Abstract
1,4-Pentadien-3-one derivatives derived from curcumin possess excellent inhibitory activity against plant viruses. On the basis of this finding, a series of novel 1,4-pentadien-3-one derivatives containing a 1,3,4-thiadiazole moiety were designed and synthesized, and their structures confirmed by IR, 1H-NMR, and 13C-NMR spectroscopy and elemental analysis. The antiviral activities of the title compounds were evaluated against tobacco mosaic virus (TMV) and cucumber mosaic virus (CMV) in vivo. The assay results showed that most of compounds had remarkable antiviral activities against TMV and CMV, among which compounds 4b, 4h, 4i, 4k, 4o, and 4q exhibited good curative, protection, and inactivation activity against TMV. Compounds 4h, 4i, 4k, 4l, 4o, and 4q exhibited excellent protection activity against TMV, with EC50 values of 105.01, 254.77, 135.38, 297.40, 248.18, and 129.87 μg/mL, respectively, which were superior to that of ribavirin (457.25 µg/mL). In addition, preliminary SARs indicated that small electron-withdrawing groups on the aromatic ring were favorable for anti-TMV activity. This finding suggests that 1,4-pentadien-3-one derivatives containing a 1,3,4-thiadiazole moiety may be considered as potential lead structures for discovering new antiviral agents.
Keywords: 1,4-pentadien-3-one derivatives; 1,3,4-thiadiazole; synthesis; anti-TMV; anti-CMV
1. Introduction
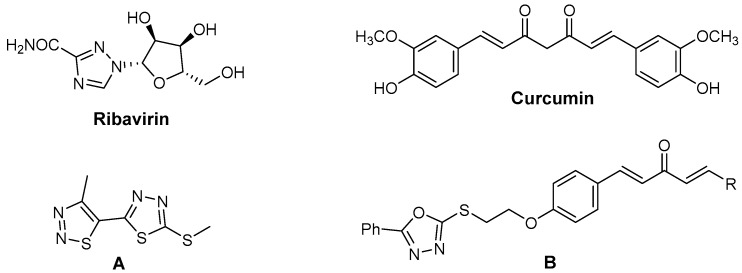
Tobacco mosaic virus (TMV) and cucumber mosaic virus (CMV) are two important plant viruses, which can infect at least 125 species of nine plant families and cause significant economic losses in various crops, including tobacco, tomato, pepper, cucumbers, and a number of ornamental flowers [1,2]. Ribavirin (Figure 1) is widely used for preventing plant viruses, however, its inhibitory activities against TMV and CMV are less than 50% at 500 μg/mL [3]. In recent years, biologists have made great efforts to develop novel and effective antiviral compounds [4,5], but few compounds with high inhibition against plant virus have been found. That is to say, there are no efficient antiviral agents that can absolutely inhibit plant virus [6], so the development of new effective antiviral agents remains a significant challenge.
Figure 1.
The structures of some antiviral agents and synthesized compounds.
Natural products are important sources for drug development, and some natural products and their derivatives, such as limonoids [7], quassinoids [8], xanthones [9], antofine and its derivatives [10,11], as well as phenanthroindolizidine and its analogues [6], show excellent antiviral activity. Therefore, it is a development trend of modern agrochemical research to design and synthesize pesticides based on natural products. Curcumin (Figure 1), a member of a small family of natural products, exhibits extensive biological activities, including antibacterial [12], anticancer [13,14], and antiviral properties [15,16]. 1,4-Pentadien-3-one derivatives derived from curcumin possess numerous potential biological activities and play an important role in the discovery of new antiviral molecules. Our group has designed and synthesized a number of such 1,4-pentadien-3-one derivatives [17,18,19,20,21,22], and most of them exhibited excellent antiviral activities against TMV and CMV.
1,3,4-Thiadiazole is a potent multi-targeting pharmacological scaffold in heterocyclic chemistry [23]. 1,3,4-Thiadiazole derivatives have various biological activities, such as antimicrobial [24], antitubercular [25], anticonvulsants [26], antibacterial [27], anti-inflammatory [28,29], anticancer [30,31], antinociceptive [32], enzyme inhibitory [33], antidepressant and anxiolytic [34] effects. In our previous work, a number of 5-(4-chlorophenyl)-1,3,4-thiadiazole sulfonamides were designed and synthesized, and these compounds showed moderate anti-TMV activities [35]. In addition, 1,3,4-thiadiazole thioether derivative A (Figure 1) was found to display remarkable antiviral activity against TMV, and the results indicated that the thioethers exhibited better antiviral activity than sulfonamides [36].
In the further development of antiviral agents, a series of novel 1,4-pentadien-3-one derivatives containing a 5-phenyl-1,3,4-oxadiazole moiety (B, Figure 1) was found to have excellent anti-TMV activity [37]. In this study, we aimed to use a phenyl-1,3,4-thiadiazole to replace the phenyl-1,3,4-oxadiazole system to build novel 1,4-pentadien-3-one derivatives containing a 1,3,4-thiadiazole moiety for the development of antiviral agents. The assay results showed that most of title compounds exhibited good antiviral activity, among which compounds 4b, 4h, 4i, 4k, 4o, and 4q exhibited good curative, protection, and inactivation activity against TMV. The structure-activity relationships (SAR) of the compounds are also discussed. To the best of our knowledge, this is the first report on the synthesis and antiviral activity evaluation of 1,4-pentadien-3-one derivatives containing a 1,3,4-thiadiazole moiety.
