Abstract
Several constituents of essential oils have been shown to be active against pathogens such as bacteria, fungi, and protozoa. This study demonstrated the in vitro action of ten compounds present in essential oils against Leishmania amazonensis promastigotes. With the exception of p-cymene, all evaluated compounds presented leishmanicidal activity, exhibiting IC50 between 25.4 and 568.1 μg mL−1. Compounds with the best leishmanicidal activity presented a phenolic moiety (IC50 between 25.4 and 82.9 μg mL−1). Alicyclic alcohols ((−)-menthol and isoborneol) and ketones ((−)-carvone) promoted similar activity against the parasite (IC50 between 190.2 and 198.9 μg mL−1). Most of the compounds showed low cytotoxicity in L929 fibroblasts. Analysis of the structure-activity relationship of these compounds showed the importance of the phenolic structure for the biological action against the promastigote forms of the parasite.
Keywords: monoterpenes, Leishmania amazonensis, leishmanicidal activity, essential oil
1. Introduction
Leishmaniasis is a disease of worldwide distribution, present in Africa, Latin America, Asia, and Europe. It is estimated that 1.3 million new cases occur annually in these continents, with approximately 20,000 to 30,000 deaths due to the disease [1]. Leishmaniasis is transmitted by sandflies and is usually associated with poverty, malnutrition, poor living conditions, and climate and environmental changes [1,2]. Leishmaniasis can be caused by different protozoa species of the genus Leishmania, and the development of the disease is influenced by factors such as parasite species, parasite-host interaction, and vulnerability of the host immune system, presenting variable clinical manifestations as cutaneous, diffuse cutaneous, mucocutaneous and visceral leishmaniasis [3,4].
Antimonials and amphotericin B are the first-choice drugs for the treatment of leishmaniasis in most countries. However, there are several limitations related to their use, such as high financial cost (drug price and hospital support/hospitalization), long term treatment (up to 30 days), high toxicity and variable efficacy (30 to 98% cure). In addition, the emergence of resistant strains, especially in endemic areas, is also a limiting factor for the use of traditional chemotherapies [5,6,7].
A vast array of essential oil-bearing plants has been used to control parasites for centuries [8,9]. For example, garlic oil is known to be active against 12 different human and nonhuman parasites [10], eugenol-containing basil and clove oils possess antiphagocytic activity, and Mentha crispa essential oil is active against Trypanosoma brucei [11]. The discovery of new drugs has been based on natural products for years, either by the synthesis of substances that mimic a natural product, by modifying an existing natural molecule, or by the use of the natural product itself [12]. Among natural products, monoterpenes stand out for their wide use in the industry, in addition to having vast biological activity already verified against fungi, bacteria, viruses, and parasites, including protozoa [13,14,15,16,17,18,19,20,21]. Thus, the present work aims to investigate the relationship between chemical structures and the activity of different monoterpenes and a phenylpropanoide, constituents of essential oils, against promastigote forms of Leishmania amazonensis.
2. Results and Discussion
Nine monoterpenes and one phenylpropanoid were selected (Table 1) to investigate the action of these compounds against L. amazonensis promastigotes and to determine the relationship between their chemical structure and biological activity.
Table 1.
In vitro leishmanicidal activity of different essential oils constituents against Leishmania amazonensis promastigotes.
| Compound | Chemical Structure | IC50 (µg mL−1) * | IC50 (µM) * | R2 |
|---|---|---|---|---|
| Carvacrol (1) | 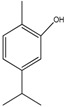 |
25.4 ± 2.4 | 169.08 ± 15.97 | 0.70 |
| Thymol (2) | 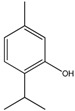 |
26.8 ± 3.7 | 178.40 ± 24.63 | 0.81 |
| 3-Carene (3) | 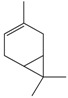 |
72.5 ± 18.5 | 532.18 ± 135.79 | 0.81 |
| Eugenol (4) | 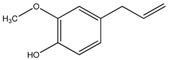 |
82.9 ± 6.2 | 504.87 ± 37.75 | 0.98 |
| Isoborneol (5) | 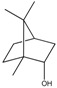 |
190.2 ± 9.8 | 1233.06 ± 63.53 | 0.97 |
| (–)-Carvone (6) | 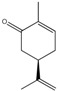 |
194.7 ± 16.9 | 1296.09 ± 112.50 | 0.94 |
| (–)-Menthol (7) | 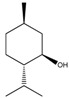 |
198.9 ± 12.0 | 1272.87 ± 76.79 | 0.96 |
| (–)-Linalool (8) | 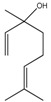 |
276.2 ± 24.0 | 1790.59 ±155.59 | 0.91 |
| 1,8-Cineole (9) | 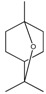 |
568.1 ± 56.5 | 3682.98 ± 366.28 | 0.89 |
| p-Cymene (10) | 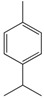 |
>1000 | >7450.45 | - |
| c Amphotericin B | - | 0.05 ± 0.01 | 0.054 ± 0.01 | - |
a IC50 = Drug concentration capable of inhibiting 50% of promastigote multiplication; b R2 = Coefficient of determination—measure for the quality of the curve fitting of sigmoidal dose-response curves; c Amphotericin B was used as positive control. * Represents the mean of three independent experiments conducted in triplicate and expressed with means plus or minus standard deviation (±SD).
