Abstract
Objective
In Australia and other countries, participation in colorectal cancer (CRC) screening using fecal occult blood testing is low. Previous research suggests that fecal sampling induces disgust, so approaches not involving feces may increase participation. This study aimed to determine population preferences for CRC screening tests that utilize different sample collections (stool, blood, and saliva) and the extent to which specific attributes (convenience, performance, and cost) impact this preference.
Materials and methods
People aged 50–74 years completed a survey. Preference for screening for CRC through stool, blood, and saliva was judged through ranking of preference and attributes critical to preference and confirmed via a discrete choice experiment (DCE) where test attributes were described as varying by performance, cost, and sample type. Participants also completed a measure of aversion to sample type.
Results
A total of 1,282 people participated in the survey. The DCE and ranking exercise confirmed that all test attributes had a statistically significant impact on respondents’ preferences (P < 0.001). Blood and saliva were equally preferred over stool; however, test performance was the most influential attribute. In multivariable analyses, those who preferred blood to stool collection exhibited higher aversion to fecal (OR = 1.17; P ≤ 0.001) and saliva (OR = 1.06; P ≤ 0.05) sampling and perceived that they had less time for home sample collection (OR = 0.72, P ≤ 0.001). Those who preferred saliva to stool had higher aversion to fecal (OR = 1.15; P ≤ 0.001) and blood (OR = 1.06, P ≤ 0.01) sampling and less time for home sample collection (OR = 0.81, P ≤ 0.5).
Conclusion
Aversion to sample type and perceived inconvenience of sample collection are significant drivers of screening preference. While blood and saliva sampling were the most preferred methods, test performance was the most important attribute of a screening test, regardless of sample type. Efforts to increase CRC screening participation should focus on a test, or combination of tests, that combines the attributes of high performance, low aversion, and convenience of use.
Keywords: quantitative study, preference, discrete choice experiment, ranking, home stool test, Australia
Introduction
It is estimated that in Australia in 2017, colorectal cancer (CRC) will be the second most diagnosed cancer and the third leading cause of cancer-related death.1 In 2015, Australia’s National Bowel Cancer Screening Program (NBCSP) provided a free fecal immunochemical test (FIT) for men and women aged 50–74 years every 5 years, and the program is being gradually expanded to a biennial offer to those aged 50–74 years by 2020.2 The majority of countries with an organized population CRC screening program also provides the opportunity to utilize some form of fecal occult blood test (FOBT), either FIT or guiaic fecal occult blood test (gFOBT).3–5 Despite evidence that screening with an FOBT can detect cancer and precancerous growth at an early stage,6–8 thereby improving prognosis and reducing mortality,9–11 participation in testing around the world is considered suboptimal with participation rates below 50% in many countries.6–8 In Australia, the participation rate in the NBCSP of just 39.0% was achieved in the year 2014–20152 although it must be recognized that one-quarter to one-third of people approached could already have been up to date with screening.12
A key element of a successful population screening program is the achievement of high participation rates because early (curable) lesions that bleed can be found only in individuals who participate in the recommended screening at the recommended intervals. Participation rates are impacted by attitudinal and practical barriers including perceptions of test accuracy, preparation requirements, complexity of the testing process, the perceived likelihood or extent of pain,13,14 perceptions of the risk reduction achieved, the interval time between rescreening,15 and embarrassment.16 Fecal aversion, a term used to describe an attitude that fecal sampling is “unhygienic” and “distasteful,” has also been identified as a contributor to poor fecal test participation.17,18 Taken together, a growing body of research suggests that screening program success is largely dependent on consumer evaluation of the requirements of a screening regimen, and fecal sampling, in particular, has to overcome “the perils of prudishness.”19 Without optimal participation, screening cannot succeed as a public health population strategy, and it is apparent that test technology influences intended and actual participation.
Currently, new approaches to population screening for CRC that do not involve fecal sampling are at various stages of development. These include markers derived from biological fluids including saliva20,21 and blood.22–24 The generally accepted attitude to these biomarkers is that they are likely to be preferred to current modalities because they would circumvent a number of barriers including fecal aversion.
Few studies to date have specifically examined, from a necessarily hypothetical perspective, the likelihood of greater population acceptance (and therefore potential for greater uptake) of alternative biomarkers as a substitute for, or complement to, the fecal test. Current evidence suggests that blood sampling may be preferred to stool,25,26 although this is not always the case,27,28 and a combination of, or choice between, stool and blood testing may be even preferred over either test individually.25 Other studies conducted in the context of preference for giving saliva, urine, or blood for medical testing have found that saliva was the preferred method over blood or urine when presented as a hypothetical possibility29 and after actual sample collection.30 It is clear that insufficient evidence exists concerning consumer preferences for screening modality, particularly when placed within plausible contexts where tests vary on critical dimensions likely to influence preferences such as test performance, method of sample collection, cost, and perceived convenience of sample collection location. In the light of this research gap, we utilized two approaches – a ranking task and a discrete choice experiment (DCE) – to examine the influence of these particular test attributes on consumer preferences for CRC screening modalities.
Ranking involves a respondent assigning a level of importance to each attribute that describes a test procedure separately. The measurement process requires an absolute judgment made in the context of a relative preference, whereas a DCE requires respondents to make trade-offs between stimuli according to underlying attributes with these presented in scenarios. DCEs are widely used in market research to determine which attributes of a product influence consumer preferences and to what extent consumers are prepared to “trade-off” one attribute against another. DCEs are also increasingly being utilized in health economics to determine patient preferences for health care options. A DCE approach to data collection and analysis provides the opportunity to identify critical attributes of a health action or service that determine preferences and their comparative importance to decision-making and to attach a monetary value to decisions.31–33 A number of studies using the DCE method have compared test preferences for existing CRC screening (colonoscopy vs flexible sigmoidoscopy vs fecal test) have been conducted,15,16,27,33–35 but none have examined hypothetical technologies or approaches.
We expanded the ranking and DCE exercises to include an investigation of demographic and psychological predictors of preferences for specific screening tests. Previous research has highlighted that aversion is negatively associated with acceptance of stool-based screening17,26 and that perception of convenience influences sample preference,26 and so we hypothesized that these two factors, aversion and convenience, influenced the acceptance of (hypothetical) blood and saliva collection for CRC screening. Thus, the aims of this study were as follows:
to identify the relative importance of the separate attributes of sample type, perceived convenience of sample collection, test performance, and cost in the decision to utilize a specific form of CRC screening via a ranking and DCE exercise;
to confirm that sample types rated as more aversive to provide would be less preferred and that perceived convenience of sample collection positively influences sample preference; and
to explore demographic associations with affective response to sampling.