2. Results and Discussion
2.1. Chemistry
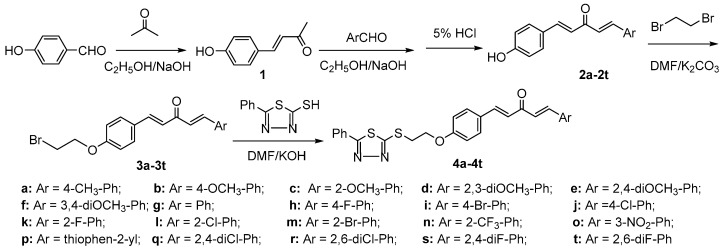
A synthetic route to 1,4-pentadien-3-one derivatives containing a 1,3,4-thiadiazole moiety was designed and is shown in Scheme 1. According to previously reported methods [38], 5-phenyl-1,3,4-thiadiazole-2-thiol could be obtained. The (1E,4E)-1-(4-(2-bromoethoxy)phenyl)-5-substitued phenylpenta-1,4-dien-3-ones 3a–3t were prepared from 4-hydroxybenzaldehyde and aromatic aldehydes via reported procedures [22,37].
Scheme 1.
Synthesis of the title compounds 4a–4t.
To obtain the target compounds in high yield, the reaction solvent, catalyst and temperature for the synthesis of compound 4a were optimized on the basis of the molar ratio of 5-phenyl-1,3,4-thiadiazole-2-thiol:base:3a = 1.05:1.2:1.0, after a reaction time of 4 h, and the results are outlined in Table 1. It was shown that the maximum yield of 4a (up to 87%) was achieved when the solvent, base and temperature were DMF, KOH and 40 °C, respectively. The other compounds were then synthesized under these conditions.
Table 1.
Effect of the different conditions for the synthesis of 4a.
| No. | Solvent | Base | Temperature (°C) | Yield (%) a |
|---|---|---|---|---|
| 1 | DMF | Et3N | 25 | 0 |
| 2 | DMF | K2CO3 | 25 | 21 |
| 3 | DMF | K2CO3 | 50 | 38 |
| 4 | acetone | KOH | 25 | 18 |
| 5 | THF | KOH | 25 | 26 |
| 6 | CH3CN | KOH | 25 | 38 |
| 7 | DMF | KOH | 25 | 56 |
| 8 | DMF | KOH | 40 | 87 |
| 9 | DMF | NaOH | 40 | 72 |
a Yields of isolated products.
The structures of the target compounds were confirmed by IR, 1H-NMR, and 13C-NMR spectroscopy and elemental analysis. The data of 4a are shown and discussed below. The IR spectra exhibited characteristic absorption bands at 3435 cm−1, which indicate the presence of a =CHCOCH= grouping. The stretching frequency at 2942–2838 cm−1 was assigned to CH vibrations. The characteristic absorptions at 1627 cm−1, 1610 cm−1, and 1598–1448 cm−1 were attributed to the presence of C=O, C=N, and C=C group vibrations, respectively. The characteristic absorption at 1108 cm−1 was assigned to CH vibrations. In the 1H-NMR, the four low-frequency downfield doublets at δ 7.71 (d, 1H, J = 16.0 Hz), 7.69 (d, 1H, J = 16.0 Hz), 7.03 (d, 1H, J = 16.0 Hz), and 6.97 (d, 1H, J = 15.0 Hz) ppm revealed the presence of four trans =C-H protons. The various absorption peaks at δ 7.89–6.98 ppm indicated the presence of phenyl protons. The triplets at 4.77 and 3.80 ppm indicated the presence of -O-CH2- and –S-CH2- absorption peaks. The presence of Ar-CH3 showed a singlet at 2.39 ppm. The typical chemical shifts near δ 189.03, 168.81, 164.50, and 160.35 ppm of 13C-NMR spectra validated the presence of C=O, C-N (two), and C-O, respectively. Meanwhile, peaks near δ 66.33 and 32.69 ppm confirmed the presence of -OCH2- and -SCH2-. The typical peak near δ 21.64 ppm also verified the presence of -ArCH3. ESI-HRMS (m/z), calcd. for C28H24O2N2NaS2 [M + Na]+ 507.11714, found 507.11646.
2.2. Antiviral Activity of Title Compounds against TMV In Vivo
The antiviral activities of the title compounds 4a–4t against TMV were evaluated by the half-leaf method [39] and the results were summarized in Table 2. It was found that most of the title compounds exhibited good antiviral activity against TMV in vivo. Compounds 4a, 4b, 4g, 4h, 4i, 4k, 4o, 4q, and 4t showed remarkable curative activity against TMV, with values of 55.8, 56.4, 56.3, 56.2, 53.7, 56.5, 51.7, 58.7 and 54.5%, respectively, which were better than that of ribavirin (37.9%). Meanwhile, compounds 4b, 4f, 4h, 4i, 4k, 4l, 4o, and 4q exhibited excellent protection activity, also superior to ribavirin (51.8%). Overall, most of the compounds except 4d indicated significant inactivation activity against TMV at 500 µg/mL.
