Abstract
Copper-64 is a useful radioisotope for positron emission tomography (PET). Due to the wide range of applications, the demand of 64Cu with low metallic impurities is increasing. Here we report a simple method for the efficient production of high specific activity 64Cu using a cyclotron for biomedical application. We designed new equipment based on the plating of enriched 64Ni as the target, and used automated ion exchange chromatography to purify copper-64 efficiently after irradiation and dissolution of the target in good radiochemical and chemical yield and purity. The 64Cu radionuclide produced using 99.32% enriched 64Ni with a density of 61.4 ± 5.0 mg/cm2, reaching a total radioactivity greater than 200 mCi, with specific activity up to 5.6 GBq/μmoL. It was further incorporated into modified monoclonal antibody DOTA-rituximab to synthesize 64Cu-DOTA-rituximab, which was used successfully for micro-PET imaging.
Keywords: copper-64, solid target, Rituximab, positron emission tomography (PET)
1. Introduction
Copper-64 (64Cu) is an attractive radionuclide of considerable interest for positron emission tomography (PET) imaging and radiotherapy due to its intrinsic physical and chemical properties. It has high spatial resolution comparable to 18F radionuclide, with comparable average free travel distance for their generated positrons (Rave.(β+) = 0.70 and 0.69 mm, respectively), due to their comparable positron energy (0.656 MeV and 0.635 MeV, respectively) [1,2]. It has a relatively long half-life of 12.7 h, compared with fluorine-18 (t1/2 = 110 min) and carbon-11 (t1/2 = 20.4 min). In addition, 64Cu also emits β− and Auger electrons, enabling it to be useful for both PET imaging and radiotherapy. Moreover, the versatile coordination chemistry of 64Cu allows for its reaction with a wide variety of chelator systems, such as DOTA, NOTA, TETA and CB-TE2A, that can be linked to antibodies, peptides and nanoparticles [3]. The 64Cu radioisotope can be used for the design and synthesis of a wide range of radio-probes, providing attractive candidates for PET imaging.
A number of radiotracers involving 64Cu as radionuclide have been applied in nuclear medicine as a means of studying their PET imaging [4,5,6]. Incorporation of 64Cu into diacetyl-bis(N4-methylthiosemicarbazone) (ATSM) ligand was used for PET hypoxia imaging, such as in head and neck cancer, and cardiac conditions [7,8]. More radiotracers are in clinical development [9,10,11,12], especially those with peptides and antibodies. For example, 64Cu-DOTA-trastuzumab was used to conduct PET imaging of HER2-positive lesions in patients with primary and metastatic breast cancer [13]. The PET image of 64Cu-DOTATATE provided superior image quality, and detected more lesions than 111In-DTPA-octreotide [14]. Grubmüller, B. et al. investigated the diagnostic potential of 64Cu-PSMA-617 in patients with prostate adenocarcinoma [15].
64Cu has been produced at many centers [16,17,18,19]. Among the nuclear reactions examined, the 64Ni (p, n) 64Cu method is the best and widely used, since high production yield of the 64Cu can be obtained with low energy protons in this route [20,21]. At Washington University, an effective method was investigated to produce high specific activity 64Cu on a small biomedical cyclotron using the 64Ni (p, n) 64Cu nuclear reaction, and 64Cu has been produced for more than 17 years by the irradiation of electroplated enriched 64Ni targets in this center [20,22]. The Turku PET Centre has been producing 64Cu since 2008 using 64Ni (p, n) 64Cu reaction, and also handles the irradiated target, radioactive liquids and gases using automated equipment [23,24]. At the University of Wisconsin, 64Cu and 61Co radionuclides have been simultaneously produced using the 64Ni (p, n) 64Cu nuclear reaction on a low energy proton-only cyclotron [25]. Ohya, T. et al. (2016) produced high-quality 64Cu for routine use via an electrodeposited 64Ni target, and successfully reduced the metallic impurities level of the 64Cu product, such as Co and Ni [21]. Other nuclear reactions examined include 64Ni (d, 2n) 64Cu, 64Zn (d, 2p) 64Cu, 64Zn (n, p) 64Cu [26,27]. In China, researchers also have a growing interest in 64Cu, and the demand of no-carrier added 64Cu has started to increase.