Materials and methods
Participant recruitment and study design
The Electoral Commission of South Australia provided a random sample of 3,000 names and addresses of men and women aged 50–74 years in selected urban and rural residential areas representing a range of socioeconomic status. No exclusion criteria were applied. Ethics approval was obtained from Flinders University Social and Behavioral Research Ethics Committee (5627 SBREC), and the study was conducted in Adelaide, Australia. All study invitees were informed about the aims of the study and its anonymous and voluntary nature, before providing their consent by the act of completing the survey.
Invitees were mailed an introductory letter 2 weeks prior to survey distribution, at which point there was an opportunity to opt out of the study. Invitees were advised that they would be asked to complete a web-based survey (SurveyMonkey, San Mateo, California, USA) but were able to request a paper version. They subsequently received a letter providing web access instructions or paper-based survey (if requested) as well as the option again to request a paper-based survey or to opt out. A complaints procedure form and a reply-paid envelope were also included. Four weeks after survey distribution, people who opted out were excluded from the study and those who had not completed the survey received a reminder letter and paper version of the survey. No further attempts were made to recruit non-respondents after this stage. Participants who completed a survey within 9 weeks of initial invitation constituted the survey sample.
Survey measures
The survey recorded responses to basic questions about demographic characteristics (age, gender, marital and employment status, educational level, country of birth, and cultural identification). The Socio-Economic Indexes for Areas (SEIFA) Index of Relative Socio-Economic Disadvantage was utilized as an index of socioeconomic status. This is a broad measure of residential area disadvantage.36 A higher score indicates a relative lack of disadvantage in general, for example, few households with low incomes, few people with no qualifications, and few people in low skilled occupations.
Self-reported fecal test use was measured by one item, “I perform home stool tests for bowel cancer” (4-point Likert scale ranging from never [1] to always [4] with a not applicable choice provided). Responses were dichotomised to “never” (no FOBT use) and “any” (prior FOBT use), so that we could determine if responses varied among those who had experience with FOBT and those who did not. Emotions associated with the thoughts of stool, blood, and saliva testing, hereinafter termed “Affective Response to Sampling,” were assessed via responses to scenarios describing the process of sample attainment for each sample collection method (Table 1). The four statements described the process as unpleasant, unhygienic, embarrassing, and uncomfortable. Each scenario was scored on a 7-point Likert scale, where (1) represented strongly disagree and (7) strongly agree; thus, scores could range from 4 to 28, with higher scores indicating greater aversion. “Perceived Convenience of Sample Collection” was assessed on the basis of response to questions on test-taking requirements, captured on a 5-point Likert rating scale, where (1) indicated difficult to find time for and (5) easy to find time. Statements rated were as follows: attendance at an appointment at the doctor, attendance at a pathology center, and completing a test at home and sending a sample in the post for the blood, saliva, and stool tests, respectively.
Table 1.
Affective response to sampling: stool, blood, and saliva collection scenarios
| Stool sample | Blood sample | Saliva sample |
|---|---|---|
| 1. Obtain a home stool test kit (from your doctor, the chemist, or other provider). It contains collection paper, two sample containers with a collection stick attached to the inside of the cap, manufacturer’s instructions, and a return envelope. 2. For the first sample, pass the bowel motion (stool) on to the collection paper placed inside the toilet bowel. 3. The collection stick is used to collect a small sample of stool (equivalent to a few grains of rice) and then to place back into the sample container. 4. The process is repeated on another bowel motion. 5. Both completed samples are placed into the return envelope supplied and posted to the testing laboratory. 6. Results are posted to you as soon as they are available. |
1. Visit a doctor or a local pathology collection center. 2. A health professional uses a needle to collect a blood sample from your arm at the doctor’s surgerya or pathology center. 3. The tube of blood is transported to a local testing laboratory. 4. Results are sent to your doctor as soon as they are available. |
1. Visit a doctor or a local pathology collection center. 2. A health professional provides you with a small container to collect a saliva sample. 3. You are required to deposit about a teaspoon of saliva from your mouth into the container. 4. The container of saliva is transported to a local testing laboratory. 5. Results are sent to your doctor as soon as they are available. |
Notes: Given the following scenarios, on a scale of 1–7 where 1 is strongly disagree and 7 is strongly agree, providing a sample would be 1) unpleasant, 2) unhygienic, 3) embarrassing, and 4) uncomfortable.
Equivalent to “doctor’s office.”
Ranking test preference
Five survey questions were developed to determine respondent preferences for each test attribute: sample type (stool, blood, and saliva); sample collection location (home, pathology center, and doctor’s office); test performance, defined as the capacity of the test to detect bowel cancer (70%, 80%, and 90% detection); cost of test ($30, $50, and $80); and most preferred test attribute – sample type, sample collection location, test performance, or test cost. Performance was included because previous research has shown test accuracy to be an important determinant of preference.32 We provided identical test accuracy options for the three sample types based on the plausible ability of the fecal test to detect bowel cancer specifically (rather than adenomas). Levi et al37 reported a 94% test sensitivity for detecting CRC, which is similar to our previous finding of 86% sensitivity for detecting CRC after a positive FIT.38 Cost test was included (notwithstanding that the cost of cancer screening in Australia is largely covered by the national health system) to ensure concordance with the DCE, which utilizes cost as a calculation of the health economics measure, willingness to pay (WTP). Respondents ranked their preferences from highest to lowest (ie, 1–3 for items 1–4 and 1–4 for item 5 with 1 indicating the top rank or the most preferred). Ties were allowed. Ranks were converted to scores, so that ranks 1, 2, 3, and 4 received corresponding scores of 4, 3, 2, and 1; missing values were allocated a numerical 0 score,39 so that means could be calculated. A mean was obtained for each sample type with a higher score indicating greater preference.
DCE
The DCE tested the relative influence of sample type (blood, feces, and saliva), test performance (70%, 80%, or 90% of cancers detected, as for the ranking exercise), and cost to the consumer for undertaking the test ($30, $50, or $80 Australian) on choice. Cost was included as an attribute to estimate the WTP; WTP is a measure of the amount of money a person is willing to sacrifice to procure a good outcome or avoid something undesirable.
Specification of three levels for each of the three attributes resulted in 27 (=33) possible scenario permutations, and a total of 351 (=27 × 26/2) possible pairwise choices. Utilization of the fractional factorial design described by Street and Burgess40 reduced the number of choice scenarios into a manageable set of 27 choices for presentation. The resulting DCE design was blocked into three survey versions of nine pairs each. This design was 100% efficient for the estimation of main effects only. The random sample of invitees (n = 3,000) were sorted alphabetically by surname, and one of the three versions of the survey was systematically assigned in such a way that the first 1,000 invitees received the first version, the next 1,000 received the second version, and the remaining 1,000 received the final version.