Table 2.
Antiviral activities of the test compounds against TMV in vivo.
| Compound | Curative Activity (%) a | Protection Activity (%) a | Inactivation Activity (%) a | EC50 of Protection Activity (µg/mL) a |
|---|---|---|---|---|
| 4a | 55.8 ± 3.1 | 55.9 ± 3.5 | 84.1 ± 4.8 | 344.23 ± 2.35 |
| 4b | 56.4 ± 1.5 | 64.5 ± 1.1 | 84.1 ± 4.8 | 319.67 ± 1.89 |
| 4c | 36.8 ± 0.6 | 52.2 ± 1.4 | 91.8 ± 3.8 | 411.42 ± 2.68 |
| 4d | 21.2 ± 1.5 | 24.2 ± 2.1 | 64.3 ± 2.6 | 1058.25 ± 2.11 |
| 4e | 45.0 ± 1.6 | 30.4 ± 2.1 | 82.4 ± 1.8 | 1042.09 ± 1.28 |
| 4f | 40.6 ± 0.8 | 61.6 ± 3.3 | 90.1 ± 0.3 | 389.46 ± 2.32 |
| 4g | 56.3 ± 2.7 | 46.3 ± 1.4 | 78.5 ± 1.9 | 592.44 ± 1.89 |
| 4h | 56.2 ± 3.5 | 70.2 ± 1.3 | 93.8 ± 1.7 | 105.01 ± 3.15 |
| 4i | 53.7 ± 1.6 | 64.3 ± 2.7 | 85.3 ± 2.0 | 254.77 ± 1.66 |
| 4j | 44.6 ± 3.2 | 53.6 ± 3.7 | 85.2 ± 2.2 | 388.31 ± 2.05 |
| 4k | 56.5 ± 2.1 | 68.4 ± 1.8 | 87.1 ± 3.6 | 135.38 ± 3.12 |
| 4l | 45.9 ± 3.1 | 63.4 ± 4.4 | 83.0 ± 1.9 | 297.40 ± 4.10 |
| 4m | 40.5 ± 1.8 | 54.8 ± 2.5 | 81.5 ± 1.2 | 334.03 ± 1.08 |
| 4n | 47.9 ± 2.5 | 59.8 ± 4.3 | 89.3 ± 2.5 | 309.09 ± 2.56 |
| 4o | 51.7 ± 2.8 | 64.8 ± 3.1 | 77.6 ± 1.3 | 248.18 ± 4.14 |
| 4p | 24.2 ± 4.5 | 50.7 ± 1.5 | 88.6 ± 1.1 | 466.15 ± 1.98 |
| 4q | 58.7 ± 3.0 | 68.4 ± 1.6 | 84.7 ± 2.6 | 129.87 ± 3.55 |
| 4r | 42.3 ± 2.4 | 56.7 ± 2.8 | 87.4 ± 2.9 | 316.77 ± 2.54 |
| 4s | 28.2 ± 3.8 | 59.0 ± 1.7 | 82.7 ± 1.6 | 316.52 ± 4.29 |
| 4t | 54.5 ± 4.4 | 54.1 ± 1.2 | 82.7 ± 1.6 | 425.71 ± 3.17 |
| Ribavirin b | 37.9 ± 1.9 | 51.8 ± 2.3 | 72.9 ± 2.4 | 457.25± 2.68 |
a Average of three replicates, at 500 μg/mL. b The commercial antiviral agent.
Based on the previous bioassays, the 50% effective concentration (EC50) values of protection activities against TMV of the title compounds were tested and are listed in Table 2. Most of the target compounds showed good antiviral activity against TMV. Compounds 4h, 4i, 4k, 4l, 4o, and 4q exhibited excellent protection activity against TMV, with the EC50 values of 105.01, 254.77, 135.38, 297.40, 248.18 and 129.87 μg/mL, respectively, which were better than that of ribavirin (457.25 µg/mL). In summary, we found that the compounds 4b, 4h, 4i, 4k, 4o, and 4q had good curative, protection, and inactivation activity against TMV.
2.3. Antiviral Activity of Title Compounds against CMV In Vivo
The antiviral activities of the title compounds 4a–4t against CMV were tested by the half-leaf method [22] and the results are summarized in Table 3. Some of the title compounds exhibited good antiviral activity against CMV in vivo. Compounds 4e, and 4f showed remarkable curative activity against CMV, with values of 55.9% and 50.2%, respectively, which were better than that of ribavirin (36.8%). Meanwhile, the protection and inactivation activity of the target compounds was similar to that of ribavirin.
Table 3.
