Abstract
Background and purpose
Acute Kidney Injury (AKI) is a severe complication affecting many hospitalized patients after cardiac surgery, with negative impacts on short- and long-term clinical outcomes and on healthcare costs. Recently, clinical interest has been aimed at defining and classifying AKI, identifying risk factors and developing diagnostic strategies to identify patients at risk early on. Achieving an early and accurate diagnosis of AKI is a crucial issue, because prevention and timely detection may help to prevent negative clinical outcomes and avoid AKI-associated costs. In this retrospective study, we evaluate the NephroCheck Test as a diagnostic tool for early detection of AKI in a high-risk population of patients undergoing cardiac surgery at the San Bortolo Hospital of Vicenza.
Methods
We assessed the ability of the NephroCheck Test to predict the probability of developing CSA-AKI (cardiac surgery-associated AKI) and evaluated its accuracy as a diagnostic test, by building a multivariate logistic regression model for CSA-AKI prediction.
Results
Based on our findings, when the results of the NephroCheck Test are included in a multivariate model its performance is substantially improved, as compared to the benchmark model, which only accounts for the other clinical factors. We also define a rule – in terms of a probability cut-off – for discriminating cases that are at higher risk of developing AKI of any stage versus those in which AKI is less likely.
Conclusions
Our study has implications in clinical practice: when a Nephrocheck Test result is >0.3 ng/dL, an automated electronic alert prompts the physician to intervene by following a checklist of preventive measures.
Keywords: Acute kidney injury, Cardiac surgery, Cell cycle arrest biomarkers
Introduction
Acute kidney injury (AKI) is a strong independent risk factor for morbidity and mortality, longer hospital stay and development of de-novo chronic kidney disease (1). New consensus criteria for diagnosis and staging of AKI (2) led to the global implementation of the RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) (3) and AKIN (Acute Kidney Injury Network) classifications (4) and, finally, to the KDIGO Clinical Practice Guidelines (5). These diagnostic criteria are based on a time-dependent change of serum creatinine (sCr) and urine output (UO). Despite their wide utilization, these criteria have several limitations: sCr is influenced by multiple nonrenal factors (sex, age, race, muscle mass, diet, certain drugs and clinical conditions). An increase of its level occurs only when more than half of kidney function is lost (6). Finally, criteria based only on sCr may neglect subclinical forms of kidney dysfunction and damage (7). Furthermore, the definition of AKI staging is retrospective and therefore precludes the possibility of a timely assessment of AKI risk and severity, which narrows the window for the implementation of preventive measures. Therefore, current markers such as sCr seem to be inadequate to capture the whole spectrum of AKI mechanisms and the continuum of the syndrome (7).
These issues have led to discovery and validation of novel biomarkers that offer additional diagnostic value in identifying patients at risk and providing early detection of kidney damage. Various molecules have emerged as promising biomarkers, but only a few of them have shown an “acceptable level of sensitivity and specificity” (8).
Recently, urine insulin-like growth factor-binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinases-2 (TIMP-2) have become commercially available. Both are soluble proteins, expressed by the kidney, thought to be involved in G1 cell-cycle arrest during the earliest phases of cellular stress and tubular injury (8, 9). Kashani et al first published a study of validation for these biomarkers (10). The study suggests that the product of the 2 biomarkers performed better than each molecule alone. The validation of the (TIMP-2)*(IGFBP7) product was reported in the Sapphire study (11) where the authors highlighted its remarkable ability to predict the development of moderate and severe AKI (stage 2 to 3, KDIGO) within 12 hours from sample collection. This study set 2 clinical cut-off values that allow a stratification of patients, distinguishing those at a lower risk from those at a greater risk of developing AKI. A cut-off >0.3 (ng/mL)2/1,000 allows early identification of most patients at risk of developing AKI (9). Therefore, 0.3 (ng/mL)2/1,000 is intended to be used in routine clinical practice as a signal to initiate preventive and protective measures. A higher cut-off >2.0 (ng/ml)2/1,000 identifies the patients with the highest risk of AKI, for whom more active kidney-sparing interventions may be appropriate. The measurement of the (TIMP-2)*(IGFBP7) product is known commercially as the NephroCheck Test.