Statistical analyses
The unidimensionality and reliability (internal consistency) of multi-item scales constructed for this study were assessed by principal components analysis (PCA) and Cronbach’s alpha co-efficient, respectively. Results and final items are summarized in Table 2. Scales were developed for affective response to sampling and perceived convenience of sample collection. Items with a moderate to large correlation value (0.50–0.90)41,42 were aggregated to create a mean total score. The “Affective Response to Sampling” scale was unidimensional (ie, all four items loaded on one component with an eigenvalue exceeding 1 and explained 76%, 72%, and 89% of the variance for stool, blood, and saliva, respectively). Reliability for stool, blood, and saliva sampling was strong with alphas of 0.89, 0.85, and 0.96, respectively.
Table 2.
List of multiscale and single items and descriptive statistics
| Scale name | Item description | Mean (SD) score | Cronbach’s alpha |
|---|---|---|---|
| Affective response to sampling – stool (n = 1,217, maximum score = 28) | Collecting a stool sample would be 1) unpleasant; 2) unhygienic; 3) embarrassing; 4) uncomfortable | 12.61 (5.68) | 0.892 |
| Affective response to sampling – blood (n = 1,232, maximum score = 28) | Collecting a blood sample would be 1) unpleasant; 2) unhygienic; 3) embarrassing; 4) uncomfortable | 9.14 (4.75) | 0.851 |
| Affective response to sampling – saliva (n = 1,236, maximum score = 28) | Collecting a saliva sample would be 1) unpleasant; 2) unhygienic; 3) embarrassing; 4) uncomfortable | 8.59 (4.97) | 0.957 |
| Perceived convenience of external sample collection (n = 1,262, maximum score = 10) | Finding the time to attend an appointment at the 1) doctor; 2) pathology center | 7.13 (2.29) | 0.833 |
| Perceived convenience of home sample collection (n = 1,262, maximum score = 10) | Finding time to 1) complete a test at home; 2) send a sample in the post | 8.49 (1.76) | 0.840 |
Four items comprising “Perceived Convenience of Sample Collection” were subjected to PCA, and two components with eigenvalues exceeding one were identified, explaining 60.2% and 25.8% of the variance, respectively, and with strong loadings. The two-factor solution, with two items loading on each factor, was supported by parallel analysis,43 and two items were termed “perceived convenience of external sample collection” and “perceived convenience of home sample collection”. Each had good reliability with an alpha of 0.72 and 0.73, respectively.
For the ranking analysis, one-way repeated measures ANOVA was conducted to compare sample ranking scores. If multivariate test results indicated that there were significant differences at the P < 0.05 level, post hoc analyses were conducted.
For the DCE analysis, data were analyzed within a random utility maximization framework using the conditional logit model.44 In this experiment, each respondent indicated their preferences between two screening scenarios differing on the three attributes (cost, test performance, and sample type) with nine choice questions in total. The empirical model to be estimated was specified as:
where U is the utility that individual receives from choosing alternative in each choice scenario, βi is a vector of coefficients reflecting the desirability of the attributes, and ε is a random term. Conditional on βi, it is assumed that ε is independent and identically distributed with Gumbel distribution.44 The cost attribute was treated as a continuous variable for the purposes of calculating WTP.45 We calculated WTP by dividing the estimated coefficients for sample type or test performance attributes by the estimated coefficient for the cost attribute. The 95% CIs were calculated using the bootstrap technique.46
We performed univariate and multivariate multinomial regression analyses with sample preference (blood and saliva) as the separate dependent variable and using stool sampling as the referent. For the regression analyses, only those individuals who expressed a clear preference for a particular sample type (blood, stool, or saliva) were utilized, ie, those with equal preferences for a sample type were not included in the analysis (resultant n = 1,194). Predictive variables included categorical demographic measures (gender, age group, previous FOBT use, education, geographic location, partner status, employment, and SEIFA status, which was assessed by dichotomising the SEIFA score at the 50th percentile and comparing participants in decile ≤5 (most disadvantaged) with those in decile ≥6 (least disadvantaged). The variables affective response to sampling, perceived convenience of external sample collection, and perceived convenience of home sample collection were included as continuous psychological measures. Results are presented as ORs. Finally, independent samples t-tests and one-way ANOVAs were used as appropriate to explore the relationship between demographic factors and affective response for each sample collection method.
Results
Descriptive analyses
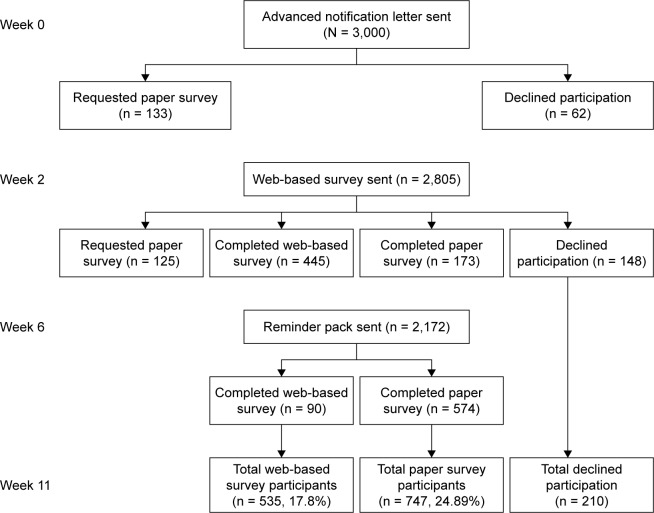
Participant numbers and attrition rates are shown in Figure 1. The survey response rate was 42.7% (1,282/3,000). Chi-square analysis indicated that there was no statistically significant association between gender and participation status (P = 0.30). There was a significant association for age (P > 0.001); further examination of each age band’s observed vs expected frequencies indicated that less people aged 50–54 years and more people aged 65–69 years participated in the survey. Table 3 provides participant demographic details for each survey version group. There were no statistically significant differences across groups for any of these characteristics. Taking the group as a whole, participation in the paper version of the survey (747/1,282, 58.3%) was higher than the web-based version (535/1,282, 41.7%). The majority of participants were living in a less disadvantaged area (742/1,282, 57.9%). Less than half the participants were in full- or part-time employment (533/1,248, 42.7%), which may reflect the fact that 43.4% (557/1,282) of participants were aged 65 years and older. Over half of the survey respondents (750/1,258, 59.6%) indicated that they had previous experience with FOBT. The majority of respondents were born in Australia.
Figure 1.
Participation flow diagram.
Table 3.