Here we report a robust, reliable and user-friendly plating vessel, which can be used for the effective preparation of the 64Ni solid target. The production of 64Cu was performed on a Sumitomo HM-20 biomedical cyclotron (20 MeV) via the 64Ni (p, n) 64Cu reaction. The γ-ray spectroscopy of the produced 64Cu solution was also measured to evaluate the radionuclide impurities. After 64Cu was purified, a labeling experiment to synthesize 64Cu-DOTA-Rituximab was performed to examine the quality of 64Cu, including labeling yield and radiochemical purity of the radiotracer, which targets the CD20 antigen, which is expressed on B cell lymphocytes and in the majority of non-Hodgkin’s lymphoma (NHL) [28]. The synthesized radiotracer was further examined by micro-PET imaging using SCID (severe combined immune deficiency) mice bearing Ramos RA1 tumors which overexpress CD20 antigen.
2. Results and Discussion
2.1. Preparation of 64Ni Target
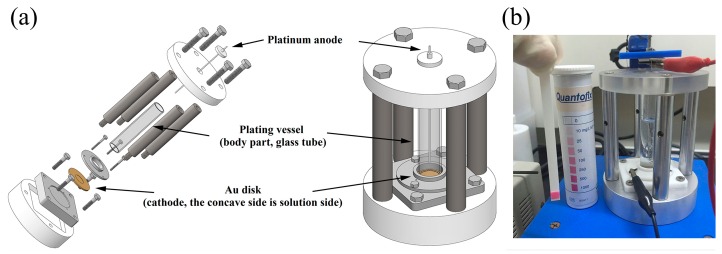
In order to make 64Cu via the nuclear reaction of 64Ni (p, n) 64Cu, we made the enriched 64Ni targets on a gold (Au) disk by electrodeposition of 64Ni from an aqueous solution of Ni(NH3)62+ at pH = 9.05, using a robust, reliable and user-friendly apparatus (Figure 1). The Au disk (30.8 mm diameter × 1.5 mm thickness) was used as a cathode, and a platinum wire was used as an anode. A cavity of 12.1 mm diameter and 0.2 mm depth was milled into the Au disk and 64Ni (99.32%) was plated into this cavity. The 64Ni electroplating was performed with a constant current of 15–25 mA at 2.4–2.6 V in the aqueous solution for 48–72 h. During the electrodeposition, the green color of the 64Ni plating solution gradually faded away and the bubbling of H2 gas was clearly visible. When the 64Ni plating solution became colorless, the electrodeposition was finished. The absence of Ni2+ was also confirmed by analytical test strips. After cleaning and drying, the plated 64Ni on Au-disk was 70.6 ± 5.8 mg and the density of the plated 64Ni was 61.4 ± 5.0 mg/cm2, assuming a uniform thickness on the disk.
Figure 1.
The 64Ni plating vessel. (a) Illustration of new 64Ni plating vessel by scheme; (b) Picture of the actual electric plating unit.
2.2. Quality Control of 64Ni Target
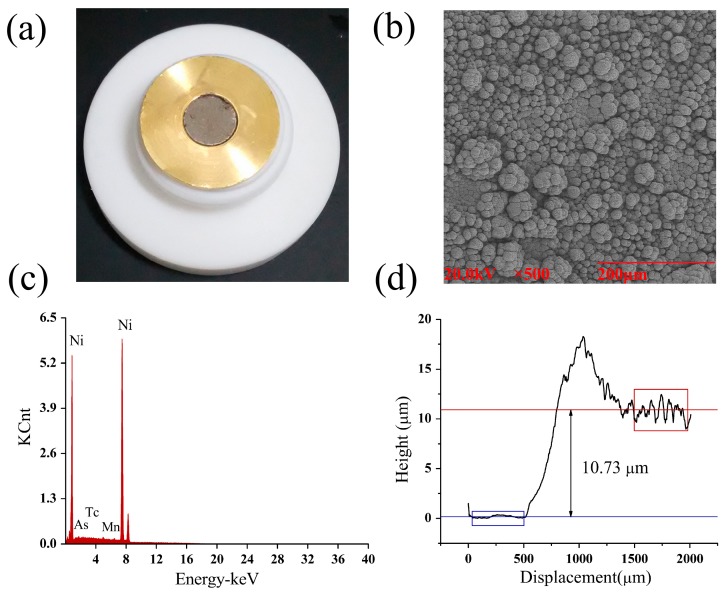
The quality of the 64Ni solid target prepared above was evaluated by a number of physical techniques, to determine the uniformity, to characterize the metallic impurities, and to measure the thickness of 64Ni layer on Au-disk. The SEM (scanning electron microscopy) image (Figure 2b) of the 64Ni solid target showed the uniform layer of Ni on Au surface. The EDS (energy dispersive X-ray spectroscopy) (Table 1 and Figure 2c) results showed no significant amount of metallic impurities in the 64Ni layer. The 64Ni target thickness on Au-disk was measured to be 10.73 μm by alpha step apparatus (Figure 2d).