Among the compounds evaluated in this study, three presented a phenolic moiety: carvacrol (IC50 25.4 μg mL−1), thymol (IC50 26.8 μg mL−1), and eugenol (IC50 82.9 μg mL−1). The positional isomers carvacrol and thymol were the most active compounds in this series, exhibiting the lowest IC50 values among evaluated compounds. In addition, these compounds presented similar leishmanicidal activity, possibly suggesting that the different positions of the hydroxyl in the aromatic ring does not influence the antiparasitic activity.
The acidity of phenolic hydroxyl groups may play an important role in explaining higher potencies of phenolic compounds in this series. Aside from the hydroxyl and the aromatic ring, eugenol also bears a hydrogen bond between the methoxyl in the ortho position and the phenolic OH, which reduces the release of protons by the OH group due to the presence of this intramolecular hydrogen bonding [22]. This molecular property might justify eugenol lower potency in relation to both phenolic compounds deprived of intramolecular hydrogen bonds, carvacrol and thymol [23].
Non-aromatic hydroxylated compounds, such as isoborneol and menthol alcohols, exhibited weaker antiparasitic action than phenolic compounds (IC50 of 190.2 μg mL−1 and 198.0 μg mL−1, respectively). Within the aliphatic alcohols tested, linalool was the least active compound (IC50 of 276.2 μg mL−1). Carvone, an α,β-unsaturated ketone, exhibited similar results to isoborneol and menthol alicyclic alcohols (IC50 of 194.7 μg m−1). The enone of carvone might play an important antiparasitic activity, as expected from the hydroxyls of alcohols, once conjugated ketones have the potential to function as a Michael acceptor by reacting with nucleophilic species of the parasite [24].
The importance of the phenolic hydroxyl group as for leishmanicidal activity is observed in carvacrol, thymol, p-cymene, and menthol in a similar manner, as previously observed by Ultee et al. [25] for monoterpenes with antibacterial action. Interestingly, p-cymene, a precursor of thymol and carvacrol, which does not have the hydroxyl group, did not exhibit any activity against L. amazonensis, unlike the two hydroxylated derivatives that exhibited better results. These results suggest that the lack of a polar hydroxyl group in the p-menthane ring template, as well as the absence of aromaticity might render monoterpenes inactive. The importance of aromatic hydroxyls for antiparasitic activity is again highlighted in the analysis of menthol. Although menthol has a hydroxyl group, the absence of the aromatic ring contributed to an IC50 well above phenolic compounds. The importance of the aromatic structure for leishmanicidal activity was also observed in the study of synthetically modified lactones [26].
Previous studies of carvacrol in the literature found an IC50 of 2.3 μg mL−1 [27] and 28.0 μg mL−1 [28] against L. chagasi promastigotes, while another study found an IC50 of 15.3 μg mL−1 against L. amazonensis promastigotes [29]. The different values of IC50, when comparing the same species and compound, are probably related to the experimental design. In a previous study, the use of longer drug-parasite incubation (72 h) and the assay development performed with the chromogen p-nitrophenol phosphate to measure viability, determined the IC50 of 15.3 μg mL−1 for carvacrol [29]. Meanwhile, in the present study (IC50 of 25.4 μg mL−1), the parasites were incubated for 24 h using the resazurin method.
Several studies have reported the action of thymol on species of Leishmania, such as L. chagasi (IC50 of 9.8 μg mL−1, 12.85 μg mL−1, and 65.2 μg mL−1) [28,30,31], L. panamensis (IC50 of 194.3 µg mL−1) [32] and L. amazonensis (IC50 of 22.6 μg mL−1) [33], presenting similar activity to the ones observed in our study. Eugenol, on the other hand, was evaluated against promastigote forms of L. chagasi (IC50 of 56.1 μg mL−1) [30] and L. amazonensis (IC50 of 500 μg mL−1 and 80.0 μg mL−1) [34,35], and the latter exhibits similar results to our experiment.
