Abstract
This study was conducted to determine the occurrence of antimicrobial resistance and enterotoxin-encoding genes (EEGs) in Staphylococcus spp. recovered from equipment used to prepare hospital meals, in a university hospital in Rio de Janeiro, Brazil. Sixty samples were collected from semi-industrial equipment (one blender and one mixer) in the hospital's kitchen. Resistance genes and SCCmec types were detected by PCR. From the 40 isolates of Staphylococcus spp. identified, 8 were Staphylococcus aureus. Thirty-two (80%) Staphylococcus spp. isolates were resistant to at least one antimicrobial agent. Resistance genetic determinants were detected: erm gene (Staphylococcus epidermidis [n = 2]; Staphylococcus hominis [n = 1]), mecA gene (S. epidermidis [n = 2]), and aa(6′)-aph(2′′) gene (Staphylococcus caprae [n = 1], S. epidermidis [n = 2], S. hominis [n = 1], Staphylococcus pausteri [n = 1], Staphylococcus simulans [n = 1], and Staphylococcus warneri [n = 1]). The presence of at least one EEG in 83% (n = 33) of the isolates was identified. Two strains of S. epidermidis were methicillin-resistant S. epidermidis (MRSE) and harboring SCCmec type IV. Staphylococcus spp. contaminated some hospital kitchen's equipment, indicating that hygiene procedures should be improved. Results also indicate that meals can be a vehicle to disseminate multiresistant Staphylococcus spp., including MRSE, and Staphylococcus with EEGs.
Keywords: : hospital meals, Staphylococcus spp, kitchen equipment, antimicrobial resistance, enterotoxin
Introduction
The importance of providing wholesome food to hospitalized patients and the deleterious effect that contaminated food may cause to the patient's recovery has been extensively studied.1–3 Foodborne outbreaks in hospitals may occur due to poor kitchen hygienic condition and lack of training of food handlers.3
Food handlers play an important role in food safety, since they may introduce pathogens in the food product during processing, distribution, and transport.4 According to Köck et al.,5 41% of nonhospitalized adults harbor Staphylococcus aureus in their noses. Borges et al.4 showed that in Brazil, S. aureus isolated from meals that were related to a single strain isolated from food handlers suggested that the reason for diet contamination may be a result of food handling.
Hospital equipment has been shown to be contaminated with nosocomial pathogens with most of them being capable of surviving for weeks on healthcare surfaces and may be transmitted directly or indirectly to patients. In some hospitals, contamination surveillance is not implemented, therefore their contamination levels are still unknown.6,7
Gram-positive cocci are still a huge threat for public health, especially with the increasing incidence of methicillin-resistant S. aureus (MRSA). Methicillin resistance is mainly conferred by the mecA gene carried in a family of mobile genetic elements called staphylococcal cassette chromosomes (SCCs). Besides promoting SCCmec acquisition, the hospital environment appears to contribute to the genetic diversity generation in the SCCmec elements.8,9
Given the rapid increase in antimicrobial resistance and the important impact of these multidrug-resistant (MDR) organisms on morbidity and mortality rates, it is paramount to set up regular and precise microbial surveillance programs to offer extensive information on antimicrobial susceptibility patterns in different geographical regions and in different time periods.10
Besides antimicrobial resistance, the pathogenicity of Staphylococcus spp. is directly related to the capacity of production of staphylococcal enterotoxin and staphylococcal enterotoxin-like. The staphylococcal enterotoxins may cause toxic-shock syndrome and food poisoning, in addition to allergic reactions and autoimmune responses. In addition, the staphylococcal enterotoxins may act as potent gastrointestinal toxins and as super-antigens that stimulate proliferation of nonspecific T cells. The staphylococcal enterotoxins are resistant to gastrointestinal proteases and conditions such as freezing, drying, heat treatment, and harsh environment.11
Considering the high number of immunocompromised patients inside hospitals, studies on antimicrobial resistance and the presence of enterotoxin-encoding genes (EEGs) in Staphylococcus spp. recovered from these environments are very important. Therefore, the aim of this study was to verify the occurrence of Staphylococcus spp. isolated from a state university hospital kitchen's equipment, to determine their antimicrobial resistance profiles and to identify the presence of EEGs.
Materials and Methods
Sample analysis
During a 15-week period, a total of 60 samples were collected from semi-industrial equipment (1 blender and 1 mixer) from the kitchen of a tertiary care teaching hospital with 525 beds located in the city of Rio de Janeiro. The kitchen is located inside the hospital and has about 80 m2. It presents 20 employees, aged between 28 and 60 years, working on alternate days. Samples were collected from surfaces of the equipment, after decontamination processes, with sterile swabs twice a week (one collection per shift) as previously described.12 Equipment is used to prepare soups, mashed potatoes, paste diets, and juices. The equipment microbiological analysis included isolation of Staphylococcus spp. in Baird-Parker Agar (BP-OXOID, Ltd., Basingstoke, Hampshire, England).
Identification of Staphylococcus spp.