Figure 2.
The 64Ni target produced in this study. (a) Photo of the 64Ni target; (b) The SEM image of the 64Ni target; (c) The EDS spectrum of the 64Ni target; (d) The thickness measurement of the 64Ni solid target.
Table 1.
The metallic impurities in the plated 64Ni target, analyzed by EDS.
| Element | Wt % | At % |
|---|---|---|
| AsL | 00.00 | 00.00 |
| TcL | 00.15 | 00.09 |
| MnK | 00.07 | 00.07 |
| NiK | 99.78 | 99.84 |
2.3. Preparation of 64Cu
After irradiation of 5 h, the 64Ni target was dissolved in 6 M hydrochloride acid, and then the solution was load to an anion exchange column to separate into different components. The 64Ni was washed out with 6 M HCl and collected for recycling. Due to the elevated cost of enriched 64Ni, recycling of the target material for re-use could reduce the production cost of 64Cu, without sacrificing the quality of subsequent 64Cu production. When the eluted was switched to 1 M HCl, the first band coming out was co-produced cobalt radioisotopes (approximately 1 mL), and the second was the 64Cu, which was collected and evaporated to dryness. The residue was dissolved in 0.1 M HCl for further use. The separation process of 64Cu took about 2.5 h after irradiation.
2.4. Quality of 64Cu
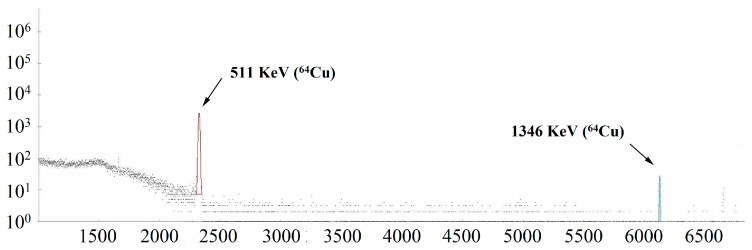
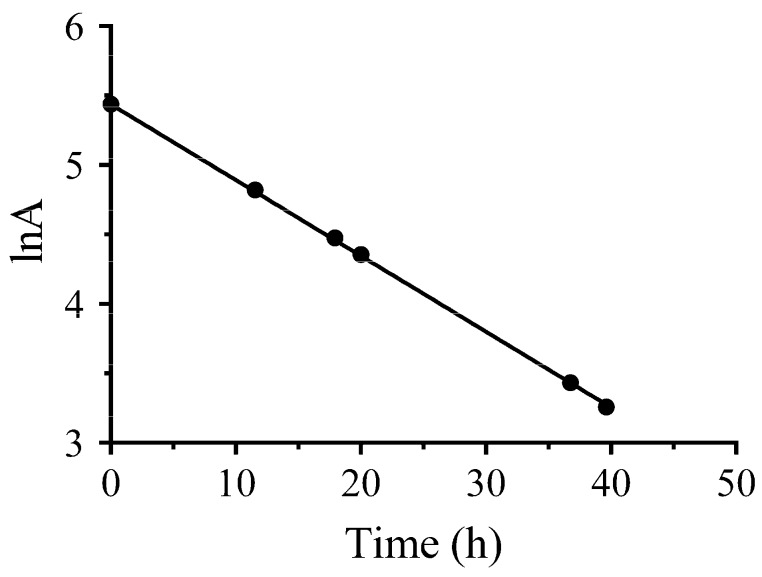
The quality of 64Cu produced was evaluated by analysis of its metallic impurities and measurement of half-life of its radioactivity. The inductively coupled plasma-mass spectrometry (ICP-MS) analysis was used to evaluate the amount of metallic impurities in a decayed 64Cu solution, and showed a concentration of 6.339 ppb of Ni (0.127 μg), 4.112 ppb of Cu (0.082 μg), 5.502 ppb of Zn (0.110 μg), 0.108 ppb of Fe (0.002 μg), 0.102 ppb of Co (0.002 μg), 0.669 ppb of Ga (0.013 μg). The gamma spectrum of the produced 64CuCl2 solution (Figure 3) showed that the 64Cu radionuclide purity was >99%. The half-life of the produced radioactivity was determined by the radioactivity measured at different time points into the following equations:
| (1) |
where A is the radioactivity of the 64Cu at time t, A0 is the radioactivity of the 64Cu at 0 h, and λ represents the constant. We obtained the following equation (Figure 4):
| (2) |
when , the
| (3) |
Figure 3.
Gamma spectra of 64CuCl2 solution after purification.
Figure 4.
