Abstract
Pharmacologic efforts to improve outcomes following aneurysmal subarachnoid hemorrhage (aSAH) remain disappointing, likely owing to the complex nature of post-hemorrhage brain injury. Previous work suggests that heparin, due to the multimodal nature of its actions, reduces the incidence of clinical vasospasm and delayed cerebral ischemia that accompany the disease. This narrative review examines how heparin may mitigate the non-vasospastic pathological aspects of aSAH, particularly those related to neuroinflammation. Following a brief review of early brain injury in aSAH and heparin’s general pharmacology, we discuss potential mechanistic roles of heparin therapy in treating post-aSAH inflammatory injury. These roles include reducing ischemia-reperfusion injury, preventing leukocyte extravasation, modulating phagocyte activation, countering oxidative stress, and correcting blood-brain barrier dysfunction. Following a discussion of evidence to support these mechanistic roles, we provide a brief discussion of potential complications of heparin usage in aSAH. Our review suggests that heparin’s use in aSAH is not only safe, but effectively addresses a number of pathologies initiated by aSAH.
Keywords: heparin, enoxaparin, subarachnoid hemorrhage, edema, brain injury, inflammation
1. Introduction
Despite decades of research, aneurysmal subarachnoid hemorrhage (aSAH) significantly compromises quality of life in those patients who survive their initial hemorrhage. Half of all surviving patients demonstrate some significant deficit of language, memory, or executive function [1,2,3,4], with only a third of survivors ultimately returning to work [5]. Consistent with these clinical findings, radiographic evaluation of survivors of aSAH demonstrates significant degrees of brain atrophy and other signs of global brain injury [6,7]. Although infarction due to vasospasm undoubtedly explains the acquisition of new focal deficits following aSAH, vasospasm-centered theories do not compellingly account for this global brain injury, especially given that effective pharmacologic prevention of vasospasm fails to improve outcomes following aSAH [8].
Modern understandings of post-SAH brain injury recognize the importance of other pathophysiological processes [9,10] emphasizing inflammation in particular as a central component of post-SAH brain injury [11]. SAH generates a wide variety of hemoglobin breakdown products capable of wide dissemination via the CSF, leading to global inflammation via activation of receptors such as toll-like receptor 4 [12,13], attraction of inflammatory phagocytes [14], and creation of a pro-oxidative environment via depletion of anti-oxidants [15]. Several clinical studies link this systemic inflammatory response to brain injury and poor outcome following aSAH [7,16,17,18,19]. Therefore, mitigating this inflammatory response presents an attractive therapeutic approach to improving outcomes following aSAH. Although specific inhibition of pro-inflammatory regulators, such as cytokines, represents a valid therapeutic strategy [20], aSAH activates multiple inflammatory pathways, suggesting that blockade of any single upstream inflammatory initiator may not adequately curb the downstream inflammatory response.
Multiple lines of clinical and pre-clinical evidence suggest that the endogenous anti-coagulant heparin effectively reduces inflammation and improves outcomes following aSAH [21,22,23,24]. Perhaps because of its clinical use as an anti-coagulant and its widely studied interactions with the coagulation cascade, clinicians remain relatively ignorant heparin’s promiscuous interaction with a number of biological processes, especially those involved with inflammation. Although this absence of specificity typically hinders the application of drugs like heparin as therapeutic agents, these pleiotropic effects may prove advantageous in the complex inflammatory milieu accompanying aSAH. This review seeks to summarize the potential benefits of heparin therapy in the context of aSAH, with a particular emphasis on its anti-inflammatory properties.
2. Early Brain Injury, Inflammation, and Blood-Brain Barrier Failure in Aneurysmal Subarachnoid Hemorrhage
Aneurysm rupture initiates two separate pathologic events that contribute to global brain injury. The initial rupture rapidly raises intracranial pressure, leading to cerebral hypoperfusion, possible cerebral circulatory arrest, and global brain injury analogous to that of cardiac arrest [25]. The second occurs gradually in the days following hemorrhage as the subarachnoid blood clot breaks down, releasing a variety of hemoglobin and coagulation by-products into the CSF, where they undergo global distribution. Although these events negatively affect nearly every aspect of the CNS, they have a number of specific consequences for the brain, of which vasospasm represents only a single component.
SAH induces a significant degree of CNS inflammation, both from activation of the brain’s endogenous microglia as well as influx of circulating leukocytes [26,27,28,29,30]. Inflammatory activation of these cells has a number of deleterious effects. Activated microglia mediate the significant neuronal apoptosis observed following experimental SAH [26]. Brain invasion by circulating neutrophils induces microvascular dysfunction [31], endothelial injury [32], and memory deficits via NMDA receptor dysfunction [29]. Perivascular invasion by leukocytes plays a role in vasospasm, as blockade of invasion via monoclonal antibody prevents vasospasm in both rabbits [33] and monkeys [34]. Generation of reactive species such as peroxynitrite via phagocyte-derived inducible nitric oxide synthase contributes to oxidative brain injury following aSAH [35]. This inflammatory state following aSAH mediates brain injury over several weeks [36]. Despite its clear role in brain injury following aSAH, this inflammatory response is not entirely detrimental. Both endogenous and circulating phagocytes mediate hemoglobin clearance following hemorrhage [37,38]. Activated microglia and their inflammatory cytokines also promote neurogenesis in non-aSAH models via activation of the brain’s stem cell compartment [39,40], a relevant finding given evidence of increased neurogenesis following human aSAH [41]. However, despite these potentially beneficial effects, the excess inflammation that follows aSAH causes more harm than benefit, especially in the early post-ictal period.
Besides directly modulating the CNS microenvironment, aSAH induces failure of the blood brain barrier with consequent vasogenic edema formation. Diverse mechanisms underlie this phenomenon, as ischemia-hypoperfusion injury and hemorrhagic products within the subarachnoid space both contribute to blood-brain barrier (BBB) dysfunction following aSAH [42,43,44]. Besides brain swelling due to vasogenic edema [45], BBB dysfunction contributes to several other pathologic processes. Perfusion studies in patients with delayed cerebral ischemia following aSAH demonstrate abnormal permeability of the BBB prior to actual infarction [46]. Experimental studies link the loss of white matter integrity to BBB disruption following SAH [47]. Finally, the BBB represents a crucial gateway for inflammatory cell infiltration of the brain [48]; loss of barrier integrity could augment this already significant neuroinflammatory response to aSAH.
3. Heparin—Physiologic and Pharmacologic Roles
The heparins refer to a class of endogenous glycosaminoglycans originally isolated from canine liver cells during investigations of endogenous coagulants (hence, the Greek root hepar meaning “liver”) [49]. Heparin molecules consist of linear polymeric chains of heavily sulfated polysaccharide chains. This high degree of sulfation imparts the highest negative charge density of any known biological macromolecule, allowing heparin to interact with a large number of proteins [50]. Given its context of discovery as well as its utility as an anticoagulant, traditional understandings of heparin’s pharmacologic actions situate it within the clotting cascade. Within this understanding, heparin’s primary role is to bind antithrombin III, inducing an allosteric activation of the latter that allows antithrombin III to inhibit the clotting Factor Xa, a phenomenon relatively independent of the size of the heparin molecule [51]. By virtue of heparin’s large size and high negative charge, the heparin-antithrombin III complex may also bind thrombin and inactivate thrombin, although this action crucially depends on sufficient chain length to bind both antithrombin III and thrombin. Low molecular weight heparins exploit this latter property to inhibit Factor Xa without appreciable anti-thrombin activity. Of note, the anti-coagulant properties of heparin depend on its negative charge to allow for both thrombin binding and allosteric activation of antithrombin III; stripping heparin molecules of their negatively charged sulfate moieties reduces heparin’s function as an anticoagulant [52].
Despite the prominence of anti-coagulation in heparin’s clinical applications, several lines of evidence suggest that anti-coagulation is not heparin’s primary physiologic role. Despite its initial discovery in mammals, heparin-like molecules exist in a wide variety of species lacking a formal hematologic system, including arthropods and echinoderms [53]. Furthermore, within mammals, mast cells and basophils represent the most abundant histologic source of heparin [54]; the importance of these cells in allergic and anti-helminthic response would seem to situate heparin within the broader schema of type 2 immune responses [55]. Finally, besides well-characterized interactions with antithrombin III, heparin promiscuously interacts with a variety of inflammatory proteins and chemokines [56]. While heparin’s anticoagulant properties undoubtedly play a role in inflammatory physiology, a simple understanding of heparin as anticoagulant neglects heparin’s broader role as a modulator of inflammatory function.
Heparin and its derivative molecules possess significant anti-inflammatory activity [57]. Heparin and heparinoids exert their anti-inflammatory effects through a wide variety of mechanisms. At the level of signal transduction, they reduce LPS-induced nuclear translocation of NF-κβ and the associated inflammatory response both in vitro and in vivo, although mechanistic explanations of this phenomenon are lacking [58,59]. Heparin molecules bind and inhibit cellular adhesion molecules such as the selectins [60,61], thereby preventing lymphocyte homing and extravasation. Heparin binds with high affinity to a number of inflammatory cytokines including IL-12 [62] and IL-2 [63], potentially sequestering them. Heparin prevents activation of the effector cells of inflammation, such as neutrophils and macrophages [64,65]. Finally, heparin directly inhibits molecular mediators of inflammation, such as elastase [66] and major basic protein [67]. Compellingly, the anti-inflammatory actions of heparin may be independent of its anti-coagulant activity [68]. Given its potent role as an anti-inflammatory agent, several clinical trials have evaluated heparin and its derivatives in diseases of inappropriate inflammation, including sepsis [69,70], and asthma [71].
Heparin’s main clinical roles in the management of aSAH, prevention of VTE and systemic heparinization during endovascular treatment of aneurysms, exploit its anticoagulant properties. However, the broader role of heparin outside the coagulation cascade argues for a broader utility in aneurysmal SAH. Several clinical studies already suggest that heparin and its low molecular weight derivative enoxaparin reduce the incidence of clinical vasospasm and delayed cerebral infarction following aneurysmal SAH [21,24,72], although the literature is not entirely consistent in this result [73]; a previous review already discussed the role of heparin in the prevention of delayed cerebral ischemia following aneurysmal SAH [74]. Beyond vasospasm, however, heparin may improve outcomes in aSAH by preventing inflammation and restoring blood-brain barrier integrity. Given the increasing recognition of inflammation, edema, and blood-brain barrier dysfunction as mediators of poor outcome following aSAH, these extravascular effects of heparin represent an even more important aspect of its therapeutic efficacy than the prevention of vasospasm.