After storage, strains were inoculated into 3 ml of Brain Heart Infusion (Difco, Becton Dickinson, MD) and incubated at 35°C ± 2°C for 18/24 hr. Growth was plated on Nutrient Agar (BP-OXOID, Ltd.) and incubated at 35°C ± 2°C for 18/24 hr. The bacteria were identified by mass spectrometry analysis (MALDI-TOF MS) according to a standard extraction protocol using formic acid, as recommended by Bruker13 and by classical methodology.14
Antimicrobial resistance
Antimicrobial susceptibility testing (AST) was determined by the disk diffusion method, according to the recommendations of the Clinical and Laboratory Standards Institute.15 The following antimicrobial drugs were tested (BP-OXOID): penicillin (P 10 U), erythromycin (E 15 μg), gentamicin (CN 10 μg), rifampin (RF 5 μg), clindamycin (DA 2 μg), trimethoprim/sulfamethoxazole (SXT 1.25 μg/23.75 μg), tetracycline (TE 30 μg), cefoxitin (FOX 30 μg), and ciprofloxacin (CIP 5 μg). Reference strain S. aureus ATCC 25923 was used as control.
Minimum inhibitory concentration (MIC) was determined by broth microdilution method using cation-adjusted Mueller–Hinton broth (Difco Laboratories) for oxacillin (OX), vancomycin (VA), gentamicin (CN), and erythromycin (E) (Sigma-Aldrich, Inc., St. Louis) for all isolates and according to CLSI guidelines.15 Reference strain S. aureus ATCC 29213 was used as control.
Detection of resistance genes
The detection of genes encoding resistance mechanisms was performed by PCR for genes, ermA, ermB, aac(6′)-aph(2′′), and mecA, as previously described.16–18 Strains isolated by Carneiro et al.19 were used as positive control.
Molecular typing
Characterization of SCCmec types I, II, III, IV, and V was carried out, for all methicillin-resistant Staphylococcus spp. (MRS) isolates, by multiplex PCR analysis as previously described.20
Detection of enterotoxin genes
The enterotoxin genes detection was determined by PCR. Specific primers were used to detect sea, seb, sec, sed, see, seg, seh, sei, sej, sek, sel, sem, sen, seo, sep, seq, ser, and seu genes.21–23 Strains FRI472 (sed, seg, sei, and sej), FRI137 (sec, seh, sel, sem, sen, seo, and seu), FRI913 (see, sek, and seq), FRI S6 (seb), FRI 361 (ser), FRI100 (sea), and HMPL 280 (sep) were used as positive controls, generously provided by D. Doro and T.C.Oliveira at Universidade Federal de Londrina, Brazil.
Results
Out of the 60 samples analyzed, 40 strains of Staphylococcus spp. were isolated and identified: 20 from the mixer and 20 from the blender. S. aureus was the most frequent species (n = 8), followed by S. caprae (n = 6), S. warneri (n = 6), S. epidermidis (n = 4), S. pausteri (n = 2), S. auricularis (n = 2), S. hominis (n = 2), S. kloosii (n = 2), S. haemolyticus (n = 2), S. saprophyticus (n = 2), S. simulans (n = 2), S. cohnii (n = 1), and S. succinus (n = 1) (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/mdr).
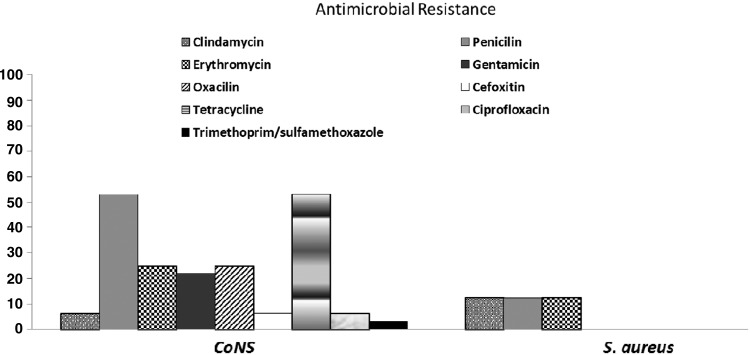
AST and MIC testing revealed that 80% (32/40) of the isolates were resistant to at least one antimicrobial drug. All S. aureus were susceptible to oxacilin. Among the coagulase negative Staphylococcus (CoNS), 25% (8/32) were resistant to oxacillin by MIC determination (Fig. 1) and mecA gene was detected in two methicillin-resistant S. epidermidis (MRSE) isolates, which also presented resistance to cefoxitin in AST (Table 1). MRSE isolates harbored SCCmec type IV. All isolates were susceptible to vancomycin on MIC testing.
FIG. 1.
Percentage of antimicrobials resistance in CoNS and Staphylococcus aureus isolated from equipment used in the kitchen of a University Hospital in Rio de Janeiro. CoNS, coagulase negative Staphylococcus.
Table 1.