Antiviral activities of the test compounds against CMV in vivo.
| Compound | Curative Activity (%) a | Protection Activity (%) a | Inactivation Activity (%) a |
|---|---|---|---|
| 4a | 29.3 ± 1.3 | 40.1 ± 2.5 | 71.3 ± 3.1 |
| 4b | 23.6 ± 2.2 | 38.9 ± 2.9 | 65.2 ± 2.5 |
| 4c | 37.9 ± 3.7 | 43.4 ± 3.1 | 61.5 ± 2.2 |
| 4d | 18.5 ± 1.8 | 33.6 ± 2.1 | 51.2 ± 3.4 |
| 4e | 55.9 ± 1.8 | 42.3 ± 1.1 | 79.6 ± 4.2 |
| 4f | 50.2 ± 2.7 | 46.6 ± 2.3 | 62.5 ± 1.9 |
| 4g | 39.8 ± 2.5 | 48.5 ± 2.7 | 66.7 ± 2.2 |
| 4h | 39.8 ± 2.5 | 36.2 ± 2.2 | 62.1 ±1.9 |
| 4i | 37.3 ± 2.5 | 35.6 ± 2.1 | 51.8 ± 1.1 |
| 4j | 40.6 ± 3.7 | 45.9 ± 1.6 | 79.1± 2.5 |
| 4k | 26.4 ± 2.3 | 35.6 ± 1.9 | 49.9± 3.6 |
| 4l | 29.7 ± 1.1 | 43.5 ± 2.5 | 66.5 ± 2.0 |
| 4m | 28.5 ± 2.3 | 42.2 ± 1.4 | 68.9 ± 2.8 |
| 4n | 18.9 ± 2.9 | 25.4 ± 1.7 | 46.2 ± 0.9 |
| 4o | 31.8 ± 2.8 | 49.9 ± 1.9 | 58.2 ± 3.2 |
| 4p | 29.5 ± 1.4 | 38.5 ± 2.1 | 59.8 ± 1.9 |
| 4q | 44.5 ± 1.8 | 48.1 ± 2.4 | 43.9 ± 1.2 |
| 4r | 26.4 ± 2.2 | 40.5 ± 1.9 | 42.5 ± 2.8 |
| 4s | 41.0 ± 1.7 | 54.7 ± 2.4 | 78.5 ± 1.9 |
| 4t | 34.8 ± 2.3 | 48.1 ± 1.6 | 69.5 ± 2.0 |
| Ribavirin b | 36.8 ± 1.6 | 47.9 ± 2.7 | 71.2 ± 1.7 |
a Average of three replicates, at 500 μg/mL. b A commercial antiviral agent.
2.4. Antiviral Activity and Structure-Activity Relationships
The antiviral bioassay results indicated that the target compounds showed excellent antiviral activity against TMV. The preliminary SAR results were deduced on the basis of the anti-TMV activity (as shown in Table 2). The results showed that when the Ar is 4-OCH3-Ph (4b), 3,4-diOCH3-Ph (4f), 4-F-Ph (4h), 4-Br-Ph (4i), 2-F-Ph (4k), 2-Cl-Ph (4l), 3-NO2-Ph (4o), and 2,4-diCl-Ph (4q) groups, the corresponding target compounds exhibited good protection activity. Moreover, the results showed that electron-withdrawing groups on aromatic rings were favorable for antiviral activity at the same position, these findings were confirmed by the following activity order 4h (Ar = 4-F-Ph) > 4i (Ar = 4-Br-Ph) > 4a (Ar = 4-CH3-Ph), 4k (Ar = 2-F-Ph) > 4l (Ar = 2-Cl-Ph) > 4c (Ar = 2-OCH3-Ph), and 4q (2,4-diCl-Ph) > 7e (2,4-diOCH3-Ph). Bulky group of aromatic rings disfavor antiviral activity, a notion supported by the activity order of 7b (Ar = 4-OCH3-Ph) > 4e (Ar = 2,4-diOCH3-Ph), 4h (Ar = 4-F-Ph) > 4i (Ar = 4-Br-Ph), and 4k (Ar = 2-F-Ph) > 4l (Ar = 2-Cl-Ph) > 4m (Ar = 2-Br-Ph).
3. Materials and Methods
3.1. General Information
Melting points of the compounds were recorded on an XT-4 binocular microscope melting point apparatus (Beijing Tech Instruments Co., Beijing, China), and are uncorrected. Proton nuclear magnetic resonance (NMR) spectra were determined at 500 and 125 MHz using an ECX 500 NMR spectrometer (JEOL, Tokyo, Japan) in CDCl3 solvent, using TMS as an internal standard. Infrared (IR) spectra were obtained on a Vector 22 Fourier transform infrared (FTIR) spectrometer (Bruker, Karlsruhe, Germany) in KBr disks. Elemental analyses were determined on an Elementar Vario-III CHN analyzer (Elementar Analysensysteme GmbH, Frankfurt, Germany). High resolution mass spectrometer (HRMS) data was conducted using a Thermo Scientific Q Exactive (Thermo, Waltham, MA, USA). Reaction progress was monitored by thin-layer chromatography (TLC) on silica gel GF254. Column chromatographic purification was carried out using silica gel (200–300 mesh, Qingdao Bangkai Hi-Tech materials Co., Ltd. Qingdao, Shandong, China). All solvents and reagents were of analytical reagent grade or chemically pure, and the solvents were dried in advance and distilled before use.