The radioactivity of the produced 64Cu measured at different time points vs. time, with fitted equation, on a logarithmic scale.
The half-life of the produced 64Cu was calculated:
The half-life calculated ( h) of the produced 64Cu is in accordance with that of the radioisotope 64Cu ( h).
2.5. Radio-Synthesis of 64Cu-DOTA-Rituximab and Micro-PET Imaging
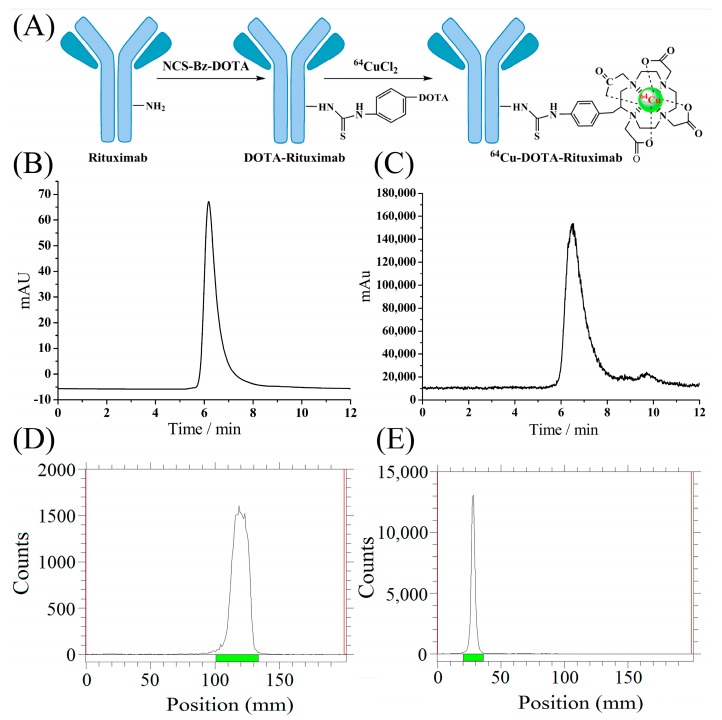
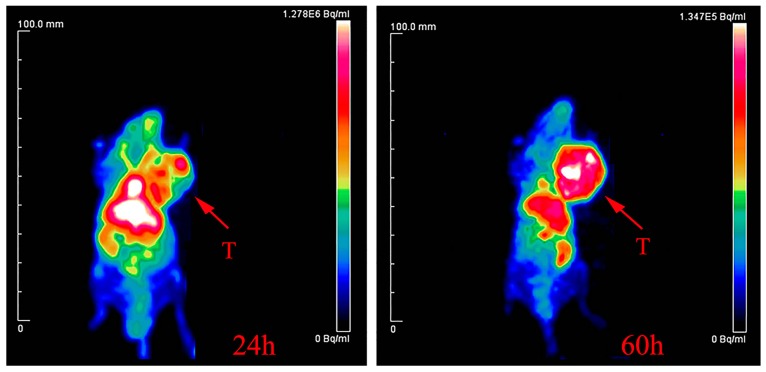
To assess the quality and quantity of the produced 64Cu, we made 64Cu-DOTA-rituximab (Figure 5), with high chemical yield, high radiochemical purity, and high specific activity. Micro-PET imaging of the radiotracer in mice bearing Ramos RA1 tumors clearly showed the tumor at 24 h and 60 h post-injection, with excellent resolution and clarity at the latter (Figure 6).
Figure 5.
Radio-synthesis of 64Cu-DOTA-rituximab. (A) Modification of Rituximab and radiolabeling by 64Cu radionuclide; (B) The Radio-HPLC (radioactive high performance liquid chromatography) chromatograph of rituximab; (C) The Radio-HPLC chromatograph of 64Cu-DOTA-rituximab after purification by PD-10 column; (D) The Radio-TLC (radioactive thin-layer chromatography) image of 64CuCl2; (E) The Radio-TLC image of 64Cu-DOTA-rituximab after purification by PD-10 column.
Figure 6.
Micro-PET image of 64Cu-DOTA-rituximab in SCID mice bearing Ramos RA1 tumors at 24 h and 60 h post-intravenous injection. The arrows indicate the location of tumor.