4. Heparin in Post-SAH Brain Injury
4.1. Ischemia-Reperfusion Injury
A hallmark of aneurysm rupture is a transient period of hypoperfusion or even frank intracranial circulatory arrest secondary to the acute rise in intracranial pressure at ictus. Although other factors, such as CSF dissemination of blood products, undoubtedly play a role in secondary brain injury, the ischemia-reperfusion injury at ictus is arguably the predominant injury following aSAH, as its clinical correlate, loss of consciousness at ictus, remains one of the best predictors of outcome following aSAH [75,76]. Multiple studies demonstrate the efficacy of heparin and its derivatives in ischemia-reperfusion injury. Several studies in rats demonstrate reduced infarct volume following heparin and heparin-derivative administration following transient cerebral arterial occlusion [77,78,79,80,81]. Both the efficacy of non-anticoagulating 2,3-O-desulfated heparin derivative in vivo [79] as well as the neuroprotection low molecular weight heparin affords against ischemia-reperfusion injury in vitro [82] suggest that heparin and its derivatives exert these effects independently of their actions on the coagulation cascade. Proposed modes of action include neutralization of oxidative species [83] and inhibition of neuronal apoptosis [84], although neither of these studies provide definitive evidence of these modes of action.
4.2. Leukocyte Extravasation
Mobilization of leukocytes from the periphery into inflamed tissue (leukocyte extravasation) is a crucial component of inflammation generally and post-SAH neuroinflammation specifically [85]. Administration of low dose heparin following experimental SAH reduces the number of inflammatory cells within the CNS [22]. Although studies of heparin in SAH do not address leukocyte extravasation explicitly, several animal models of neuroinflammatory injury suggest that heparins directly inhibit this process. Administration of unfractionated heparin following murine TBI reduces leukocyte extravasation as demonstrated by in vivo microscopy [86]. Administration of low molecular weight heparin yields similar results [87]. Compellingly, an in vivo microscopy study of experimental meningitis also demonstrates a reduction in leukocyte extravasation with heparin administration, a phenomenon attributable to both a reduction in leukocyte sticking and leukocyte rolling to the endothelium [88]. These findings of reduced leukocyte adhesion and leukocyte extravasation following heparin administration in disparate neuroinflammatory conditions suggests a common mechanism of action. Although the movement of leukocytes from the vascular to the parenchymal compartment is a complex process, adhesion of leukocytes to the vessel wall critically depends on interactions between cell surface glycoproteins and selectins, surface lectins expressed by both leukocytes (L-selectin) and endothelial cells (E-selectin) [89]. Given current knowledge of heparin’s effects on endothelium-leukocyte interactions, inhibition of leukocyte expressed selectin [90] provides a plausible candidate mechanism to explain its effects on leukocyte extravasation. Several studies demonstrate that heparin-mediated inhibition of selectins occurs independently of anti-coagulation [60,91]. Consistent with these observations, studies of heparin in experimental TBI [86] note equivalent efficacy of low, non-anticoagulating heparin doses compared to higher doses with regard to leukocyte extravasation.
4.3. Inflammatory Activation
Although heparin’s effects on leukocyte extravasation mediate some aspects of its anti-inflammatory processes, heparin may also reduce injury by modulating inflammatory cell activation. A key effector mechanism in the pathological neuroinflammatory response following SAH is phagocyte activation, resulting in production of direct mediators of pathology such as peroxynitrite [35] and matrix metalloproteinase-9 [47]. Given that heparin’s effects on leukocyte extravasation do not readily account for the reduction in endogenous microglial activation observed with heparinization following SAH [22], heparin’s effects on activation of inflammatory cells appear relevant to SAH. Given heparin’s wide array of interactions with inflammatory mediators, no single mechanism of heparin induced immunoquiescence likely accounts for its full pleiotropic effects. Nevertheless, several interesting candidate mechanisms bear further discussion.
The receptor for advanced glycation end-products (RAGE) expressed on phagocytes mediates a number of inflammatory effects via activation of NF-κB [92]; SAH induces RAGE expression throughout the cortex by both neurons and microglia [93]. Furthermore, SAH induces the expression and release of a number of RAGE ligands, including HMGB1 [93,94,95,96,97,98] and S100B [99,100,101,102], within the CNS. Consistent with its inflammatory role, inhibition of RAGE signaling reduces inflammation and improves functional outcomes following experimental SAH [103,104,105]. Heparins, including low molecular weight heparins and non-anticoagulating derivatives such as 2,3-O-desulfated heparin, inhibit interactions between RAGE and its ligands with a high degree of specificity [65,106,107,108,109,110]. Although not well studied in SAH, pharmacologic blockade of HMGB1 duplicates some of the beneficial effects of enoxaparin following experimental TBI, including reduction of leukocyte extravasation, suggesting that heparin mediates some of its effects via modulating RAGE’s interactions with its ligands [111]. Blockade of RAGE inhibition may account for some of heparin’s effects on inflammatory cell activation.
Another potential mechanism of inflammatory modulation focuses on macrophage polarization. Although overly simplistic, macrophages and microglia roughly partition into two distinct phenotypes [112]. The M1 phenotype promotes anti-cellular immune responses important to viral and tumor defense. Consistent with this function, M1 phagocytes mediate a variety of biological effects relevant to post-SAH brain injury including peroxynitrite production via upregulation of inducible nitric oxide synthase and production of inflammatory cytokines including IL-1β, processes that contribute to early brain injury following SAH [35,113]. M2 phagocytes, in contrast, have opposite effects, reducing nitric oxide via arginine consumption and producing anti-inflammatory cytokines. Modulation of phagocyte polarization from a M1 to a M2 phenotype appears protective in SAH [114]. As mentioned previously, mast cells and basophils are the predominant sources of physiologic heparin [115]. This association strongly suggests that heparin plays a role in allergic and anti-helminthic responses. A common murine marker of M2 phenotype, the Ym1 receptor appears necessary for generation of a Type II immune response [116,117]. Compellingly, heparin appears to be a physiologic ligand of Ym1 [118]. Although direct evaluations of heparin’s physiologic effects on phagocyte polarization are lacking, a study of oral enoxaparin in experimental ulcerative colitis demonstrates modulation of colonic macrophages from an M1 to an M2 phenotype with enoxaparin administration [119], a finding consistent with this hypothesized physiologic role. Further studies are required, however, to establish whether heparin modulates phagocyte polarization in aSAH.
4.4. Oxidative Stress
Production of reactive oxidative species (ROS) by activated phagocytes mediates significant injury following SAH [35]. Strategies to reduce oxidative stress following experimental SAH demonstrate numerous beneficial effects [120,121,122,123]. Heparin demonstrates a number of anti-oxidant effects. Extracellular superoxide dismutase (EC-SOD), a powerful anti-oxidant enzyme, demonstrates specific heparin binding [124,125]. From a physiologic standpoint, binding of EC-SOD to cell surface heparan sulfate sequesters EC-SOD to the cell surface, as mutations in the heparin-binding domain of SOD or administration of IV heparin results in release of EC-SOD into circulating fluids [126,127]. Aside from increasing circulating levels of EC-SOD, heparin also appears to induce its synthesis [128]. This release of EC-SOD into circulating fluids increases its activity, as heparin administration into the CSF of rabbits overexpressing EC-SOD results in a 27-fold increase in SOD activity within the CSF [129]. Although not studied in subarachnoid hemorrhage, systemic administration of enoxaparin enhances brain SOD activity in experimental cerebral ischemia-reperfusion injury [83], confirming the relevance of this phenomenon to intracranial pathology. Thioredoxin reductase, another enzyme thought protective in oxidative brain injury [130,131], also demonstrates high affinity binding to heparin [132], although the significance of this interaction is unclear. Several in vitro studies demonstrate direct antioxidant actions of the heparin molecule itself independent of any associated co-enzyme [133,134]. Taken together, these findings suggest that heparin may ameliorate the oxidative injury generated following aSAH.
4.5. Blood-Brain Barrier Dysfunction and Vasogenic Edema
Edema formation following SAH portends a poor prognosis [42]. Blood-brain barrier dysfunction and its associated vasogenic edema therefore present highly plausible therapeutic targets in aSAH. Multiple lines of evidence, in both aSAH and other injuries, suggest that heparin reduces blood-brain barrier dysfunction and its associated vasogenic edema. In a murine endovascular perforation model of SAH, pretreatment with low dose heparin reduced edema formation at 24 h following injury, with improved early behavioral outcome [23]. Heparin administration in other forms of experimental brain injury reduces blood-brain barrier dysfunction and edema formation in models as diverse as TBI [86,87,111,135], ischemic stroke [78,136], intracerebral hemorrhage [137,138], and meningitis [88]. A human study of TBI patients corroborates these findings, demonstrating more rapid resolution of pathologic CT imaging features, especially edema, with early enoxaparin administration [139].
Given the intimate association between inflammation and edema, heparin’s anti-edema effects most likely derive from its anti-inflammatory properties. However, heparin demonstrates significant interaction with two molecules relevant to edema formation following aSAH, vascular endothelial growth factor (VEGF) and bradykinin (BK) [42]. VEGF, an angiogenic protein initially identified as a vascular permeability factor, binds heparin [140]. The effects of heparin on VEGF-endothelial cell interactions are complex. At low concentrations, heparin appears to enhance binding of VEGF to its receptor; however, at progressively higher concentrations heparin inhibits VEGF binding [141]. This inhibitory effect is sensitive to both the sulfation [141] and the size of heparin molecules employed [142]. Given the role of VEGF in SAH-mediated edema specifically and brain edema generally, heparin inhibition of VEGF presents a plausible albeit uninvestigated mode of edema reduction. Like VEGF, the interactions between heparin and bradykinin appear complex. While mast cell-derived heparin is crucial to both bradykinin formation and vascular permeability in allergic conditions [143], other studies suggest that intravenous heparin inhibits bradykinin induced vascular permeability [144]. Furthermore, bradykinin-mediated edema in aSAH occurs very early following hemorrhage [145]. Taken together, these findings suggests that heparin’s interactions with bradykinin may not be relevant to its effects on edema.