In this pilot retrospective study, we evaluated the predictive ability of the NephroCheck Test for early AKI diagnosis in a cardiac surgery (CS) population, as compared to current methods based on sCr. We also built a model for AKI prediction to define a rule for selecting cases in which (TIMP-2)*(IGFBP7) should be appropriately used.
Methods
Study design and population
We conducted a pilot observational, retrospective, single-center study in the intensive care unit (ICU) of the Cardiac Surgery Department at San Bortolo Hospital of Vicenza, Italy. The Ethical Committee of the hospital approved the study design. From July 2014 to December 2015, adult patients undergoing CS were retrospectively included in the study. The inclusion criteria consisted of selecting patients (a) aged ≥18 years (b) who underwent CS procedures. Patients were excluded from the study if (c) they underwent a surgery procedure of the TAVI type (via transcatheter aortic valve implantation) or (d) they were already receiving dialysis treatment at the hospitalization.
We considered 2 cohorts: the first (2014, only sCr and clinical evaluation but no NephroCheck Test) included 332 patients; the second cohort (2015, standard evaluation + NephroCheck Test cohort) enrolled 110 patients. The NephroCheck Test was performed in urine samples collected at several pre- and postoperative time points. In the 2015 cohort, patients that underwent emergency surgery were not included.
The primary endpoint was to generate a logistic regression model to predict AKI, and identify whether (TIMP-2)*(IGFBP7) might improve the model's predictive value as compared to a benchmark model without incorporation of biomarkers. Data collection and laboratory data are shown in the online supplements (see supplementary Tables I to V and supplementary Fig. 1 available online as supplementary material at www.artificial-organs.com - cohort baseline demographics and surgical data, cohort comparisons, sensitivity and specificity and ROC curves, etc.).
Fig. 1.
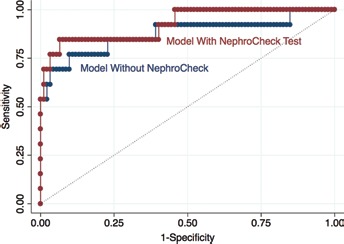
Figure displays the improvement in the area under the ROC curve from 87.54% (blue line) to 92.47% (red line), due to the inclusion of Nephrocheck results in the clinical model.
AKI adjudication and definition
AKI was diagnosed as determined by an adjudication committee of 3 expert nephrologists who were blinded to the biomarker results, based on the KDIGO criteria (5) using the peak postoperative sCr within 10 days after surgery and excluding sCr at admission to postoperative ICU. Baseline sCr was defined as the usual value of sCr preceding the admission within at least 7 days (12) or as the nadir sCr after recovery of renal function (13).
Statistical analysis
We ran a 2-sample t-test, for continuous variables, or a 2-sample test of proportions, for categorical variables, to check whether the 2 cohorts of patients were comparable. According to primary endpoint, our outcome variable is dichotomous (AKI vs. non-AKI) and the covariates were selected among all the available parameters (anamnestic and preoperative information as well as data on intra- and postoperative therapies). To improve the specification of the benchmark model, the selection of covariates took place on the sample including both cohorts together and the method of purposeful selection of covariates was applied (14). At the beginning, a wide group of variables of interest was selected because they were thought to be risk factors or to correlate with AKI, based on the literature. The association of every potential predictor with the outcome was analyzed through univariate logistic regression. A multiple regression model was fit with all the potential predictors, even if not significant in the univariate analysis. In the multivariate model, some predictors turned out to be statistically not significant. To avoid dropping crucial parameters, the decision was based on the likelihood ratio test and on clinical considerations. We performed regression diagnostics, aiming at checking the selected model fit. Quality of fit was assessed through the Hosmer-Lemeshow statistic, splitting the sample into 10 groups of covariate patterns according to the strategy suggested by Hosmer, Lemeshow and Sturdivant (15). Secondly, we ran a specification error test to ensure that no important variables had been omitted from the model and that the correct link function (namely, the logit function) had been chosen.