3. Materials and Methods
3.1. Materials and Reagents
High purity reagents were used for production of 64Cu in this study. Isotopically enriched 64Ni (99.32% 64Ni; 0.13% 58Ni; 0.07% 60Ni; 0.01% 61Ni; 0.47% 62Ni) was from Isoflex Company (San Francisco, CA, USA); (NH4)2SO4 (99.999% metals basis) from Alfa Aesar (Ward Hill, MA, USA); concentrated HCl (99.999% trace metals basis), HNO3 (99.999% trace metals basis), and NH4OH (99.99% trace metals basis) were purchased from Sigma-Aldrich (St. Louis, MO, USA); AG®1-X8 ion exchange resin was from Bio-Rad Laboratories (Hercules, CA, USA); platinum wire (99.997% metals basis) from Alfa Aesar (Ward Hill, MA, USA); Ni2+ analytical test strips from Qtantofix (Sigma-Aldrich, St. Louis, MO, USA); disposable PD-10 desalting columns were from GE Healthcare (Piscataway, NJ, USA).
3.2. Equipment
The alpha step apparatus (Alpha-step IQ, KLA-Tencor, Milpitas, CA, USA), scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDS) (1910FE, AMRAY, Pawtucket, RI, USA) were used to characterize the quality of the 64Ni solid target produced in this study. The irradiation experiments were performed using a Sumitomo HM-20 cyclotron (20 MeV, Sumitomo Heavy Industries, Ltd., Tokyo, Japan). The separation of 64Cu was performed using a 64Cu separation system (Industrial Equipment Division, Sumitomo Heavy Industries, Ltd., Tokyo, Japan). Inductively coupled plasma-mass spectrometry (ICP-MS) (ELEMENT XR mass spectrometer, Thermo Fisher, Bremen, Germany) was used to analyze the purity of the 64Cu sample. The Agilent Technologies 1200 series of high performance liquid chromatography (HPLC) system (Agilent, Lake Forest, CA, USA) equipped with both a UV absorption detector and a B-Fc-1000 HPLC radioactivity detector (Bioscan, Washington, DC, USA) and the radioactive thin-layer chromatography scanner (Radio-TLC) (Bioscan, IAR-2000, Washington, DC, USA) were used to analyze the radiochemical purity of tracers.
3.3. Plating Solution
The 64Ni electroplating solution was prepared as reported previously with some modifications [25]. The enriched 64Ni metal (65–80 mg) was dissolved in 5 mL of warm 6 M HNO3. After the metal was completely dissolved, the solution was then evaporated to dryness under vacuum. The green-colored residue was dissolved in 300 µL of concentrated H2SO4 and the solution then diluted with 2 mL of 18 MΩ·cm water (Milli-Q Waters, Millipore Corporation, Billerica, MA, USA) slowly and carefully. The pH of the solution was adjusted to 9.05 ± 0.05 by adding about 1.5 mL concentrated NH4OH. To this solution, ~300 mg of (NH4)2SO4 was added and the volume of the solution was adjusted to 5 mL with 18 MΩ·cm water. The final solution was transferred to the electroplating cell for target plating.
3.4. Characterizations of Ni-64 Target
After electroplating, the 64Ni solid target was examined by measuring 64Ni thickness, composition and structure. The thickness was measured by alpha step apparatus, the composition was measured by scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDS), and the structure was characterized by scanning electron microscopy (SEM). Meanwhile, the identity of metallic impurities of the 64Ni target was further analyzed by energy dispersive X-ray spectroscopy (EDS).
3.5. Preparation of 64Ni Target and Irradiation
The 64Ni targets were prepared by electrodeposition of the enriched 64Ni (99.32%) solution prepared as described above. Electroplating of 64Ni was realized using a plating vessel of our own design and manufacture (Figure 1), where the Au disk was used as a cathode and platinum wire as an anode. The electrodeposition of 64Ni was achieved at 2.4–2.6 V and 15–25 mA with the platinum anode at ~1 cm from the Au disk electrode. This process took 48–72 h. The enriched 64Ni target was irradiated at 12.5 MeV (which decreased by Al) with 20 μA current on a Sumitomo HM-20 cyclotron (20 MeV) for about 5–7 h. 64Cu was produced from the 64Ni (p, n) 64Cu nuclear reaction.