The potent behavior of thymol and carvacrol identified in the present study, when compared with other monoterpenes, has already been observed previously in bacteria. Dorman and Deans [36] evaluated the action of six essential oils and their major compounds, with 21 monoterpenes and the phenylpropanoid eugenol, against 25 different bacterial species, and identified that thymol, carvacrol, and eugenol were much more potent than the other monoterpenes. These authors also discussed a value of the phenolic hydroxyl present in these compounds for this biological activity. Nazzaro et al. [37] still emphasized the importance of the delocalization of electrons, besides the phenolic hydroxyl, as important characteristics for the antibacterial activity of carvacrol and thymol. However, bacteria and protozoa are microorganisms, thus they are relatively different in structural and molecular terms.
Isoborneol, a bicyclic alcohol, was previously evaluated against promastigotes of L. infantum, L. tropica, and L. major. No leishmanicidal activity was found, even at a maximum dose of 400.0 μg mL−1 [38]. To date, there are no reports of isoborneol activity against L. amazonensis. In our study, isoborneol exhibited an IC50 of 190.2 μg mL−1 as leishmanicidal against L. amazonensis. In this study, linalool, an acyclic tertiary alcohol, exhibited an IC50 of 276.2 μg mL−1, considerably higher than the value of 4.3 ng mL−1 found by ROSA et al. [39], which was also obtained on L. amazonensis promastigotes. Meanwhile, Dutra et al. [35] did not identify changes in the viability of promastigotes of L. infantum chagasi treated with linalool up to the dose of 750 μg mL−1. These authors believe that the difference between their results and those observed by ROSA et al. [39] may be related to different concentrations of enantiomers. The literature does not mention the action of menthol, a cyclic alcohol, in L. amazonensis or other species of Leishmania. In our results, an IC50 of 198.0 μg mL−1 was obtained for this compound.
Among the monoterpenes, two hydrocarbons 3-carene and p-cymene were tested. No report was found in the literature of the leishmanicidal activity of the bicyclic hydrocarbon 3-carene on L. amazonensis. In this study, an IC50 of 72.5 μg mL−1 was found for 3-carene against L. amazonensis. However, on L. donovani promastigotes, 3-carene exhibited an IC50 of 27.0 μg mL−1 [40]. p-Cymene, an aromatic hydrocarbon, was evaluated on L. chagasi and found to have an IC50 of 149.1 μg mL−1 [28]. On the other hand, in our experiments, no effect on the viability of L. amazonensis promastigotes was found in concentrations up to 1000.0 μg mL−1.
(−)-Carvone was previously evaluated on L. chagasi promastigotes and exhibited an IC50 > 300.0 μg mL−1 [28]. However, no IC50 for carvone was found in the literature against L. amazonensis. In our trial, an IC50 of 194.7 μg mL−1 was obtained for (−)-carvone against L. amazonensis. Similar results were also obtained for the alicyclic alcohols isoborneol and menthol.
Machado et al. [38] evaluated the cyclic ether 1,8-cineole on promastigotes of L. infantum, L. tropica, and L. major in increasing doses up to 400 μg mL−1. However, no leishmanicidal activity was detected. On the other hand, Camargos et al. [41] obtained an IC50 of 4697.0 µM against L. amazonensis promastigotes, which is equivalent to 724.0 μg mL−1, similar to that obtained in the present study (568.1 μg mL−1).
The replacement of the hydroxyl group in menthol by the ether function found in the 1,8-cineole p-menthane ring resulted in a lower toxic effect (IC50 = 568.1 μg mL−1 as compared to 198.9 μg mL−1 of menthol), against the assessed species of Leishmania. This result corroborates the importance of the aromatic ring and the hydroxyl group to yield more potent compounds.
The interpretation of the results and comparison between studies should also consider similar species of Leishmania and life stage, such as promastigote or amastigote forms of the parasite, since different species of the parasite and evolutionary forms have specific biological characteristics that can produce discordant results [42,43,44]. In addition, the results are dependent on the experimental model being used [38].
In general, the compounds showed low cytotoxicity on L929 fibroblasts at the concentrations evaluated, 50.0 and 100.0 μg mL−1. The results of the tests are expressed as a percentage of viability (Table 2).
Table 2.
