Y, Kurokawa Y, Miyazaki Y, Takahashi T, Yamasaki M, et al. Monitoring gastric cancer progression with circulating tumour DNA. Br J Cancer. 2015;112(2):352. doi: 10.1038/bjc.2014.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tavangar SM, Larijani B, Mahta A, Hosseini SMA, Mehrazine M, Bandarian F. Craniopharyngioma: a clinicopathological study of 141 cases. Endocr Pathol. 2004;15(4):339–344. doi: 10.1385/ep:15:4:339. [DOI] [PubMed] [Google Scholar]
- 12.Pantel K, Alix-Panabières C. Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res. 2013;73(21):6384–6388. doi: 10.1158/0008-5472.CAN-13-2030. [DOI] [PubMed] [Google Scholar]
- 13.Costs AA. Outcomes comparison of tissue and blood based biopsies for the purpose of biomarker testing. Value Health. 2016;19(3):A143–A1A4. [Google Scholar]
- 14.Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472–484. doi: 10.1038/nrclinonc.2013.110. [DOI] [PubMed] [Google Scholar]
- 15.Fisher R, Pusztai L, Swanton C. Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer. 2013;108(3):479. doi: 10.1038/bjc.2012.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.X-x S, Yu Q. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacol Sin. 2015;36(10):1219. doi: 10.1038/aps.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2010;1805(1):105–117. doi: 10.1016/j.bbcan.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murtaza M, Dawson S-J, Pogrebniak K, Rueda OM, Provenzano E, Grant J, et al. Multifocal clonal evolution characterized using circulating tumour DNA in a case of metastatic breast cancer. Nat Commun. 2015;6:8760. doi: 10.1038/ncomms9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malapelle U, de-Las-Casas CM, Rocco D, Garzon M, Pisapia P, Jordana-Ariza N, et al. Development of a gene panel for next-generation sequencing of clinically relevant mutations in cell-free DNA from cancer patients. Br J Cancer. 2017;116(6):802–810. doi: 10.1038/bjc.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett CW, Berchem G, Kim YJ, El-Khoury V, Cell-free DNA. Next-generation sequencing in the service of personalized medicine for lung cancer. Oncotarget. 2016;7(43):71013. doi: 10.18632/oncotarget.11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cani AK, Hovelson DH, Demirci H, Johnson MW, Tomlins SA, Rao RC. Next generation sequencing of vitreoretinal lymphomas from small-volume intraocular liquid biopsies: new routes to targeted therapies. Oncotarget. 2017;8(5):7989–7998. doi: 10.18632/oncotarget.14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14(9):531–548. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 23.Gerdes L, Iwobi A, Busch U, Pecoraro S. Optimization of digital droplet polymerase chain reaction for quantification of genetically modified organisms. Biomolecular detection and quantification. 2016;7:9–20. doi: 10.1016/j.bdq.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jovelet C, Madic J, Remon J, Honoré A, Girard R, Rouleau E, et al. Crystal digital droplet PCR for detection and quantification of circulating EGFR sensitizing and resistance mutations in advanced non-small cell lung cancer. PLoS One. 2017;12(8):e0183319. doi: 10.1371/journal.pone.0183319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt MW, Kennedy SR, Salk JJ, Fox EJ, Hiatt JB, Loeb LA. Detection of ultra-rare mutations by next-generation sequencing. Proc Natl Acad Sci. 2012;109(36):14508–14513. doi: 10.1073/pnas.1208715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shu Y, Wu X, Tong X, Wang X, Chang Z, Mao Y, et al. Circulating tumor DNA mutation profiling by targeted next generation sequencing provides guidance for personalized treatments in multiple Cancer types. Sci Rep. 2017;7.1:583. doi: 10.1038/s41598-017-00520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bratman SV, Newman AM, Alizadeh AA, Diehn M. Potential clinical utility of ultrasensitive circulating tumor DNA detection with CAPP-Seq. Taylor Francis; 2015. [DOI] [PMC free article] [PubMed]
- 28.Chaudhuri A, Lovejoy A, Chabon J, Newman A, Stehr H, Say C, et al. CAPP-Seq circulating tumor DNA analysis for early detection of tumor progression after definitive radiation therapy for lung Cancer. International journal of radiation oncology• biology. Physics. 2016;96(2):S41–SS2. [Google Scholar]
- 29.Mandel P. Les acides nucleiques du plasma sanguin chez l'homme. CR Acad Sci Paris. 1948;142:241–243. [PubMed] [Google Scholar]
- 30.Komatsubara KM, Sacher AG. Circulating Tumor DNA as a Liquid Biopsy: Current Clinical Applications and Future Directions. Oncology (Williston Park, NY). 2017;31(8). [PubMed]
- 31.Leon S, Shapiro B, Sklaroff D, Yaros M, Free DNA. In the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37(3):646–650. [PubMed] [Google Scholar]
- 32.Stroun M, Anker P, Maurice P, Lyautey J, Lederrey C, Beljanski M. Neoplastic characteristics of the DNA found in the plasma of cancer patients. Oncology. 1989;46(5):318–322. doi: 10.1159/000226740. [DOI] [PubMed] [Google Scholar]
- 33.Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch R-D, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61(4):1659–1665. [PubMed] [Google Scholar]
- 34.Anker P, Stroun M, Maurice PA. Spontaneous extracellular synthesis of DNA released by human blood lymphocytes. Cancer Res. 1976;36(8):2832–2839. [PubMed] [Google Scholar]
- 35.Diehl F, Li M, Dressman D, He Y, Shen D, Szabo S, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci U S A. 2005;102(45):16368–16373. doi: 10.1073/pnas.0507904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mouliere F, Rosenfeld N. Circulating tumor-derived DNA is shorter than somatic DNA in plasma. Proc Natl Acad Sci. 2015;112(11):3178–3179. doi: 10.1073/pnas.1501321112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allis CD, Jenuwein T, Reinberg D. Epigenetics: CSHL Press; 2007.