5. Complications of Heparin Therapy in aSAH
5.1. Heparin Induced Thrombocytopenia (HIT)
Heparin-induced thrombocytopenia is arguably the most feared heparin related complication. An autoimmune phenomenon, HIT results from antibody formation to the heparin-platelet factor 4 complex, resulting in both platelet depletion and a paradoxical pro-thrombotic state with significant risk of both arterial and venous thromboembolic complications [146]. Although studies suggest that 3 to 5 percent of patients undergoing intravenous unfractionated heparin will develop HIT, the incidence of symptomatic HIT following SAH may be much higher, with a reported incidence between 5% and 15% [147,148]. Although general studies associate LMWH with a reduced risk of symptomatic HIT, the one comparison available to date finds no difference in rates of HIT between LMWH and UFH in SA [148]. Despite its associated thrombocytopenia, ischemic complications represent a particular concern with symptomatic HIT. Several large series identify increased risk of stroke, death, and poor outcome in SAH patients with HIT [147,149]. Risk factors identified for HIT in a multivariate analysis of patients with aSAH included female gender, clip treatment of aneurysm, and greater number of vasospasm treatments [149]. This last association may reflect either increased risk of HIT due to use of catheter heparinization in the treatment of vasospasm or, alternatively, an increased risk of cerebral ischemia due to a HIT-induced pro-thrombotic state. In our own reported series of low dose infusion for aneurysmal SAH, none of the forty-three treated patients developed symptomatic HIT [21], despite use of clip treatment and prolonged (fourteen day) heparin exposure. This lower than expected incidence could reflect the use of a low dose (12 U/kg/h), reduced auto-antibody production due to heparin’s anti-inflammatory properties, a reduced incidence of vasospasm, or an inadequate number of subjects to assess HIT in this population. Since publication of this initial experience, two incidences of symptomatic HIT (2/150 patients) have developed in patients undergoing low dose heparin infusion, requiring cessation of their heparin infusion. Both patients required multiple interventions for vasospasm, with one experiencing a good recovery; the other died secondary to cerebral infarction. Although the low incidence of HIT in this population and excellent preliminary results of patients treated with low dose heparin outweigh this low risk of HIT, the apparently increased risk of cerebral ischemia from HIT in aSAH mandates vigilant screening for this condition.
5.2. Hemorrhagic Complications
Aside from HIT, fear of bleeding forms the other principal concern with heparin administration following aSAH. Patients with aSAH experience increased brain hemorrhage risk due to a variety of circumstances: aneurysm re-rupture prior to definitive treatment, periprocedural bleeding from surgery or a ventriculostomy, or hemorrhage into infarcted brain tissue. Although some experiences with systemic heparinization during endovascular treatment of ruptured aneurysms suggests that this practice does not increase the risk of hemorrhage or aneurysm re-bleeding [150,151], others suggest a significant risk of brain hemorrhage with this practice [152]. Ventriculostomy (EVD) placement followed by systemic heparinization for aSAH appears to be relatively safe, with multiple studies finding a low risk of significant EVD hemorrhage with systemic heparinization [151,153,154]. However, heparinization does appear to increase the risk of minor EVD hemorrhage [154]. In most reports of heparin or its derivatives as a therapeutic modality for vasospasm, hemorrhagic complications are either rare or non-existent [21,24,72], although one study noted an increased risk of non-significant bleeding in patients receiving enoxaparin compared to controls [73]. This low reported risk of hemorrhage is especially striking given the early (less than 12 h) post-operative initiation of heparin in at least one of these studies study [21]. The relatively low rate of hemorrhagic complications in these therapeutic studies of heparin most likely derive from a combination of low, non-anticoagulating doses of heparin as well as the relative safety of heparin use even following procedural interventions. Thus, the available evidence generally does not support the fears of hemorrhagic complications with heparinization following aneurysmal SAH.
6. Heparin Derivatives
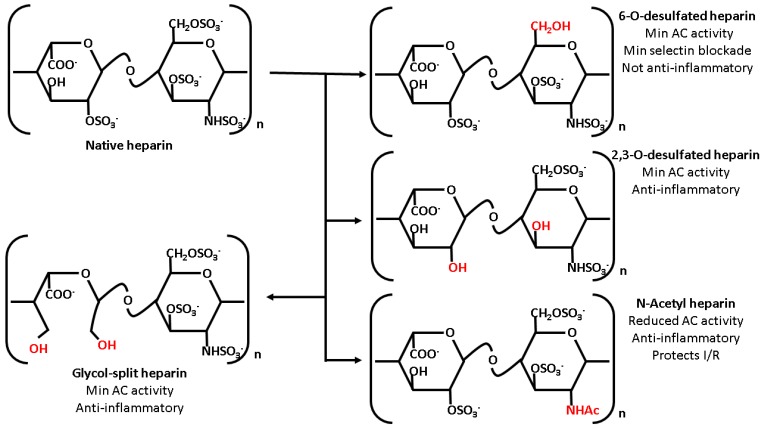
Although unfractionated heparin is perhaps the best studied member of the heparin family in the context of aSAH, its theoretical associated risk of hemorrhagic complications might preclude its use in certain patients, such as those with difficult to secure aneurysms. However, as briefly discussed earlier, heparin’s interactions with other molecular partners depends crucially on both the size and sulfation of the heparin molecule; modification of either of these parameters has significant effects on heparin’s pharmacologic effects. As previously discussed, the unfractionated heparin used clinically consists of polysaccharide polymers of significantly variable length. Cleavage of these polymers using either chemical or enzymatic digestion yields the low-molecular weight heparins. Low molecular weight heparins such as enoxaparin provide several advantages over standard unfractionated heparin, including more predictable clinical dosing and relatively specific anti-factor Xa activity [51]. Aside from digestion of unfractionated heparin, chemical synthesis of heparin’s anti-thrombin binding pentasaccharide motif yields fondaparinux, a potent anti-coagulant with highly specific inhibition of factor Xa without significant risk of heparin induced thrombocytopenia [155]. Aside from modification of polymer length, several other strategies change heparin’s pharmacological effects via chemical modification. Due to its high degree of sulfation, heparin possesses a significant high negative charge density, allowing for ionic interactions with a wide variety of molecular partners, including anti-thrombin III. Chemical desulfation reduces heparin’s anticoagulant properties while retaining its interactions with other molecular pathways. 2,3-O-desulfated heparin, prepared via cold alkaline hydrolysis of native heparin, demonstrates markedly reduced anti-thrombin III binding with a concurrent reduction in anti-coagulant activity while still retaining native heparin’s desirable anti-inflammatory effects such as selectin binding, RAGE blockade, and protease inhibition [108]. Replacement of the N-sulfate group with an acetyl side chain similarly reduces anticoagulant activity without compromising anti-inflammatory and anti-protease effects [156,157]. Although 6-O-desulfated heparin demonstrates reduced anti-coagulant activity with retained blockade of inflammatory receptors such as RAGE, loss of the 6-O-sulfate moiety also results in decreased selectin inhibition with compromised anti-inflammatory activity [158]. Finally, periodate cleavage of the 2,3 vicinal diols in uronate residues yield so-called glycol-split heparins, a class of heparin with reduced anticoagulant activity despite retained sulfate moieties. These glycol-split heparins demonstrate reduced anticoagulant activity while retaining useful features of native heparin, such as elastase inhibition and cytokine binding [157]. Although a full review of chemically modified heparinoids lies well-beyond the scope of this article, several of these molecules, especially 2,3-O-desulfated heparin, demonstrate a desirable blend of anti-inflammatory features with reduced anti-coagulant activity that might prove useful in aneurysmal SAH. Figure 1 depicts a summary of these modified heparin species along with salient characteristics.
Figure 1.
Summary of heparin chemical derivatives. Chemical modification of unfractionated heparin modulates its pharmacology. Although all derivatives discussed demonstrate reduced anticoagulant activity, chemical modification also affects heparin’s other pharmacologic properties in a regiospecific manner. 2,3-O-desulfated heparin demonstrates nearly retained selecting and RAGE inhibition; 6-O-desulfated heparin, despite numerous sulfated residues, fails to bind selectins and does not demonstrate significant anti-inflammatory effects in vivo. Although less well studied in inflammation, N-acetyl heparin does demonstrate evidence of efficacy in ischemia-reperfusion (I/R) injury despite reduced anticoagulant activity. Finally, glycol split heparin retains the ability to inhibit proteases such as elastase despite reduced anti-coagulant activity.
7. Conclusions
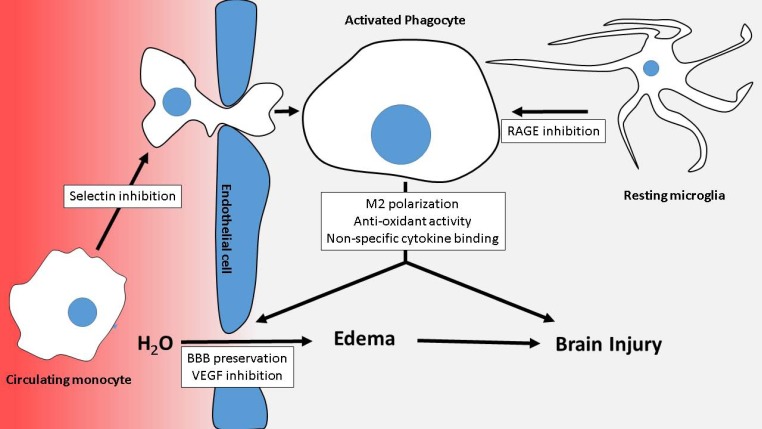
Given the complexity of brain injury following aneurysmal subarachnoid hemorrhage, no single therapeutic modality is likely to address all aspects relevant to its treatment. Given the multimodal nature of heparin’s interactions with mediators of post-SAH brain injury, as summarized in the accompanying figure (Figure 2), heparin and heparin derivatives may form a significantly better class of therapeutic for aSAH than more well-defined pharmacologic agents. Although initial clinical and experimental work suggests the efficacy of heparin in aSAH, these studies are relatively preliminary, with further clinical work required to confirm the utility of heparin. Furthermore, the absence of clear mechanistic insight into heparin’s therapeutic effects mandates further experimental work, as a better understanding of heparin’s mechanism of action could lead to improved and safer heparin derivatives. Nevertheless, heparin for aSAH represents a promising way forward in a disease much in need of effective therapy.
Figure 2.
Summary of heparin’s modes of action. Several confluent processes combine to injure the brain including extravasation of circulating cells into the brain parenchyma, activation of microglia, production of harmful molecules and cytokines by activated phagocytes, and blood-brain barrier breakdown with subsequent vasogenic edema formation. Heparin antagonizes these processes, with heparin’s mechanisms of action indicated in the white boxes.