3.2. Chemistry
3.2.1. General Procedure for Preparation of Intermediates 3a–3t
(E)-4-(4-hydroxyphenyl) but-3-en-2-one (1) was synthesized from 4-hydroxybenzaldehyde (20 mmol) and acetone (20 mL) via a room temperature Claisen-Schmidt aldol condensation. (1E,4E)-1-(4-Hydroxyphenyl)-5-substitued phenylpenta-1,4-dien-3-ones 2 were synthesized from 1 (4 mmol) and an aromatic aldehyde (4 mmol) at room temperature. Thus, to a solution of the corresponding 2 (2 mmol) in DMF (3 mL), potassium carbonate (4 mmol) was added, and the resulting solution was stirred at room temperature for 1 h. Then 1,2-dibromoethane (10 mmol) was added to the mixture, warmed to 80 °C and stirred for 6 h. Upon reaction completion (as indicated by TLC), the solid was removed by filtration, and N,N-dimethylformamide and excess 1,2-dibromoethane were evaporated under vacuum. The crude product was purified by silica-gel column chromatography using petroleum ether/ethyl acetate (5:1/v:v) as the eluant to give (1E,4E)-1-(4-(2-bromoethoxy)phenyl)-5-substitued phenylpenta-1,4-dien-3-ones 3a–3t as faint yellow powders [22,37].
3.2.2. General Procedure for Preparation of Title Compounds 4a–4t
A mixture of 5-phenyl-1,3,4-thiadiazole-2-thiol (1.05 mmol) and potassium hydroxide (1.2 mmol) in DMF (4 mL) was stirred at room temperature for 1 h, and then a solution of the appropriate (1E,4E)-1-(4-(2-bromoethoxy)phenyl)-5-substitued phenylpenta-1,4-dien-3-one 3 (1.0 mmol) in DMF (4 mL) was added. The mixture was warmed to 50 °C and stirred continuously for 4 h to 6 h. Upon reaction completion (as indicated by TLC), after dropwise addition of cold brine, the solid was filtered off, and washed with cold water. Then the crude product was recrystallized from ethyl acetate, filtered, washed, and dried to obtain the title 1,4-pentadien-3-one derivatives. The physical characteristics, IR, 1H-NMR, 13C-NMR, and elemental analysis data, for all the synthesized compounds are provided in Supporting Information and the representative data of 4a are listed below.
(1E,4E)-1-(4-(2-((5-Bhenyl-1,3,4-thiadiazol-2-yl)thio)ethoxy)phenyl)-5-(p-tolyl)penta-1,4-dien-3-one (4a). Faint yellow powder; m.p. 125–126 °C; yield 87%; IR (KBr, cm−1): ν 3435 (=CHCOCH=), 2942–2838 (CH), 1627 (C=O), 1610 (C=N), 1598–1448 (C=C and benzene), 1108 (-C-O-C); 1H-NMR: δ 7.89 (d, 2H, J = 8.6 Hz, 5’-Ar-2, 6-H), 7.71 (d, 1H, J = 16.0 Hz, 5-H), 7.69 (d, 1H, J = 16.0 Hz, 1-H), 7.57 (d, 2H, J = 8.5 Hz, 1-Ar-2, 6-H), 7.51 (d, 2H, J = 8.0 Hz, 5-Ar-2, 6-H), 7.49–7.48 (m, 3H, 5’-Ar-3, 4, 5-H), 7.22 (d, 2H, J = 8.0 Hz, 5-Ar-3, 5-H), 7.03 (d, 1H, J = 16.0 Hz, 2-H), 6.98 (d, 2H, J = 8.5 Hz, 1-Ar-3, 5-H), 6.97 (d, 1H, J = 15.0 Hz, 4-H), 4.47 (t, 2H, J = 6.5 Hz, -CH2O-), 3.80 (t, 2H, J = 6.5 Hz, -CH2S-), 2.39 (s, 3H, CH3); 13C-NMR: δ 189.03, 168.81, 164.50, 160.35, 143.08, 142.84, 141.00, 132.24, 131.28, 130.26, 130.26, 129.79, 129.79, 129.34, 129.34, 128.47, 128.47, 128.14, 127.82, 127.82, 124.78, 123.67, 115.15, 115.15, 66.33, 32.69, 21.64.; Anal. Calcd. for C28H24N2O2S2: C, 69.39; H, 4.99; N, 5.78; Found: C, 69.30; H, 5.01; N, 5.81. ESI-HRMS (m/z), calcd. for C28H24O2N2NaS2 [M + Na]+ 507.11714, found 507.11646.
3.3. Antiviral Biological Assay
3.3.1. Purification of TMV and CMV
TMV and CMV were inoculated in Nicotiana tabacum cv. K326, and purified by the Gooding method [40].
3.3.2. Curative Activities of Compounds against TMV and CMV In Vivo
TMV and CMV (at a concentration of 6 μg/mL) were inoculated on the whole leaves of the same growing leaves of Nicotiana tabacum L. After 1 h, the leaves were washed with water, and after drying, the compound solution was smeared on the left side of leaf and the solvent was smeared on the right side for control. The local lesion numbers were recorded 3 to 4 days after inoculation [22,39].
3.3.3. Protective Activities of Compounds against TMV and CMV In Vivo
The solution of the compound was smeared on the left side of leaf, while the solvent was served as control on the right side of leaf. The leaves were inoculated with 6 μg/mL TMV and CMV after 12 h. Then, the leaves were washed with water. The number of local lesions numbers was counted after 3 to 4 days [22,39].