Demographic characteristics of survey participants by DCE version allocated
| Demographic characteristics | Version 1, n (%) |
Version 2, n (%) |
Version 3, n (%) |
Total N |
|---|---|---|---|---|
| Sample | 438 | 420 | 424 | 1,282a |
| Survey type | ||||
| Web | 176 (40.2) | 170 (40.5) | 189 (44.6) | 535 (41.7) |
| Paper | 262 | 250 | 235 | 747 |
| Gender | ||||
| Male | 207 (47.3) | 197 (46.9) | 207 (48.8) | 611 (47.7) |
| Female | 231 | 223 | 217 | 671 |
| Age, years (n = 1,282) | ||||
| 50–54 | 72 (16.4) | 72 (17.1) | 73 (17.2) | 217 (16.9) |
| 55–59 | 88 (20.1) | 71 (16.9) | 82 (19.3) | 241 (18.8) |
| 60–64 | 89 (20.3) | 85 (20.2) | 93 (21.9) | 267 (20.8) |
| 65–69 | 96 (21.9) | 95 (22.6) | 98 (23.1) | 289 (22.6) |
| 70–74 | 93 (22.2) | 97 (23.1) | 78 (18.4) | 268 (20.9) |
| Location | ||||
| Urban | 336 (76.7) | 319 (76.0) | 325 (76.7) | 980 (76.4) |
| Rural | 102 | 101 | 99 | 302 |
| Marital status | ||||
| Yes | 339 (77.4) | 327 (77.9) | 305 (71.9) | 971 (75.7) |
| No | 86 | 79 | 105 | 270 |
| Employment status | ||||
| In workforce | 176 (40.2) | 173 (41.2) | 184 (43.4) | 533 (41.6) |
| Not in workforce | 253 | 235 | 227 | 715 |
| Education level | ||||
| <Year 12 | 190 (43.4) | 176 (41.9) | 179 (42.2) | 545 (42.5) |
| ≥Year 12 | 236 | 231 | 230 | 697 |
| SEIFAb | ||||
| ≤5 | 185 (42.2) | 175 (41.7) | 180 (42.5) | 540 (42.1) |
| ≥6 | 253 | 245 | 244 | 742 |
| Born in Australia | ||||
| Yes | 322 (73.5) | 316 (75.2) | 308 (72.6) | 946 (73.8) |
| No | 102 | 91 | 103 | 296 |
| Prior FOBT experience | ||||
| Yes | 248 (56.6) | 258 (61.4) | 244 (57.5) | 750 (58.5) |
| No | 185 | 155 | 168 | 508 |
Notes:
Not all questions were completed by all participants; the numbers indicated above are not based on the total number of respondents.
SEIFA: decile ≤5, most disadvantaged; decile ≥6, least disadvantaged.
Abbreviations: DCE, discrete choice experiment; FOBT, fecal occult blood test; SEIFA, Socio-Economic Indexes for Areas.
Relative importance of screening attributes: ranking results
Results of ranking attributes (means, SDs, and effect sizes) are shown in Table 4. One-way repeated measures ANOVA was used to compare scores for each attribute item; all attributes showed statistically significant differences (P < 0.001) with medium to large effect sizes. Post hoc analyses indicated that for sample type saliva and blood were equally preferred, and stool was the least preferred. For convenience of sample collection location, collection at both home and the doctor were equally preferred and collection at a pathology center was less preferred. Test performance of 90% was the most preferred, followed by 80% and lastly by 70%. A test cost of $30 was the most preferred, followed by $50 and lastly a cost of $80 per test. Mean scores for perceived importance of test attribute (sample type, convenience, performance, and cost) indicated that test performance was regarded as the most important attribute of a screening test, followed equally by convenience and sample type and lastly test cost.
Table 4.
Preference scores for screening test attributes, transformed from rankings
| Attribute (score range) | Item | Mean score (SD) | df | F | η2 |
|---|---|---|---|---|---|
| Sample type (0–3) | Saliva | 2.18 (0.87) | 2, 1,280 | 152.88a | 0.193 |
| Blood | 2.12 (0.81) | ||||
| Stool | 1.56 (0.87) | ||||
| Sample collection location (0–3) | Home | 2.08 (0.97) | 2, 1,280 | 68.71a | 0.097 |
| Doctor’s surgery | 2.06 (0.80) | ||||
| Pathology center | 1.70 (0.84) | ||||
| Test performance (0–3) | 90% | 2.84 (0.60) | 2, 1,280 | 3,951.35a | 0.861 |
| 80% | 1.94 (0.45) | ||||
| 70% | 1.03 (0.40) | ||||
| Test cost (0–3) | $30 | 2.52 (0.87) | 2, 1,280 | 744.97a | 0.538 |
| $50 | 2.04 (0.58) | ||||
| $80 | 1.23 (0.71) | ||||
| Test attributes (0–4) | Performance | 3.55 (0.92) | 3, 1,279 | 905.49a | 0.680 |
| Convenience | 2.37 (0.94) | ||||
| Sample type | 2.34 (1.10) | ||||
| Cost | 1.71 (1.04) |
Note:
P < 0.001.
Relative importance of screening attributes: DCE results
Ninety-six percent of respondents (1,231/1,282) answered all nine pairs of choice scenarios and were included in the conditional logit regression analysis, results from which are presented in Table 5. All attributes were statistically significant. The sign and magnitude of the coefficients attached to test performance and the cost attributes indicated that respondents would prefer screening tests with higher performance and lower cost. Analysis confirmed that the results were largely uninfluenced by demographic characteristics, mode of survey administration, previous test experience, and attitude toward the CRC screening test.
Table 5.
Conditional logit estimates for individual’s preferences for colorectal cancer screening test and marginal rates of substitution with respect to cost (N = 1,231)
| Attributes | Levels | Coefficient | Cluster robust SE | WTP (AUD) | 95% CI (lower) | 95% CI (upper) |
|---|---|---|---|---|---|---|
| Sample type | Stool | −0.353 | 0.023a | −20.950 | −25.188 | −17.163 |
| Saliva | 0.134 | 0.022a | 7.965 | 5.399 | 10.634 | |
| Blood | 0.219 | 0.023a | 12.985 | 9.942 | 16.419 | |
| Test | 70% | −1.495 | 0.024a | −88.768 | −98.414 | −80.636 |
| performance | 80% | 0.023 | 0.011b | 1.365 | 0.126 | 2.653 |
| 90% | 1.472 | 0.038a | 87.403 | 79.292 | 97.028 | |
| Cost | −0.017 | 0.001a | – | – | – | |
| Log likelihood | −4,312.525 | |||||
| Observations | 11,079 |
Notes: Conditional logit estimates reported in the table. Cost attribute was included as a continuous variable; all other attributes were effect coded. CIs estimated using bootstrap method (with 10,000 replications).
P < 0.01;
P < 0.05.
Abbreviations: AUD, Australian dollar; SE, standard error; WTP, willingness to pay.
The WTP estimates for the full sample are also reported in Table 5. Participants were willing to pay $13 on average for a blood sample test and $8 for saliva; in contrast, the negative coefficient for the stool sample type suggests that respondents required a payment of $21 to participate in a stool test.
