Abstract
The Fritillaria genus, including different kinds of medicinal and edible plants belonging to the Liliaceae family which have the function of treating and relieving a cough and eliminating phlegm, is widely planted in Xinjiang (China). There are few comprehensive studies reporting on the characterization of the chemical constituents of Fritillaria from Xinjiang, and to date, no work describing the quantitative differences between the components in Fritillaria from Xinjiang and related species. The purpose of this study was to develop qualitative and quantitative analytical methods by Ultra Performance Liquid Chromatography-Quadrupole Time-of-flight Mass Spectrometry (UPLC-QTOF-MS) for the rapid quantification and quantitation of alkaloids in wild and cultivated Xinjiang Fritillaria, which could be used in the quality control of medicine based on this natural herb. Using the UPLC-QTOF-MS method, the chemical constituents of Xinjiang Fritillaria were identified by fragmentation information and retention behavior, and were compared to reference standards. Furthermore, a quantitative comparision of four major alkaloids in wild and cultivated Xinjiang Fritillaria was conducted by determining the content of Sipeimine-3β-d-glucoside, Sipeimine, Peimisine, and Yibeinoside A, respectively. A total of 89 characteristic peaks, including more than 40 alkaloids, were identified in the chromatographic results of Fritillaria. Four main alkaloids were quantified by using a validated method based on UPLC-QTOF-MS. The relative contents of Sipeimine-3β-d-glucoside, Sipeimine, Peimisine, and Yibeinoside A varied from 0.0013%~0.1357%, 0.0066%~0.1218%, 0.0033%~0.0437%, and 0.0019%~0.1398%, respectively. A rough separation of wild and cultivated Fritillaria could be achieved by the cluster analysis method.
Keywords: UPLC-QTOF-MS, Fritillariae, qualitative and quantitative analysis, alkaloids
1. Introduction
Fritillaria represents the bulbs of various plants from the Fritillaria genus in the Liliaceae family, which have been used in traditional Chinese medicine for a long period of time because of their effects of clearing heat, moistening the lung, resolving phlegm, and relieving coughs, for the treatment of a cough caused by lung heat and dryness, a low sputum dry cough, a cough due to a yin deficiency, and sputum with blood [1,2,3]. Various bioactive chemical components have been found in Fritillaria, mainly consisting of alkaloids, saponins, terpenes, and glycosides. Studies on the chemical constituents and pharmacological actions have shown that the active ingredients resulting in the cough-curing and phlegm-reducing effects were alkaloids [3,4]. Normally, there are five kinds of Fritillaria, including Fritillaria Cirrhosa, Fritillaria ussuriensis, Fritillaria pallidiflora, Fritillaria thunbergii, and Fritillaria hupehensis. Among them, the wild species of Fritillaria pallidiflora Schrenk is only distributed in the regions north of the Tianshan Mountains (Xinjiang, China) [5]. With the increasing demand of Fritillaria Pallidiflora Schrenk, traditional wild medicine could not satisfy the market demand and cultivated species were introduced. However, whether the quality of cultivated Fritillaria Pallidiflora Schrenk possesses a similar effect as the wild medicine has required scientists to discover effective methods for the quality control of the specimens, including the approaches for determining the four main alkaloids in Fritillaria: Sipeimine-3β-d-glucoside, Sipeimine, Peimisine, and Yibeinoside A.
Several studies have been reported to determinate the content of alkaloids as the evaluating criteria by adopting methods based on HPLC-UV, HPLC-ELSD, precolumn derivatization HPLC-UV, TLC scanning, spectrophotometry, and LC-MS [6,7,8,9,10,11]. However, it is difficult to directly analyze Fritillaria pallidiflora Schrenk by these methods due to the lack of UV chromophores on steroidal alkaloids (for example, classic Sipeimine-3β-d-glucosid, Sipeimine, Peimisine, and Yibeinoside). The treatment of pre-column derivatization HPLC-UV is normally useful, but is very complicated, and HPLC-ELSD suffers from a low sensitivity [12]. With the advantages of a short analysis cycle, strong separating capability, high resolution and sensibility, accurate molecular mass measurement of compounds, and rapid identification of the composition, UPLC/Q-TOF-MS has been widely applied in the study of the material base of natural products, due to its high efficiency in analyzing the chemical components and metabolomics of specimens [13,14,15]. The advantage of a mass spectrometric detector in relation to the selectiveness and sensitivity reveals that it may be effective in analyzing alkaloids in Fritillaria.
In this study, an ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS) method was established to rapidly analyze the chemical components of Fritillaria from both wild and cultivated resources for a detailed comparision, especially for alkaloids with the above reported activities, which provided a reference for medicinal quality control and material basis research. Then, a specific, accurate, and reproducible quantitative method was developed based on UPLC-QTOF-MS, to determine the content of four alkaloids (Sipeimine-3β-d-glucoside, Sipeimine, Peimisine, and Yibeinoside A) in Fritillaria from 23 different sources, which is useful for the quality control of Fritillaria. The result was analyzed by SPSS (Statistical Product and Service Solutions) 22 software. To the best of our knowledge, no analysis method based on UPLC-QTOF-MS for the quantitative and qualitative determination of alkaloids in Fritillaria has been reported.
2. Results
2.1. Characterization of Chemical Constituents
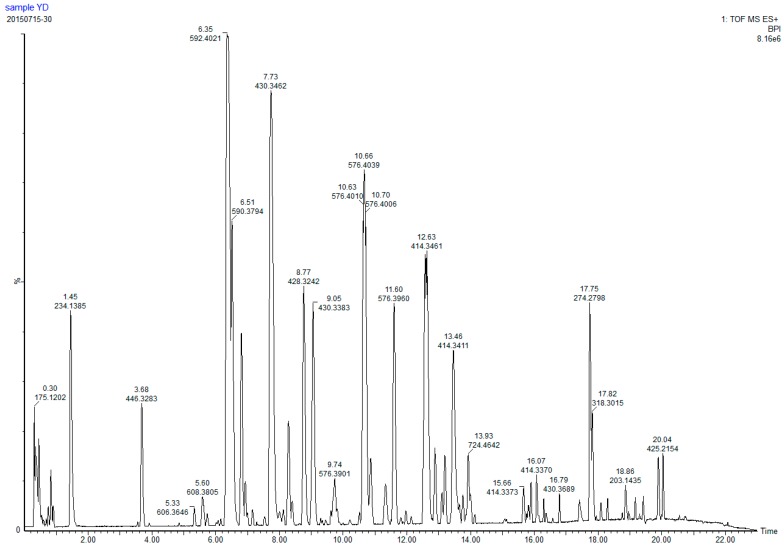
The components of both wild and artificial cultivated Fritillaria were analyzed by UPLC-QTOF-MS and relative information is listed in Figure 1. The accurate molecular mass of the positive molecular ion peaks of each chromatogram was obtained, and possible molecular formulas were calculated by MassLynx MS software. A total of 89 and 85 characteristic peaks are elucidated in wild and artifical cultivated Fritillaria, respectively, showing that the number of components is similar. By analysing the chromatographic peaks according to the fragment ion peak information and reference data, more than 40 alkaloids were identified (as shown in Table 1 and the structural formula in Figure 2). The value of the mass weight measured by mass spectroscopy is well matched with the value calculated.
Figure 1.
Base peak chromatogram of Fritillaria ingredients.
Table 1.