3.3.4. Inactivation Activities of Compounds aga inst TMV and CMV In Vivo
TMV and CMV was inhibited by mixing with the compound solution at the same volume for 30 min, respectively. Then, it was inoculated on the left side of leaf, and the right side of the leaf was inoculated with solvent and virus mixture as the control. The local lesion numbers were recorded 3 to 4 days after inoculation [22,39]. The in vivo inhibition rates of the compound were calculated based on the following formula (“av” means average). Inhibition rate (%) = [(av local lesion no. of control − av local lesion no. smeared with drugs)/av local lesion no. of control] × 100%. Three replications were conducted for each compound.
4. Conclusions
In summary, a serial of 1,4-pentadien-3-one derivatives containing a 1,3,4-thiadiazole moiety were designed for the development of antiviral agents, and twenty novel compounds were synthesized using optimized reaction conditions. The assay results demonstrated that most of compounds exhibited remarkable antiviral activities against TMV and CMV, among which compounds 4b, 4h, 4i, 4k, 4o, and 4q exhibited good curative, protection, and inactivation activity against TMV. Especially, compounds 4h, 4i, 4k, 4l, 4o, and 4q displayed excellent protection activity against TMV, with the EC50 values of 105.01, 254.77, 135.38, 297.40, 248.18, and 129.87 μg/mL, respectively, which were better than that of ribavirin (457.25 µg/mL). Preliminary SARs illuminated that small electron-withdrawing groups on the aromatic ring were favorable for antiviral activity at the same position. This finding suggests that 1,4-pentadien-3-one derivatives containing a 1,3,4-thiadiazole moiety may be used as potential lead structures for development of new antiviral agents.
Acknowledgments
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (Nos. 21362004 and 21562013) and Subsidy Project for Outstanding Key Laboratory of Guizhou Province in China (20154004) and Collaborative Innovation Center for Natural Products and Biological Drugs of Yunnan for supporting the project.
Supplementary Materials
The following are available online. The data and spectrogram of compounds 4a–4t.
Author Contributions
Deyu Hu conceived and designed the experiments. Lu Yu and Xiuhai Gan performed the experiments and analyzed the data; Dagui Zhou and Fangcheng He evaluated the antiviral activities of the title compounds. Song Zeng provided the material for evaluating the antiviral activities. All authors contributed to this study, read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 4a–4t are available from the authors.
References
- 1.Bos L. 100 years of virology: From vitalism via molecular biology to genetic engineering. Trends Microbiol. 2000;8:82–87. doi: 10.1016/S0966-842X(99)01678-9. [DOI] [PubMed] [Google Scholar]
- 2.Jacquemond M. Cucumber mosaic virus. Adv. Virus Res. 2012;84:439–504. doi: 10.1016/B978-0-12-394314-9.00013-0. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z.W., Wang L., Ma S., Liu Y.X., Wang L.Z., Wang Q.M. Design, synthesis, antiviral activity, and SARs of 1,4-Aminophenanthroindolizidines. J. Agric. Food Chem. 2012;60:5825–5831. doi: 10.1021/jf3013376. [DOI] [PubMed] [Google Scholar]
- 4.Sidwell R.W., Huffman J.H., Khare G.P., Allen L.B., Witkowski J.T., Robins R.K. Broad-spectrum antiviral activity of virazole: 1-β-d-Ribofuranosyl-1,2,4-triazole-3- carboxamide. Science. 1972;177:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- 5.Li Y.M., Zhang Z.K., Jia Y.T., Shen Y.M., He H.P., Fang R.X., Chen X.Y., Hao X.J. 3-Acetonyl-3-hydroxyoxindole: A new inducer of systemic acquired resistance in plants. Plant Biotechnol. J. 2008;6:301–308. doi: 10.1111/j.1467-7652.2008.00322.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z.W., Wei P., Wang L.Z., Wang Q.M. Design, synthesis, and anti-tobacco mosaic virus (TMV) activity of phenanthroindolizidines and their analogues. J. Agric. Food Chem. 2012;60:10212–10219. doi: 10.1021/jf303550a. [DOI] [PubMed] [Google Scholar]
- 7.Ge Y.H., Liu K.X., Zhang J.X., Mu S.Z., Hao X.J. The limonoids and their antitobacco mosaic virus (TMV) activities from Munronia unifoliolata Oliv. J. Agric. Food Chem. 2012;60:4289–4295. doi: 10.1021/jf205362d. [DOI] [PubMed] [Google Scholar]