- 38.Abbott DW, Ivanova VS, Wang X, Bonner WM, Ausió J. Characterization of the stability and folding of H2A. Z chromatin particles implications for transcriptional activation. J Biol Chem. 2001;276(45):41945–41949. doi: 10.1074/jbc.M108217200. [DOI] [PubMed] [Google Scholar]
- 39.Anderson J, Thåström A, Widom J. Spontaneous access of proteins to buried nucleosomal DNA target sites occurs via a mechanism that is distinct from nucleosome translocation. Mol Cell Biol. 2002;22(20):7147–7157. doi: 10.1128/MCB.22.20.7147-7157.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jansen A, Verstrepen KJ. Nucleosome positioning in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 2011;75(2):301–320. doi: 10.1128/MMBR.00046-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snyder MW, Kircher M, Hill AJ, Daza RM, Shendure J. Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin. Cell. 2016;164(1):57–68. doi: 10.1016/j.cell.2015.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.genomics WDC. A nucleosome footprint reveals the source of cfDNA. Nat Rev Genet. 2016;17(3):125. doi: 10.1038/nrg.2016.3. [DOI] [PubMed] [Google Scholar]
- 43.Ma X, Zhu L, Wu X, Bao H, Wang X, Chang Z, et al. Cell-free DNA provides a good representation of the tumor genome despite its biased fragmentation patterns. PLoS One. 2017;12(1):e0169231. doi: 10.1371/journal.pone.0169231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleischhacker M, Schmidt B. Circulating nucleic acids (CNAs) and cancer—a survey. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2007;1775(1):181–232. doi: 10.1016/j.bbcan.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Yong E. Cancer biomarkers: written in blood. Nature. 2014;511:524–526. doi: 10.1038/511524a. [DOI] [PubMed] [Google Scholar]
- 46.Heidary M, Auer M, Ulz P, Heitzer E, Petru E, Gasch C, et al. The dynamic range of circulating tumor DNA in metastatic breast cancer. Breast Cancer Res. 2014;16(4):421. doi: 10.1186/s13058-014-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo N, Lou F, Ma Y, Li J, Yang B, Chen W, et al. Circulating tumor DNA detection in lung cancer patients before and after surgery. Sci Rep. 2016;6 [DOI] [PMC free article] [PubMed]
- 48.Wei Z, Wang W, Zitan Shu XZ, Zhang Y. Correlation between circulating tumor DNA levels and response to tyrosine kinase inhibitors (TKI) treatment in non-small cell lung Cancer. Medical science monitor: international medical journal of experimental and clinical research. 2017;23:3627–3634. doi: 10.12659/MSM.902265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O’Connell A, Feeney N, et al. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA oncology. 2016;2(8):1014–1022. doi: 10.1001/jamaoncol.2016.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.af Hällström TM, Puhka M, Kallioniemi O. Circulating tumor DNA in early-stage breast cancer: personalized biomarkers for occult metastatic disease and risk of relapse? EMBO Molecular Medicine. 2015;7(8):994–995. doi: 10.15252/emmm.201505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khatami F, Aghayan HR, Sanaei M, Heshmat R, Tavangar SM, Larijani B. The potential of circulating tumor cells in personalized management of breast cancer: a systematic review. Acta Medica Iranica. 2017;55(3):175–193. [PubMed] [Google Scholar]
- 52.Olsson E, Winter C, George A, Chen Y, Howlin J, Tang MHE, et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO molecular medicine. 2015;7(8):1034–1047. doi: 10.15252/emmm.201404913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early-and late-stage human malignancies. Science translational medicine. 2014;6(224):224ra24-ra24. [DOI] [PMC free article] [PubMed]