Result of chromatographic peak identification of wild Fritillaria (Gongliu County, Yili) (S1).
| Peak | tR/min | Formula | Experiment Value m/z | Theroretical Value m/z | Error | Fragments | Compound | Relative Mass Fraction % | References | Structural Formula | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mDa | ppm | ||||||||||
| 1 | 0.3 | C6H14N4O2 | 175.1202 | 175.1195 | 0.7 | 4 | 0.61 | ||||
| 2 | 0.35 | C6H14N4O2 | 175.1198 | 175.1195 | 0.3 | 1.7 | 1.47 | ||||
| 3 | 0.45 | C9H17NO8 | 268.1047 | 268.1046 | 0.1 | 0.4 | 136.0632, 119.036 | Adenosine | 0.97 | [16,17,18] | |
| 4 | 0.54 | C12H21NO6 | 276.1445 | 276.1447 | −0.2 | −0.7 | 0.24 | ||||
| 5 | 0.6 | C10H23NO6 | 254.1617 | 254.1617 | 0 | 0 | 0.10 | ||||
| 6 | 0.68 | C9H15NO4 | 202.1081 | 202.1079 | 0.2 | 1 | 0.09 | ||||
| 7 | 0.75 | C6H9NO3 | 144.0663 | 144.0661 | 0.2 | 1.4 | 0.20 | ||||
| 8 | 0.83 | C8H9N | 120.0818 | 120.0813 | 0.5 | 4.2 | 0.60 | ||||
| 9 | 0.9 | C15H35NO10 | 390.2355 | 390.2355 | 0.1 | 0.3 | 0.22 | ||||
| 10 | 1.45 | C10H19NO5 | 234.1385 | 234.1341 | 4.4 | 3.2 | 3.17 | ||||
| 11 | 3.56 | C33H51NO9 | 606.3644 | 606.3642 | 1.2 | 2 | 0.10 | ||||
| 12 | 3.68 | C27H43NO4 | 446.3283 | 446.3284 | 0.4 | 0.9 | yibeisine | 1.65 | [6,19] | Figure 2D | |
| 13 | 3.92 | C33H51NO10 | 622.3589 | 622.3605 | −1.6 | −2.6 | 0.09 | ||||
| 14 | 4.85 | C33H51NO9 | 606.3632 | 606.3642 | −1 | −1.6 | 0.08 | ||||
| 15 | 4.93 | C33H51NO9 | 606.364 | 606.3642 | −0.2 | −0.3 | 0.05 | ||||
| 16 | 5.33 | C33H51NO9 | 606.3646 | 606.3642 | 0.4 | 0.7 | 0.25 | ||||
| 17 | 5.6 | C33H53NO9 | 608.3805 | 608.3799 | 0.5 | 0.8 | 0.51 | ||||
| 18 | 5.73 | C34H55NO8 | 606.4001 | 606.4006 | −0.5 | −0.8 | 0.22 | ||||
| 19 | 6.02 | C39H63NO13 | 754.4374 | 754.4378 | −1.7 | −2.3 | 0.07 | ||||
| 20 | 6.08 | C39H63NO13 | 754.438 | 754.4378 | 0.2 | 0.3 | 0.14 | ||||
| 21 | 6.17 | C33H53NO8 | 592.385 | 592.385 | 0 | 0 | 138.1285, 574.3744, 412.3216, 394.3110, | Sipeimine-3β-d-glucoside | 0.11 | [20,21] | Figure 2A |
| 22 | 6.35 | C33H53NO8 | 592.4021 | 592.4015 | 0.6 | 1 | 138.1285, 574.3744, 412.3216, 394.3110 | Sipeimine-3β-d-glucoside its isomers | 11.95 | [20,21] | Figure 2A |
| 23 | 6.51 | C33H51NO8 | 590.3693 | 590.3588 | 0.6 | 1 | 428.3167, 412.3211, 114.0921, 142.0782 | Peimisine-3-O-β-d-glucopyranoside its isomers | 4.68 | [22] | |
| 24 | 6.81 | C33H53NO8 | 592.3846 | 592.3849 | −0.3 | −0.5 | 138.1285, 574.3744, 412.3216, 394.3110, | Sipeimine-3β-d-glucoside its isomers | 2.53 | [20,21] | Figure 2A |
| 25 | 6.93 | C33H55NO8 | 594.4009 | 594.4006 | 0.2 | 0.3 | 576.3896, 414.3364, 138.1285 | Peiminoside | 0.62 | [23] | |
| 26 | 6.99 | C33H51NO8 | 590.3691 | 590.3693 | −0.2 | −0.3 | 428.3167, 412.3211, 114.0921, 142.0782 | Peimisine-3-O-β-d-glucopyranoside its isomers | 0.22 | [22] | |
| 27 | 7.15 | C27H41NO4 | 444.3121 | 444.3114 | 0.5 | 1.1 | 428.3167, 412.3211, 114.0921, 142.0782 | Yibeissine isomers | 0.27 | [24] | |
| 28 | 7.29 | C27H41NO4 | 444.3139 | 444.3127 | 2.5 | 5.6 | 428.3167, 412.3211, 114.0921, 142.0782 | Yibeissine isomers | 0.13 | [24] | |
| 29 | 7.54 | C27H43NO4 | 446.3274 | 446.327 | 0.4 | 0.9 | Yibeinine its isomers | 0.21 | [6,19] | Figure 2D | |
| 30 | 7.73 | C27H43NO3 | 430.3462 | 430.3342 | 2.1 | 4.9 | 412.3279, 138.1301 | Sipeimine | 9.46 | [23] | Figure 2B |
| 31 | 8.00 | C27H43NO3 | 430.3316 | 430.3321 | −0.6 | −1.4 | 412.3279, 138.1301 | Sipeimine its isomers | 0.07 | [23] | Figure 2B |
| 32 | 8.12 | C27H43NO3 | 430.3313 | 430.3321 | −0.8 | −1.9 | 412.3279, 138.1301 | Sipeimine its isomers | 0.13 | [23] | Figure 2B |
| 33 | 8.29 | C27H43NO3 | 430.3323 | 430.3321 | 0.2 | 0.5 | 412.3279, 138.1301 | Sipeimine its isomers | 1.55 | [23] | Figure 2B |
| 34 | 8.4 | C27H45NO3 | 432.3474 | 432.3478 | −0.4 | −0.9 | 414.3366, 398.3054, 138.1284 | Verticine its isomers | 0.42 | [23] | |
| 35 | 8.77 | C27H41NO3 | 428.3165 | 428.3165 | 0 | 0 | 412.3211, 114.0921, 142.0782 | Peimisine | 4.13 | [20,23,25] | Figure 2E |
| 36 | 9.05 | C27H43NO3 | 430.3383 | 430.3321 | 0.1 | 0.2 | 412.3279, 138.1301 | Sipeimine | 3.73 | [23] | Figure 2B |
| 37 | 9.3 | C23H33NO | 340.2599 | 340.2581 | 0.8 | 2.4 | 0.17 | ||||
| 38 | 9.35 | C27H41NO3 | 428.3165 | 428.3165 | 0 | 0 | 412.3211, 114.0921, 142.0782 | Peimisine its isomers | 0.15 | [20,23,25] | Figure 2E |
| 39 | 9.45 | C27H43NO4 | 446.3271 | 446.3271 | 0 | 0 | Yibeinine its isomers | 0.21 | [6,19] | Figure 2D | |
| 40 | 9.62 | C34H55NO8 | 606.4005 | 606.4006 | −0.1 | −0.2 | 0.28 | ||||
| 41 | 9.74 | C33H53NO7 | 576.3902 | 576.3901 | 0.1 | 0.2 | 414.3369, 396.3263 | Yibeinoside A its isomers | 1.01 | [25] | Figure 2F |
| 42 | 9.81 | C33H55NO7 | 578.4049 | 578.4057 | −0.7 | −1.2 | 416.3516, 398.3409 | hupeheninoside | 0.45 | [25] | |