- 8.Chen J., Yan X.H., Dong J.H., Sang P., Fang X., Di Y.T., Zhang Z.K., Hao X.J. Tobacco mosaic virus (TMV) inhibitors from Picrasma quassioides Benn. J. Agric. Food Chem. 2009;57:6590–6595. doi: 10.1021/jf901632j. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y.P., Zhao W., Xia Z.Y., Kong G.H., Lu X.P., Hu Q.F., Gao X.M. Three novel xanthones from Garcinia paucinervis and their anti-TMV activity. Molecules. 2013;18:9663–9669. doi: 10.3390/molecules18089663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q.M., Yao Y.C., Huang R.Q., Fan Z.J., Li G.R., Yu X.S. Antiviral activity of antofine from Cynanchum komarovii. Agrochemicals. 2007;46:425–427. [Google Scholar]
- 11.Wang Z.W., Wei P., Xu X.Z., Liu Y.X., Wang L.Z., Wang Q.M. Design, synthesis, and antiviral activity evaluation of phenanthrene-based antofine derivatives. J. Agric. Food Chem. 2012;60:8544–8551. doi: 10.1021/jf302746m. [DOI] [PubMed] [Google Scholar]
- 12.Mishra S., Narain U., Mishra R., Misra K. Design, development and synthesis of mixed bioconjugates of piperic acid-glycine, curcumin-glycine/alanine and curcumin-glycine-piperic acid and their antibacterial and antifungal properties. Bioorg. Med. Chem. 2005;13:1477–1486. doi: 10.1016/j.bmc.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborti S., Das L., Kapoor N., Das A., Dwivedi V., Poddar A., Chakraborti G., Janik M., Basu G., Panda D., et al. Curcumin recognizes a unique binding site of tubulin. J. Med. Chem. 2011;54:6183–6196. doi: 10.1021/jm2004046. [DOI] [PubMed] [Google Scholar]
- 14.Venkateswarlu S., Ramachandra M.S., Subbaraju G.V. Synthesis and biological evaluation of polyhydroxycurcuminoids. Bioorg. Med. Chem. 2005;13:6374–6380. doi: 10.1016/j.bmc.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 15.Burke T.R., Jr., Fesen M., Mazumder A., Yung J., Wang J., Carothers A.M., Grunberger D., Driscoll J., Pommier Y., Kohn K. Hydroxylated aromatic inhibitors of HIV-1 integrase. J. Med. Chem. 1995;38:4171–4178. doi: 10.1021/jm00021a006. [DOI] [PubMed] [Google Scholar]
- 16.Mazumder A., Wang S.M., Neamati N., Nicklaus M., Sunder S., Chen J.L., Milne G.W.A., Rice W.G., Burke T.R., Jr., Pommier Y. Antiretroviral agents as inhibitors of both human immunodeficiency virus type 1 integrase and protease. J. Med. Chem. 1996;39:2472–2481. doi: 10.1021/jm960074e. [DOI] [PubMed] [Google Scholar]
- 17.Luo H., Liu J.J., Jin L.H., Hu D.Y., Chen Z., Yang S., Wu J., Song B.A. Synthesis and antiviral bioactivity of novel (1E,4E)-1-aryl-5-(2-(quinazolin-4-yloxy)phenyl)-1,4-pentadien-3-one derivatives. Eur. J. Med. Chem. 2013;63:662–669. doi: 10.1016/j.ejmech.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 18.Ma J., Li P., Li X.Y., Shi Q.C., Wan Z.H., Hu D.Y., Jin L.H., Song B.A. Synthesis and antiviral bioactivity of novel 3-((2-((1E,4E)-3-oxo-5-arylpenta-1,4-dien-1-yl)phenoxy)methyl)-4(3H)-quinazolinone Derivatives. J. Agric. Food Chem. 2014;62:8928–8934. doi: 10.1021/jf502162y. [DOI] [PubMed] [Google Scholar]
- 19.Han Y., Ding Y., Xie D.D., Hu D.Y., Li P., Li X.Y., Xue W., Jin L.H., Song B.A. Design, synthesis and antiviral activity of novel rutin derivatives containing 1,4-pentadien-3-one moiety. Eur. J. Med. Chem. 2015;92:732–737. doi: 10.1016/j.ejmech.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Long C.W., Li P., Chen M.H., Dong L.R., Hu D.Y., Song B.A. Synthesis, anti-tobacco mosaic virus and cucumber mosaic virus activity, and 3D-QSAR study of novel 1,4-pentadien-3-one derivatives containing 4-thioquinazoline moiety. Eur. J. Med. Chem. 2015;102:639–647. doi: 10.1016/j.ejmech.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Chen M.H., Hu D.Y., Li X.Y., Yang S., Zhang W.Y., Li P., Song B.A. Antiviral activity and interaction mechanisms study of novel glucopyranoside derivatives. Bioorg. Med. Chem. Lett. 2015;25:3840–3844. doi: 10.1016/j.bmcl.2015.07.068. [DOI] [PubMed] [Google Scholar]
- 22.Wu F., Li P., Hu D.Y., Song B.A. Design, synthesis, and antiviral activity of novel purine derivatives containing 1,4-pentadien-3-one moiety. Res. Chem. Intermed. 2016;42:7153–7168. doi: 10.1007/s11164-016-2524-9. [DOI] [Google Scholar]
- 23.Hu Y., Li C.Y., Wang X.M., Yang Y.H., Zhu H.L. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014;114:5572–5610. doi: 10.1021/cr400131u. [DOI] [PubMed] [Google Scholar]
- 24.Farghaly T.A., Abdallah M.A., Abdel Aziz M.R. Synthesis and antimicrobial activity of aome new 1,3,4-thiadiazole derivatives. Molecules. 2012;17:14625–14636. doi: 10.3390/molecules171214625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oruc E.E., Rollas S., Kandemirli F., Shvets N., Dimoglo A.S. 1,3,4-thiadiazole derivatives. synthesis, structure elucidation, and structure-antituberculosis activity relationship investigation. J. Med. Chem. 2004;47:6760–6767. doi: 10.1021/jm0495632. [DOI] [PubMed] [Google Scholar]