Cytotoxic activity of the compounds on L929 fibroblasts.
| Compounds | Viability (%) | |
|---|---|---|
| 50 µg mL−1 | 100 µg mL−1 | |
| 3-Carene | 84.1 ± 6.4 ** | 48.7 ± 6.7 * |
| Carvacrol | 51.3 ± 3.0 | 46.1 ± 2.9 * |
| (−)-Carvone | 65.1 ± 4.7 | 58.2 ± 4.2 |
| 1,8-Cineole | 71.4 ± 0.7 | 66.9 ± 7.8 |
| Eugenol | 78.1 ± 7.0 | 63.1 ± 1.7 |
| Isoborneol | 73.3 ± 9.4 | 72.9 ± 7.5 |
| (−)-Linalool | 65.7 ± 8.2 | 66.7 ± 7.8 |
| (−)-Menthol | 83.8 ± 8.0 ** | 81.2 ± 2.4 ** |
| Thymol | 64.5 ± 4.0 | 58.5 ± 6.7 |
| p-Cymene | 91.2 ± 6.6 ** | 87.1 ± 6.7 ** |
Low cytotoxicity (viability between >50% and <80%); * Moderate cytotoxicity (viability between >30% and <50%); and ** Non-cytotoxic (viability >80%).
An intensity scale based on the methods of Rodrigues et al. [45] was used to classify the cytotoxicity of the compounds. At 50.0 μg mL−1, 3-carene, menthol and p-cymene were considered non-cytotoxic. The remaining compounds showed low cytotoxicity. At the concentration of 100.0 μg mL−1, only menthol was not considered cytotoxic. Most compounds showed low cytotoxicity, whereas carvacrol and 3-carene showed moderate cytotoxicity to the cells. Additionally, essential oil components, such as carvacrol, thymol, 1,8-cineole, isoborneol, menthol, carvone, linalool, and p-cymene are considered to be safe food additives, an indication of low mammalian toxicity [46].
The structure-activity relationships are important not only to understand how these compounds act on the parasite, but also to guide future studies on these molecules. Although most of the compounds used in this work had an IC50 higher than 100.0 μg mL−1, the tests are still preliminary, performed only on L. amazonensis promastigotes. Some studies show a greater sensitivity to intracellular amastigote forms of Leishmania, with an IC50 about 50% lower than that found for promastigotes [47,48]. Thus, a more detailed evaluation of these compounds in the intracellular form of the parasite and in other mammalian cells is needed to better evaluate the toxicity of these compounds.
3. Materials and Methods
3.1. Compounds
The following compounds were used: 3-carene (96.1% purity) (IUPAC (International Union of Pure and Applied Chemistry) name: 4,7,7-trimethylbicyclo[4.1.0]hept-3-ene), carvacrol (99.9%) (IUPAC name: 2-methyl-5-propan-2-ylphenol), (−)-carvone (99.4%) (IUPAC name: (5R)-2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-one), 1,8-cineole (99.7%) (IUPAC name: 2,2,4-trimethyl-3-oxabicyclo[2.2.2]octan-6-ol), p-cymene (99.7%) (IUPAC name: 1-methyl-4-propan-2-ylbenzene), isoborneol (99.0%) (IUPAC name (1R,3R,4R)-4,7,7-trimethylbicyclo[2.2.1]heptan-3-ol), (−)-linalool (99.1%) (IUPAC name: (3R)-3,7-dimethylocta-1,6-dien-3-ol) e (−)-menthol (99.4%) (IUPAC name: (1R,2S,5R)-5-methyl-2-propan-2-ylcyclohexan-1-ol) purchased from Sigma-Aldrich (St. Louis, MO, USA), eugenol (99.0%) (IUPAC name: 2-methoxy-4-prop-2-enylphenol) purchased from Biodinâmica (Ibiporã, PR, Brazil) and thymol (99.0%) (IUPAC name: 5-methyl-2-propan-2-ylphenol) purchased from Synth (Diadema, SP, Brazil), all with analytical purity.
3.2. Parasites
Culture of L. amazonensis (LTCP 9667 obtained by Giudice et al. [44]) promastigotes were maintained at 24 °C in Schneider′s Drosophila medium (Sigma-Aldrich, St. Louis, MO, USA; pH 6.7) supplemented with 10% (v/v) inactivated fetal bovine serum (FBS, Gibco by Thermo Fisher Scientific, Carlsbad, CA, USA), ampicillin 500 mg m−1, 1% and gentamicin 40 mg mL−1, 0.1% (Sigma-Aldrich, St. Louis, MO, USA).
3.3. Fibroblasts
Culture of the mouse fibroblast cell line (L929, ATCC CCL-1) were maintained in Dulbecco′s Modified Eagle Medium (DMEM, Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% heat-inactivated FBS and 1% streptomycin/penicillin (5000 units + 5 mg mL−1, Sigma-Aldrich, St. Louis, MO, USA), and kept in a humid atmosphere at 37 °C and 5% CO2.