- 54.Leary RJ, Kinde I, Diehl F, Schmidt K, Clouser C, Duncan C, et al. Development of personalized tumor biomarkers using massively parallel sequencing. Sci Transl Med 2010;2(20):20ra14-20ra14. [DOI] [PMC free article] [PubMed]
- 55.Dawson S-J, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin S-F, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199–1209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 56.Murtaza M, Dawson S-J, Tsui DW, Gale D, Forshew T, Piskorz AM, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497(7447):108. doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- 57.Sidransky D. Nucleic acid-based methods for the detection of cancer. Science. 1997;278(5340):1054–1058. doi: 10.1126/science.278.5340.1054. [DOI] [PubMed] [Google Scholar]
- 58.Heitzer E, Auer M, Ulz P, Geigl JB, Speicher MR. Circulating tumor cells and DNA as liquid biopsies. Genome medicine. 2013;5(8):73. doi: 10.1186/gm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61(1):112–123. doi: 10.1373/clinchem.2014.222679. [DOI] [PubMed] [Google Scholar]
- 60.Mohammadi-asl J, Larijani B, Khorgami Z, Tavangar SM, Haghpanah V, Kheirollahi M, et al. Qualitative and quantitative promoter hypermethylation patterns of the P16, TSHR, RASSF1A and RARβ2 genes in papillary thyroid carcinoma. Med Oncol. 2011;28(4):1123–1128. doi: 10.1007/s12032-010-9587-z. [DOI] [PubMed] [Google Scholar]
- 61.Garcia-Murillas I, Beanney M, Epstein M, Howarth K, Lawson A, Hrebien S, et al. Abstract 2743: comparison of enhanced tagged-amplicon sequencing and digital PCR for circulating tumor DNA analysis in advanced breast cancer. Cancer Res. 2017;77(13 Supplement):2743. [Google Scholar]
- 62.Tzanikou E, Markou A, Politaki E, Koytsodontis G, Psyrri A, Georgoulias V, et al. Abstract 1725: Detection of <em>ESR1</em> D538G mutation in circulating tumor cells (CTCs) and paired circulating tumor DNA (ctDNA) samples of breast cancer patients. Cancer Research. 2017;77(13 Supplement):1725-.
- 63.Schiavon G, Hrebien S, Garcia-Murillas I, Pearson A, Tarazona N, Lopez-Knowles E, et al. Abstract 926: ESR1 mutations evolve during the treatment of metastatic breast cancer, and detection in ctDNA predicts sensitivity to subsequent hormone therapy. Cancer Res. 2015;75(15 Supplement):926. [Google Scholar]
- 64.Turner NC, Jiang Y, O'Leary B, Hrebien S, Cristofanilli M, Andre F, et al. Efficacy of palbociclib plus fulvestrant (P+F) in patients (pts) with metastatic breast cancer (MBC) and ESR1 mutations (mus) in circulating tumor DNA (ctDNA). Journal of Clinical Oncology. 2016;34(15_suppl):512-.
- 65.Baird RD, Rossum AV, Oliveira M, Beelen K, Garcia-Corbacho J, Mandjes IAM, et al. POSEIDON trial phase 1b results: Safety and preliminary efficacy of the isoform selective PI3K inhibitor taselisib (GDC-0032) combined with tamoxifen in hormone receptor (HR) positive, HER2-negative metastatic breast cancer (MBC) patients (pts) - including response monitoring by plasma circulating tumor (ct) DNA. Journal of Clinical Oncology. 2016;34(15_suppl):2520-.
- 66.Baselga J, Im S-A, Iwata H, Clemons M, Ito Y, Awada A, et al. Abstract S6–01: <em>PIK3CA</em> status in circulating tumor DNA (ctDNA) predicts efficacy of buparlisib (BUP) plus fulvestrant (FULV) in postmenopausal women with endocrine-resistant HR+/HER2– advanced breast cancer (BC): First results from the randomized, phase III BELLE-2 trial. Cancer Res 2016;76(4 upplement):S6–01-S6-.