| 43 | 10.00 | C33H53NO7 | 576.39 | 576.39 | 0 | 0 | 414.3369, 396.3263 | Yibeinoside A its isomers | 0.08 | [25] | Figure 2F |
| 44 | 10.21 | C33H53NO8 | 592.3848 | 592.3849 | −0.2 | −0.3 | 138.1285, 574.3744, 412.3216, 394.3110 | Sipeimine-3β-d-glucoside its isomers | 0.30 | [20,21] | Figure 2A |
| 45 | 10.51 | C27H41NO | 396.8019 | 396.8019 | 0 | 0 | 0.25 | ||||
| 46 | 10.66 | C33H53NO7 | 576.391 | 576.39 | 1 | 1.7 | 414.3369, 396.3263 | Yibeinoside A | 6.78 | [20] | Figure 2F |
| 47 | 10.87 | C33H53NO7 | 576.3898 | 576.39 | −0.2 | −0.3 | 414.3369, 396.3263 | Yibeinoside A its isomers | 1.05 | [20] | Figure 2F |
| 48 | 11.33 | C33H53NO7 | 576.39 | 576.39 | 0 | 0 | 414.3369, 396.3263 | Yibeinoside A its isomers | 0.87 | [20] | Figure 2F |
| 49 | 11.6 | C33H53NO7 | 576.396 | 576.4076 | −3.6 | −6.2 | 414.3369, 396.3263 | Yibeinoside A its isomers | 3.56 | [20] | Figure 2F |
| 50 | 11.81 | C33H53NO7 | 576.396 | 576.396 | 0 | 0 | 414.3369, 396.3263 | Yibeinoside A its isomers | 0.12 | [20] | Figure 2F |
| 51 | 11.97 | C28H45NO2 | 428.3163 | 428.3165 | −0.2 | −0.5 | puqietinedinone its isomers | 0.30 | [26] | ||
| 52 | 12.14 | C33H53NO8 | 592.3859 | 592.3849 | 1 | 1.7 | 138.1285, 574.3744, 412.3216, 394.3110, | Sipeimine-3β-d-glucoside its isomers | 0.21 | [20,21] | Figure 2A |
| 53 | 12.63 | C27H43NO2 | 414.3461 | 414.6438 | 0.9 | 1.6 | 396.3253, 105.0697 | puqiedinoneits isomers | 8.84 | [20,23] | Figure 2C |
| 54 | 12.89 | C33H53NO7 | 576.3909 | 576.3901 | 0.9 | 1.6 | 414.3369, 396.3263 | Yibeinoside A | 1.11 | [20] | Figure 2F |
| 55 | 13.1 | C40H67NO12 | 754.4739 | 754.4742 | −0.3 | −0.2 | 0.55 | ||||
| 56 | 13.19 | C33H53NO7 | 576.3902 | 576.3901 | 0.2 | 0.3 | 414.3369, 396.3263 | Yibeinoside A its isomers | 0.93 | [20] | Figure 2F |
| 57 | 13.46 | C27H43NO2 | 414.3411 | 414.3472 | 3.9 | 9.4 | 396.3253, 105.0697 | puqiedinone its isomers | 4.03 | [20,23] | Figure 2C |
| 58 | 13.64 | C34H57NO8 | 608.4161 | 608.4162 | −0.1 | −0.2 | pingbeininoside | 0.39 | [24] | ||
| 59 | 13.76 | C33H53NO8 | 592.385 | 592.385 | 0 | 0 | 138.1285, 574.3744, 412.3216, 394.3110 | Sipeimine-3β-d-glucoside its isomers | 0.51 | [20,21] | Figure 2A |
| 60 | 13.93 | C39H65NO11 | 724.4642 | 724.4636 | 0.6 | 0.8 | 1.11 | ||||
| 61 | 14 | C27H43NO3 | 430.3323 | 430.3321 | 0.2 | 0.5 | 412.3279, 138.1301 | Sipeimine its isomers | 0.64 | [23] | Figure 2B |
| 62 | 14.13 | C39H65NO12 | 740.4592 | 740.4585 | 0.7 | 0.9 | 0.24 | ||||
| 63 | 15.07 | C27H45NO2 | 416.3525 | 416.3529 | −0.5 | −1.2 | 398.3418 | Songbeinine | 0.27 | [23] | |
| 64 | 15.13 | C33H55NO7 | 578.406 | 578.4059 | 0.1 | 0.2 | 416.3516, 398.3409 | hupeheninoside | 0.19 | [20] | |
| 65 | 15.66 | C27H43NO2 | 414.3373 | 414.3373 | 0 | 0 | 396.3253, 105.0697 | puqiedinone its isomers | 0.58 | [20,23] | Figure 2C |
| 66 | 15.75 | C28H47NO2 | 430.3636 | 430.3685 | −4.9 | −11.4 | puqietinone | 0.23 | [27] | ||
| 67 | 15.82 | C28H45NO2 | 428.3527 | 428.3529 | −0.2 | −0.5 | puqietinedinone its isomers | 0.39 | [27] | ||
| 68 | 15.9 | C33H55NO6 | 562.4106 | 562.4108 | −0.2 | −0.4 | 0.49 | ||||
| 69 | 16.07 | C27H43NO2 | 414.337 | 414.3372 | −0.3 | −0.7 | 396.3253, 105.0697 | puqiedinone its isomers | 0.63 | [20,23] | Figure 2C |
| 70 | 16.30 | C27H43NO4 | 446.3635 | 446.3634 | 0 | 0 | Yibeinine its isomers | 0.31 | [6,19] | Figure 2D | |
| 71 | 16.37 | C14H31NO2 | 246.2432 | 246.2433 | −0.1 | −0.4 | 0.44 | ||||
| 72 | 16.58 | C16H35NO3 | 290.2693 | 290.2695 | −0.2 | −0.7 | 0.24 | ||||
| 73 | 16.79 | C28H47NO2 | 430.3689 | 430.3685 | 0.4 | 0.9 | puqietinone | 0.35 | [27] | ||
| 74 | 17.41 | C13H10O | 183.081 | 183.081 | 0 | 0 | 1.00 | ||||
| 75 | 17.75 | C16H35NO2 | 274.2798 | 274.2759 | 1.3 | 4.7 | 3.04 | ||||
| 76 | 17.82 | C18H39NO3 | 318.3015 | 318.3008 | 0.7 | 2.2 | 1.50 | ||||
| 77 | 17.94 | C16H33NO2 | 272.2589 | 272.259 | −0.2 | −0.7 | 0.18 | ||||
| 78 | 18.09 | C16H35N | 242.2849 | 242.2848 | 0.1 | 0.4 | Thymidine | 0.36 | [18] | ||
| 79 | 18.29 | C21H30O2 | 359.2323 | 359.2324 | −0.1 | −0.3 | 0.32 | ||||
| 80 | 18.76 | C18H39NO2 | 302.3057 | 302.3059 | −0.2 | −0.7 | 0.20 | ||||
| 81 | 18.86 | C14H18O | 203.1437 | 203.1437 | 0.1 | 0.5 | 0.68 | ||||
| 82 | 18.96 | C18H30O2 | 279.2321 | 279.2324 | −0.3 | −1.1 | 0.25 | ||||
| 83 | 19.17 | C17H24O3 | 277.1801 | 277.1804 | −0.3 | −1.1 | 0.37 | ||||
| 84 | 19.3 | C21H37N | 304.3001 | 304.3004 | −0.3 | −1 | 0.20 | ||||
| 85 | 19.42 | C18H20O4 | 301.1417 | 301.144 | −2.3 | −7.6 | 0.43 | ||||
| 86 | 19.89 | C15H26O | 223.2063 | 223.2062 | 0.3 | 1.3 | Patchouli alcohol | 0.92 | [26] | ||
| 87 | 20.04 | C22H32O8 | 425.2154 | 425.2175 | −1.6 | −3.8 | 0.80 | ||||
| 88 | 20.55 | C16H33NO | 256.264 | 256.264 | 0 | 0 | 0.18 | ||||
| 89 | 20.73 | C18H35NO | 282.2795 | 282.2797 | −0.6 | −2.1 | 0.26 | ||||
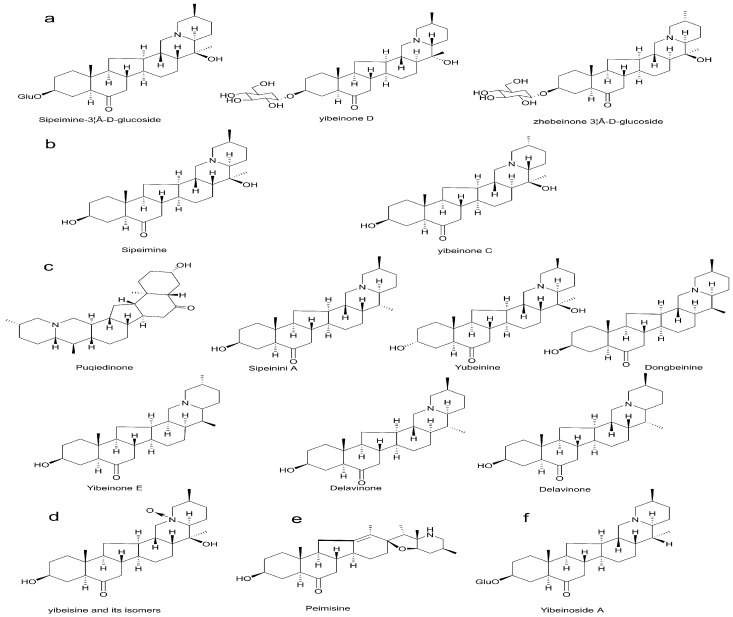
Figure 2.