3.4. Evaluation of Leishmanicidal Activity
Promastigotes of L. amazonensis in log-phase growth were distributed in a 96-well plate (5 × 105 cells/well) and treated with the evaluated compounds solubilized in dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA) and diluted in Schneider′s medium in different concentrations (between 0.0 and 1000.0 μg mL−1) and incubated for 24 h at 24 °C in a biochemical oxygen demand (BOD) incubator. Promastigotes incubated in the absence of test compounds were used as a negative control. Promastigotes treated with Amphotericin B (Sigma-Aldrich, St. Louis, MO, USA) were used as a positive control. The cellular viability of the parasites was evaluated using the colorimetric method of resazurin, adapted from Kulshrestha et al. [49]. Briefly, after the treatment time, 50 μL of resazurin (2 mM mL−1 in phosphate-buffered saline (PBS)) (Sigma-Aldrich, St. Louis, MO, USA) was added per well and the plates were again incubated for 6 h at 24 °C, then read in a spectrophotometer (Synerg H1, Biotek, Winooski, VT, USA) at 570 and 595 nm. Absorbance was used to calculate cell viability based on the following equation:
| (1) |
wherein:
| (2) |
The IC50 values were obtained by a non-linear regression analysis from the viability values using the GraphPad Prism 5.0 program. All experiments were performed in triplicate from three independent experiments and the data were expressed as mean ± standard deviation (±SD).
3.5. Cytotoxicity
Fibroblasts were distributed in 96-well plates (2 × 104 cells/well) and incubated for 24 h in a 5% CO2 atmosphere at 37 °C. After this period, the medium was removed and the adhered cells were treated with the compounds at concentrations of 50.0 and 100.0 μg mL−1 for 24 h under the same incubation conditions. Untreated cells were used as controls and considered with 100% cell viability. After the treatment period, cell viability was determined by MTT assay as described in ISO 10993-5 [50], with modifications. For this, the cell monolayer was washed twice with PBS (pH 7.4), and then 200 μl MTT (0.5 mg mL−1 in PBS, Sigma-Aldrich, St. Louis, MO, USA) was added to each well. The plates were again incubated under the same conditions as listed above, for a period of 3 h. After the incubation time, the MTT was aspirated and the formazan crystals were solubilized in 200 μL of DMSO. After 10 min, the optical density (OD) was measured on a microplate reader at the wavelength of 570 nm. The results were expressed as percentage of viability according to the following equation:
| (3) |
Each experiment was conducted in quadruplicate and repeated at least three times. Data were expressed as mean ± standard deviation (±SD).
4. Conclusions
In this work, the compounds with phenolic moiety carvacrol, thymol, and eugenol were the most potent against L. amazonensis promastigotes. In addition, the evaluated compounds exhibited low cytotoxicity on L929 fibroblasts.
Studies with a clinically more relevant intracellular amastigote form will have to be conducted in order to further evaluate the potential of these compounds. Even though the overall activity level of the investigated compounds is low compared to amphotericin B, the results presented in this work demonstrate there are activity differences among the essential oil constituents distinguishing between more and less potent compounds that may serve as a basis for the planning of more promising antileishmanial agents.
Acknowledgments
We gratefully acknowledge financial support received from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Apoio à Pesquisa e Inovação Tecnológica do Estado de Sergipe (FAPITEC).
Author Contributions
S.S.D. and R.S. conceived and designed the experiments; A.R.S.T.S. and F.V.S. performed the experiments; A.R.S.T.S., R.S., C.B.C., S.S.D. and R.T.F. analyzed the data; S.R.F., S.C.H.C., L.L.B., R.J.A., D.P.S., R.T.F. contributed to manuscript writing; A.R.S.T.S. and S.S.D. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Footnotes
Sample Availability: Not available.