Acknowledgments
This work was supported by grants to JMS from the National Institute of Neurological Disorders and Stroke (NS060801; NS061808) and the National Heart, Lung and Blood Institute (HL082517).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Ogden J.A., Utley T., Mee E.W. Neurological and psychosocial outcome 4 to 7 years after subarachnoid hemorrhage. Neurosurgery. 1997;41:25–34. doi: 10.1097/00006123-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Hackett M.L., Anderson C.S. Health outcomes 1 year after subarachnoid hemorrhage: An international population-based study. The australian cooperative research on subarachnoid hemorrhage study group. Neurology. 2000;55:658–662. doi: 10.1212/wnl.55.5.658. [DOI] [PubMed] [Google Scholar]
- 3.Kreiter K.T., Copeland D., Bernardini G.L., Bates J.E., Peery S., Claassen J., Du Y.E., Stern Y., Connolly E.S., Mayer S.A. Predictors of cognitive dysfunction after subarachnoid hemorrhage. Stroke. 2002;33:200–208. doi: 10.1161/hs0102.101080. [DOI] [PubMed] [Google Scholar]
- 4.Al-Khindi T., Macdonald R.L., Schweizer T.A. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41:e519–e536. doi: 10.1161/STROKEAHA.110.581975. [DOI] [PubMed] [Google Scholar]
- 5.Haley E.C., Jr. Measuring cognitive outcome after subarachnoid hemorrhage. Ann. Neurol. 2006;60:502–504. doi: 10.1002/ana.21040. [DOI] [PubMed] [Google Scholar]
- 6.Kivisaari R.P., Salonen O., Servo A., Autti T., Hernesniemi J., Ohman J. Mr imaging after aneurysmal subarachnoid hemorrhage and surgery: A long-term follow-up study. AJNR Am. J. Neuroradiol. 2001;22:1143–1148. [PMC free article] [PubMed] [Google Scholar]
- 7.Tam A.K., Kapadia A., Ilodigwe D., Li Z., Schweizer T.A., Macdonald R.L. Impact of global cerebral atrophy on clinical outcome after subarachnoid hemorrhage. J. Neurosurg. 2013;119:198–206. doi: 10.3171/2013.3.JNS121950. [DOI] [PubMed] [Google Scholar]
- 8.Macdonald R.L., Higashida R.T., Keller E., Mayer S.A., Molyneux A., Raabe A., Vajkoczy P., Wanke I., Bach D., Frey A., et al. Randomised trial of clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid hemorrhage undergoing surgical clipping (conscious-2) Acta Neurochir. Suppl. 2013;115:27–31. doi: 10.1007/978-3-7091-1192-5_7. [DOI] [PubMed] [Google Scholar]
- 9.Cossu G., Messerer M., Oddo M., Daniel R.T. To look beyond vasospasm in aneurysmal subarachnoid haemorrhage. Biomed. Res. Int. 2014;2014:628597. doi: 10.1155/2014/628597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowland M.J., Hadjipavlou G., Kelly M., Westbrook J., Pattinson K.T. Delayed cerebral ischaemia after subarachnoid haemorrhage: Looking beyond vasospasm. Br. J. Anaesth. 2012;109:315–329. doi: 10.1093/bja/aes264. [DOI] [PubMed] [Google Scholar]
- 11.Lucke-Wold B.P., Logsdon A.F., Manoranjan B., Turner R.C., McConnell E., Vates G.E., Huber J.D., Rosen C.L., Simard J.M. Aneurysmal subarachnoid hemorrhage and neuroinflammation: A comprehensive review. Int. J. Mol. Sci. 2016;17:497. doi: 10.3390/ijms17040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin S., Yin Q., Zhong Q., Lv F.L., Zhou Y., Li J.Q., Wang J.Z., Su B.Y., Yang Q.W. Heme activates tlr4-mediated inflammatory injury via myd88/trif signaling pathway in intracerebral hemorrhage. J. Neuroinflamm. 2012;9:46. doi: 10.1186/1742-2094-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon M.S., Woo S.K., Kurland D.B., Yoon S.H., Palmer A.F., Banerjee U., Iqbal S., Ivanova S., Gerzanich V., Simard J.M. Methemoglobin is an endogenous toll-like receptor 4 ligand-relevance to subarachnoid hemorrhage. Int. J. Mol. Sci. 2015;16:5028–5046. doi: 10.3390/ijms16035028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallia G.L., Tamargo R.J. Leukocyte-endothelial cell interactions in chronic vasospasm after subarachnoid hemorrhage. Neurol. Res. 2006;28:750–758. doi: 10.1179/016164106X152025. [DOI] [PubMed] [Google Scholar]
- 15.Ayer R.E., Zhang J.H. Oxidative stress in subarachnoid haemorrhage: Significance in acute brain injury and vasospasm. Acta Neurochir. Suppl. 2008;104:33–41. doi: 10.1007/978-3-211-75718-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou S.H., Feske S.K., Atherton J., Konigsberg R.G., De Jager P.L., Du R., Ogilvy C.S., Lo E.H., Ning M. Early elevation of serum tumor necrosis factor-alpha is associated with poor outcome in subarachnoid hemorrhage. J. Investig. Med. 2012;60:1054–1058. doi: 10.2310/JIM.0b013e3182686932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanafy K.A., Morgan Stuart R., Fernandez L., Schmidt J.M., Claassen J., Lee K., Sander Connolly E., Mayer S.A., Badjatia N. Cerebral inflammatory response and predictors of admission clinical grade after aneurysmal subarachnoid hemorrhage. J. Clin. Neurosci. 2010;17:22–25. doi: 10.1016/j.jocn.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon C.J., Hopkins S., Vail A., King A.T., Smith D., Illingworth K.J., Clark S., Rothwell N.J., Tyrrell P.J. Inflammation as a predictor for delayed cerebral ischemia after aneurysmal subarachnoid haemorrhage. J. Neurointerv. Surg. 2013;5:512–517. doi: 10.1136/neurintsurg-2012-010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tam A.K., Ilodigwe D., Mocco J., Mayer S., Kassell N., Ruefenacht D., Schmiedek P., Weidauer S., Pasqualin A., Macdonald R.L. Impact of systemic inflammatory response syndrome on vasospasm, cerebral infarction, and outcome after subarachnoid hemorrhage: Exploratory analysis of conscious-1 database. Neurocrit. Care. 2010;13:182–189. doi: 10.1007/s12028-010-9402-x. [DOI] [PubMed] [Google Scholar]
- 20.Jedrzejowska-Szypulka H., Larysz-Brysz M., Kukla M., Snietura M., Lewin-Kowalik J. Neutralization of interleukin-1beta reduces vasospasm and alters cerebral blood vessel density following experimental subarachnoid hemorrhage in rats. Curr. Neurovasc. Res. 2009;6:95–103. doi: 10.2174/156720209788185669. [DOI] [PubMed] [Google Scholar]
- 21.Simard J.M., Aldrich E.F., Schreibman D., James R.F., Polifka A., Beaty N. Low-dose intravenous heparin infusion in patients with aneurysmal subarachnoid hemorrhage: A preliminary assessment. J. Neurosurg. 2013;119:1611–1619. doi: 10.3171/2013.8.JNS1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simard J.M., Tosun C., Ivanova S., Kurland D.B., Hong C., Radecki L., Gisriel C., Mehta R., Schreibman D., Gerzanich V. Heparin reduces neuroinflammation and transsynaptic neuronal apoptosis in a model of subarachnoid hemorrhage. Transl. Stroke Res. 2012;3:155–165. doi: 10.1007/s12975-012-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altay O., Suzuki H., Hasegawa Y., Sorar M., Chen H., Tang J., Zhang J.H. Effects of low-dose unfractionated heparin pretreatment on early brain injury after subarachnoid hemorrhage in mice. Acta Neurochir. Suppl. 2016;121:127–130. doi: 10.1007/978-3-319-18497-5_22. [DOI] [PubMed] [Google Scholar]
- 24.Bruder M., Won S.Y., Kashefiolasl S., Wagner M., Brawanski N., Dinc N., Seifert V., Konczalla J. Effect of heparin on secondary brain injury in patients with subarachnoid hemorrhage: An additional “h“ therapy in vasospasm treatment. J. Neurointerv. Surg. 2017 doi: 10.1136/neurintsurg-2016-012925. neurintsurg–2016–012925. [DOI] [PubMed] [Google Scholar]
- 25.Grote E., Hassler W. The critical first minutes after subarachnoid hemorrhage. Neurosurgery. 1988;22:654–661. doi: 10.1227/00006123-198804000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Hanafy K.A. The role of microglia and the tlr4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. J. Neuroinflamm. 2013;10:83. doi: 10.1186/1742-2094-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackowski A., Crockard A., Burnstock G., Russell R.R., Kristek F. The time course of intracranial pathophysiological changes following experimental subarachnoid haemorrhage in the rat. J. Cereb. Blood Flow Metab. 1990;10:835–849. doi: 10.1038/jcbfm.1990.140. [DOI] [PubMed] [Google Scholar]
- 28.Moraes L., Grille S., Morelli P., Mila R., Trias N., Brugnini A., Luberas L.N., Biestro A., Lens D. Immune cells subpopulations in cerebrospinal fluid and peripheral blood of patients with aneurysmal subarachnoid hemorrhage. Springerplus. 2015;4:195. doi: 10.1186/s40064-015-0970-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Provencio J.J., Swank V., Lu H., Brunet S., Baltan S., Khapre R.V., Seerapu H., Kokiko-Cochran O.N., Lamb B.T., Ransohoff R.M. Neutrophil depletion after subarachnoid hemorrhage improves memory via nmda receptors. Brain Behav. Immun. 2016;54:233–242. doi: 10.1016/j.bbi.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Provencio J.J., Fu X., Siu A., Rasmussen P.A., Hazen S.L., Ransohoff R.M. Csf neutrophils are implicated in the development of vasospasm in subarachnoid hemorrhage. Neurocrit. Care. 2010;12:244–251. doi: 10.1007/s12028-009-9308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H., Testai F.D., Valyi-Nagy T., M N.P., Zhai F., Nanegrungsunk D., Paisansathan C., Pelligrino D.A. vap-1 blockade prevents subarachnoid hemorrhage-associated cerebrovascular dilating dysfunction via repression of a neutrophil recruitment-related mechanism. Brain Res. 2015;1603:141–149. doi: 10.1016/j.brainres.2015.01.047. [DOI] [PubMed] [Google Scholar]