Structure of (a) Sipeimine-3β-d-glucoside and its isomers; (b) Sipeimineand and its isomers; (c) Puqiedinone and its isomers; (d) Yibeisine and its isomers; (e) Peimisine, and (f) Yibeinoside A.
2.2. Quantitative Analysis
In order to conduct an accurate quantitation of the alkaloids in Fritillaria, four alkaloids with a high abundance and good resolution in the chromatogram (sipeimine-3β-d-glucoside, sipeimine, peimisine, and Yibeinoside A) were separated from the 40 compounds and were used as the marker of quantitation. By using the area normalization method, the content of the main ingredients could be ascertained and are listed in Table 2. The ingredients from wild Fritillaria with a higher relative mass fraction included Sipeimine-3β-d-glucoside and its isomers (15.61%), Sipeimine-3β-d-glucoside and its isomers (15.58%), puqiedinone and its isomers (14.08%), and Peimisine-3-O-β-d-glucopyranoside and its isomers (4.9%). The ingredients from cultivated Fritillaria with a higher relative mass fraction included Sipeimine-3β-d-glucoside and its isomers (17.49%), puqiedinone and its isomers (12.85%), Sipeimine-3β-d-glucoside and its isomers (12.71%), Yibeinoside A and its isomers (11.55%), Peimisine-3-O-β-d-glucopyranoside and its isomers (6.28%), and Peimisine and its isomers (5.15%), revealing certain differences between wild and cultivated Fritillaria. Hence, we chose sipeimine-3β-d-glucoside, sipeimine, peimisine, and Yibeinoside A for the further quantitation of the alkaloids in Fritillaria.
Table 2.
The content of four alkaloids in 23 Fritillaria from different sources (%, n = 3).
| Number | Sipeimine-3β-d-glucoside | Sipeimine | Peimisine | Yibeinoside A |
|---|---|---|---|---|
| S1 | 0.0576 | 0.0573 | 0.0155 | 0.0349 |
| S2 | 0.0565 | 0.0395 | 0.0087 | 0.0337 |
| S3 | 0.0084 | 0.0275 | 0.0338 | 0.0236 |
| S4 | 0.0665 | 0.0141 | 0.012 | 0.1398 |
| S5 | 0.0044 | 0.0105 | 0.0096 | 0.0034 |
| S6 | 0.0375 | 0.0453 | 0.0096 | 0.0202 |
| S7 | 0.0099 | 0.0216 | 0.0079 | 0.0038 |
| S8 | 0.0338 | 0.0493 | 0.0099 | 0.018 |
| S9 | 0.0427 | 0.1218 | 0.0343 | 0.0087 |
| S10 | 0.0749 | 0.0319 | 0.0083 | 0.0357 |
| S11 | 0.1033 | 0.0337 | 0.0073 | 0.0499 |
| S12 | 0.0018 | 0.0113 | 0.0033 | 0.0038 |
| S13 | 0.0815 | 0.0337 | 0.0073 | 0.0558 |
| S14 | 0.0763 | 0.0413 | 0.0126 | 0.0494 |
| S15 | 0.0545 | 0.0471 | 0.0093 | 0.0366 |
| S16 | 0.0039 | 0.0358 | 0.0437 | 0.0132 |
| S17 | 0.0083 | 0.0091 | 0.0113 | 0.0203 |
| S18 | 0.0013 | 0.0066 | 0.0094 | 0.0019 |
| S19 | 0.007 | 0.0152 | 0.0124 | 0.0051 |
| S20 | 0.0737 | 0.1086 | 0.0071 | 0.008 |
| S21 | 0.0882 | 0.0913 | 0.0058 | 0.0076 |
| S22 | 0.0088 | 0.0755 | 0.0206 | 0.0075 |
| S23 | 0.1357 | 0.1019 | 0.0067 | 0.0116 |
2.2.1. Method Validation
Three reference standards (Sipeimine-3β-d-glucoside (99.1%, Batch No.110767-201208, Beijing, China), Sipeimine (96.4%, Batch No.111917-201202, Beijing, China), and Peimisine (≥98%, Batch No. 111892-201402, Beijing, China) were brought from National Institutes for Food and Drug Control, and Standard of Yibeinoside A (>98%, Batch No.141009, Chengdu, China) was used to test the method of validation of the quantitative analysis of alkaloids in Fritillaria. Standard curves of sipeimine-3β-d-glucoside, sipeimine, peimisine, and Yibeinoside A were produced with the methods mentioned in Section 4.3.2. As listed in Table S1, all of the regression equations were larger than 0.999, and both the LODs and LOQs were of a nanogram grade, suggesting that the proposed method had a high precision and was available for quantitation. The precision and repeatability of the method built were investigated by continuously analyzing the same sample solution six times and the results are listed in Table S2. The RSD in the precision degree of the four target compounds were 4.89%, 1.26%, 1.37%, and 2.39%, indicating that the precision was very good. The RSD value of the repeatability experiment for the four compounds was satisfactory, with values of 6.53%, 5.26%, 5.14%, and 6.95%, respectively.
The experiments were relatively stable and reliable for 24 h, because the values of RSD in the stability experiment were all below 6.28%. Five doses of sample 8 were prepared to test the solution and were injected to analyze the contents of the four compounds (see results in Table S2, indicating a good reproducibility of the method). Based on the record in Chinese Pharmacopoeia (2015) (Part 3) [28], the accuracy of the method should be investigated prior to measurement. Hence, standard addition recovery was conducted to investigate the accuracy of the method. The recoveries of the standard solution at low, mediate, and high concentration levels were calculated according to the equivalent compound content of 80%, 100%, and 120%, respectively. As shown in Table S3, the method possessed a good recovery rate, ranging from 99.8% to 103.5%, indicating the satisfactory accuracy of this method.
2.2.2. Quantitative Investigations
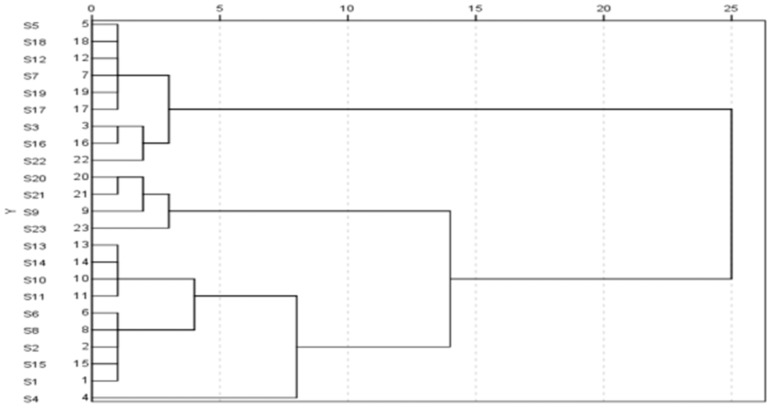
The contents of the four alkaloids in 23 samples were analyzed by external standard calibration and the results are presented in Table 2. SPSS 22 software was used as the ward’s method. The measurement range was the square Euclidean distance. The parent sample was analyzed by cluster analysis. In Figure 3, a relationship between the contents of the four alkaloids and sources, as well as the producing areas, can be seen. The differences between the wild and cultivated Fritillaria Pallidiflora Schrenk are obvious. S3, S5, S7, S16, S17, S18, S19, and S22 were all wild Fritillaria Pallidiflora Schrenk, which could be grouped together. The others could be placed in another group. The content of the four alkaloids in Wild Fritillaria S5, S7, S18, S19, and S22 was relatively closer and grew in the Tacheng area. Similarly, the content of the four alkaloids in S20, S21, and S23 was relatively closer and grew in Kazakhstan. The content of the four alkaloids in S1, S2, S4, S10, S11, S13, S14, and S15 was relatively closer, which were wild or cultivated species growing in the Yili area. The cluster analysis results showed the influence of the different area on the medicinal content.