- 67.Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor–positive breast cancer. Science translational medicine. 2015;7(283):283ra51-ra51. [DOI] [PMC free article] [PubMed]
- 68.Oshiro C, Kagara N, Naoi Y, Shimoda M, Shimomura A, Maruyama N, et al. PIK3CA mutations in serum DNA are predictive of recurrence in primary breast cancer patients. Breast Cancer Res Treat. 2015;150(2):299–307. doi: 10.1007/s10549-015-3322-6. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Murillas I, Schiavon G, Weigelt B, Ng C, Hrebien S, Cutts RJ, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med 2015;7(302):302ra133-302ra133. [DOI] [PubMed]
- 70.Ma C, Bose R, Gao F, Freedman R, Telli M, Kimmick G, et al. Abstract CT011: Circulating tumor DNA (ctDNA) sequencing for <em>HER2</em> mutation (<em>HER2</em><sup>mut</sup>) screening and response monitoring to neratinib in metastatic breast cancer (MBC). Cancer Res 2017;77(13 Supplement):CT011-CT.
- 71.Qiu M, Wang J, Xu Y, Ding X, Li M, Jiang F, et al. Circulating tumor DNA is effective for the detection of EGFR mutation in non–small cell lung Cancer: a meta-analysis. Cancer Epidemiology Biomarkers & Prevention. 2014. [DOI] [PubMed]
- 72.Goto T, Hirotsu Y, Amemiya K, Nakagomi T, Shikata D, Yokoyama Y, et al. Distribution of circulating tumor DNA in lung cancer: analysis of the primary lung and bone marrow along with the pulmonary venous and peripheral blood. Oncotarget. 2017;8(35):59268–59281. doi: 10.18632/oncotarget.19538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirsch FR, Varella-Garcia M, Bunn Jr PA, Franklin WA, Dziadziuszko R, Thatcher N, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non–small-cell lung cancer. J Clin Oncol. 2006;24(31):5034–5042. doi: 10.1200/JCO.2006.06.3958. [DOI] [PubMed] [Google Scholar]
- 74.Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 75.Bai H, Wang Z, Chen K, Zhao J, Lee JJ, Wang S, et al. Influence of chemotherapy on EGFR mutation status among patients with non–small-cell lung cancer. J Clin Oncol. 2012;30(25):3077–3083. doi: 10.1200/JCO.2011.39.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Overman MJ, Modak J, Kopetz S, Murthy R, Yao JC, Hicks ME, et al. Use of research biopsies in clinical trials: are risks and benefits adequately discussed? J Clin Oncol. 2012;31(1):17–22. doi: 10.1200/JCO.2012.43.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Normanno N, Denis MG, Thress KS, Ratcliffe M, Reck M. Guide to detecting epidermal growth factor receptor (EGFR) mutations in ctDNA of patients with advanced non-small-cell lung cancer. Oncotarget. 2017;8(7):12501–12516. doi: 10.18632/oncotarget.13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henick BS, Goldberg SB, Narayan A, Rossi C, Rodney S, Kole AJ, et al. Circulating tumor DNA (ctDNA) to monitor treatment response and progression in patients treated with tyrosine kinase inhibitors (TKIs) and immunotherapy for EGFR-mutant non-small cell lung cancer (NSCLC). Proc Am Soc Clin Oncol. 2017;
- 79.De Mattos-Arruda L, Cortes J, Santarpia L, Vivancos A, Tabernero J, Reis-Filho JS, et al. Circulating tumour cells and cell-free DNA as tools for managing breast cancer. Nat Rev Clin Oncol. 2013;10(7):377–389. doi: 10.1038/nrclinonc.2013.80. [DOI] [PubMed] [Google Scholar]
- 80.Wang S, Han X. Hu X, Wang X, Zhao L, tang L, et al. clinical significance of pretreatment plasma biomarkers in advanced non-small cell lung cancer patients. Clin Chim Acta. 2014;430:63–70. doi: 10.1016/j.cca.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 81.Jing C-W, Wang Z, Cao H-X, Ma R, Wu J-Z. High resolution melting analysis for epidermal growth factor receptor mutations in formalin-fixed paraffin-embedded tissue and plasma free DNA from non-small cell lung cancer patients. Asian Pac J Cancer Prev. 2013;14(11):6619–6623. doi: 10.7314/apjcp.2013.14.11.6619. [DOI] [PubMed] [Google Scholar]
- 82.Zhang H, Liu D, Li S, Zheng Y, Yang X, Li X, et al. Comparison of EGFR signaling pathway somatic DNA mutations derived from peripheral blood and corresponding tumor tissue of patients with advanced non-small-cell lung cancer using liquidchip technology. The Journal of Molecular Diagnostics. 2013;15(6):819–826. doi: 10.1016/j.jmoldx.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 83.Yeung KT, More S, Woodward B, Velculescu V, Husain H. Circulating tumor DNA for mutation detection and identification of mechanisms of resistance in non-small cell lung Cancer. Molecular Diagnosis & Therapy. 2017;21(4):375–384. doi: 10.1007/s40291-017-0260-5. [DOI] [PubMed] [Google Scholar]
- 84.Audibert C, Shea M, Glass D, Kozak M, Caze A, Hohman R, et al. Use of FDA-approved vs. lab-developed tests in advanced non-small cell lung cancer. Proc Am Soc Clin Oncol. 2016;e20532. 10.1200/JCO.2016.34.15_suppl.e20532.