- 32.Satoh S., Yamamoto Y., Toshima Y., Ikegaki I.I., Asano T., Suzuki Y., Shibuya M. Fasudil, a protein kinase inhibitor, prevents the development of endothelial injury and neutrophil infiltration in a two-haemorrhage canine subarachnoid model. J. Clin. Neurosci. 1999;6:394–399. doi: 10.1016/S0967-5868(99)90034-6. [DOI] [PubMed] [Google Scholar]
- 33.Pradilla G., Wang P.P., Legnani F.G., Ogata L., Dietsch G.N., Tamargo R.J. Prevention of vasospasm by anti-cd11/cd18 monoclonal antibody therapy following subarachnoid hemorrhage in rabbits. J. Neurosurg. 2004;101:88–92. doi: 10.3171/jns.2004.101.1.0088. [DOI] [PubMed] [Google Scholar]
- 34.Clatterbuck R.E., Gailloud P., Ogata L., Gebremariam A., Dietsch G.N., Murphy K.J., Tamargo R.J. Prevention of cerebral vasospasm by a humanized anti-cd11/cd18 monoclonal antibody administered after experimental subarachnoid hemorrhage in nonhuman primates. J. Neurosurg. 2003;99:376–382. doi: 10.3171/jns.2003.99.2.0376. [DOI] [PubMed] [Google Scholar]
- 35.Iqbal S., Hayman E.G., Hong C., Stokum J.A., Kurland D.B., Gerzanich V., Simard J.M. Inducible nitric oxide synthase (nos-2) in subarachnoid hemorrhage: Regulatory mechanisms and therapeutic implications. Brain Circ. 2016;2:8–19. doi: 10.4103/2394-8108.178541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kooijman E., Nijboer C.H., van Velthoven C.T., Mol W., Dijkhuizen R.M., Kesecioglu J., Heijnen C.J. Long-term functional consequences and ongoing cerebral inflammation after subarachnoid hemorrhage in the rat. PLoS ONE. 2014;9:e90584. doi: 10.1371/journal.pone.0090584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schallner N., Pandit R., LeBlanc R., Thomas A.J., Ogilvy C.S., Zuckerbraun B.S., Gallo D., Otterbein L.E., Hanafy K.A. Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase-1. J. Clin. Investig. 2015;125:2609–2625. doi: 10.1172/JCI78443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matz P., Turner C., Weinstein P.R., Massa S.M., Panter S.S., Sharp F.R. Heme-oxygenase-1 induction in glia throughout rat brain following experimental subarachnoid hemorrhage. Brain Res. 1996;713:211–222. doi: 10.1016/0006-8993(95)01511-6. [DOI] [PubMed] [Google Scholar]
- 39.Bernardino L., Agasse F., Silva B., Ferreira R., Grade S., Malva J.O. Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells. 2008;26:2361–2371. doi: 10.1634/stemcells.2007-0914. [DOI] [PubMed] [Google Scholar]
- 40.Shigemoto-Mogami Y., Hoshikawa K., Goldman J.E., Sekino Y., Sato K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci. 2014;34:2231–2243. doi: 10.1523/JNEUROSCI.1619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sgubin D., Aztiria E., Perin A., Longatti P., Leanza G. Activation of endogenous neural stem cells in the adult human brain following subarachnoid hemorrhage. J. Neurosci. Res. 2007;85:1647–1655. doi: 10.1002/jnr.21303. [DOI] [PubMed] [Google Scholar]
- 42.Hayman E.G., Wessell A., Gerzanich V., Sheth K.N., Simard J.M. Mechanisms of global cerebral edema formation in aneurysmal subarachnoid hemorrhage. Neurocrit. Care. 2017;26:301–310. doi: 10.1007/s12028-016-0354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simard J.M., Geng Z., Woo S.K., Ivanova S., Tosun C., Melnichenko L., Gerzanich V. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2009;29:317–330. doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Germano A., Avella D., Imperatore C., Caruso G., Tomasello F. Time-course of blood-brain barrier permeability changes after experimental subarachnoid haemorrhage. Acta Neurochir. (Wien) 2000;142:575–580; discussion 580–581. doi: 10.1007/s007010050472. [DOI] [PubMed] [Google Scholar]
- 45.Claassen J., Carhuapoma J.R., Kreiter K.T., Du E.Y., Connolly E.S., Mayer S.A. Global cerebral edema after subarachnoid hemorrhage: Frequency, predictors, and impact on outcome. Stroke. 2002;33:1225–1232. doi: 10.1161/01.STR.0000015624.29071.1F. [DOI] [PubMed] [Google Scholar]
- 46.Ivanidze J., Kesavabhotla K., Kallas O.N., Mir D., Baradaran H., Gupta A., Segal A.Z., Claassen J., Sanelli P.C. Evaluating blood-brain barrier permeability in delayed cerebral infarction after aneurysmal subarachnoid hemorrhage. AJNR Am. J. Neuroradiol. 2015;36:850–854. doi: 10.3174/ajnr.A4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egashira Y., Zhao H., Hua Y., Keep R.F., Xi G. White matter injury after subarachnoid hemorrhage: Role of blood-brain barrier disruption and matrix metalloproteinase-9. Stroke. 2015;46:2909–2915. doi: 10.1161/STROKEAHA.115.010351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carvey P.M., Hendey B., Monahan A.J. The blood-brain barrier in neurodegenerative disease: A rhetorical perspective. J. Neurochem. 2009;111:291–314. doi: 10.1111/j.1471-4159.2009.06319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wardrop D., Keeling D. The story of the discovery of heparin and warfarin. Br. J. Haematol. 2008;141:757–763. doi: 10.1111/j.1365-2141.2008.07119.x. [DOI] [PubMed] [Google Scholar]
- 50.Capila I., Linhardt R.J. Heparin-protein interactions. Angew. Chem. Int. Ed. Engl. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::AID-ANIE390>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 51.Gray E., Mulloy B., Barrowcliffe T.W. Heparin and low-molecular-weight heparin. Thromb. Haemost. 2008;99:807–818. doi: 10.1160/TH08-01-0032. [DOI] [PubMed] [Google Scholar]
- 52.Wei M., Gao Y., Tian M., Li N., Hao S., Zeng X. Selectively desulfated heparin inhibits p-selectin-mediated adhesion of human melanoma cells. Cancer Lett. 2005;229:123–126. doi: 10.1016/j.canlet.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 53.Medeiros G.F., Mendes A., Castro R.A., Bau E.C., Nader H.B., Dietrich C.P. Distribution of sulfated glycosaminoglycans in the animal kingdom: Widespread occurrence of heparin-like compounds in invertebrates. Biochim. Biophys. Acta. 2000;1475:287–294. doi: 10.1016/S0304-4165(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 54.Ronnberg E., Melo F.R., Pejler G. Mast cell proteoglycans. J. Histochem. Cytochem. 2012;60:950–962. doi: 10.1369/0022155412458927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pulendran B., Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pomin V.H., Mulloy B. Current structural biology of the heparin interactome. Curr. Opin. Struct. Biol. 2015;34:17–25. doi: 10.1016/j.sbi.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Lever R., Page C.P. Non-anticoagulant effects of heparin: An overview. Handb. Exp. Pharmacol. 2012:281–305. doi: 10.1007/978-3-642-23056-1_12. [DOI] [PubMed] [Google Scholar]
- 58.Li X., Li Z., Zheng Z., Liu Y., Ma X. Unfractionated heparin ameliorates lipopolysaccharide-induced lung inflammation by downregulating nuclear factor-kappab signaling pathway. Inflammation. 2013;36:1201–1208. doi: 10.1007/s10753-013-9656-5. [DOI] [PubMed] [Google Scholar]
- 59.Li X., Zheng Z., Li X., Ma X. Unfractionated heparin inhibits lipopolysaccharide-induced inflammatory response through blocking p38 mapk and nf-kappab activation on endothelial cell. Cytokine. 2012;60:114–121. doi: 10.1016/j.cyto.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 60.Koenig A., Norgard-Sumnicht K., Linhardt R., Varki A. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J. Clin. Investig. 1998;101:877–889. doi: 10.1172/JCI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stevenson J.L., Choi S.H., Varki A. Differential metastasis inhibition by clinically relevant levels of heparins—Correlation with selectin inhibition, not antithrombotic activity. Clin. Cancer Res. 2005;11:7003–7011. doi: 10.1158/1078-0432.CCR-05-1131. [DOI] [PubMed] [Google Scholar]
- 62.Hasan M., Najjam S., Gordon M.Y., Gibbs R.V., Rider C.C. Il-12 is a heparin-binding cytokine. J. Immunol. 1999;162:1064–1070. [PubMed] [Google Scholar]
- 63.Najjam S., Gibbs R.V., Gordon M.Y., Rider C.C. Characterization of human recombinant interleukin 2 binding to heparin and heparan sulfate using an elisa approach. Cytokine. 1997;9:1013–1022. doi: 10.1006/cyto.1997.0246. [DOI] [PubMed] [Google Scholar]
- 64.Cohen-Mazor M., Mazor R., Kristal B., Kistler E.B., Ziv I., Chezar J., Sela S. Heparin interaction with the primed polymorphonuclear leukocyte cd11b induces apoptosis and prevents cell activation. J. Immunol. Res. 2015;2015:751014. doi: 10.1155/2015/751014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L., Ling Y., Huang M., Yin T., Gou S.M., Zhan N.Y., Xiong J.X., Wu H.S., Yang Z.Y., Wang C.Y. Heparin inhibits the inflammatory response induced by lps and hmgb1 by blocking the binding of hmgb1 to the surface of macrophages. Cytokine. 2015;72:36–42. doi: 10.1016/j.cyto.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 66.Spencer J.L., Stone P.J., Nugent M.A. New insights into the inhibition of human neutrophil elastase by heparin. Biochemistry. 2006;45:9104–9120. doi: 10.1021/bi060338r. [DOI] [PubMed] [Google Scholar]
- 67.Swaminathan G.J., Myszka D.G., Katsamba P.S., Ohnuki L.E., Gleich G.J., Acharya K.R. Eosinophil-granule major basic protein, a c-type lectin, binds heparin. Biochemistry. 2005;44:14152–14158. doi: 10.1021/bi051112b. [DOI] [PubMed] [Google Scholar]