Figure 3.
Cluster analysis dendrogram of sample.
2.2.3. Producing Area Selection
A total of 23 samples of medicinal Fritillaria Pallidiflora Schrenk, including wild and cultivated species, whose plant sources are Fritillariae pallidiflorae bulbus, Fritillariae walujewii, Fritillariae pallidiflora Schrenk, Fritillaria tortifolia, Yumin Fritillary Bulb, Fritillaria meleagria Linn., Fritillaria maximowiczii, Fritillaria imperialis, and whose origin include Gongliu County Yili, Nalati Xinyuan County, Yili Tekes County, Toli County Tacheng, Tacheng Yumin County, and Jimunai County Aletai, were included in this investigation. In addition, there three Fritillaria samples from Kazakhstan are mentioned in this study. The qualitative result shows that the category of alkaloids found among them is accordance with the results listed in Table 1. Compared with the result of qualification, the quantitation method based on four main alkaloids was more important, which presented the effect of the planting place of Fritillaria.
From Kazakhstan, where the climate is similar to Xinjiang, a chemical composition analysis of wild and cultivated species showed the content differences among all kinds of Fritillaria from different places. The retention time, molecular ion peak, and relative molecular mass of 23 components of Fritillaria were compared with the four alkaloids, which laid a foundation for the quality control and material basis of Fritillaria.
2.2.4. Index Components Selection
Various bioactive constituents have been found in Fritillaria, and alkaloids were the active ingredients relative to the cough-curing and phlegm-reducing effects. The alkaloids of Fritillaria pallidiflora were normally steroidal alkaloids, like Sipeimine-3β-d-glucoside, Sipeimine, Peimisine, and Yibeinoside A/B. The Chinese Pharmacopoeia of the 2015 Edition, which includes Fritillaria pallidiflora, used Sipeimine-3β-d-glucoside and Sipeimine as index components for the content determination of no less than 0.07%. Considering the diversity of chemical components and compounds of a higher relative mass fraction in chemical analysis, this work added Peimisine and Yibeinoside A as index components to increase the quality control level of Fritillaria pallidiflora.
2.2.5. Sample Pretreatment
Alkaloids mostly exist in the plant cells as salt, but only free alkaloids are fat-soluble. Therefore, they must first be alkalized to form their free form by lime milk, sodium carbonate solution, or dilute ammonia water, and then extracted with a lipophilic-organic-solvent like chloroform, dichloromethane, or benzene [27]. Briefly, 0.25 g of Fritillaria powder was put into a 50 mL conical flask, and 25 mL of methanol–water (6:4, v/v) mixture was added. Then, the suspension was sonicated (100 W, 35 kHz) for 30 min. Methanol–water (6:4, v/v) mixture was then added. After being filtered and centrifugated (5000 rpm), the supernatant was collected and dried, and the residues were re-dissolved in 5 mL methanol prior to analysis. The result of the sample recovery shows that this method had a good extraction efficiency for Sipeimine-3β-d-glucoside, Sipeimine, Yibeinoside A, and Peimisine.
2.2.6. Result Analysis of the Content Determination
The four alkaloids from the Fritillaria samples from 23 sources exhibited large variations, and the relative contents of Sipeimine-3β-d-glucoside, Sipeimine, Peimisine, and Yibeinoside A were 0.0013% (S18)~0.1357% (S23), 0.0066% (S18)~0.1218% (S9), 0.0033% (S12)~0.0437% (S16), and 0.0019% (S18)~0.1398% (S4), respectively. A rough separation of wild and cultivated Fritillaria could be achieved by cluster analysis on 23 samples using the content results as cluster variables.
3. Discussion
An approach based on the use UPLC-QTOF-MS was developed to investigate the chemical constituents of wild and artificial cultivated Fritillaria, and to conduct a comparison of the results, which was expected to be useful in the foundation of medicinal quality control and material basis research. After analysing the accurate molecular mass and molecular fragments, 89 and 85 components were found in wild and artifical cultivated Fritillaria, and 40 alkaloids were elucidated. Based on the experience reported in an earlier study, the product ion at m/z 114 (C6H12NO) was formed by the cleavage of the E-ring with the loss of C21H30O2, owing to the presence of an O bridge between the 17 and 23-position, with an electronic induction effect. The product ion at m/z 414 corresponded to the loss of a d-glucopyranoside unit (162 Da), with further peaks appearing at m/z 138.12 (C9H16N), produced by the loss of C18H28O2.
Compound 3 (tR = 0.45 min) produced [M + H]+ ions at m/z 268, and highly abundant fragment ions at m/z 136 [M + H − 132]+ and m/z 119 [M + H − 149]+ in the MS2 experiment, suggesting that adenine was present, and the loss of 149 (132 + 17) Da was attributed to the release of a nucleosides moiety and hydroxyl molecule. According to the previous reports [16,17,18], compound 1 was identified as Adenosine. Compound 12 (tR = 3.68 min) produced [M + H]+ ions at m/z 446. According to the previous reports [20,27], compound 2 was identified as yibeisine. Compound 21 (tR = 6.17 min) produced [M + H]+ ions at m/z 592, and highly abundant fragment ions at m/z 138 [M + H − 454]+ , m/z 574 [M + H − 18]+, m/z 412 [M + H − 180]+, and m/z 394 [M + H − 198]+ in the MS2 experiment, suggesting that gallic acid and the loss of 18 Da could be attributed to the release of a water molecule, and the loss of 180 Da (162 + 18) was attributed to the release of a glucose moiety and water molecule. Moreover, Sterolsc was present. According to the previous reports [20,21], the retention time of the reference standard, compound 21, was identified as Sipeimine-3β-d-glucoside. Compounds 22, 24, 44, and 59 possessed the same mass weight and fragments as compound 21, and could be regarded as the isomers of Sipeimine-3β-d-glucoside.
Compounds 23 and 26 (tR = 6.51 min, 6.99 min) produced [M + H]+ ions at m/z 590, and highly abundant fragment ions at m/z 428 [M + H − 162]+, m/z 574 [M + H − 16]+, m/z 412 [M + H − 178]+, and m/z 394 [M + H − 196]+ in the MS2 experiment, suggesting that the loss of 162 Da was attributed to the release of a glucose moiety, the loss of 16 Da was attributed to the release of an Oxygen atom, and the loss of 196 Da was attributed to the release of Α-d-(+)-glucopyranose. According to the previous reports [22], compounds 23 and 26 were tentatively identified as the isomers of Peimisine-3-O-β-d-glucopyranoside.
Compound 25 (tR = 6.93 min) produced [M + H]+ ions at m/z 594, and highly abundant fragment ions at m/z 576 [M + H − 18]+, m/z 414 [M + H − 180]+, and m/z 138 [M + H − 456]+ in the MS2 experiment, suggesting that the loss of 18 Da was attributed to the release of a water molecule and the loss of 180 Da (162 + 18) was attributed to the release of a glucose moiety and water molecule. According to the previous reports [23], compound 25 was identified as Peiminoside.
Compounds 27 and 28 (tR = 7.15 min, 7.29 min) produced [M + H]+ ions at m/z 444, and highly abundant fragment ions at m/z 428 [M + H − 16]+ , m/z 114 [M + H − 330]+, m/z 412 [M + H − 32]+, and m/z 142 [M + H − 302]+ in the MS2 experiment, suggesting that the release of an oxygen atom was an indicator of the presence of xylose and/or arabinose and a glucose group. According to the previous reports [24], compound 27 was tentatively identified as yibeissine.