3.6. Radiochemical Separation
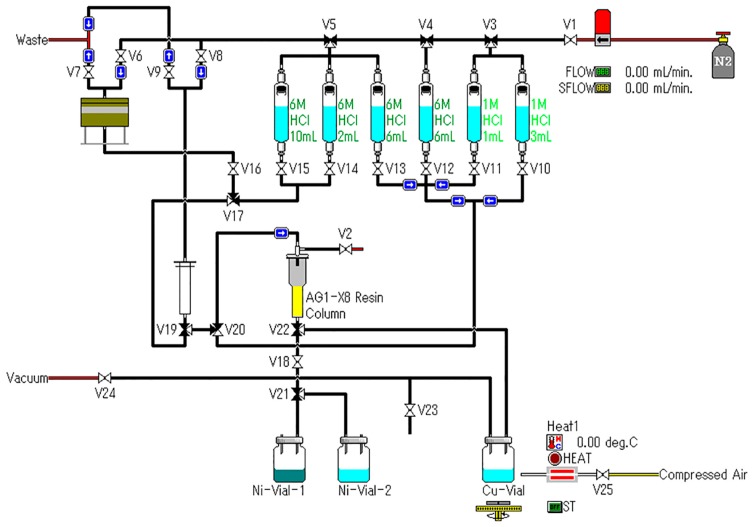
After irradiation, the 64Ni target was placed into the dissolving bath of 6 M HCl. The complete dissolution of the target material took about 40 min under heating, and then the solution was loaded on an anion exchange column (AG 1-X8) pretreated with 18 MΩ·cm water and 6 M HCl, sequentially. The enriched 64Ni was eluted with 6 M HCl and collected for recycling, and the 64Cu fraction was eluted with 1 M HCl, which was further evaporated to dryness. The residue was finally dissolved in 0.1 M HCl for further use. All of these procedures were performed using an automated system and carried out in a hot cell with remote control (Figure 7, Sumitomo Heavy Industries, Ltd., Tokyo, Japan).
Figure 7.
Schematic representation of the automated 64Cu separation system.
3.7. Radio-nuclide Analysis
The radionuclide identity and purity of the produced 64CuCl2 solution were measured using γ-ray spectroscopy (HTA Co., Ltd., Beijing, China). In addition, the identity of radioactivity of the produced 64Cu was further confirmed by measurement of its half-life, by measuring radioactivity at different time points. A decayed sample from the produced 64Cu was also analyzed by inductively coupled plasma-mass spectrometry (ICP-MS) for traces of metallic impurities.
3.8. Radiolabeling of DOTA-Rituximab and Micro-PET Imaging
To assess the quality of the produced 64Cu (quantity, specific activity and purity), the synthesis of a radiotracer, 64Cu-DOTA-rituximab, was performed. Here, the site-specific modification of monoclonal antibody DOTA-rituximab which contains two DOTA chelator in each antibody was used as we previously reported [29]. The 64CuCl2 solution prepared above was reacted with DOTA-rituximab in a solution of pH = 5.5 at RT for 30 min. After incubation, the radiotracer was purified using a PD-10 desalting column, and characterized by Radio-TLC and Radio-HPLC, to determine the labeling yield and radiochemical purity. The identity of the radiotracer, 64Cu-DOTA-rituximab, was further evaluated by PET imaging, which was carried out on a micro-PET rodent model scanner as reported earlier [29]. After formulation, 64Cu-DOTA-rituximab (0.5 mCi) was injected intravenously into the tail vein of the mice bearing Ramos RA1 tumors (n = 3), which overexpress CD20 antigen, and the animals were imaged with micro-PET at both 24 and 60 h post-injection.
4. Conclusions
In this study, we presented the improved method for the preparation of 64Cu, especially the improved efficiency of electroplating of 64Ni. The 64Ni solid target on an Au-disk has a uniform surface, with the thickness of 10.73 μm, and no metallic impurities. The total plated 64Ni was 70.6 ± 5.8 mg and the density of the plated 64Ni was 61.4 ± 5.0 mg/cm2 (assuming a uniform thickness). After irradiation of the target and purification, the gamma spectrum of the produced 64Cu showed its radionuclide purity to be >99%, with both peaks at 511 keV and 1346 keV. In addition, the produced 64CuCl2 solution has high specific activity up to 5.6 GBq/μmoL. It was further incorporated into the modified monoclonal antibody DOTA-rituximab to synthesize 64Cu-DOTA-rituximab, which was further used successfully for micro-PET imaging.
Acknowledgments
This work was supported, in part, by the National Natural Science Foundation of China (No. 81371592, No. 81401467, No. 81501519, No. 81571705 and No. 81671733), Beijing Natural Science Foundation (No. 7162041 and No. 7171002), Beijing Municipal Commission of Health and Family Planning (215 backbone program), and 2017 Beijing Science & Technology Nova Star Program (xx2017016).
Author Contributions
Q.X., H.Z., X.M., Q.R., C.X. and Z.Y. conceived and designed the experiments; Q.X., H.Z., F.W. and X.M. performed the experiments; Q.X., H.Z. and X.M. analyzed the data; Q.X. and H.Z. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Sample of the rituximab is available from the authors.