Test performance trade-offs were consistent with the hypothesis; on average, respondents were willing to pay $87 and $1 for a 90% and 80% cancer detection rate, respectively. In the context of better performance being available, the negative coefficient on the 70% detection rate suggests that respondents required a payment of $89 to have this test.
The relationship of demographic and psychological characteristics to relative preference for blood and saliva sampling compared to FIT
To address Aim 2 and test our hypothesis that affective responses to a sample and convenience of sample collection are significant psychological predictors of sample preference, we conducted univariate and multinomial regression analyses with FIT sampling, “usual care” within Australia, as the referent. Results, presented as ORs, are summarized in Table 6. Fully adjusted multivariate models showed that higher negative affective response to FIT and saliva sample collection, and lower perceived convenience of home collection, resulted in a preference for blood testing. Similarly, negative affective response to FIT and blood sample collection and lower perceived convenience of home sample collection resulted in a preference for saliva collection.
Table 6.
Associations between demographic and psychological characteristics with preference for blood and saliva testing compared to FOBT
| Demographic characteristics | Sample
|
Preference for blood (univariate)
|
Preference for blood (multivariate)
|
Preference for saliva (univariate)
|
Preference for saliva (multivariate)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | OR | 95% CI | β | OR | 95% CI | β | OR | 95% CI | β | OR | 95% CI | β | |
| 50–59 years | 423 | 35.4 | 0.70 | 0.45–1.10 | −0.351 | 0.73 | 0.43–1.25 | −0.316 | 1.46 | 0.94–2.26 | 0.376 | – | – | – |
| 60–69 years | 524 | 43.9 | 0.59c | 0.39–0.90 | −0.522 | 0.63 | 0.39–1.02 | −0.465 | 0.91 | 0.60–1.39 | −0.091 | – | – | – |
| 70+ years | 247 | 20.7 | 1 | – | – | – | – | – | 1 | – | – | – | – | – |
| Female | 630 | 52.8 | 1 | – | – | – | – | – | 1 | – | – | – | – | – |
| Male | 564 | 47.2 | 0.80 | 0.58–1.09 | −0.230 | – | – | – | 0.90 | 0.67–1.22 | −0.106 | – | – | – |
| No previous FOBT screening | 472 | 40.3 | 2.18a | 1.55–3.05 | 0.777 | 1.35 | 0.92–1.99 | 0.300 | 1.64b | 1.18–2.27 | 0.492 | 1.17 | 0.81–1.69 | 0.154 |
| Previous FOBT screening | 700 | 59.7 | 1 | – | – | – | – | – | 1 | – | – | 1 | – | – |
| <Year 12 education | 514 | 44.0 | 1.24 | 0.90–1.70 | 0.212 | – | – | – | 0.64b | 0.47–0.87 | −0.455 | 0.732 | 0.52–1.04 | −0.312 |
| ≥Year 12 education | 653 | 56.0 | 1 | – | – | – | – | – | 1 | – | – | – | – | – |
| Urban living | 911 | 76.3 | 0.80 | 0.56–1.15 | −0.222 | – | – | – | 1.26 | 0.88–1.81 | 0.230 | – | – | – |
| Rural living | 283 | 23.7 | 1 | – | – | – | – | – | 1 | – | – | – | – | – |
| Partner | 915 | 78.6 | 1 | – | – | – | – | – | 1 | – | – | – | – | – |
| No partner | 249 | 21.4 | 0.55 | 0.61–1.30 | −0.117 | – | – | – | 0.81 | 0.56–1.16 | −0.215 | – | – | – |
| Employed | 508 | 43.4 | 1 | – | – | – | – | – | 1 | – | – | – | – | – |
| Not employed | 662 | 56.6 | 1.36 | 0.98–1.89 | 0.308 | – | – | – | 0.80 | 0.59–1.09 | −0.219 | – | – | – |
| SEIFA most disadvantaged | 495 | 41.5 | 1.09 | 0.79–1.49 | 0.083 | – | – | – | 0.72c | 0.53–0.98 | −0.332 | 1.09 | 0.75–1.58 | 0.087 |
| SEIFA least disadvantaged | 699 | 58.5 | 1 | – | – | – | – | – | 1 | – | – | – | – | – |
| Affective response to FIT collection | 1,139 | 1.17a | 1.13–1.21 | 0.153 | 1.17a | 1.12–1.22 | 0.155 | 1.13a | 1.09–1.16 | 0.117 | 1.15a | 1.10–1.20 | 0.139 | |
| Affective response to blood collection | 1,154 | 1.00 | 0.96–1.03 | −0.003 | – | – | – | 1.05b | 1.02–1.09 | 0.049 | 1.06b | 1.01–1.12 | 0.059 | |
| Affective response to saliva collection | 1,157 | 1.07a | 1.01–1.12 | 0.071 | 1.06c | 1.01–1.12 | 0.062 | 0.99 | 0.95–1.02 | −0.014 | – | – | – | |
| Perceived convenience of home sample collection | 1,180 | 0.69a | 0.61–0.77 | −0.371 | 0.72a | 0.62–0.83 | −0.330 | 0.76a | 0.68–0.85 | −0.278 | 0.81c | 0.71–0.94 | −0.206 | |
| Perceived convenience of external sample collection | 1,178 | 1.00 | 0.94–1.08 | 0.004 | – | – | – | 0.91b | 0.85–0.97 | −0.095 | 1.03 | 0.94–1.12 | 0.027 | |
Notes: Analysis – multinomial logistic regression.
P < 0.001.
P < 0.01.
P ≤ 0.05.
Abbreviations: FIT, fecal immunochemical test; FOBT, fecal occult blood test; SEIFA, Socio-Economic Indexes for Areas.
The relationship of demographic characteristics to affective response to sampling
To address Aim 3 and explore demographic associations with affective response to sampling, participants were asked to indicate their level of negative affective response to each sampling mode based on sample collection scenarios (Table 1). Bivariate analyses indicated significant associations for some but not all demographic variables. For stool testing, those who had not previously screened with FIT were more averse to stool testing (mean = 14.3, SD = 5.8) than those who had previously screened (mean = 11.5, SD = 5.3, P < 0.001). For blood collection, men were more averse (mean = 9.5, SD = 4.9) than women (mean = 8.9, SD = 4.6, P < 0.05), as were people who lived in a less disadvantaged area (mean = 9.6, SD = 4.8 vs 8.7, SD = 4.7, P < 0.01). For saliva collection, unemployed participants were more averse compared to those who were employed (mean = 9.0, SD = 5.3 vs mean = 8.1, SD = 4.5, P < 0.01), and participants aged between 70 and 74 years (mean = 9.4, SD = 5.6) were significantly more averse than younger participants aged 50–59 years (mean = 8.1, SD = 4.6, P < 0.05). All significant results were very small effects, with the largest (0.6) reported for the relationship between no prior FOBT screening and higher negative affective response to stool testing.