Compounds 29, 39 and 70 (tR = 7.54 min, 9.45 min, 16.3 min) produced [M + H]+ ions at m/z 446. According to the previous reports [6,19], compound 11 was identified as Yibeinine and its isomers.
Compound 30 (tR = 7.73 min) produced [M + H]+ ions at m/z 430, and highly abundant fragment ions at m/z 412 [M + H − 18]+ and m/z 138 [M + H − 292]+ in the MS2 experiment, suggesting the release of a water molecule and the presence of a glucose group. According to the previous reports [23] and on the basis of the retention time of the reference standard, compound 30 was identified as Sipeimine. Compounds 31, 32, 33, and 36 possessed the same mass weight and fragments as compound 30, which could be regarded as the isomers of Sipeimine.
Compound 34 (tR = 8.40 min) produced [M + H]+ ions at m/z 432, and highly abundant fragment ions at m/z414 [M + H − 18]+, m/z 398 [M + H − 34]+, and m/z 138 [M + H − 294]+ in the MS2 experiment, suggesting the release of a water molecule, the loss of 18 Da and two ammonia molecules, and the presence of a glucose group. According to the previous reports [23], compound 34 was tentatively identified as Verticine and its isomers.
Compound 35 (tR = 8.77 min) produced [M + H]+ ions at m/z 428, and highly abundant fragment ions at m/z 412 [M + H − 16]+, m/z 114 [M + H − 314]+, and m/z 142 [M + H − 286]+ in the MS2 experiment, suggesting the loss of an oxygen atom, an indicator of the presence of xylose and/or arabinose and a glucose group. Based on the retention time of the reference standard and previous reports [20,23,25], compound 35 was identified as Peimisine. Compound 38 possessed the same mass weight and fragments as compound 35, which could be regarded as the isomers of Peimisine.
Compound 41 (tR = 9.74 min, 10.00 min) produced [M + H]+ ions at m/z 592. The product ion at m/z 574.3744 corresponded to the loss of a H2O molecule (−18 Da), with further peaks appearing at m/z 412 and 394, produced by the loss of a glucose unit (162 Da) and a water molecule (18 Da). According to the previous reports [20], compound 41 was tentatively identified as Yibeinoside A and its isomers.
Compound 54 (tR = 12.89 min), produced [M + H]+ ions at m/z 576. The product ion at m/z 414, 396, corresponded to the loss of a moiety of glucose and a water molecule. Compared with the retention time of the reference standard, compound 54 was identified as Yibeinoside A. Compounds 41, 43, 46, 47, 48, 49, 50, and 56 possessed the same fragments and mass weight as compound 54, and were tentatively identified as the isomers of Yibeinoside A.
Compounds 65 and 69 (tR = 15.66, 16.07 min) produced [M + H]+ ions at m/z 414, and highly abundant fragment ions at m/z 105 [M + H − 309]+ and m/z 396 [M + H − 18]+ in the MS2 experiment, suggesting that gallic acid and a water molecule were released. According to the previous reports [20,23], compound 65 was tentatively identified as puqiedinone and its isomers.
Compounds tentatively identified by the accurate mass weight included compound 66 (tR = 15.75 min), which produced [M + H]+ ions at m/z 608. According to the previous reports [23], compound 66 was identified as puqietinone. Compound 73 was also identified as puqietinone. Compound 78 (tR = 18.09 min) produced [M + H]+ ions at m/z 242. According to the previous reports [18], compound 78 was identified as Thymidine. Compound 86 (tR = 19.89 min) produced [M + H]+ ions at m/z 223. According to the previous reports [26], compound 86 was identified as Patchouli alcohol. The alkaloids were closely connected with the bio-activities in Fritillaria. As mentioned above, the alkaloids in Fritillaria exhibit a wide range of bioactivities in many aspects, such as anti-micobial, antiumor, antihypertensive, and antiussive activities. In this study, the recognized 40 alkaloids matched well with the biological alkaloids identified in the earlier reports on Fritillaria [29], indicating that no wild and cultivated Fritillaria possessed the same potential for treating relative diseases due to the same kinds of efficient components.
Four typical alkaloids, namely Sipeimine-3β-d-glucoside, Sipeimine, Peimisine, and Yibeinoside A, were chosen as the standard to build a simple, rapid, accurate, sensible, and reproducible method for the quality control of Fritillaria. After analysing the components of Fritillaria planted in different places, SPSS was adopted to roughly classify Fritillaria, which could be used to screen the substitute of Fritillaria with similar components and bioactivities through the identification of the quality and authenticity in Fritillaria, providing a reference on the quality evaluation, and playing a significant role in the sufficient utilization and protection of Fritillaria medicinal plant resources.
4. Materials and Methods
4.1. Materials and Reagents
A total of 23 samples of Fritillaria herbs were collected during 2013 to 2015 from Xinjiang and a herbal medicine market, respectively. They were identified by Sulaiman Halike, a senior pharmacist from the Xinjiang Uygur Autonomous Region Institute for Food and Drug Control (for details, see Table 3). For the species from Kazakhstan, the herbs were collected from the village of Urunkhayka near Lake Markakol in East Kazakhstan. Relative herbs were smashed and dried under a vacuum at 80 oC, and were then sieved (80 mesh) and deposited in an ampubottle before use. The numbers of the herbs were 20130001-1, 20140001-2, and 20150001-6, as stored in the Xinjiang Uygur Autonomous Region Institute for Food and Drug Control. Each sample was crushed, sifted through a 60 mesh sieve, mixed thoroughly, and sealed in a bag, avoiding light.
Table 3.
Fritillaria source.
| Sampel No. | Provenance | Origin | |
|---|---|---|---|
| S1 | (Wild) | F. pallidiflora | Gongliu County, Yili |
| S2 | (Cultivate) | F. pallidiflora | Gongliu County, Yili |
| S3 | (Wild) | F. walujewii | Xinyuan County, Yili |
| S4 | (Wild) | F. pallidiflora | Huocheng County, Yili |
| S5 | (Wild) | F. tortifolia | Toli County, Tacheng |
| S6 | (Cultivate) | F. yuminensis | Yumin County, Tacheng |
| S7 | (Wild) | F. yuminensis | Yumin County, Tacheng |
| S8 | (Cultivate) | F. tortifolia | Toli County, Tacheng |
| S9 | (Cultivate) | F. walujeweii | Altai, Xinjiang |
| S10 | (Wild) | F. pallidiflora | Tekes County, Yili |
| S11 | (Wild) | F. pallidiflora | Tekes County, Yili |
| S12 | (Cultivate) | Fritillaria | Kazakhstan |
| S13 | (Cultivate) | F. pallidiflora | Yili, Xinjiang |
| S14 | (Cultivate) | F. pallidiflora | Yili, Xinjiang |
| S15 | (Wild) | F. pallidiflora | Yili, Xinjiang |
| S16 | (Wild) | F. walujeweii | Altai, Xinjiang |
| S17 | (Wild) | F. verticillata | Jeminay County,Altai |
| S18 | (Wild) | F. tortifolia | Toli County, Tacheng |
| S19 | (Wild) | F. yuminensis | Yumin County, Tacheng |
| S20 | (Wild) | Fritillaria | Kazakhstan |
| S21 | (Wild) | Fritillaria | Kazakhstan |
| S22 | (Wild) | F. yuminensis | Yumin County, Tacheng |
| S23 | (Wild) | Fritillaria | Kazakhstan |
Standard substances of Sipeimine-3β-d-glucoside (99.1%, Batch No.110767-201208), Sipeimine (96.4%, Batch No.111917-201202, Beijing, China), and Peimisine (≥98%, Batch No. 111892-201402, Beijing, China) were brought from the National Institute for Food and Drug Control. Standard of Yibeinoside A (>98%, Batch No.141009, Pufei De Biotech Co., Ltd., Chengdu, China) was brought from Chengdu Pufei De Biotech Co., Ltd. Ammonia water (Kelong Chemical Reagent Factory, 20120310, Chengdu, China), chloroform ( Fu Yu Fine Chemical Co., Ltd 20151002, Tianjin, China), and formic acid (Tianjin Kwangfu Fine Chemical Industry Research Institute, 20140629) were all of an analytical grade. Methanol (Fisher, 143714, New York, NY, USA) and acetonitrile (Fisher, 155753, New York, NY, USA) were of a Mass spectrometry grade. The water used was ultrapure water.
