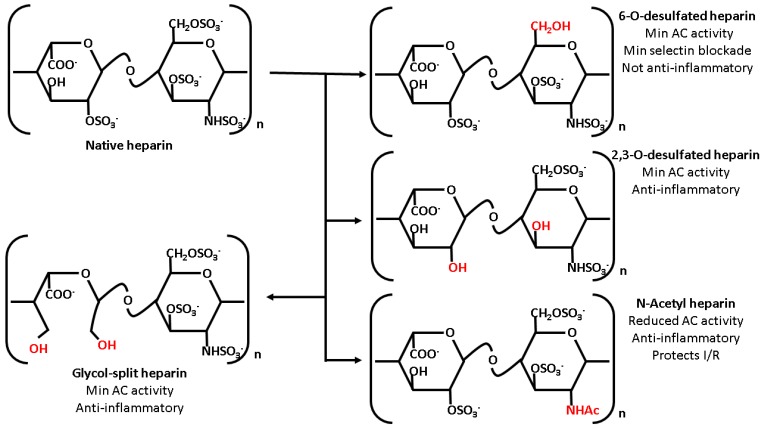
Figure 1.
Summary of heparin chemical derivatives. Chemical modification of unfractionated heparin modulates its pharmacology. Although all derivatives discussed demonstrate reduced anticoagulant activity, chemical modification also affects heparin’s other pharmacologic properties in a regiospecific manner. 2,3-O-desulfated heparin demonstrates nearly retained selecting and RAGE inhibition; 6-O-desulfated heparin, despite numerous sulfated residues, fails to bind selectins and does not demonstrate significant anti-inflammatory effects in vivo. Although less well studied in inflammation, N-acetyl heparin does demonstrate evidence of efficacy in ischemia-reperfusion (I/R) injury despite reduced anticoagulant activity. Finally, glycol split heparin retains the ability to inhibit proteases such as elastase despite reduced anti-coagulant activity.