- 68.Shastri M.D., Stewart N., Horne J., Zaidi S.T., Sohal S.S., Peterson G.M., Korner H., Gueven N., Patel R.P. Non-anticoagulant fractions of enoxaparin suppress inflammatory cytokine release from peripheral blood mononuclear cells of allergic asthmatic individuals. PLoS ONE. 2015;10:e0128803. doi: 10.1371/journal.pone.0128803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang C., Chi C., Guo L., Wang X., Guo L., Sun J., Sun B., Liu S., Chang X., Li E. Heparin therapy reduces 28-day mortality in adult severe sepsis patients: A systematic review and meta-analysis. Crit. Care. 2014;18:563. doi: 10.1186/s13054-014-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chande N., MacDonald J.K., Wang J.J., McDonald J.W. Unfractionated or low molecular weight heparin for induction of remission in ulcerative colitis: A cochrane inflammatory bowel disease and functional bowel disorders systematic review of randomized trials. Inflamm. Bowel Dis. 2011;17:1979–1986. doi: 10.1002/ibd.21776. [DOI] [PubMed] [Google Scholar]
- 71.Duong M., Cockcroft D., Boulet L.P., Ahmed T., Iverson H., Atkinson D.C., Stahl E.G., Watson R., Davis B., Milot J., et al. The effect of ivx-0142, a heparin-derived hypersulfated disaccharide, on the allergic airway responses in asthma. Allergy. 2008;63:1195–1201. doi: 10.1111/j.1398-9995.2008.01707.x. [DOI] [PubMed] [Google Scholar]
- 72.Wurm G., Tomancok B., Nussbaumer K., Adelwohrer C., Holl K. Reduction of ischemic sequelae following spontaneous subarachnoid hemorrhage: A double-blind, randomized comparison of enoxaparin versus placebo. Clin. Neurol. Neurosurg. 2004;106:97–103. doi: 10.1016/j.clineuro.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 73.Siironen J., Juvela S., Varis J., Porras M., Poussa K., Ilveskero S., Hernesniemi J., Lassila R. No effect of enoxaparin on outcome of aneurysmal subarachnoid hemorrhage: A randomized, double-blind, placebo-controlled clinical trial. J. Neurosurg. 2003;99:953–959. doi: 10.3171/jns.2003.99.6.0953. [DOI] [PubMed] [Google Scholar]
- 74.Simard J.M., Schreibman D., Aldrich E.F., Stallmeyer B., Le B., James R.F., Beaty N. Unfractionated heparin: Multitargeted therapy for delayed neurological deficits induced by subarachnoid hemorrhage. Neurocrit. Care. 2010;13:439–449. doi: 10.1007/s12028-010-9435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J., Alotaibi N.M., Akbar M.A., Ayling O.G., Ibrahim G.M., Macdonald R.L., Noble A., Molyneux A., Quinn A., Schatlo B., et al. Loss of consciousness at onset of aneurysmal subarachnoid hemorrhage is associated with functional outcomes in good-grade patients. World Neurosurg. 2017;98:308–313. doi: 10.1016/j.wneu.2016.10.099. [DOI] [PubMed] [Google Scholar]
- 76.Suwatcharangkoon S., Meyers E., Falo C., Schmidt J.M., Agarwal S., Claassen J., Mayer S.A. Loss of consciousness at onset of subarachnoid hemorrhage as an important marker of early brain injury. JAMA Neurol. 2016;73:28–35. doi: 10.1001/jamaneurol.2015.3188. [DOI] [PubMed] [Google Scholar]
- 77.Quartermain D., Li Y.S., Jonas S. The low molecular weight heparin enoxaparin reduces infarct size in a rat model of temporary focal ischemia. Cerebrovasc. Dis. 2003;16:346–355. doi: 10.1159/000072556. [DOI] [PubMed] [Google Scholar]
- 78.Li P.A., He Q.P., Siddiqui M.M., Shuaib A. Posttreatment with low molecular weight heparin reduces brain edema and infarct volume in rats subjected to thrombotic middle cerebral artery occlusion. Brain Res. 1998;801:220–223. doi: 10.1016/S0006-8993(98)00559-9. [DOI] [PubMed] [Google Scholar]
- 79.Mocco J., Shelton C.E., Sergot P., Ducruet A.F., Komotar R.J., Otten M.L., Sosunov S.A., Macarthur R.B., Kennedy T.P., Connolly E.S., Jr. O-desulfated heparin improves outcome after rat cerebral ischemia/reperfusion injury. Neurosurgery. 2007;61:1297–1303; discussion 1303–1304. doi: 10.1227/01.neu.0000306109.55174.e6. [DOI] [PubMed] [Google Scholar]
- 80.Smith D.R., Ducker T.B., Kempe L.G. Temporary experimental intracranial vascular occlusion. Effect of massive doses of heparin on brain survival. J. Neurosurg. 1969;30:537–544. doi: 10.3171/jns.1969.30.5.0537. [DOI] [PubMed] [Google Scholar]
- 81.Yanaka K., Spellman S.R., McCarthy J.B., Oegema T.R., Jr., Low W.C., Camarata P.J. Reduction of brain injury using heparin to inhibit leukocyte accumulation in a rat model of transient focal cerebral ischemia. I. Protective mechanism. J. Neurosurg. 1996;85:1102–1107. doi: 10.3171/jns.1996.85.6.1102. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Z.G., Lu T.S., Yuan H.Y. Neuroprotective effects of ultra-low-molecular-weight heparin in vitro and vivo models of ischemic injury. Fundam. Clin. Pharmacol. 2011;25:300–303. doi: 10.1111/j.1472-8206.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Z.G., Zhang Q.Z., Cheng Y.N., Ji S.L., Du G.H. Antagonistic effects of ultra-low-molecular-weight heparin against cerebral ischemia/reperfusion injury in rats. Pharmacol. Res. 2007;56:350–355. doi: 10.1016/j.phrs.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Z.G., Sun X., Zhang Q.Z., Yang H. Neuroprotective effects of ultra-low-molecular-weight heparin on cerebral ischemia/reperfusion injury in rats: Involvement of apoptosis, inflammatory reaction and energy metabolism. Int. J. Mol. Sci. 2013;14:1932–1939. doi: 10.3390/ijms14011932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu H.L., Garcia M., Testai F., Vetri F., Barabanova A., Pelligrino D.A., Paisansathan C. Pharmacologic blockade of vascular adhesion protein-1 lessens neurologic dysfunction in rats subjected to subarachnoid hemorrhage. Brain Res. 2014;1586:83–89. doi: 10.1016/j.brainres.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 86.Nagata K., Kumasaka K., Browne K.D., Li S., St-Pierre J., Cognetti J., Marks J., Johnson V.E., Smith D.H., Pascual J.L. Unfractionated heparin after tbi reduces in vivo cerebrovascular inflammation, brain edema and accelerates cognitive recovery. J. Trauma Acute Care Surg. 2016;81:1088–1094. doi: 10.1097/TA.0000000000001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li S., Marks J.A., Eisenstadt R., Kumasaka K., Samadi D., Johnson V.E., Holena D.N., Allen S.R., Browne K.D., Smith D.H., et al. Enoxaparin ameliorates post-traumatic brain injury edema and neurologic recovery, reducing cerebral leukocyte endothelial interactions and vessel permeability in vivo. J. Trauma Acute Care Surg. 2015;79:78–84. doi: 10.1097/TA.0000000000000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weber J.R., Angstwurm K., Rosenkranz T., Lindauer U., Freyer D., Burger W., Busch C., Einhaupl K.M., Dirnagl U. Heparin inhibits leukocyte rolling in pial vessels and attenuates inflammatory changes in a rat model of experimental bacterial meningitis. J. Cereb. Blood Flow Metab. 1997;17:1221–1229. doi: 10.1097/00004647-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 89.McEver R.P. Selectins: Initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc. Res. 2015;107:331–339. doi: 10.1093/cvr/cvv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nelson R.M., Cecconi O., Roberts W.G., Aruffo A., Linhardt R.J., Bevilacqua M.P. Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood. 1993;82:3253–3258. [PubMed] [Google Scholar]
- 91.Sudha T., Phillips P., Kanaan C., Linhardt R.J., Borsig L., Mousa S.A. Inhibitory effect of non-anticoagulant heparin (s-nach) on pancreatic cancer cell adhesion and metastasis in human umbilical cord vessel segment and in mouse model. Clin. Exp. Metastasis. 2012;29:431–439. doi: 10.1007/s10585-012-9461-9. [DOI] [PubMed] [Google Scholar]
- 92.Byun K., Yoo Y., Son M., Lee J., Jeong G.B., Park Y.M., Salekdeh G.H., Lee B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017 doi: 10.1016/j.pharmthera.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 93.Li H., Wu W., Sun Q., Liu M., Li W., Zhang X.S., Zhou M.L., Hang C.H. Expression and cell distribution of receptor for advanced glycation end-products in the rat cortex following experimental subarachnoid hemorrhage. Brain Res. 2014;1543:315–323. doi: 10.1016/j.brainres.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 94.Zhao X.D., Mao H.Y., Lv J., Lu X.J. Expression of high-mobility group box-1 (hmgb1) in the basilar artery after experimental subarachnoid hemorrhage. J. Clin. Neurosci. 2016;27:161–165. doi: 10.1016/j.jocn.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 95.Chang C.Z., Wu S.C., Kwan A.L., Lin C.L. 4′-O-beta-d-glucosyl-5-O-methylvisamminol, an active ingredient of saposhnikovia divaricata, attenuates high-mobility group box 1 and subarachnoid hemorrhage-induced vasospasm in a rat model. Behav. Brain Funct. 2015;11:28. doi: 10.1186/s12993-015-0074-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Sokol B., Wozniak A., Jankowski R., Jurga S., Wasik N., Shahid H., Grzeskowiak B. Hmgb1 level in cerebrospinal fluid as a marker of treatment outcome in patients with acute hydrocephalus following aneurysmal subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 2015;24:1897–1904. doi: 10.1016/j.jstrokecerebrovasdis.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 97.Sun Q., Wu W., Hu Y.C., Li H., Zhang D., Li S., Li W., Li W.D., Ma B., Zhu J.H., et al. Early release of high-mobility group box 1 (hmgb1) from neurons in experimental subarachnoid hemorrhage in vivo and in vitro. J. Neuroinflamm. 2014;11:106. doi: 10.1186/1742-2094-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakahara T., Tsuruta R., Kaneko T., Yamashita S., Fujita M., Kasaoka S., Hashiguchi T., Suzuki M., Maruyama I., Maekawa T. High-mobility group box 1 protein in csf of patients with subarachnoid hemorrhage. Neurocrit. Care. 2009;11:362–368. doi: 10.1007/s12028-009-9276-y. [DOI] [PubMed] [Google Scholar]