References
- 1.Nayak T.K., Brechbiel M.W. Radioimmunoimaging with longer-lived positron-emitting radionuclides: Potentials and challenges. Bioconjug. Chem. 2009;20:825–841. doi: 10.1021/bc800299f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland J.P., Sheh Y., Lewis J.S. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 2009;36:729–739. doi: 10.1016/j.nucmedbio.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wadas T.J., Wong E.H., Weisman G.R., Anderson C.J. Copper chelation chemistry and its role in copper radiopharmaceuticals. Curr. Pharm. Des. 2007;13:3–16. doi: 10.2174/138161207779313768. [DOI] [PubMed] [Google Scholar]
- 4.Natarajan A., Arksey N., Iagaru A., Chin F.T., Gambhir S.S. Validation of 64Cu-dota-rituximab injection preparation under good manufacturing practices: A Pet tracer for imaging of B-cell non-Hodgkin lymphoma. Mol. Imaging. 2015;14:1–11. doi: 10.2310/7290.2014.00055. [DOI] [PubMed] [Google Scholar]
- 5.Kurihara H., Hamada A., Yoshida M., Shimma S., Hashimoto J., Yonemori K., Tani H., Miyakita Y., Kanayama Y., Wada Y., et al. 64Cu-DOTA-trastuzumab PET imaging and HER2 specificity of brain metastases in HER2-positive breast cancer patients. EJNMMI Res. 2015;5:8. doi: 10.1186/s13550-015-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanghera B., Wood K., Sonoda L.I., Gogbashian A., Lowe G., Nunes A., Stirling J., Shepherd C., Beynon G., Wong W.L. Pre-clinical positron emission tomography reconstruction algorithm effect on Cu-64 ATSM lesion hypoxia. Mol. Imaging Radionucl. Ther. 2016;25:19–25. doi: 10.4274/mirt.18189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grassi I., Nanni C., Cicoria G., Blasi C., Bunkheila F., Lopci E., Colletti P.M., Rubello D., Fanti S. Usefulness of 64Cu-ATSM in head and neck cancer: A preliminary prospective study. Clin. Nucl. Med. 2014;39:59–63. doi: 10.1097/RLU.0b013e3182a756f0. [DOI] [PubMed] [Google Scholar]
- 8.Handley M.G., Medina R.A., Mariotti E., Kenny G.D., Shaw K.P., Yan R., Eykyn T.R., Blower P.J., Southworth R. Cardiac hypoxia imaging: Second-generation analogues of 64Cu-ATSM. J. Nucl. Med. 2014;55:488–494. doi: 10.2967/jnumed.113.129015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfeifer A., Knigge U., Binderup T., Mortensen J., Oturai P., Loft A., Berthelsen A.K., Langer S.W., Rasmussen P., Elema D., et al. 64Cu-DOTATATE PET for neuroendocrine tumors: A prospective head-to-head comparison with 111In-DTPA-octreotide in 112 patients. J. Nucl. Med. 2015;56:847–854. doi: 10.2967/jnumed.115.156539. [DOI] [PubMed] [Google Scholar]
- 10.Anderson C.J., Dehdashti F., Cutler P.D., Schwarz S.W., Laforest R., Bass L.A., Lewis J.S., Mccarthy D.W. 64Cu-Teta-octreotide as a pet imaging agent for patients with neuroendocrine tumors. J. Nucl. Med. 2001;42:213–221. [PubMed] [Google Scholar]
- 11.Chang A.J., Grigsby P.W. Differences in uptake and distribution of 64Cu-Atsm and 18 F-FDG in primary tumors of patients with squamous cell carcinoma of the cervix. Fuel Energy Abstr. 2010;78:S144. [Google Scholar]
- 12.Chang A.J. Intratumoral heterogeneity of 64Cu-ATSM uptake is a prognostic indicator in patients with cervical cancer. Omics J. Radiol. 2013;2:545–553. doi: 10.4172/2167-7964.1000130. [DOI] [Google Scholar]
- 13.Tamura K., Kurihara H., Yonemori K., Tsuda H., Suzuki J., Kono Y., Honda N., Kodaira M., Yamamoto H., Yunokawa M., et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 2013;54:1869–1875. doi: 10.2967/jnumed.112.118612. [DOI] [PubMed] [Google Scholar]
- 14.Pfeifer A., Knigge U., Mortensen J., Oturai P., Berthelsen A.K., Loft A., Binderup T., Rasmussen P., Elema D., Klausen T.L., et al. Clinical PET of neuroendocrine tumors using 64Cu-DOTATATE: First-in-humans study. J. Nucl. Med. 2012;53:1207–1215. doi: 10.2967/jnumed.111.101469. [DOI] [PubMed] [Google Scholar]