We then applied the selected multivariate model to the 2015 cohort, in order to include the NephroCheck Test as a covariate and assess any improvement in AKI prediction. Two different models were run: 1 containing test results as a binary variable, based on a 0.3 cut-off, while the other a continuous variable. A p value lower than 0.05 was considered significant for all statistical tests.
The results of the fitted regression model and, in particular, its performance in terms of classification were then assessed by means of constructing a classification table that uses the estimated probability to predict group membership. We selected the probability cut-off based on the sensitivity and specificity curves.
Analyses were performed using STATA version 13.1 software (Stata Corporation).
Results
The descriptive analysis of both cohorts are shown in the online supplementary material. We built the AKI prediction model based on the sample size that included both cohorts. The dependent variable was the occurrence of AKI within 10 days after cardiac surgery (AKIPOST). The multiple regression model included all predictors that were statistically significant in the univariate analysis, while some covariates, even when not statistically significant according to the p value of their Wald statistic, improved the model goodness of fit or were included because of their importance from a clinical viewpoint.
At first, we built a model combining both cohorts without the NephroCheck Test results (Tab. I). The Hosmer-Lemeshow statistic was 3.81 with an associated p value of 0.87, indicating that the model fit well. The regression diagnostics based on the specification error test confirmed that no relevant variables were omitted from the model and that the logit function was applicable (see supplementary Tab. IV, available online as supplementary material). The base model needed to be compared to the model including the NephroCheck Test results as a covariate in order to assess the improvement in the model's performance in terms of AKI prediction. Therefore, the base model had to be applied to the same cohort on which the model that included the NephroCheck Test variable had been run. Table II displays the results of the base model computed on the 2015 cohort only.
Afterwards, we built a separate regression model to assess whether inclusion of the NephroCheck Test, administered 12 hours after surgery, improves AKI risk prediction in the 2015 cohort. NephroCheck Test was added as a binary variable (nc12hcut03, (TIMP-2)*(IGFBP7) ≤0.3 and >0.3 (ng/ml)2/1,000, results displayed in Tab. Ill), then as a continuous variable (nc12h; Tab. IV).
Regarding the NephroCheck Test results as a binary variable (nc12hcut03), the coefficient of nc12hcut03 was positive and statistically significant (p value 0.014). The results we obtained can be better understood by comparing both models (with and without nc12hcut03), in particular by looking at the respective classification tables: the NephroCheck Test improved the performance of the model by discriminating patients who developed AKI from those who did not (Tab. V). The probability cut-off was selected at 15%, corresponding to the point where the specificity and sensitivity curves cross (see supplementary Fig.1). The model without nc12hcut03 provided a good discrimination for classifying the patient into the AKI or non-AKI group (84.76% of patients were correctly classified); however, after nc12hcut03 inclusion, the model improved substantially in terms of sensitivity (from 76.92% to 84.62%) without losing anything in terms of specificity. In other words, the true positive rate increased while the false negative rate decreased, leading to a higher percentage of patients who were correctly classified (86.67%) (Tab. V). The area under the ROC curve improved after including the NephroCheck results, from 87.54% to 92.47% (Fig. 1).
Afterwards, the continuous variable nc12h replaced nc12hcut03 in the logit model (Tab. V). In terms of classification performance at a 15% probability cut-off, the model with the continuous variable nc12h was less sensitive (76.92%, as compared to 84.62% of the model with the binary variable nc12hcut03) and the number of patients falsely classified as non-AKI slightly increased, however, the false positive rate decreased (specificity increased to 88.04%). Overall, the proportion of patients correctly classified was 86.67%, comparable to the model with nc12hcut03 (see supplementary Tab. V).