4.2. Method
4.2.1. Characterization of Chemical Constituents
Liquid Chromatography separation was performed using a Waters ACQUITY UPLC I-Class system (Waters Corporation, Milford, MA, USA) composed of a quaternary pump, an on-line degasser, an autosampler, and a column temperature controller. Then, separations were achieved on a Waters BEH-C18 column (50 mm × 2.1 mm, 1.7 μm) (Waters Corporation, Milford, MA, USA) using an ACQUITYTM Ultra performance Liquid Chromatography system (Waters, Milford, MA, USA). The column temperature was maintained at 40 °C. The mobile phase was a binary mobile phase consisting of 0.1% formic acid-water solution (A) and acetonitrile (B) with an elution gradient of 0~15 min (95%→75% A), 15~22 min (75%→0% A), 22~23 min (0% A), and 23~23.1 min (0%→95% A), while the flow rate was 0.4 mL min−1 and the inject volume was 2 μL.
A Waters Xevo G2-S Q-TOF Mass Spectrometer (Waters corporation, Manchester, UK) with an electrospray ionization (ESI) interface was connected with the liquid chromatography. The ESI source was operated in a positive ionization mode with a scanning range of m/z 50~1200. The ion source parameters were optimized as follows: capillary voltage, 0.5 kV; ion source temperature, 100 °C; desolvation temperature, 450 °C; cone voltage, 40 V; extraction cone voltage, 80 V; cone gas flow, 50 L h−1; and solvent-removing flow (N2), 800 L h−1. Leucine-enkephalin ([M + H]+ = 556.2771) was used for real-time correction. All of the operations, data acquisition, and analysis were controlled by MassLynxVer.4.0 software (Waters Corporation, Manchester, UK).
4.2.2. Quantitative Analysis
Chromatographic separation was achieved using a Waters ACQUITY-UPLC BEH-C18 column (100 mm × 2.1 mm, 1.7 μm) (Milford, MA, USA). The column temperature was maintained at 40 °C. The mobile phase was a mixture of 0.1% formic acid-water solution and acetonitrile. Elution gradients of 0~5 min (95%→87% A) and 5~15 min (87%→78% A) were used, while the flow rate was 0.4 mL·min−1 and the injected volume was 2 μL. Mass spectrometry was performed in a positive mode with a scanning range of m/z 50~1200. The ion source parameters were optimized as follows: capillary voltage, 0.5 kV; ion source temperature, 100 °C; desolvation temperature, 450 °C; cone voltage, 40 V; extraction cone voltage, 80 V; cone gas flow, 50 L·h−1; and solvent-removing flow (N2).
4.3. Preparation of Sample Solutions
4.3.1. Characterization of Chemical Constituents
A total of 0.25 g of Fritillaria powder was weighed precisely and put into a 50 mL conical flask with a plug, and 25 mL of methanol–water (6:4, v/v) mixture was then added. The suspension was weighed and extracted under sonication (100 W, 35 kHz) for 30 min, before being cooled, weighed again. It was then added to a methanol–water (6:4, v/v) mixture to make up for the weight, and was filtered and centrifuged (5000 rpm) for 10 min. The supernatant was collected and dried in an evaporation pan, and the residues were re-dissolved in methanol and transferred to a 5 mL volumetric flask, before methanol was added. The solvent was filtered through a 0.22 μm organic membrane before analysis.
4.3.2. Quantitative Analysis
In order to conduct an effective qualification of the alkaloids in Fritillaria, the selectivity of the compounds was very important. Based on the high resolution of the chromatogram and the high abundance of the content, four compounds, including Sipeimine-3β-d-glucoside, Sipeimine, Peimisine, and Yibeinoside A, were finally selected as a marker for the qualification of alkaloids. Stock solutions of Sipeimine-3β-d-glucoside, Sipeimine, Peimisine, and Yibeinoside A were prepared in methanol at a concentration of 1 mg mL−1 and mixed for preparing stock standard solutions for Sipeimine-3β-d-glucoside, Sipeimine, Peimisine, and Yibeinoside A with concentrations of 4.028, 1.015, 4.08, 2.212 μg mL−1, respectively. Then, the mixed stock standard solutions were separately diluted with methanol to obtain the standard working solutions. The concentrations of the calibration standard solutions for Sipeimine-3β-d-glucoside, Sipeimine, Peimisine, Yibeinoside A, and Peimisine were 7.867~4028 ng·mL−1, 1.982~1015 ng·mL−1, 4.320~2212 ng·mL−1, and 7.969~4080 ng·mL−1, respectively.
A total of 0.25 g of Fritillaria powder was precisely weighed and put into a 50 mL conical flask with a plug, before being alkalized with 2 mL ammonia water (25%) for 1 h. Then, 25 mL chloroform–methanol (8:2, v/v) was added and mixed to perform an ultrasonic extraction (100 W, 35 kHz) for 30 min. After being filtered and dried in an evaporation pan, the residues were re-dissolved in methanol and transferred to a 10 mL volumetric flask, where methanol was added for a constant volume. A total of 1 mL of the solution was transferred into another 10 mL volumetric flask for diluting. The solvent was filtered through 0.22 μm organic membranes before analysis.
4.4. Quantitative Method Validation
A total of 2 μL of different concentrations of the standard working item 4.3.2 was weighed precisely to analyze the chromatographic and mass spectrometry conditions mentioned in Section 4.2.2, and linear regression was carried out between the peak area of the standard sample (Y) and the corresponding concentration, to obtain the regression equation, correlation coefficient, and linearity range. Stepwise dilution was used on the lowest mass concentration of standard reserving liquid mixture. The quantization limit was a signal to noise ratio of 10 and the detection limit was a signal to noise ratio of three.
4.5. Standard Addition Recovery Experiments
The recovery was investigated at low, mediate, and high concentration levels. Sample powders (S8, 0.125 g) were put into a 50 mL conical flask, which was used as the background. Then, the standard solution with a content of the four standards of 80%, 100%, and 120% was added, respectively, and then analyzed under the chromatographic and mass spectrometry conditions mentioned above. The relative results are presented in Table S3.
5. Conclusions
An approach based on the use of UPLC-QTOF-MS was developed to investigate the chemical constituents of wild and artificial cultivated Fritillaria, and to conduct a comparison of the results, which was expected to be useful in the foundation of medicinal quality control and material basis research. After analysing the accurate molecular mass and molecular fragments, the 89 and 85 components were found in wild and artifical cultivated Fritillaria, and 40 alkaloids were elucidated. Four typical alkaloids, namely Sipeimine-3β-d-glucoside, Sipeimine, Peimisine, and Yibeinoside A, were chosen as the standard to build a simple, rapid, accurate, sensible, and reproducible method for the quality control of Fritillaria. After analysing the components of Fritillaria planted in different places, SPSS was adopted to roughly classify the Fritillaria specimens, which could be used to screen a substitute of Fritillaria with similar components and bioactivities through the identification of the quality and authenticity of Fritillaria, providing a reference on the quality evaluation and playing a significant role in the sufficient utilization and protection of Fritillaria medicinal plant resources.
Acknowledgments
This work was supported by the China National Natural Science Foundation (Grant No. U1403201).
Supplementary Materials
The following are available online, Table S1: Regression equation, LODs and LOQs of quantitative method for four alkaloid, Table S2: The results of precision, stability and repetition experiment, Table S3: The results of recovery experiments.