- 99.Murakami K., Koide M., Dumont T.M., Russell S.R., Tranmer B.I., Wellman G.C. Subarachnoid hemorrhage induces gliosis and increased expression of the pro-inflammatory cytokine high mobility group box 1 protein. Transl. Stroke Res. 2011;2:72–79. doi: 10.1007/s12975-010-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kellermann I., Kleindienst A., Hore N., Buchfelder M., Brandner S. Early csf and serum s100b concentrations for outcome prediction in traumatic brain injury and subarachnoid hemorrhage. Clin. Neurol. Neurosurg. 2016;145:79–83. doi: 10.1016/j.clineuro.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 101.Brandner S., Xu Y., Schmidt C., Emtmann I., Buchfelder M., Kleindienst A. Shunt-dependent hydrocephalus following subarachnoid hemorrhage correlates with increased S100B levels in cerebrospinal fluid and serum. Acta Neurochir. Suppl. 2012;114:217–220. doi: 10.1007/978-3-7091-0956-4_42. [DOI] [PubMed] [Google Scholar]
- 102.Stranjalis G., Korfias S., Psachoulia C., Kouyialis A., Sakas D.E., Mendelow A.D. The prognostic value of serum S-100B protein in spontaneous subarachnoid haemorrhage. Acta Neurochir. (Wien) 2007;149:231–237; discussion 237–238. doi: 10.1007/s00701-006-1106-9. [DOI] [PubMed] [Google Scholar]
- 103.Haruma J., Teshigawara K., Hishikawa T., Wang D., Liu K., Wake H., Mori S., Takahashi H.K., Sugiu K., Date I., et al. Anti-high mobility group box-1 (hmgb1) antibody attenuates delayed cerebral vasospasm and brain injury after subarachnoid hemorrhage in rats. Sci. Rep. 2016;6:37755. doi: 10.1038/srep37755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hao G., Dong Y., Huo R., Wen K., Zhang Y., Liang G. Rutin inhibits neuroinflammation and provides neuroprotection in an experimental rat model of subarachnoid hemorrhage, possibly through suppressing the rage-nf-kappab inflammatory signaling pathway. Neurochem. Res. 2016;41:1496–1504. doi: 10.1007/s11064-016-1863-7. [DOI] [PubMed] [Google Scholar]
- 105.Li H., Yu J.S., Zhang D.D., Yang Y.Q., Huang L.T., Yu Z., Chen R.D., Yang H.K., Hang C.H. Inhibition of the receptor for advanced glycation end-products (rage) attenuates neuroinflammation while sensitizing cortical neurons towards death in experimental subarachnoid hemorrhage. Mol. Neurobiol. 2017;54:755–767. doi: 10.1007/s12035-016-9703-y. [DOI] [PubMed] [Google Scholar]
- 106.Myint K.M., Yamamoto Y., Doi T., Kato I., Harashima A., Yonekura H., Watanabe T., Shinohara H., Takeuchi M., Tsuneyama K., et al. Rage control of diabetic nephropathy in a mouse model: Effects of rage gene disruption and administration of low-molecular weight heparin. Diabetes. 2006;55:2510–2522. doi: 10.2337/db06-0221. [DOI] [PubMed] [Google Scholar]
- 107.Liu R., Mori S., Wake H., Zhang J., Liu K., Izushi Y., Takahashi H.K., Peng B., Nishibori M. Establishment of in vitro binding assay of high mobility group box-1 and s100a12 to receptor for advanced glycation endproducts: Heparin‘s effect on binding. Acta Med. Okayama. 2009;63:203–211. doi: 10.18926/AMO/31812. [DOI] [PubMed] [Google Scholar]
- 108.Rao N.V., Argyle B., Xu X., Reynolds P.R., Walenga J.M., Prechel M., Prestwich G.D., MacArthur R.B., Walters B.B., Hoidal J.R., et al. Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of rage with its ligands. Am. J. Physiol. Cell Physiol. 2010;299:C97–C110. doi: 10.1152/ajpcell.00009.2010. [DOI] [PubMed] [Google Scholar]
- 109.Ling Y., Yang Z.Y., Yin T., Li L., Yuan W.W., Wu H.S., Wang C.Y. Heparin changes the conformation of high-mobility group protein 1 and decreases its affinity toward receptor for advanced glycation endproducts in vitro. Int. Immunopharmacol. 2011;11:187–193. doi: 10.1016/j.intimp.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 110.Takeuchi A., Yamamoto Y., Munesue S., Harashima A., Watanabe T., Yonekura H., Yamamoto H., Tsuchiya H. Low molecular weight heparin suppresses receptor for advanced glycation end products-mediated expression of malignant phenotype in human fibrosarcoma cells. Cancer Sci. 2013;104:740–749. doi: 10.1111/cas.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li S., Eisenstadt R., Kumasaka K., Johnson V.E., Marks J., Nagata K., Browne K.D., Smith D.H., Pascual J.L. Does enoxaparin interfere with hmgb1 signaling after tbi? A potential mechanism for reduced cerebral edema and neurologic recovery. J. Trauma Acute Care Surg. 2016;80:381–387; discussion 387–389. doi: 10.1097/TA.0000000000000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Loane D.J., Kumar A. Microglia in the tbi brain: The good, the bad, and the dysregulated. Exp. Neurol. 2016;275(Pt 3):316–327. doi: 10.1016/j.expneurol.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sozen T., Tsuchiyama R., Hasegawa Y., Suzuki H., Jadhav V., Nishizawa S., Zhang J.H. Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2009;40:2519–2525. doi: 10.1161/STROKEAHA.109.549592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.You W., Wang Z., Li H., Shen H., Xu X., Jia G., Chen G. Inhibition of mammalian target of rapamycin attenuates early brain injury through modulating microglial polarization after experimental subarachnoid hemorrhage in rats. J. Neurol. Sci. 2016;367:224–231. doi: 10.1016/j.jns.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 115.Farrugia B.L., Lord M.S., Melrose J., Whitelock J.M. Can we produce heparin/heparan sulfate biomimetics using “mother-nature“ as the gold standard? Molecules. 2015;20:4254–4276. doi: 10.3390/molecules20034254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arora M., Chen L., Paglia M., Gallagher I., Allen J.E., Vyas Y.M., Ray A., Ray P. Simvastatin promotes th2-type responses through the induction of the chitinase family member ym1 in dendritic cells. Proc. Natl. Acad. Sci. USA. 2006;103:7777–7782. doi: 10.1073/pnas.0508492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cai Y., Kumar R.K., Zhou J., Foster P.S., Webb D.C. ym1/2 promotes th2 cytokine expression by inhibiting 12/15(s)-lipoxygenase: Identification of a novel pathway for regulating allergic inflammation. J. Immunol. 2009;182:5393–5399. doi: 10.4049/jimmunol.0803874. [DOI] [PubMed] [Google Scholar]
- 118.Chang N.C., Hung S.I., Hwa K.Y., Kato I., Chen J.E., Liu C.H., Chang A.C. A macrophage protein, Ym1, transiently expressed during inflammation is a novel mammalian lectin. J. Biol. Chem. 2001;276:17497–17506. doi: 10.1074/jbc.M010417200. [DOI] [PubMed] [Google Scholar]
- 119.Lean Q.Y., Eri R.D., Randall-Demllo S., Sohal S.S., Stewart N., Peterson G.M., Gueven N., Patel R.P. Orally administered enoxaparin ameliorates acute colitis by reducing macrophage-associated inflammatory responses. PLoS ONE. 2015;10:e0134259. doi: 10.1371/journal.pone.0134259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu H., Zhang D.M., Chen H.L., Lin Y.X., Hang C.H., Yin H.X., Shi J.X. N-acetylcysteine suppresses oxidative stress in experimental rats with subarachnoid hemorrhage. J. Clin. Neurosci. 2009;16:684–688. doi: 10.1016/j.jocn.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 121.Endo H., Nito C., Kamada H., Yu F., Chan P.H. Reduction in oxidative stress by superoxide dismutase overexpression attenuates acute brain injury after subarachnoid hemorrhage via activation of akt/glycogen synthase kinase-3beta survival signaling. J. Cereb. Blood Flow Metab. 2007;27:975–982. doi: 10.1038/sj.jcbfm.9600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yamaguchi M., Zhou C., Heistad D.D., Watanabe Y., Zhang J.H. Gene transfer of extracellular superoxide dismutase failed to prevent cerebral vasospasm after experimental subarachnoid hemorrhage. Stroke. 2004;35:2512–2517. doi: 10.1161/01.STR.0000145198.07723.8e. [DOI] [PubMed] [Google Scholar]
- 123.Froehler M.T., Kooshkabadi A., Miller-Lotan R., Blum S., Sher S., Levy A., Tamargo R.J. Vasospasm after subarachnoid hemorrhage in haptoglobin 2-2 mice can be prevented with a glutathione peroxidase mimetic. J. Clin. Neurosci. 2010;17:1169–1172. doi: 10.1016/j.jocn.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 124.Sandstrom J., Carlsson L., Marklund S.L., Edlund T. The heparin-binding domain of extracellular superoxide dismutase c and formation of variants with reduced heparin affinity. J. Biol. Chem. 1992;267:18205–18209. [PubMed] [Google Scholar]
- 125.Adachi T., Yamnamoto M., Hara H. Heparin-affinity of human extracellular-superoxide dismutase in the brain. Biol. Pharm. Bull. 2001;24:191–193. doi: 10.1248/bpb.24.191. [DOI] [PubMed] [Google Scholar]
- 126.Sandstrom J., Nilsson P., Karlsson K., Marklund S.L. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J. Biol. Chem. 1994;269:19163–19166. [PubMed] [Google Scholar]