Discussion
In this preliminary study, we investigated the utility of the NephroCheck Test as a diagnostic tool for the early identification of AKI, or at least of patients at risk of AKI, in order to plan a subsequent multidisciplinary algorithm of intervention (16). In recent years, the spectrum of AKI has been extended: the syndrome is described as a continuum from increased susceptibility of the kidney to catastrophic kidney failure. Biomarkers have refined the diagnostic criteria, making it possible to detect Acute Kidney Stress and to define a preinjury phase that potentially leads to overt clinical AKI (17, 18). The term “Subclinical AKI” therefore describes a condition in which kidney injury exists, without the “classic” KDIGO dysfunction criteria (increase sCr and oliguria) (7, 19). The necessity to categorize different AKI pictures as a continuum highlights the innate limit of sCr in identifying the whole spectrum of AKI conditions.
As mentioned earlier, the Sapphire study identified 2 NephroCheck test cut-offs that are associated with an increased risk of developing mild to severe AKI (10).
The ability of the NephroCheck Test to predict and rule out AKI risk strongly affects its potential for routine adoption and clinical application. Most AKI biomarkers have lost their rationale of use because of low specificity and sensitivity, which result in marginal or absent clinical implications. Obviously, the routine adoption of new biomarkers requires a careful analysis of the cost-benefit ratio. Levante and Ronco (20) outlined how a multidisciplinary process might justify adoption of new biomarkers, provided that the short- and long-term benefits for the patients, the payers, the decision-makers and the other stakeholders are highlighted. In our Health Public System, the cost of this biomarker could be justified based on 2 aspects: firstly, to prevent the requirement of renal replacement therapy (RRT) in critically ill patients and, secondly, to limit the high risk of AKI progression toward CKD, so as to ward off the costs of chronic dialysis care to patients. Indeed, the negative impact of AKI is not limited to the hospitalization period, since it might extend over the long run and is associated with a substantial increase in health-care costs. It was estimated that the annual AKI-associated costs, including inpatient care and postdischarge care, is around £1.2 billion in England, which represents a substantial component of the National Health System budget (21). Additionally, AKI is likely to have adverse long-term clinical effects, even when patients recover their renal function. Pannu et al (22) found that 30.8% of patients who had developed AKI (KDIGO stage 2 or 3) died and 2.1% progressed to kidney failure (CKD stage 5) requiring dialysis within a 34-month period. Since there is no specific therapy for AKI, in order to recognize its onset early on, AKI prevention and limiting or avoiding its progression represent the only strategy that is available.
Recently, 2 different studies evaluated the ability of the NephroCheck Test to predict AKI in CS patients. Wetz et al documented that the use of (TIMP-2)*(IGFBP-7) could be a good predictor for AKI only in patients with high risk for AKI on the first postoperative day but not in the early postoperative phase (23). Toufic Finge et al observed that the measurement of early postoperative urinary (TIMP-2)*(IGFBP-7) (within 3-hour postoperative period) cannot accurately predict the occurrence of AKI within the first 48 hours after CS (24).
These data are in contrast with those of Meersch et al (25), which identified the NephroCheck test as a specific and early predictor of CSA-AKI, with an AUC of 0.90 when considering the maximum value in the first 24 postoperative hours, and an AUC of 0.81 when considering the 4-hours postoperative value.
The possible explanation of these conflicting results could be in the different patients and surgical settings; in fact Meersch and colleagues included patients with high preoperative and surgical risk (long CBP and ACC times) for AKI, conversely Wetz et al have included only patients undergoing CABG surgery (shorter CBP time) while Toufic Finge et al have not differentiated patients based on the risk of preoperative AKI.
We built a multiple logistic regression model to predict the probability of developing CSA-AKI on a heterogeneous sample of patients who underwent CS in 2014. We implemented the routine adoption of the NephroCheck Test as an additional diagnostic tool on a cohort of patients undergoing CS during 2015. The individuals in the 2 cohorts (2014 no NephroCheck Test vs. 2015 with NephroCheck Test) had comparable epidemiological and clinical characteristics. The multiple regression model included variables describing patients' clinical background, surgical procedures and early postsurgical features. The selected model was then applied to the 2015 cohort and, subsequently, the results of the NephroCheck Test administered 12 hours after surgery were included as a covariate, in order to assess improvement in AKI risk prediction through the model.