- 85.Nygaard AD, Garm Spindler K-L, Pallisgaard N, Andersen RF, Jakobsen A. The prognostic value of KRAS mutated plasma DNA in advanced non-small cell lung cancer. Lung Cancer. 2013;79(3):312–317. doi: 10.1016/j.lungcan.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 86.Sherwood JL, Corcoran C, Brown H, Sharpe AD, Musilova M, Kohlmann A. Optimised pre-analytical methods improve KRAS mutation detection in circulating tumour DNA (ctDNA) from patients with non-small cell lung cancer (NSCLC) PLoS One. 2016;11(2):e0150197. doi: 10.1371/journal.pone.0150197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paweletz CP, Oxnard GR, Feeney N, Hilton JF, Gandhi L, Do KT, et al. Abstract 3157: Serial droplet digital PCR (ddPCR) of plasma cell-free DNA (cfDNA) as pharmacodynamic (PD) biomarker in Phase 1 clinical trials for patients (pts) with KRAS mutant non-small cell lung cancer (NSCLC). Cancer Res. 2016;76(14 Supplement):3157-.
- 88.Bardelli A. Medical research: personalized test tracks cancer relapse. Nature. 2017;545(7655):417–418. doi: 10.1038/545417a. [DOI] [PubMed] [Google Scholar]
- 89.Geva S, Rozenblum AB, Ilouze M, Roisman L, Twito T, Dvir A, et al. P2.03b-047 the clinical impact of multiplex ctDNA Gene analysis in lung cancer. Journal of Thoracic Oncology. 12(1):S964.
- 90.Chabon JJ, Simmons AD, Lovejoy AF, Esfahani MS, Newman AM, Haringsma HJ, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun. 2016;7:11815. doi: 10.1038/ncomms11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Merriott DJ, Chaudhuri AA, Jin M, Chabon JJ, Newman A, Stehr H, et al. circulating tumor dna quantitation for early response assessment of immune checkpoint inhibitors for lung cancer. Int J Radiat Oncol Biol Phys. 99(2):S20–S1.
- 92.Khatami F, Larijani B, Heshmat R, Keshtkar A, Mohammadamoli M, Teimoori-Toolabi L, et al. Meta-analysis of promoter methylation in eight tumor-suppressor genes and its association with the risk of thyroid cancer. PLoS One. 2017;12(9):e0184892. doi: 10.1371/journal.pone.0184892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cai X, Gao Y, Shen H, Laird P, Fan, J-B, Xu W, et al. Non-invasive diagnosis of early-stage lung cancer via targeted high-throughput DNA methylation sequencing of circulating tumor DNA (ctDNA). AACR; 2017. [DOI] [PMC free article] [PubMed]
- 94.Pantel K, Alix-Panabieres C. Liquid biopsy in 2016: circulating tumour cells and cell-free DNA in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2017;14(2):73–74. doi: 10.1038/nrgastro.2016.198. [DOI] [PubMed] [Google Scholar]
- 95.Yan W, Zhang A, Powell MJ. Genetic alteration and mutation profiling of circulating cell-free tumor DNA (cfDNA) for diagnosis and targeted therapy of gastrointestinal stromal tumors. Chinese journal of cancer. 2016;35(1):68. doi: 10.1186/s40880-016-0131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sotoudeh M, Derakhshan MH, Abedi-Ardakani B, Nouraie M, Yazdanbod A, Tavangar SM, et al. Critical role of helicobacter pylori in the pattern of gastritis and carditis in residents of an area with high prevalence of gastric cardia cancer. Dig Dis Sci. 2008;53(1):27–33. doi: 10.1007/s10620-007-9817-1. [DOI] [PubMed] [Google Scholar]