Author Contributions
Aziz Mohammat, Ablumiti Yili, and Haji Akbar Aisa conceived and designed the experiments; Aziz Mohammat, performed the experiments; Aziz Mohammat, and Ablumiti Yili analyzed the data; Haji Akbar Aisa contributed the reagents/materials/analysis tools; Aziz Mohammat wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.China Pharmacopoeia Committee . Part 1. Pharmacopoeia of the People’s Republic of China. China Chemical Industry Press; Beijing, China: 2015. pp. 141–142. [Google Scholar]
- 2.Wang D., Wang S., Chen X., Xu X., Zhu J., Nie L., Long X. Antitussive, expectorant and anti-inflammatory activities of four alkaloids isolated from Bulbus of Fritillaria wabuensis. J. Ethnopharmacol. 2012;139:189–193. doi: 10.1016/j.jep.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Wang D., Yang J., Du Q., Li H., Wang S. The total alkaloid fraction of bulbs of Fritillaria cirrhosa displays anti-inflammatory activity and attenuates acute lung injury. J. Ethnopharmacol. 2016;193:150–158. doi: 10.1016/j.jep.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y.J., Sheng P., Yao L., Shi H., Zhang X. ISSR Analysis on genetic diversity of 8 Species of plants in Fritillaria L. from Xinjiang. Chin. Wild Plant Resour. 2015;4:1–6. [Google Scholar]
- 5.Xu X.Q., Xu X.W. Review on the research of Fritillaria. Qiu Yi Wen Yao. 2013;11:319–320. (In Chinese) [Google Scholar]
- 6.Hao D.C., Gu X.J., Xiao P.G. Phytochemical and biological research of Fritillaria medicinal resources. Chin. J. Nat. Med. 2013;11:330–344. doi: 10.3724/SP.J.1009.2013.00330. [DOI] [PubMed] [Google Scholar]
- 7.Gan J.S., Ma Y., Wang Z.Y., Liu X.S., Liu Y. Analysis on chemical constituents in Epimedii Herba by UPLC/Q-TOF-MS. Drugs Clin. 2014;29:349–352. [Google Scholar]
- 8.Zhou N., Guo D.Q., Shen L., Chen Q.Y., Qin Y. Comparative contents of four alkaloids in bulbs of Fritillaria taipaiensis and Fritillaria unibracteata. Food Sci. 2014;35:133–136. [Google Scholar]
- 9.Peng R., Tan J., Ma P. Study on HPLC fingerprint of alkaloids in Fritillaria taipaiensis. Mod. Tradit. Chin. Med. Materia Medica-World Sci. Technol. 2015;17:152–155. [Google Scholar]
- 10.Wang Y., Zhang Q.L., Chen X.Y., Tang Z.H., Wu Z. Quantitative determination of peiminine in Bulbus Fritillaria available on market by HPLC. Chin. Tradit. Herb. Drugs. 2001;32:24–25. [Google Scholar]
- 11.Chen M., Liu M.Y., Hu C. Determination of Peiminine in Wenglitong tablets by TLC. Chin. Pharm. 2008;12:936–937. [Google Scholar]
- 12.Zhou J., Li P., Li H., Jiang Y., Ren M., Liu Y. Development and validation of a liquid chromatography/electrospray ionization time-of-flight mass spectrometry method for relative and absolute quantification of steroidal alkaloids in Fritillaria species. J. Chromatogr. A. 2008;1177:126–137. doi: 10.1016/j.chroma.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J., Liu W., Zeng X., Chen B. Fingerprint analysis of Fritillaria thunbergii using rapid resolution liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Chin. J. Chin. Mater. Med. 2013;38:2832–2837. [PubMed] [Google Scholar]
- 14.Klockmann S., Reiner E., Bachmann R., Hackl T., Fischer M. Food fingerprinting: metabolomic approaches for geographical origin discrimination of hazelnuts (Corylus avellana) by UPLC-QTOF-MS. J. Agric. Food Chem. 2016;64:9253–9262. doi: 10.1021/acs.jafc.6b04433. [DOI] [PubMed] [Google Scholar]
- 15.In G., Seo H.K., Park H.W., Jang K.H. A Metabolomic approach for the discrimination of red ginseng root parts and targeted validation. Molecules. 2017;22:471. doi: 10.3390/molecules22030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan B., Wang L., Dai X., Huang L., Yang M., Chen S. Identification and quantitative analysis of nucleosides and nucleobases in aqueous extracts of Fritillaria Cirrhosa D. Don. Using HPLC–DAD and HPLC-ESI-MS. Anal. Lett. 2011;44:2491–2502. doi: 10.1080/00032719.2011.551856. [DOI] [Google Scholar]
- 17.Liu M., Xu W., Xu C., Chen D., Wang J. Two new steroidal alkaloids from bulbs of Fritillaria pallidiflora. Chin. Tradit. Herb. Drugs. 2016;47:876–880. [Google Scholar]
- 18.Xu W., Liu M., Chen D., Wang J. Chemical constituents from bulbs of Fritillaria pallidiflora Schrenk. Biochem. Syst. Ecol. 2014;57:198–202. doi: 10.1016/j.bse.2014.08.021. [DOI] [Google Scholar]
- 19.Pan B., Su X., Hu B., Yang N., Chen Q., Wu W. Fusarium redolens 6WBY3, an endophytic fungus isolated from Fritillaria unibracteata var. wabuensis, produces peimisine and imperialine-3β-d-glucoside. Fitoterapia. 2015;103:213–221. doi: 10.1016/j.fitote.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Wang L. Ph.D. Thesis. Chinese Academy of Medical Sciences; Peking Union Medical College, Beijing, China: 2013. Quality Study on Fritillariae Cirrhosae Bulbus. [Google Scholar]
- 21.Li Y., Zhang L., Wu H., Wu X., Ju L., Zhang Y. Metabolomic study to discriminate the di erent Bulbus Fritillariae species using rapid resolution liquid chromatography-quadrupole time-of- ight mass spectrometry coupled with multivariate statistical analysis. Anal. Methods. 2014;6:2247–2259. doi: 10.1039/c3ay41928b. [DOI] [Google Scholar]
- 22.Yan L., Abulimiti Y., Jun L., Aziz M., Haji A.A. New isosteroidal alkaloids with tracheal relaxant effect from the bulbs of Fritillaria pallidiflora Schrenk. Bioorg. Med. Chem. Lett. 2016;26:1983–1987. doi: 10.1016/j.bmcl.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y., Dai J., Xiao W., Zhao L. Isolation and purification of peimisine from Fritillaria taipaiensis bulbs by High-speed Counter-current Chromatography. Chem. Ind. For. Prod. 2015;35:86–90. [Google Scholar]
- 24.Liang J., Cao X., Jian W., Ren H., Wu S. Analysis of volatile components of flowers of Fritillaria thunbergii by GC-TOF-MS. Zhongguo Zhong Yao Za Zhi. 2011;36:2689–2692. (In Chinese) [PubMed] [Google Scholar]
- 25.Zhang L., Sun J., Wen Q., Ma Y., Feng L. LC-MSn Analysis of the components in bulbus Fritilariae Ussuriensis and the urine of rats after oral administered bulbus Fritillariae Ussuriensis. Chin. J. Pharmacovigil. 2014;11:203–205. [Google Scholar]
- 26.Shang Y., Li S., Xiao L. Review on extraction technology of alkaloids from plants. Mod. Chem. Ind. 2002;22:51–54. [Google Scholar]
- 27.Lu A., Wu H. Research progress of nucleosides in traditional Chinese Medicine. Chin. J. Inf. Tradit. Chin. Med. 2006;13:94. [Google Scholar]
- 28.China Pharmacopoeia Committee . Part 3. Pharmacopoeia of the People’s Republic of China. China Chemical Industry Press; Beijing, China: 2015. pp. 213–214. [Google Scholar]
- 29.Zhou J.L., Xin G.Z., Shi Z.Q., Ren M.T., Qi L.W., Li H.J., Li P. Characterization and identification of steroidal alkaloids in Fritillaria species using liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A. 2010;1217:7109–7122. doi: 10.1016/j.chroma.2010.09.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.