- 26.Chapleo C.B., Myers P.L., Smith A.C., Tulloch I.F., Walter D.S. Substituted 1,3,4-thiadiazolewith anticonvulsant activity. J. Med. Chem. 1987;30:951–954. doi: 10.1021/jm00388a038. [DOI] [PubMed] [Google Scholar]
- 27.Li P., Shi L., Yang X., Yang L., Chen X.W., Wu F., Shi Q.C., Xu W.M., He M., Hu D.Y., et al. Design, synthesis, and antibacterial activity against rice bacterial leaf blightand leaf streak of 2,5-substituted-1,3,4-oxadiazole/thiadiazolesulfonederivative. Bioorg. Med. Chem. Lett. 2014;24:1677–1680. doi: 10.1016/j.bmcl.2014.02.060. [DOI] [PubMed] [Google Scholar]
- 28.Rebolledo C.L., Sotelo-Hitschfeld P., Brauchi S., Olavarría M.Z. Design and synthesis of conformationally restricted capsaicin analogues based in the 1,3,4-thiadiazole heterocycle reveal a novel family of transient receptor potential vanilloid 1 (TRPV1) antagonists. Eur. J. Med. Chem. 2013;66:193–203. doi: 10.1016/j.ejmech.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Moise M., Sunel V., Profire L., Popa M., Desbrieres J., Peptu C. Synthesis and biological activity of some new 1,3,4-thiadiazole and 1,2,4-triazole compounds containing a phenylalanine moiety. Molecules. 2009;14:2621–2631. doi: 10.3390/molecules14072621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomha S.M., Kheder N.A., Abdelhamid A.O., Mabkhot Y.N. One pot single step synthesis and biological evaluation of some novel bis(1,3,4-thiadiazole) derivatives as potential cytotoxic agents. Molecules. 2016;21:1532. doi: 10.3390/molecules21111532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flefel E.M., El-Sayed W.A., Mohamed A.M., El-Sofany W.I., Awad H.M. Synthesis and anticancer activity of new 1-thia-4-azaspiro[4.5]decane, their derived thiazolopyrimidine and 1,3,4-thiadiazole thioglycosides. Molecules. 2017;22:170. doi: 10.3390/molecules22010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altıntop M.D., Can Ö.D., Özkay Ü.D., Kaplancıklı U.A. Synthesis and evaluation of new 1,3,4-thiadiazole derivatives as antinociceptive agents. Molecules. 2016;21:1004. doi: 10.3390/molecules21081004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Sadek M.M., Hassan S.Y., Abdelwahab H.E., Yacout G.A. Synthesis of new 1,3,4-thiadiazole and 1,2,3,4-oxathiadiazole derivatives from carbohydrate precursors and study of their effect on tyrosinase enzyme. Molecules. 2012;17:8378–8396. doi: 10.3390/molecules17078378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clerici F., Pocar D., Maddalena G., Loche A., Perlini V., Brufani M. Synthesis of 2-amino-5-sulfanyl-1,3,4-thiadiazole derivatives and evaluation of their antidepressant and anxiolytic activity. J. Med. Chem. 2001;44:931–936. doi: 10.1021/jm001027w. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z., Xu W.M., Liu K.M., Yang S., Fan H.T., Bhadury P.S., Hu D.Y., Zhang Y.P. Synthesis and antiviral activity of 5-(4-chlorophenyl)-1,3,4-thiadiazole sulfonamides. Molecules. 2010;15:9046–9056. doi: 10.3390/molecules15129046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu W.M., Li S.Z., He M., Yang S., Li X.Y., Li P. Synthesis and bioactivities of novel thioether/sulfone derivatives containing 1,2,3-thiadiazole and 1,3,4-oxadiazole/thiadiazole moiety. Bioorg. Med. Chem. Lett. 2013;23:5821–5824. doi: 10.1016/j.bmcl.2013.08.107. [DOI] [PubMed] [Google Scholar]
- 37.Gan X.H., Hu D.Y., Li P., Wu J., Chen X.W., Xue W., Song B.A. Design, synthesis, antiviral activity and three-dimensional quantitative structure-activity relationship study of novel 1,4-pentadien-3-one derivatives containing the 1,3,4-oxadiazole moiety. Pest Manag. Sci. 2016;72:534–543. doi: 10.1002/ps.4018. [DOI] [PubMed] [Google Scholar]
- 38.Sauer A.C., Leal J., Stefanello S.T., Leite M.T.B., Souza M.B., Soares F.A.A., Rodrigues O.E.D., Dornelles L. Synthesis and antioxidant properties of organosulfur and organoselenium compounds derived from 5-substituted-1,3,4-oxadiazole/thiadiazole-2-thiols. Tetrahedron Lett. 2017;58:87–91. doi: 10.1016/j.tetlet.2016.11.106. [DOI] [Google Scholar]
- 39.Song B.A., Zhang H.P., Wang H., Yang S., Jin L.H., Hu D.Y., Pang L.L., Xue W. Synthesis and antiviral activity of novel chiral cyanoacrylate derivatives. J. Agric. Food. Chem. 2005;53:7886–7891. doi: 10.1021/jf051050w. [DOI] [PubMed] [Google Scholar]
- 40.Gooding G.V., Jr., Hebert T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology. 1967;57:1285–1287. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.