The NephroCheck Test is statistically significant as a covariate in the multiple logistic regression model and an increase in test results signals a higher probability of developing CSA-AKI, all other factors being equal. Once the NephroCheck Test is included, the area under the curve (AUC) improves from 87.54% to 92.47%, with the main contribution given in terms of sensitivity (increasing from 76.92% to 84.62%, at the selected probability cut-off of 15%). This means that the model correctly identifies 84 out of 100 patients at risk of developing AKI, but the remaining portion of patients at risk could go undetected. In our opinion, the combination of clinical and surgical covariates might limit the zone of uncertainty in which the test is not informative (24). Otherwise, our use of the Nephrocheck Test (to identify KDIGO >0) mirrors that used by Toufic Finge (24) and Wetz (23), but it differs from the initial validation of the biomarker in ICU patients (KDIGO >1). The question arose whether the biomarker might be better applicable for predicting high-grade AKI, whether new cut-off should be identified for KDIGO 1 or in the low-risk CS population.
The results presented in this study must be evaluated in light of some considerations. Firstly, the NephroCheck Test was employed on a specific and rather homogeneous population: patients undergoing cardiac surgery. Further research would be necessary to validate its use on a more heterogeneous population.
Analysis and comparison of the classification tables of the models show that the NephroCheck Test improves early AKI detection with respect to a model that accounts only for other clinical parameters. Nevertheless, an integrated approach could probably represent the most successful diagnostic strategy, combining existing diagnostics (sCr and urine output) and clinical risk scores with biomarker-based diagnostics (the NephroCheck Test) in relation to patient conditions. In particular, it might not be optimal to use biomarker testing routinely in a more heterogeneous patient context, but it could instead be more appropriate to identify a subset of patients in whom testing would be utilized more efficiently (26).
The retrospective design might be a limit of our study. However, this preliminary evaluation was performed to integrate the Nephrocheck Test in emergency room protocols, much like troponin is used for acute myocardial infarction diagnosis (27).
Conclusions
Our study has practical implications in clinical practice. As an immediate consequence, a Nephrocheck >0.3 ng/dL leads today to an automated electronic alert that prompts the physician on duty to intervene by filling out a checklist of preventive/protective measures. This Rapid Response team is activated any time a patient at risk of AKI is identified in the ICU. A bundle of actions (28) is then applied in AKI risk patients (16).
Supplementary Material
Disclosures
Financial support: No grants or funding have been received for this study.
Conflict of interest: None of the authors has financial interest related to this study to disclose. Claudio Ronco received speaker's honoraria from Astute Medical, OCD, GE, and is consulting for OCD, Baxter and Jaffron.