- 127.Adachi T., Yamada H., Futenma A., Kato K., Hirano K. Heparin-induced release of extracellular-superoxide dismutase form (V) to plasma. J. Biochem. 1995;117:586–590. doi: 10.1093/oxfordjournals.jbchem.a124748. [DOI] [PubMed] [Google Scholar]
- 128.Adachi T., Hara H., Yamada H., Yamazaki N., Yamamoto M., Sugiyama T., Futenma A., Katagiri Y. Heparin-stimulated expression of extracellular-superoxide dismutase in human fibroblasts. Atherosclerosis. 2001;159:307–312. doi: 10.1016/S0021-9150(01)00512-3. [DOI] [PubMed] [Google Scholar]
- 129.Nakane H., Chu Y., Faraci F.M., Oberley L.W., Heistad D.D. Gene transfer of extracellular superoxide dismutase increases superoxide dismutase activity in cerebrospinal fluid. Stroke. 2001;32:184–189. doi: 10.1161/01.STR.32.1.184. [DOI] [PubMed] [Google Scholar]
- 130.Chen T.Y., Tsai K.L., Lee T.Y., Chiueh C.C., Lee W.S., Hsu C. Sex-specific role of thioredoxin in neuroprotection against iron-induced brain injury conferred by estradiol. Stroke. 2010;41:160–165. doi: 10.1161/STROKEAHA.109.562850. [DOI] [PubMed] [Google Scholar]
- 131.Baratz-Goldstein R., Deselms H., Heim L.R., Khomski L., Hoffer B.J., Atlas D., Pick C.G. Thioredoxin-mimetic-peptides protect cognitive function after mild traumatic brain injury (mtbi) PLoS ONE. 2016;11:e0157064. doi: 10.1371/journal.pone.0157064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu S.Y., Stadtman T.C. Heparin-binding properties of selenium-containing thioredoxin reductase from hela cells and human lung adenocarcinoma cells. Proc. Natl. Acad. Sci. USA. 1997;94:6138–6141. doi: 10.1073/pnas.94.12.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ross M.A., Long W.F., Williamson F.B. Inhibition by heparin of fe(II)-catalysed free-radical peroxidation of linolenic acid. Biochem. J. 1992;286((Pt 3)):717–720. doi: 10.1042/bj2860717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Albertini R., Rindi S., Passi A., Pallavicini G., De Luca G. Heparin protection against Fe2+ -and Cu2+ -mediated oxidation of liposomes. FEBS Lett. 1996;383:155–158. doi: 10.1016/0014-5793(96)00253-0. [DOI] [PubMed] [Google Scholar]
- 135.Wahl F., Grosjean-Piot O., Bareyre F., Uzan A., Stutzmann J.M. Enoxaparin reduces brain edema, cerebral lesions, and improves motor and cognitive impairments induced by a traumatic brain injury in rats. J. Neurotrauma. 2000;17:1055–1065. doi: 10.1089/neu.2000.17.1055. [DOI] [PubMed] [Google Scholar]
- 136.Pratt J., Boudeau P., Uzan A., Imperato A., Stutzmann J. Enoxaparin reduces cerebral edemaafter photothrombotic injury in the rat. Haemostasis. 1998;28:78–85. doi: 10.1159/000022416. [DOI] [PubMed] [Google Scholar]
- 137.Xi G., Wagner K.R., Keep R.F., Hua Y., de Courten-Myers G.M., Broderick J.P., Brott T.G., Hoff J.T. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke. 1998;29:2580–2586. doi: 10.1161/01.STR.29.12.2580. [DOI] [PubMed] [Google Scholar]
- 138.Gong Y., Xi G.H., Keep R.F., Hoff J.T., Hua Y. Complement inhibition attenuates brain edema and neurological deficits induced by thrombin. Acta Neurochir. Suppl. 2005;95:389–392. doi: 10.1007/3-211-32318-x_79. [DOI] [PubMed] [Google Scholar]
- 139.Kim L., Schuster J., Holena D.N., Sims C.A., Levine J., Pascual J.L. Early initiation of prophylactic heparin in severe traumatic brain injury is associated with accelerated improvement on brain imaging. J. Emerg. Trauma Shock. 2014;7:141–148. doi: 10.4103/0974-2700.136846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bruce J.N., Criscuolo G.R., Merrill M.J., Moquin R.R., Blacklock J.B., Oldfield E.H. Vascular permeability induced by protein product of malignant brain tumors: Inhibition by dexamethasone. J. Neurosurg. 1987;67:880–884. doi: 10.3171/jns.1987.67.6.0880. [DOI] [PubMed] [Google Scholar]
- 141.Tessler S., Rockwell P., Hicklin D., Cohen T., Levi B.Z., Witte L., Lemischka I.R., Neufeld G. Heparin modulates the interaction of vegf165 with soluble and cell associated flk-1 receptors. J. Biol. Chem. 1994;269:12456–12461. [PubMed] [Google Scholar]
- 142.Marchetti M., Vignoli A., Russo L., Balducci D., Pagnoncelli M., Barbui T., Falanga A. Endothelial capillary tube formation and cell proliferation induced by tumor cells are affected by low molecular weight heparins and unfractionated heparin. Thromb. Res. 2008;121:637–645. doi: 10.1016/j.thromres.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 143.Oschatz C., Maas C., Lecher B., Jansen T., Bjorkqvist J., Tradler T., Sedlmeier R., Burfeind P., Cichon S., Hammerschmidt S., et al. Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity. 2011;34:258–268. doi: 10.1016/j.immuni.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 144.Carr J. The anti-inflammatory action of heparin: Heparin as an antagonist to histamine, bradykinin and prostaglandin e1. Thromb. Res. 1979;16:507–516. doi: 10.1016/0049-3848(79)90097-5. [DOI] [PubMed] [Google Scholar]
- 145.Thal S.C., Sporer S., Schmid-Elsaesser R., Plesnila N., Zausinger S. Inhibition of bradykinin b2 receptors before, not after onset of experimental subarachnoid hemorrhage prevents brain edema formation and improves functional outcome. Crit. Care Med. 2009;37:2228–2234. doi: 10.1097/CCM.0b013e3181a068fc. [DOI] [PubMed] [Google Scholar]
- 146.Gupta S., Tiruvoipati R., Green C., Botha J., Tran H. Heparin induced thrombocytopenia in critically ill: Diagnostic dilemmas and management conundrums. World J. Crit. Care Med. 2015;4:202–212. doi: 10.1097/01.ccm.0000474632.76542.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hoh B.L., Aghi M., Pryor J.C., Ogilvy C.S. Heparin-induced thrombocytopenia type II in subarachnoid hemorrhage patients: Incidence and complications. Neurosurgery. 2005;57:243–248; discussion 243–248. doi: 10.1227/01.NEU.0000166539.02280.E5. [DOI] [PubMed] [Google Scholar]
- 148.Kim G.H., Hahn D.K., Kellner C.P., Komotar R.J., Starke R., Garrett M.C., Yao J., Cleveland J., Mayer S.A., Connolly E.S. The incidence of heparin-induced thrombocytopenia type II in patients with subarachnoid hemorrhage treated with heparin versus enoxaparin. J. Neurosurg. 2009;110:50–57. doi: 10.3171/2008.3.17480. [DOI] [PubMed] [Google Scholar]
- 149.Mehta B.P., Sims J.R., Baccin C.E., Leslie-Mazwi T.M., Ogilvy C.S., Nogueira R.G. Predictors and outcomes of suspected heparin-induced thrombocytopenia in subarachnoid hemorrhage patients. Interv. Neurol. 2014;2:160–168. doi: 10.1159/000362189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shimamura N., Naraoka M., Matsuda N., Ohkuma H. Safety of preprocedural antiplatelet medication in coil embolization of ruptured cerebral aneurysms at the acute stage. Interv. Neuroradiol. 2014;20:413–417. doi: 10.15274/INR-2014-10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hoh B.L., Nogueira R.G., Ledezma C.J., Pryor J.C., Ogilvy C.S. Safety of heparinization for cerebral aneurysm coiling soon after external ventriculostomy drain placement. Neurosurgery. 2005;57:845–849; discussion 845–849. doi: 10.1227/01.NEU.0000180814.95032.07. [DOI] [PubMed] [Google Scholar]
- 152.Egashira Y., Yoshimura S., Enomoto Y., Ishiguro M., Asano T., Iwama T. Ultra-early endovascular embolization of ruptured cerebral aneurysm and the increased risk of hematoma growth unrelated to aneurysmal rebleeding. J. Neurosurg. 2013;118:1003–1008. doi: 10.3171/2012.11.JNS12610. [DOI] [PubMed] [Google Scholar]
- 153.Zachariah J., Snyder K.A., Graffeo C.S., Khanal D.R., Lanzino G., Wijdicks E.F., Rabinstein A.A. Risk of ventriculostomy-associated hemorrhage in patients with aneurysmal subarachnoid hemorrhage treated with anticoagulant thromboprophylaxis. Neurocrit. Care. 2016;25:224–229. doi: 10.1007/s12028-016-0262-x. [DOI] [PubMed] [Google Scholar]
- 154.Bruder M., Schuss P., Konczalla J., El-Fiki A., Lescher S., Vatter H., Seifert V., Guresir E. Ventriculostomy-related hemorrhage after treatment of acutely ruptured aneurysms: The influence of anticoagulation and antiplatelet treatment. World Neurosurg. 2015;84:1653–1659. doi: 10.1016/j.wneu.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 155.Bauer K.A. Fondaparinux: Basic properties and efficacy and safety in venous thromboembolism prophylaxis. Am. J. Orthop. (Belle Mead NJ) 2002;31:4–10. [PubMed] [Google Scholar]
- 156.Zhang Y., Zhao Z., Guan L., Mao L., Li S., Guan X., Chen M., Guo L., Ding L., Cong C., et al. N-acetyl-heparin attenuates acute lung injury caused by acid aspiration mainly by antagonizing histones in mice. PLoS ONE. 2014;9:e97074. doi: 10.1371/journal.pone.0097074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Veraldi N., Hughes A.J., Rudd T.R., Thomas H.B., Edwards S.W., Hadfield L., Skidmore M.A., Siligardi G., Cosentino C., Shute J.K., et al. Heparin derivatives for the targeting of multiple activities in the inflammatory response. Carbohydr. Polym. 2015;117:400–407. doi: 10.1016/j.carbpol.2014.09.079. [DOI] [PubMed] [Google Scholar]
- 158.Wang L., Brown J.R., Varki A., Esko J.D. Heparin‘s anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of l- and p-selectins. J. Clin. Investig. 2002;110:127–136. doi: 10.1172/JCI0214996. [DOI] [PMC free article] [PubMed] [Google Scholar]