Discussion
Given the suboptimal participation rates in current population-based CRC screening tests in Australia (ie, stool-based FOBT) and emerging evidence of the utility of potential alternative CRC biomarkers such as blood and saliva, the purpose of this study was to determine whether population preferences exist for CRC screening tests that vary on various test attributes. In particular, we tested the influence of aversion to sample type and perception of convenience, expressed as finding time to provide a sample. In addition, we measured how performance, cost, and sample impacted discrete choices.
The results from the DCE demonstrate that all three attributes, cost, test performance, and sample type, significantly impacted on respondents’ preferences. Results from the WTP analysis further suggest that test performance is a more influential attribute than sample type. These results were consistent with the preference ranking exercise, which indicated that overall the most highly ranked attribute, whatever the sampling method, was test performance. These results are consistent with other studies13,47 even though previous work has compared more invasive tests (eg, FOBT and/or flexible sigmoidoscopy or colonoscopy). At the time this study was conducted, few data were available relating to the effectiveness of blood and saliva testing to detect CRC, and so we presented hypothetical performance levels for blood and saliva based on plausible levels of FIT sensitivity. Since that time, additional support for the FIT performance levels chosen has been provided by an analysis conducted by Australia’s NBCSP, which indicated 83% FIT sensitivity for CRC over the years 2006–2008.6 Recent research48 examining the utility of blood and saliva to detect CRC has shown comparable CRC sensitivity levels for fecal and blood tests (64% and 62%, respectively), and Sazanov et al49 in 2017 reported that microRNA-21 expression in saliva had a sensitivity of 97% for detection of CRC. These results suggest that the hypothetical test performance levels for all three screening modalities were within real-world detection possibilities.
To the best of our knowledge, this is one of the few studies of this kind to incorporate sample type as an attribute for consideration in a test of choice of CRC screening approaches. Consistent with our own past research,17,50 fecal aversion was shown to impact FIT acceptability, but our findings also highlighted the importance of attention to other test attributes. When the impact of attributes is considered together, results from the DCE and ranking exercise confirmed highest acceptability for blood sampling followed by saliva although the difference between these two was reduced to non-significance in the ranking task. This finding is consistent with our previous research,28 where we showed that when a group of community volunteers and South Australian electoral roll registrants were offered a choice of blood or stool test, the majority preferred a blood test (79.6% vs 20.4%, respectively, from 1,561 respondents). Although there was evidence that, consistent with past research,26 previous use of a FIT resulted in lower fecal aversion, in multivariate analysis, previous FIT screening was not a significant predictor of preference for stool sampling.
An explanation for the preference for blood testing likely rests with a number of characteristics of this approach. Although blood sampling could be described as the most physically invasive of the three procedures under consideration, it is both familiar and trusted because it is utilized for a variety of medical diagnostic purposes. It requires little from the person in terms of active participation, particularly where fasting is not required. Nonetheless, not all population groups were equally accepting; in our study, men were more averse to blood collection than women, albeit with a small effect size. This observation confirms previous findings by us,28 where univariate analysis of data from n = 1,561 indicated that men were more likely to prefer provision of a stool sample over a hypothetical blood test. It is interesting to note that, notwithstanding these findings, male participation in the NBCSP has been consistently lower than female participation,51–53 and our results point to the possibility that male-specific barriers to screening need to be addressed in the context of stool-based screening as much as for blood.54–57
A limitation of the results is that they are based on the largely hypothetical nature of the alternate screening technologies described. Although the respondents were given a detailed description of the proposed test procedure, preferences were decided based on individuals constructing a schema for how testing “might” work. For blood testing, this was not difficult, given the ubiquitous nature of this procedure currently. This contrasts with saliva, which is much less collected as a biological specimen, although it is becoming increasingly utilized for medical tests, particularly those involving DNA sampling.58
Notwithstanding this limitation, the results suggest that, in the current environment, people exhibit higher aversion to fecal sampling, even given previous FIT screening. This suggests that CRC screening participation rates might be improved if sampling moved away from feces. Extensive work is currently being completed around the world to validate blood sampling for CRC screening.59,60 In addition, collection of saliva to identify genetic lifetime risk for CRC is also showing potential for use as a screen for surveillance program enrolment eligibility.61,62 Future research should evaluate whether an adjusted choice of test performance levels for blood and saliva testing, based on discrete results, alters patient preference, Alternatively, the use of blood testing as a second-line adjunct for FIT non-participants has been shown to increase overall screening rates.63
Conclusion
The findings of this research confirm our expectation that affective response (aversion) to sample type and perceived inconvenience of sample collection (in particular home sample collection) are significant drivers of screening preference. Test performance was the most important attribute of a screening test, regardless of sample preference. Further research should address the potential for the development of a test, or combination of tests, that combine the attributes of high performance, low aversion, and convenience of use to increase screening participation rates.