References
- 1.World Health Organization . Leishmaniasis-Fact Sheet. WHO; Geneva, Switzerland: [(accessed on 14 May 2016)]. Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ [Google Scholar]
- 2.Savoia D. Recent updates and perspectives on leishmaniasis. J. Infect. Dev. Ctries. 2015;9:588–596. doi: 10.3855/jidc.6833. [DOI] [PubMed] [Google Scholar]
- 3.Kevric I., Cappel M.A., Keeling J.H. New World and Old World Leishmania. Infections A Practical Review. Dermatol. Clin. 2015;33:579–593. doi: 10.1016/j.det.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Vries H.J.C., Reedijk S.H., Henk S.D.F.H. Cutaneous Leishmaniasis: Recent developments in diagnosis and management. Am. J. Clin. Dermatol. 2015;16:99–109. doi: 10.1007/s40257-015-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ejazi S.A., Ali N. Developments in diagnosis and treatment of visceral leishmaniasis during the last decade and future prospects. Expert Rev. Anti Infect. Ther. 2013;11:79–98. doi: 10.1586/eri.12.148. [DOI] [PubMed] [Google Scholar]
- 6.Kobets T., Grekov I., Lipoldová M. Leishmaniasis: Prevention, parasite detection and treatment. Curr. Med. Chem. 2012;19:1443–1474. doi: 10.2174/092986712799828300. [DOI] [PubMed] [Google Scholar]
- 7.Goto H., Lindoso J.A.L. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev. Anti Infect. Ther. 2010;8:419–433. doi: 10.1586/eri.10.19. [DOI] [PubMed] [Google Scholar]
- 8.Jones F.A. Herbs–useful plants. Their role in history and today. Eur. J. Gastroenterol. Hepatol. 1996;8:1227–1231. doi: 10.1097/00042737-199612000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Bahmani M.P., Bahmani M., Shahsavari S., Naghdi N., Ezatpour B., Moradniani M., Rafieian-Kopaei M., Sari M. A review of the antiparasitic medicinal plants used in ethnobotany of different regions of Iran. Der. Pharm. Chem. 2016;8:134–138. [Google Scholar]
- 10.Anthony J.P., Fyfe L., Smith H. Plant active components—A resource for antiparasitic agents? Trends Parasitol. 2005;21:462–468. doi: 10.1016/j.pt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Sousa D.P., Cardoso T.L., Steverding D. Evaluation of antiparasitc activity of Mentha. crispa essential oil, its major constituent rotundifolone and analogues against Trypanosoma brucei. Planta Med. 2016;82:1346–1350. doi: 10.1055/s-0042-107082. [DOI] [PubMed] [Google Scholar]
- 12.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 13.Mafud A.C., Silva M.P., Monteiro D.C., Oliveira M.F., Resende J.G., Coelho M.L., De Sousa D.P., Mendonça R.Z., Pinto P.L., Freitas R.M., et al. Structural parameters, molecular properties, and biological evaluation of some terpenes targeting Schistosoma. mansoni parasite. Chem. Biol. Interact. 2016;244:129–139. doi: 10.1016/j.cbi.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Peixoto M.G., Costa-Júnior L.M., Blank A.F., Lima A.S., Menezes T.S.A., Santos D.A., Alves P.B., Cavalcanti S.C., Bacci L., Arrigoni-Blank M.F. Acaricidal activity of essential oils from Lippia. alba genotypes and its major components carvone, limonene, and citral against Rhipicephalus. microplus. Vet. Parasitol. 2015;210:118–122. doi: 10.1016/j.vetpar.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez C., Aznar R., Sánchez G. The effect of carvacrol on enteric viruses. Int. J. Food Microbiol. 2015;192:72–76. doi: 10.1016/j.ijfoodmicro.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Koziol A., Stryjewska A., Librowski T., Salat K., Gawel M., Moniczewski A., Lochyński S. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Rev. Med. Chem. 2014;14:1156–1168. doi: 10.2174/1389557514666141127145820. [DOI] [PubMed] [Google Scholar]
- 17.Reis S.L., Mantello A.G., Rossete E.A.G., Cardoso A.M., Beleboni R.O. Insecticidal and repellent activity of typical monoterpenes from plant essential oils against Callosobruchus. maculatus (Fabr. 1775) BMC Proc. 2014;8:115. doi: 10.1186/1753-6561-8-S4-P115. [DOI] [Google Scholar]
- 18.Moraes J., Almeida A.A.C., Brito M.R.M., Marques T.H.C., Lima T.C., de Sousa D.P., Nakano E., Mendonça R.Z., Freitas R.M. Anthelmintic Activity of the Natural Compound (+)-Limonene Epoxide against Schistosoma. mansoni. Planta Med. 2013;79:253–258. doi: 10.1055/s-0032-1328173. [DOI] [PubMed] [Google Scholar]
- 19.Hui X., Yan G., Tian F.L., Li H., Gao W.Y. Antimicrobial mechanism of the major active essential oil compounds and their structure-activity relationship. Med. Chem. Res. 2017;26:442–449. doi: 10.1007/s00044-016-1762-0. [DOI] [Google Scholar]