- 97.Malekzadeh R, Sotoudeh M, Derakhshan M, Mikaeli J, Yazdanbod A, Merat S, et al. Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the northwest of Iran. J Clin Pathol. 2004;57(1):37–42. doi: 10.1136/jcp.57.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Szpechcinski A, Chorostowska-Wynimko J, Struniawski R, Kupis W, Rudzinski P, Langfort R, et al. Cell-free DNA levels in plasma of patients with non-small-cell lung cancer and inflammatory lung disease. Br J Cancer. 2015;113(3):476. doi: 10.1038/bjc.2015.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shapiro B, Chakrabarty M, Cohn EM, Leon SA. Determination of circulating DNA levels in patients with benign or malignant gastrointestinal disease. Cancer. 1983;51(11):2116–2120. doi: 10.1002/1097-0142(19830601)51:11<2116::aid-cncr2820511127>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 100.De Mattos-Arruda L, Olmos D, Tabernero J. Prognostic and predictive roles for circulating biomarkers in gastrointestinal cancer. Future Oncol. 2011;7(12):1385–1397. doi: 10.2217/fon.11.122. [DOI] [PubMed] [Google Scholar]
- 101.Howell JA, Khan SA, Knapp S, Thursz MR, Sharma R. The clinical role of circulating free tumor DNA in gastrointestinal malignancy. Transl Res. 2017;183(Supplement C):137–154. doi: 10.1016/j.trsl.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 102.Nasseri-Moghaddam S, Malekzadeh R, Sotoudeh M, Tavangar M, Azimi K, Sohrabpour AA, et al. Lower esophagus in dyspeptic Iranian patients: a prospective study. J Gastroenterol Hepatol. 2003;18(3):315–321. doi: 10.1046/j.1440-1746.2003.02969.x. [DOI] [PubMed] [Google Scholar]
- 103.Schwarzenbach H, Stoehlmacher J, Pantel K, Goekkurt E. Detection and monitoring of cell-free DNA in blood of patients with colorectal Cancer. Ann N Y Acad Sci. 2008;1137(1):190–196. doi: 10.1196/annals.1448.025. [DOI] [PubMed] [Google Scholar]
- 104.Boni L, Cassinotti E, Canziani M, Dionigi G, Rovera F, Dionigi R. Free circulating DNA as possible tumour marker in colorectal cancer. Surg Oncol. 2007;16:29–31. doi: 10.1016/j.suronc.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 105.Frattini M, Gallino G, Signoroni S, Balestra D, Battaglia L, Sozzi G, et al. Quantitative analysis of plasma DNA in colorectal cancer patients. Ann N Y Acad Sci. 2006;1075(1):185–190. doi: 10.1196/annals.1368.025. [DOI] [PubMed] [Google Scholar]
- 106.Frattini M, Gallino G, Signoroni S, Balestra D, Lusa L, Battaglia L, et al. Quantitative and qualitative characterization of plasma DNA identifies primary and recurrent colorectal cancer. Cancer Lett. 2008;263(2):170–181. doi: 10.1016/j.canlet.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 107.Danese E, Montagnana M, Minicozzi AM, De Matteis G, Scudo G, Salvagno GL, et al. Real-time polymerase chain reaction quantification of free DNA in serum of patients with polyps and colorectal cancers. Clin Chem Lab Med. 2010;48(11):1665–1668. doi: 10.1515/CCLM.2010.301. [DOI] [PubMed] [Google Scholar]
- 108.Hedtke M, Haselmann V, Brechtel I, Duda A, Neumaier M. Use of liquid profiling/liquid biopsy to detect Ras mutations in cfdna of patients with metastatic colorectal cancer (mcrc). Clinical Chemistry and Laboratory Medicine. 2016;54(10):eA441.