Acknowledgments
The authors would like to acknowledge the contribution of Dr Leonie Burgess for providing the orthogonal design template used to distribute the scenarios and attribute levels across the three versions of the discrete choice experiment. The authors would also like to thank Stephen Cole for his insightful advice regarding the design of the project. This research was supported in part by a Foundation Daw Park Grant (2010/2011) awarded to JMO, CJW, and GPY. IF, CJW, GC, JR, and GPY were supported by a grant funded by the financial support of the Cancer Council SA’s Beat Cancer Project on behalf of its donors and the State Government of South Australia, through the Department of Health together with the support of the Flinders Foundation and its donors and partners.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Australian Institute of Health and Welfare . Cancer in Australia. Canberra: AIHW; 2017. [Google Scholar]
- 2.Australian Institute of Health and Welfare . National Bowel Cancer Screening Program: Monitoring Report 2017. Canberra: AIHW; 2017. [Google Scholar]
- 3.Benson VS, Patnick J, Davies AK, Nadel MR, Smith RA, Atkin WS. Colorectal cancer screening: A comparison of 35 initiatives in 17 countries. Int J Cancer. 2008;122(6):1357–1367. doi: 10.1002/ijc.23273. [DOI] [PubMed] [Google Scholar]
- 4.Benson VS, Atkin WS, Green J, et al. Toward standardizing and reporting colorectal cancer screening indicators on an international level: The International Colorectal Cancer Screening Network. Int J Cancer. 2012;130(12):2961–2973. doi: 10.1002/ijc.26310. [DOI] [PubMed] [Google Scholar]
- 5.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–1649. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 6.Australian Institute of Health and Welfare . Analysis of Bowel Cancer Outcomes for the National Bowel Cancer Screening Program. Canberra: AIHW; 2014. [Google Scholar]
- 7.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343(22):1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 8.Ventura L, Mantellini P, Grazzini G, et al. The impact of immunochemical faecal occult blood testing on colorectal cancer incidence. Dig Liver Dis. 2014;46(1):82–86. doi: 10.1016/j.dld.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 10.Garborg K, Holme Ø, Løberg M, Kalager M, Adami HO, Bretthauer M. Current status of screening for colorectal cancer. Ann Oncol. 2013;24(8):1963–1972. doi: 10.1093/annonc/mdt157. [DOI] [PubMed] [Google Scholar]
- 11.Cole SR, Tucker GR, Osborne JM, et al. Shift to earlier stage at diagnosis as a consequence of the National Bowel Cancer Screening Program. Med J Aust. 2013;198(6):327–330. doi: 10.5694/mja12.11357. [DOI] [PubMed] [Google Scholar]
- 12.Zajac IT, Flight I, Turnbull D, Young G, Cole S, Wilson C. Self-reported bowel screening rates in older Australians and the implications for public health screening programs. Australas Med J. 2013;6(8):411–417. doi: 10.4066/AMJ.2013.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall DA, Johnson FR, Phillips KA, Marshall JK, Thabane L, Kulin NA. Measuring patient preferences for colorectal cancer screening using a choice-format survey. Value Health. 2007;10(5):415–430. doi: 10.1111/j.1524-4733.2007.00196.x. [DOI] [PubMed] [Google Scholar]
- 14.Mansfield C, Tangka FK, Ekwueme DU, et al. Stated Preference for Cancer Screening: A Systematic Review of the Literature, 1990–2013. Prev Chronic Dis. 2016;13:E27. doi: 10.5888/pcd13.150433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dam L, Hol L, de Bekker-Grob EW, Steyerberg E, et al. What determines individuals’ preferences for colorectal cancer screening programmes? A discrete choice experiment. Eur J Cancer. 2010;46(1):150–159. doi: 10.1016/j.ejca.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Hol L, de Jonge V, van Leerdam ME, et al. Screening for colorectal cancer: comparison of perceived test burden of guaiac-based faecal occult blood test, faecal immunochemical test and flexible sigmoidoscopy. Eur J Cancer. 2010;46(11):2059–2066. doi: 10.1016/j.ejca.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Cole SR, Zajac I, Gregory T, et al. Psychosocial variables associated with colorectal cancer screening in South Australia. Int J Behav Med. 2011;18(4):302–309. doi: 10.1007/s12529-010-9101-1. [DOI] [PubMed] [Google Scholar]
- 18.Chapple A, Ziebland S, Hewitson P, Mcpherson A. What affects the uptake of screening for bowel cancer using a faecal occult blood test (FOBt): a qualitative study. Soc Sci Med. 2008;66(12):2425–2435. doi: 10.1016/j.socscimed.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Kronborg O, Regula J. Population screening for colorectal cancer: advantages and drawbacks. Dig Dis. 2007;25(3):270–273. doi: 10.1159/000103899. [DOI] [PubMed] [Google Scholar]
- 20.Fábryová H, Celec P. On the origin and diagnostic use of salivary RNA. Oral Dis. 2014;20(2):146–152. doi: 10.1111/odi.12098. [DOI] [PubMed] [Google Scholar]
- 21.Spielmann N, Wong DT. Saliva: diagnostics and therapeutic perspectives. Oral Dis. 2011;17(4):345–354. doi: 10.1111/j.1601-0825.2010.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganepola GA, Nizin J, Rutledge JR, Chang DH. Use of blood-based biomarkers for early diagnosis and surveillance of colorectal cancer. World J Gastrointest Oncol. 2014;6(4):83–97. doi: 10.4251/wjgo.v6.i4.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yörüker EE, Holdenrieder S, Gezer U. Blood-based biomarkers for diagnosis, prognosis and treatment of colorectal cancer. Clin Chim Acta. 2016;455:26–32. doi: 10.1016/j.cca.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Heichman KA. Blood-based testing for colorectal cancer screening. Mol Diagn Ther. 2014;18(2):127–135. doi: 10.1007/s40291-013-0074-z. [DOI] [PubMed] [Google Scholar]
- 25.Benning TM, Dellaert BG, Dirksen CD, Severens JL. Preferences for potential innovations in non-invasive colorectal cancer screening: A labeled discrete choice experiment for a Dutch screening campaign. Acta Oncol. 2014;53(7):898–908. doi: 10.3109/0284186X.2013.877159. [DOI] [PubMed] [Google Scholar]
- 26.Osborne JM, Wilson C, Moore V, Gregory T, Flight I, Young GP. Sample preference for colorectal cancer screening tests: Blood or stool? Open J Prev Med. 2012;2(3):326–331. [Google Scholar]
- 27.Nayaradou M, Berchi C, Dejardin O, Launoy G. Eliciting population preferences for mass colorectal cancer screening organization. Med Decis Making. 2010;30(2):224–233. doi: 10.1177/0272989X09342747. [DOI] [PubMed] [Google Scholar]
- 28.Zajac IT, Duncan A, Turnbull D, Wilson C, Flight I. Blood-based screening for bowel cancer may not resolve suboptimal screening participation in Australia. Aust N Z J Public Health. 2016;40(4):337–341. doi: 10.1111/1753-6405.12523. [DOI] [PubMed] [Google Scholar]
- 29.Koka S, Beebe TJ, Merry SP, et al. The preferences of adult outpatients in medical or dental care settings for giving saliva, urine or blood for clinical testing. J Am Dent Assoc. 2008;139(6):735–740. doi: 10.14219/jada.archive.2008.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhima M, Salinas TJ, Wermers RA, Weaver AL, Koka S. Preference changes of adult outpatients for giving saliva, urine and blood for clinical testing after actual sample collection. J Prosthodont Res. 2013;57(1):51–56. doi: 10.1016/j.jpor.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Viney R, Lancsar E, Louviere J. Discrete choice experiments to measure consumer preferences for health and healthcare. Expert Rev Pharmacoecon Outcomes Res. 2002;2(4):319–326. doi: 10.1586/14737167.2.4.319. [DOI] [PubMed] [Google Scholar]