- 20.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils–A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 21.Trombetta D., Castelli F., Sarpietro M.G., Venuti V., Cristani M., Daniele C., Saija A., Mazzanti G., Bisignano G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob. Agents Chemother. 2005;49:2474–2478. doi: 10.1128/AAC.49.6.2474-2478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olbert-Majkut A., Wierzejewska M. Conformational Study of Eugenol by Density Functional Theory Method and Matrix-Isolation Infrared Spectroscopy. J. Phys. Chem. A. 2008;112:5691–5699. doi: 10.1021/jp801430d. [DOI] [PubMed] [Google Scholar]
- 23.Arfa A.B., Combes S., Preziosi-Belloy L., Gontard N., Chalie P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006;43:149–154. doi: 10.1111/j.1472-765X.2006.01938.x. [DOI] [PubMed] [Google Scholar]
- 24.Pizzolitto R.P., Herrera J.M., Zaio Y.P., Dambolena J.S., Zunino M.P., Gallucci M.N., Zygadlo J.A. Bioactivities of Ketones Terpenes: Antifungal Effect on F. verticillioides and Repellents to Control Insect Fungal Vector, S. zeamais. Microorganisms. 2015;3:851–865. doi: 10.3390/microorganisms3040851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ultee A., Bennik M.H.J., Moezelaar R. The Phenolic Hydroxyl Group of Carvacrol Is Essential for Action against the Food-Borne Pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002:1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castaño M., Cardona W., Quiñones W., Robledo S., Echeverri F. Leishmanicidal Activity of Aliphatic and Aromatic Lactones: Correlation Structure-Activity. Molecules. 2009;14:2491–2500. doi: 10.3390/molecules14072491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melo J.O., Bitencourt T.A., Fachin A.L., Cruz E.M.O., Jesus H.C.R., Alves P.B., Arrigoni-Blank M.F., Franca S.C., Beleboni R.O., Fernandes R.P.M., et al. Antidermatophytic and antileishmanial activities of essential oils from Lippia. gracilis Schauer. genotypes. Acta Trop. 2013;128:110–115. doi: 10.1016/j.actatropica.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Escobar P., Leal S.M., Herrera L.V., Martinez J.R., Stashenko E. Chemical composition and antiprotozoal activities of Colombian Lippia. spp essential oils and their major componentes. Mem. Inst. Oswaldo Cruz. 2010;105:184–190. doi: 10.1590/S0074-02762010000200013. [DOI] [PubMed] [Google Scholar]
- 29.Monzote L., Stamberg W., Staniek K., Gille L. Toxic effects of carvacrol, caryophyllene oxide, and ascaridole from essential oil of Chenopodium. ambrosioides on mitochondria. Toxicol. Appl. Pharm. 2014;240:337–347. doi: 10.1016/j.taap.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Morais S.M., Vila-Nova N.S., Bevilaqua C.M.L., Rondon F.C., Lobo C.H., Moura A.A.A.N., Sales A.D., Rodrigues A.P., de Figuereido J.R., Campello C.C., et al. Thymol and eugenol derivatives as potential antileishmanial agentes. Bioorg. Med. Chem. 2014;22:6250–6255. doi: 10.1016/j.bmc.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farias-Junior P.A., Rios M.C., Moura T.A., Almeida R.P., Alves P.B., Blank A.F., Fernandes R.P.M., Scher R. Leishmanicidal activity of carvacrol-rich essential oil from Lippia. sidoides Cham. Biol. Res. 2012;45:399–402. doi: 10.4067/S0716-97602012000400012. [DOI] [PubMed] [Google Scholar]
- 32.Osorio E., Arango G., Robledo S., Muñoz D., Jaramillo L., Vélez I. Antileishmanial and cytotoxic activity of synthetic aromatic monoterpens. Acta Farm. Bonaer. 2006;25:405–413. [Google Scholar]
- 33.Medeiros M.G.F., Silva A.C., Citó A.M.G.L., Borges A.R., Lima S.G., Lopes J.A.D., José A.D.L., Regina C.B.Q.F. In vitro antileishmanial activity and cytotoxicity of essential oil from Lippia sidoides Cham. Parasitol. Int. 2011;60:237–241. doi: 10.1016/j.parint.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Ueda-Nakamura T., Mendonça-Filho R.R., Morgado-Díaz J.A., Maza P.K., Dias Filho B.P., Cortez D.A.G., Alviano D.S., Nakamura C.V. Antileishmanial activity of Eugenol-rich essential oil from Ocimum. gratissimum. Parasitol. Int. 2006;55:99–105. doi: 10.1016/j.parint.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Dutra F.L., Oliveira M.M., Santos R.S., Silva W.S., Alviano D.S., Vieira D.P., Lopes A.H. Effects of linalool and eugenol on the survival of Leishmania. (L.) infantum chagasi within macrophages. Acta Trop. 2016;164:69–76. doi: 10.1016/j.actatropica.