- 109.Tavangar SM, Shariftabrizi A, Soroush AR. Her–2/neu over-expression correlates with more advanced disease in Iranian colorectal cancer patients. Med Sci Monit. 2005;11(3):CR123–CRCR6. [PubMed] [Google Scholar]
- 110.Pereira AAL, Morelli MP, Overman M, Kee B, Fogelman D, Vilar E, et al. Clinical utility of circulating cell-free DNA in advanced colorectal cancer. PLoS One. 2017;12(8):e0183949. doi: 10.1371/journal.pone.0183949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spindler K-LG, Pallisgaard N, Vogelius I, Jakobsen A, Quantitative cell free DNA. KRAS and BRAF mutations in plasma from patients with metastatic colorectal cancer during treatment with cetuximab and irinotecan. Clinical Cancer research. 2012:clincanres. 2011:0564. [DOI] [PubMed]
- 112.Reinert T, Schøler LV, Thomsen R, Tobiasen H, Vang S, Nordentoft I, et al. Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut. 2016;65(4):625–634. doi: 10.1136/gutjnl-2014-308859. [DOI] [PubMed] [Google Scholar]
- 113.Lipson EJ, Velculescu VE, Pritchard TS, Sausen M, Pardoll DM, Topalian SL, et al. Circulating tumor DNA analysis as a real-time method for monitoring tumor burden in melanoma patients undergoing treatment with immune checkpoint blockade. Journal for immunotherapy of cancer. 2014;2(1):42. doi: 10.1186/s40425-014-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, et al. The incidences and mortalities of major cancers in China, 2009. Chinese journal of cancer. 2013;32(3):106. doi: 10.5732/cjc.013.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang W. TCGA divides gastric cancer into four molecular subtypes: implications for individualized therapeutics. Chinese journal of cancer. 2014;33(10):469. doi: 10.5732/cjc.014.10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nishida T, Biological HS. Clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000;15(4):1293–1301. doi: 10.14670/HH-15.1293. [DOI] [PubMed] [Google Scholar]
- 118.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 119.Lourenço N, Hélias-Rodzewicz Z, Bachet J-B, Brahimi-Adouane S, Jardin F, van Nhieu JT, et al. Copy-neutral loss of heterozygosity and chromosome gains and losses are frequent in gastrointestinal stromal tumors. Mol Cancer. 2014;13(1):246. doi: 10.1186/1476-4598-13-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Astolfi A, Nannini M, Pantaleo MA, Di Battista M, Heinrich MC, Santini D, et al. A molecular portrait of gastrointestinal stromal tumors: an integrative analysis of gene expression profiling and high-resolution genomic copy number. Lab Investig. 2010;90(9):1285–1294. doi: 10.1038/labinvest.2010.110. [DOI] [PubMed] [Google Scholar]
- 121.Nannini M, Astolfi A, Urbini M, Indio V, Santini D, Heinrich MC, et al. Integrated genomic study of quadruple-WT GIST (KIT/PDGFRA/SDH/RAS pathway wild-type GIST) BMC Cancer. 2014;14(1):685. doi: 10.1186/1471-2407-14-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gronchi A. Risk stratification models and mutational analysis: keys to optimising adjuvant therapy in patients with gastrointestinal stromal tumour. Eur J Cancer. 2013;49(4):884–892. doi: 10.1016/j.ejca.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 123.Şendur MAN, Özdemir NY, Akıncı MB, Uncu D, Zengin N, Aksoy S. Is exon mutation analysis needed for adjuvant treatment of gastrointestinal stromal tumor? World J Gastroenterol: WJG. 2013;19(1):144–146. doi: 10.3748/wjg.v19.i1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wada N, Kurokawa Y, Takahashi T, Hamakawa T, Hirota S, Naka T, et al. Detecting secondary C-KIT mutations in the peripheral blood of patients with imatinib-resistant gastrointestinal stromal tumor. Oncology. 2016;90(2):112–117. doi: 10.1159/000442948. [DOI] [PubMed] [Google Scholar]
- 125.Boonstra PA, At E, Tibbesma M, Mathijssen RH, Atrafi F, Fv C, et al. Abstract 4951: dynamics of KIT exon 11 mutations in cell free plasma DNA of patients treated for advanced gastrointestinal stromal tumors: results from the Dutch GIST bio-databank. Cancer Res. 2017;77(13 Supplement):4951. [Google Scholar]
- 126.Maier J, Lange T, Kerle I, Specht K, Bruegel M, Wickenhauser C,et al. Detection of mutant free circulating tumor DNA in the plasma of patients with gastrointestinal stromal tumor harboring activating mutations of CKIT or PDGFRA. Clin Cancer Res. 2013;0765. 10.1158/1078-0432.CCR-13-0765. [DOI] [PubMed]
- 127.Lan Y-T, Chen M-H, Fang W-L, Hsieh C-C, Lin C-H, Jhang F-Y, et al. Clinical relevance of cell-free DNA in gastrointestinal tract malignancy. Oncotarget. 2017;8(2):3009–3017. doi: 10.18632/oncotarget.13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fallahi P, Giannini R, Miccoli P, Antonelli A, Basolo F. Molecular diagnostics of fine needle aspiration for the presurgical screening of thyroid nodules. Current genomics. 2014;15(3):171–177. doi: 10.2174/1389202915999140404100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tavangar SM, Monajemzadeh M, Larijani B, Haghpanah V. Immunohistochemical study of oestrogen receptors in 351 human thyroid glands. Singap Med J. 2007;48(8):744–747. [PubMed] [Google Scholar]