- 15.Grubmüller B., Baum R.P., Capasso E., Singh A., Ahmadi Y., Knoll P., Floth A., Righi S., Zandieh S., Meleddu C., et al. 64Cu-PSMA-617 PET/CT imaging of prostate adenocarcinoma: First in-human studies. Cancer Biother. Radiopharm. 2016;31:277–286. doi: 10.1089/cbr.2015.1964. [DOI] [PubMed] [Google Scholar]
- 16.Matarrese M., Bedeschi P., Scardaoni R., Sudati F., Savi A., Pepe A., Masiello V., Todde S., Gianolli L., Messa C., et al. Automated production of copper radioisotopes and preparation of high specific activity [(64)Cu]Cu-ATSM for PET studies. Appl. Radiat. Isot. 2010;68:5–13. doi: 10.1016/j.apradiso.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Burke P., Golovko O., Clark J.C., Aigbirhio F.I. An automated method for regular productions of copper-64 for PET radiopharmaceuticals. Inorg. Chim. Acta. 2010;363:1316–1319. doi: 10.1016/j.ica.2010.01.038. [DOI] [Google Scholar]
- 18.Rajec P., Csiba V., Leporis M., Štefečka M., Pataky E.L., Reich M., Ometáková J. Preparation and characterization of nickel targets for cyclotron production of 64Cu. J. Radioanal. Nucl. Chem. 2010;286:665–670. doi: 10.1007/s10967-010-0736-9. [DOI] [Google Scholar]
- 19.Thisgaard H., Jensen M., Elema D.R. Medium to large scale radioisotope production for targeted radiotherapy using a small PET cyclotron. Appl. Radiat. Isot. 2011;69:1–7. doi: 10.1016/j.apradiso.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Kume M., Carey P.C., Gaehle G., Madrid E., Voller T., Margenau W., Welch M.J., Lapi S.E. A semi-automated system for the routine production of copper-64. Appl. Radiat. Isot. 2012;70:1803–1806. doi: 10.1016/j.apradiso.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Ohya T., Nagatsu K., Suzuki H., Fukada M., Minegishi K., Hanyu M., Fukumura T., Zhang M.R. Efficient preparation of high-quality 64Cu for routine use. Nucl. Med. Biol. 2016;43:685–691. doi: 10.1016/j.nucmedbio.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Mccarthy D.W., Shefer R.E., Klinkowstein R.E., Bass L.A., Margeneau W.H., Cutler C.S., Anderson C.J., Welch M.J. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl. Med. Biol. 1997;24:35–43. doi: 10.1016/S0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- 23.Elomaa V.V., Jurttila J., Rajander J., Solin O. Automation of 64Cu production at turku PET centre. Appl. Radiat. Isot. 2014;89:74–78. doi: 10.1016/j.apradiso.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Rajander J., Schlesinger J., Avila-Rodriguez M.A., Solin O. Increasing specific activity in Cu-64 production by reprocessing the Ni-64 target material. J. Label. Compd. Radiopharm. 2009;52:S234. [Google Scholar]
- 25.Avila-Rodriguez M.A., Nye J.A., Nickles R.J. Simultaneous production of high specific activity 64Cu and 61Co with 11.4 MeV protons on enriched 64Ni nuclei. Appl. Radiat. Isot. 2007;65:1115–1120. doi: 10.1016/j.apradiso.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Ometáková J., Rajec P., Csiba V., Leporis M. Automated production of 64Cu prepared by 18 MeV cyclotron. J. Radioanal. Nucl. Chem. 2012;293:217–222. doi: 10.1007/s10967-012-1757-3. [DOI] [Google Scholar]
- 27.Kim J.Y., Park H., Lee J.C., Kim K.M., Lee K.C., Ha H.J., Choi T.H., An G.I., Cheon G.J. A simple Cu-64 production and its application of Cu-64 ATSM. Appl. Radiat. Isot. 2009;67:1190–1194. doi: 10.1016/j.apradiso.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 28.Hagemeister F. Rituximab for the treatment of non-hodgkin′s lymphoma and chronic lymphocytic leukaemia. Drugs. 2010;70:261–272. doi: 10.2165/11532180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y., Zhu H., Zhang B., Liu F., Chen J., Wang Y., Wang Y., Zhang Z., Wu L., Si L., et al. Synthesis of site-specific radiolabeled antibodies for radioimmunotherapy via genetic code expansion. Bioconjug. Chem. 2016;27:2460–2468. doi: 10.1021/acs.bioconjchem.6b00412. [DOI] [PubMed] [Google Scholar]