- 32.Wortley S, Wong G, Kieu A, Howard K. Assessing stated preferences for colorectal cancer screening: a critical systematic review of discrete choice experiments. Patient. 2014;7(3):271–282. doi: 10.1007/s40271-014-0054-3. [DOI] [PubMed] [Google Scholar]
- 33.Pignone MP, Brenner AT, Hawley S, et al. Conjoint analysis versus rating and ranking for values elicitation and clarification in colorectal cancer screening. J Gen Intern Med. 2012;27(1):45–50. doi: 10.1007/s11606-011-1837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salkeld G, Solomon M, Short L, Ryan M, Ward JE. Evidence-based consumer choice: a case study in colorectal cancer screening. Aust N Z J Public Health. 2003;27(4):449–455. doi: 10.1111/j.1467-842x.2003.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 35.Pignone MP, Crutchfield TM, Brown PM, et al. Using a discrete choice experiment to inform the design of programs to promote colon cancer screening for vulnerable populations in North Carolina. BMC Health Serv Res. 2014;14:611. doi: 10.1186/s12913-014-0611-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Australian Bureau of Statistics . Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2011. Canberra: ABS; 2013. [Google Scholar]
- 37.Levi Z, Rozen P, Hazazi R, et al. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med. 2007;146(4):244–255. doi: 10.7326/0003-4819-146-4-200702200-00003. [DOI] [PubMed] [Google Scholar]
- 38.Lane JM, Chow E, Young GP, et al. Interval fecal immunochemical testing in a colonoscopic surveillance program speeds detection of colorectal neoplasia. Gastroenterology. 2010;139(6):1918–1926. doi: 10.1053/j.gastro.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Abeyasekera S. Analysis Approaches in Participatory Work Involving Ranks or Scores. Reading, UK: University of Reading, Natural Resources Institute, UK; 2001. [Google Scholar]
- 40.Street D, Burgess L. The Construction of Optimal Stated Choice Experiments: Theory and Methods. Hoboken, NJ: Wiley; 2007. [Google Scholar]
- 41.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ, USA: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 42.Tavakol M, Dennick R. Making sense of Cronbach’s alpha. Int J Med Educ. 2011;2:53–55. doi: 10.5116/ijme.4dfb.8dfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monte Carlo PCA for Parallel Analysis [computer program] State College, PA, USA: Psych Associates; 2000. [Google Scholar]
- 44.Ryan M, Gerard K, Amaya-Amaya M, Bateman I, editors; Bateman I, editor. Using Discrete Choice Experiments to Value Health and Health Care. Dordrecht, The Netherlands: Springer; 2007. [Google Scholar]
- 45.Bech M, Gyrd-Hansen D. Effects coding in discrete choice experiments. Health Econ. 2005;14(10):1079–1083. doi: 10.1002/hec.984. [DOI] [PubMed] [Google Scholar]
- 46.Hole AR. A comparison of approaches to estimating confidence intervals for willingness to pay measures. Health Econ. 2007;16(8):827–840. doi: 10.1002/hec.1197. [DOI] [PubMed] [Google Scholar]
- 47.Marshall DA, Johnson FR, Kulin NA, et al. How do physician assessments of patient preferences for colorectal cancer screening tests differ from actual preferences? A comparison in Canada and the United States using a stated-choice survey. Health Econ. 2009;18(12):1420–1439. doi: 10.1002/hec.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Symonds EL, Pedersen SK, Baker RT, et al. A blood test for methy-BCAT1 and IKZF1 vs. a fecal immunochemical test for detection of colorectal neoplasia. Clin Transl Gastroenterol. 2016;7(1):e137. doi: 10.1038/ctg.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sazanov A, Kiselyova E, Zakharenko A, Romanov M, Zaraysky M. Plasma and saliva miR-21 expression in colorectal cancer patients. Hum Genet. 2017;58:231–237. doi: 10.1007/s13353-016-0379-9. [DOI] [PubMed] [Google Scholar]
- 50.Worthley DL, Cole SR, Esterman A, et al. Screening for colorectal cancer by faecal occult blood test: why people choose to refuse. Intern Med J. 2006;36(9):607–610. doi: 10.1111/j.1445-5994.2006.01155.x. [DOI] [PubMed] [Google Scholar]
- 51.Australian Institute of Health and Welfare National Bowel Cancer Screening Program: Monitoring Report 2013–14 Canberra: AIHW; 2015 [Google Scholar]
- 52.Australian Institute of Health and Welfare . National Bowel Cancer Screening Program Monitoring Report: 2012–2013. Canberra: AIHW; 2014. [Google Scholar]
- 53.Australian Institute of Health and Welfare . National Bowel Cancer Screening Program Monitoring Report: Phase 2, July 2008–June 2011. Canberra: AIHW; 2012. [Google Scholar]
- 54.Oster C, Mcguiness C, Duncan A, Turnbull D. Masculinity and men’s participation in colorectal cancer screening. Psychol Men Masc. 2015;16(3):254–263. [Google Scholar]
- 55.Ritvo P, Myers RE, Paszat L, Serenity M, Perez DF, Rabeneck L. Gender differences in attitudes impeding colorectal cancer screening. BMC Public Health. 2013;13(1):500. doi: 10.1186/1471-2458-13-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duncan A, Zajac I, Flight I, Stewart BJ, Wilson C, Turnbull D. Comparison of mailed invitation strategies to improve fecal occult blood test participation in men: protocol for a randomized controlled trial. Trials. 2013;14:239. doi: 10.1186/1745-6215-14-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zajac IT, Duncan AC, Flight I, et al. Theory-based modifications of an advanced notification letter improves screening for bowel cancer in men: A randomised controlled trial. Soc Sci Med. 2016;165:1–9. doi: 10.1016/j.socscimed.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 58.Lawrence HP. Salivary markers of systemic disease: noninvasive diagnosis of disease and monitoring of general health. J Can Dent Assoc. 2002;68(3):170–174. [PubMed] [Google Scholar]
- 59.Mitchell SM, Ross JP, Drew HR, et al. A panel of genes methylated with high frequency in colorectal cancer. BMC Cancer. 2014;14:54. doi: 10.1186/1471-2407-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedersen SK, Baker RT, Mcevoy A, et al. A two-gene blood test for methylated DNA sensitive for colorectal cancer. PLoS One. 2015;10(4):e0125041. doi: 10.1371/journal.pone.0125041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colorectal Cancer Association of Canada-CCAC [webpage on the Internet] Screening and Diagnostics: A Guide to Screening Tests: Colorectal Cancer Screening. 2017. [Accessed November 3, 2017]. Available from: http://www.colorectal-cancer.ca/en/screening/screening-tests/
- 62.Basak S, Srivatsan E. Salivary biomarkers in early diagnosis and monitoring of cancer. In: Barh D, Carpi A, Verma M, Gunduz M, editors. Cancer Biomarkers: Minimal and Noninvasive Early Diagnosis and Prognosis. Boca Raton: CRC Press, Taylor and Francis Group; 2014. pp. 159–198. [Google Scholar]
- 63.Symonds EL, Pedersen S, Cole SR, et al. Improving participation in colorectal cancer screening: a randomised controlled trial of sequential offers of faecal then blood based non-invasive tests. Asian Pac J Cancer Prev. 2015;16(18):8455–8460. doi: 10.7314/apjcp.2015.16.18.8455. [DOI] [PubMed] [Google Scholar]