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 36.Dorman H.J.D., Deans S.G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 37.Nazzaro F., Fratianni F., De Martino L., Coppola R., De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013;6:1451–1474. doi: 10.3390/ph6121451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Machado M., Dinis A.M., Santos-Rosa M., Alves V., Salgueiro L., Cavaleiro C., Sousa M.C. Activity of Thymus capitellatus volatile extract, 1,8-cineole and borneol against Leishmania. species. Vet. Parasitol. 2014;200:39–49. doi: 10.1016/j.vetpar.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Rosa M.S.S., Mendonça-Filho R.R., Bizzo H.R., Rodrigues I.A., Soares R.M.A., Souto-Padrón T., Alviano C.S., Lopes A.H. Antileishmanial Activity of a Linalool-Rich Essential Oil from Croton cajucara. Antimicrob. Agents Chemother. 2003;47:1895–1901. doi: 10.1128/AAC.47.6.1895-1901.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheljazkov V.D., Cantrell C.L., Tekwani B., Khan S.I. Content, composition, and bioactivity of the essential oils of three basil genotypes function of harvesting. J. Agric. Food Chem. 2008;56:380–385. doi: 10.1021/jf0725629. [DOI] [PubMed] [Google Scholar]
- 41.Camargos H.S., Moreira R.A., Mendanha S.A., Fernandes K.S., Dorta M.L., Alonso A. Terpenes increase the lipid dynamics in the Leishmania plasma membrane at concentrations similar to their IC50 values. PLoS ONE. 2014;9:e104429. doi: 10.1371/journal.pone.0104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rios M.C., Silva W.R.T., Azevedo A.F., Santos P.L., Teixeira S.A., Muscará M.N., Thomazzi S.M., Almeida R.P., Fernandes R.P.M., Scher R. Expression of glyceraldehyde 3-phosphate dehydrogenase is enhanced in Leishmania. spp. naturally resistant to nitric oxide. Genet. Mol. Res. 2015;14:7113–7121. doi: 10.4238/2015.June.29.4. [DOI] [PubMed] [Google Scholar]
- 43.Azevedo A.F.A., Dutra J.L.L., Santos M.L.B., Santos D.A., Alves P.B., Moura T.R., Almeida R.P., Fernandes M.F., Scher R., Fernandes R.P.M. Fatty acid profiles in Leishmania. spp. isolates with natural resistance to nitric oxide and trivalent antimony. Parasitol. Res. 2014;113:19–27. doi: 10.1007/s00436-013-3621-y. [DOI] [PubMed] [Google Scholar]
- 44.Giudice A., Camada I., Leopoldo P.T.G., Pereira J.M.B., Riley L.W., Wilson M.E., John L.H., de Jesus A.R., Edgar M.C., Roque P.A. Resistance of Leishmania. (Leishmania.) amazonensis and Leishmania. (Viannia.) braziliensis to nitric oxide correlates with disease severity in Tegumentary Leishmaniasis. BMC Infect. Dis. 2007;7:1–12. doi: 10.1186/1471-2334-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodrigues F.A.R., Bomfim I.S., Cavalcanti B.C., Pessoa C., Goncalves R.S.B., Wardel J.L., Wardell S.M., de Souza M.V. Mefloquine–Oxazolidine Derivatives: A New Class of Anticancer Agents. Chem. Biol. Drug Des. 2014;83:126–131. doi: 10.1111/cbdd.12210. [DOI] [PubMed] [Google Scholar]
- 46.Winter R. A Consumer's Dictionary of Food Additives-Descriptions in Plain English of More than 12,000 Ingredients both Harmful and Desirable Found in Foods. 7th ed. Three Rivers Press; New York, NY, USA: 2009. pp. 142–528. [Google Scholar]
- 47.Robledo S., Osorio E., Muñoz D., Jaramillo L.M., Restrepo A., Arango G., Vélez I. In Vitro and In Vivo Cytotoxicities and Antileishmanial Activities of Thymol and Hemisynthetic Derivatives. Antimicrob. Agents Chemother. 2005;49:1652–1655. doi: 10.1128/AAC.49.4.1652-1655.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arruda D.C., Miguel D.C., Yokoyama-Yasunaka J.K., Katzin A.M., Uliana S.R. Inhibitory activity of limonene against Leishmania parasites in vitro and in vivo. Biomed. Pharmacother. 2009;63:643–649. doi: 10.1016/j.biopha.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Kulshrestha A., Bhandari V., Mukhopadhyay R., Ramesh V., Sundar S., Maes L. Validation of a simple resazurin-based promastigote assay for the routine monitoring of miltefosine susceptibility in clinical isolates of Leishmania. donovani. Parasitol. Res. 2013;112:825–828. doi: 10.1007/s00436-012-3212-3. [DOI] [PubMed] [Google Scholar]
- 50.International Standard ISO 10993–5 . Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. 3rd ed. International Organization for Standardization Press; Geneva, Switzerland: 2009. MTT Cytotoxicity Test. [Google Scholar]