References
- 1.Hoste E.A., Bagshaw S.M., Bellomo R. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015; 41(8): 1411–1423. [DOI] [PubMed] [Google Scholar]
- 2.Kashani K., Ronco C. Acute Kidney Injury Electronic Alert for Nephrologist: Reactive versus Proactive? Blood Purif. 2016; 42(4): 323–328. [DOI] [PubMed] [Google Scholar]
- 3.Bellomo R., Ronco C., Kellum J.A., Mehta R.L., Palevsky P.; Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8(4): R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta R.L., Kellum J.A., Shah S.V. et al. Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007; 11(2): R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012; 2(1): 1–138. [Google Scholar]
- 6.Ronco C., Kellum J.A., Haase M. Subclinical AKI is still AKI. Crit Care. 2012; 16(3): 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronco C. Acute kidney injury: from clinical to molecular diagnosis. Crit Care. 2016; 20(1): 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wetz A.J., Richardt E.M., Wand S. et al. Quantification of urinary TIMP-2 and IGFBP-7: an adequate diagnostic test to predict acute kidney injury after cardiac surgery? Crit Care. 2015; 19: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronco C. Cell Cycle Arrest Biomarkers: New Weapons for A New Battle. Blood Purif. 2014; 38(3-4): I–III. [DOI] [PubMed] [Google Scholar]
- 10.Kashani K., Al-Khafaji A., Ardiles T. et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013; 17(1): R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bihorac A., Chawla L.S., Shaw A.D. et al. Validation of Cell-Cycle Arrest Biomarkers for Acute Kidney Injury Using Clinical Adjudication. Am J Respir Crit Care Med. 2014; 189(8): 932–939. [DOI] [PubMed] [Google Scholar]
- 12.Chawla L.S., Bellomo R., Bihorac A. et al. Acute Disease Quality Initiative Workgroup 16. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017; 13(4): 241–257. [DOI] [PubMed] [Google Scholar]
- 13.De Rosa S., Samoni S., Ronco C. Creatinine-based definitions: from baseline creatinine to serum creatinine adjustment in intensive care. Crit Care. 2016; 20: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosmer D.W., Lemeshow S., Sturdivant R.X. Applied Logistic Regression. 3rd ed., Hoboken, NJ: John Wiley and Sons; 2013. [Google Scholar]
- 15.Kidney Disease; Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013; 3: 1–150. [Google Scholar]
- 16.Ronco C., Rizo-Topete L., Serrano-Soto M., Kashani K. Pro: Prevention of acute kidney injury: time for teamwork and new biomarkers. Nephrol Dial Transplant. 2017; 32(3): 408–413. [DOI] [PubMed] [Google Scholar]
- 17.Katz N., Ronco C. Acute kidney stress – a useful term based on evolution in the understanding of acute kidney injury. Crit Care. 2016; 20: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronco C. Kidney attack: overdiagnosis of acute kidney injury or comprehensive definition of acute kidney syndromes? Blood Purif. 2013; 36(2): 65–68. [DOI] [PubMed] [Google Scholar]
- 19.Kellum J.A., Bellomo R., Ronco C. Kidney attack. JAMA. 2012; 307(21): 2265–2266. [DOI] [PubMed] [Google Scholar]
- 20.Levante C., Ronco C. The Imperative for Health Economics Assessment in Acute Kidney Injury. Blood Purif. 2016; 42(2): I–VI. [DOI] [PubMed] [Google Scholar]
- 21.Kerr M., Bedford M., Matthews B., ODonoghue D. The economic impact of acute kidney injury in England. Nephrol Dial Transplant. 2014; 29(7): 1362–1368. [DOI] [PubMed] [Google Scholar]
- 22.Pannu N., James M., Hemmelgarn B., Klarenbach S.; Alberta Kidney Disease Network. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol. 2013; 8(2): 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wetz A.J., Richardt E.M., Wand S. et al. Quantification of urinary TIMP-2 and IGFBP-7: an adequate diagnostic test to predict acute kidney injury after cardiac surgery? Crit Care. 2015; 19: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finge T., Bertran S., Roger C. et al. Interest of Urinary (TIMP-2) x (IGFBP-7) for Predicting the Occurrence of Acute Kidney Injury After Cardiac Surgery: A Gray Zone Approach. Anesth Analg. 2017; 125(3): 762–769. [DOI] [PubMed] [Google Scholar]
- 25.Meersch M., Schmidt C., Van Aken H. et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014; 9(3): e93460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu R.K., Gist K., Wheeler D.S. Improving acute kidney injury diagnostics using predictive analytics. Curr Opin Crit Care. 2015; 21(6): 473–478. [DOI] [PubMed] [Google Scholar]
- 27.Rizo-Topete L., Ronco C. Critical Care Nephrology: A Multidisciplinary Approach. Blood Purif. 2017; 43(1-3): 53–56. [DOI] [PubMed] [Google Scholar]
- 28.Rizo-Topete L.M., Rosner M.H., Ronco C. Acute Kidney Injury Risk Assessment and the Nephrology Rapid Response Team. Blood Purif. 2017; 43(1-3): 82–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