- 130.Saffar H, Sanii S, Emami B, Heshmat R, Panah VH, Azimi S, et al. Evaluation of MMP2 and Caspase-3 expression in 107 cases of papillary thyroid carcinoma and its association with prognostic factors. Pathol Res Pract. 2013;209(3):195–199. doi: 10.1016/j.prp.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 131.Sanii S, Tavangar SM. Cutaneous metastasis of medullary thyroid carcinoma as the initial manifestation of an otherwise limited malignancy: a case report. Am J Dermatopathol. 2011;33(7):716–718. doi: 10.1097/DAD.0b013e3181fd7ada. [DOI] [PubMed] [Google Scholar]
- 132.Haghpanah V, Abbas SI, Mahmoodzadeh H, Shojaei A, Soleimani A, Larijani B, et al. Paraplegia as initial presentation of follicular thyroid carcinoma. Journal of the College of Physicians and Surgeons--Pakistan : JCPSP. 2006;16(3):233–234. [PubMed] [Google Scholar]
- 133.Janku F, Huang HJ, Ramzanali NM, Hong DS, Karp DD. Fu S, et al. ultra-deep next-generation sequencing of plasma cell-free (cf) DNA from patients with advanced cancers. Proc Am Soc Clin Oncol. 2015;
- 134.Sandulache VC, Williams MD, Lai SY, Lu C, William WN, Busaidy NL, et al. Real-time genomic characterization utilizing circulating cell-free DNA in patients with anaplastic thyroid carcinoma. Thyroid. 2017;27(1):81–87. doi: 10.1089/thy.2016.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bible KC, Ryder M. Evolving molecularly targeted therapies for advanced-stage thyroid cancers. Nat Rev Clin Oncol. 2016;13(7):403–416. doi: 10.1038/nrclinonc.2016.19. [DOI] [PubMed] [Google Scholar]
- 136.Cote GJ, Evers C, Hu MI, Grubbs EG, Williams MD, Hai T, et al. Prognostic significance of circulating RET M918T mutated tumor DNA in patients with advanced medullary thyroid carcinoma. The Journal of Clinical Endocrinology & Metabolism. 2017;102(9):3591–3599. doi: 10.1210/jc.2017-01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, et al. Vemurafenib in patients with BRAF V600E-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. The Lancet Oncology. 2016;17(9):1272–1282. doi: 10.1016/S1470-2045(16)30166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Khatami F, Larijani B, Tavangar SM. Circulating tumor BRAF mutation and personalized thyroid Cancer treatment. Asian Pacific journal of cancer prevention: APJCP. 2017;18(2):293–294. doi: 10.22034/APJCP.2017.18.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patel KB. Detection of circulating thyroid tumor DNA in patients with thyroid nodules. 2015. [Google Scholar]
- 140.Dakubo GD. Endocrine Cancer biomarkers in circulation. Cancer Biomarkers in Body Fluids: Springer; 2017. pp. 457–480. [Google Scholar]
- 141.Pereira E, Camacho-Vanegas O, Anand S, Sebra R, Camacho SC, Garnar-Wortzel L, et al. Personalized circulating tumor DNA biomarkers dynamically predict treatment response and survival in gynecologic cancers. PLoS One. 2015;10(12):e0145754. doi: 10.1371/journal.pone.0145754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kajbafzadeh A-M, Payabvash S, Salmasi AH, Monajemzadeh M, Tavangar SM. Smooth muscle cell apoptosis and defective neural development in congenital ureteropelvic junction obstruction. J Urol. 2006;176(2):718–723. doi: 10.1016/j.juro.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 143.Kamat AA, Sood AK, Dang D, Gershenson DM, Simpson JL, Bischoff FZ. Quantification of total plasma cell-free DNA in ovarian cancer using real-time PCR. Ann N Y Acad Sci. 2006;1075(1):230–234. doi: 10.1196/annals.1368.031. [DOI] [PubMed] [Google Scholar]
- 144.Harris FR, Kovtun IV, Smadbeck J, Multinu F, Jatoi A, Kosari F, et al. Quantification of somatic chromosomal rearrangements in circulating cell-free DNA from ovarian cancers. Sci Rep. 2016;6:29831. doi: 10.1038/srep29831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sarmadi S, Izadi-Mood N, Sotoudeh K, Tavangar SM. Altered PTEN expression; a diagnostic marker for differentiating normal, hyperplastic and neoplastic endometrium. Diagn Pathol. 2009;4(1):41. doi: 10.1186/1746-1596-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Arend RC, Londono AI, Alvarez RD, Huh WK, Bevis KS, Leath CA, et al., editors. Circulating cell-free DNA: The future of personalized medicine in ovarian cancer management. Journal of Clinical Oncology; 2016: AMER SOC CLINICAL ONCOLOGY 2318 MILL ROAD, STE 800, ALEXANDRIA, VA 22314 USA.
- 147.Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. The Lancet Oncology. 2016;17(4):425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]