Minimum Inhibitory Concentration, Antimicrobial Susceptibility, and Presence of Genes Encoding to Erythromycin, Gentamicin, and Methicillin Resistance in Strains of Staphylococcus spp. Isolated from Kitchen of a University Hospital in Rio de Janeiro
| Antimicrobials | |||||||
|---|---|---|---|---|---|---|---|
| Erythromycin | Cefoxitin | Gentamicin | |||||
| Strains | Species | MIC (μg/ml) | AST (mm) | AST (mm) | MIC (μg/ml) | AST (mm) | Genes |
| 3A | S. caprae | 1 | 30 | 30 | 8* | 12 | aa(6′)-aph(2′′) |
| 7 | S. epidermidis | 64 | 6 | 19 | 128 | 12 | aa(6′)-aph(2′′), mecA, ermA |
| 19 | S. epidermidis | 64 | 6 | 21 | 16 | 10 | aa(6′)-aph(2′′) mecA, ermB |
| 2 | S. hominis subsp. hominis | 1 | 30 | 35 | 128 | 6 | aa(6′)-aph(2′′) |
| 3B | S. hominis subsp. hominis | >128 | 6 | 25 | <0.25 | 20 | ermB |
| 16A | S. pausteri | 0.5 | 20 | 26 | 16 | 8 | aa(6′)-aph(2′′) |
| 4 | S. simulans | 0.5 | 30 | 33 | 32 | 13 | aa(6′)-aph(2′′) |
| 35 | S. warneri | 1 | 28 | 30 | 16 | 10 | aa(6′)-aph(2′′) |
Bold values represent antimicrobial resistance.
MIC, minimum inhibitory concentration; AST, antimicrobial susceptibility testing.
Gentamicin resistance was observed in 22% (7/32) of CoNS isolates (Fig. 1). Staphylococcus spp. resistant to gentamicin, presented this phenotype in both AST and MIC testing and aac(6′)-aph(2′′) gene was detected in all these isolates (Table 1).
Twenty percent of the isolates (8/40) showed resistance to erythromycin in both tests, however, one S. aureus isolate showed resistance to this antimicrobial only in AST and one S. kloosii isolate only in MIC testing (Supplementary Table S1).
The presence of ermA gene was observed in one S. epidermidis isolate validated by both tests. The ermB gene was observed in one S. epidermidis and one S. hominis subsp. hominis isolate, results confirmed by MIC testing and the AST (Table 1).
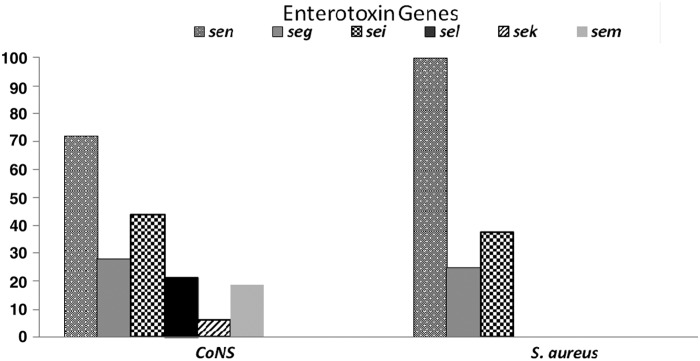
There was at least one EEG in 83% (33/40) of the isolates. All S. aureus isolates (n = 8) showed at least one of the EEGs investigated. Two S. aureus isolates presented, simultaneously, three enterotoxin genes and five CoNS isolates alike showed more than five enterotoxin genes (Supplementary Table S1). The seg, sei, sek, sel, sem, and sen genes were detected in 9, 14, 2, 7, 6, and 22 CoNS isolates, respectively (Fig. 2). The seg, sei, and sen genes were detected in two, three, and eight S. aureus isolates, respectively (Fig. 2).
FIG. 2.
Percentage of enterotoxin-encoding genes in strains of CoNS and S. aureus isolated from equipment used in the kitchen of a University Hospital in Rio de Janeiro.
Discussion
Among the Gram-positive bacteria, staphylococci, streptococci, and enterococci are important causes of both community- and hospital-acquired infections. In Brazil, The SENTRY Antimicrobial Surveillance Program was conducted in four Brazilian cities (São Paulo, Porto Alegre, Brasília, and Florianópolis) and revealed that Gram-positive organisms most frequently isolated from bloodstream infections were S. aureus, followed by CoNS.24,25 The most prevalent species isolated from blood cultures are S. epidermidis, S. haemolyticus, and S. hominis.25,26 In general, 31.0% of S. aureus strains were resistant to oxacillin (MRSA) and the vast majority of MRSA strains were also resistant to clindamycin, ciprofloxacin, and levofloxacin. Almost 80% of CoNS strains were resistant to oxacillin. This organism showed high rates of resistance to most antimicrobial agents.25
Another study in Brazil evaluated 160 CoNS isolated between 2002 and 2009 from blood cultures of patients hospitalized in different wards of a teaching hospital in São Paulo. Out of the 160 isolates analyzed, 111 were identified as S. epidermidis, followed by S. haemolyticus (n = 16), S. hominis subsp. novobiosepticus (n = 13), S. saprophyticus (n = 9), Staphylococcus capitis subsp. urealyticus (n = 5), Staphylococcus schleiferi subsp. schleiferi (n = 3), and Staphylococcus xylosus, S. warneri, and S. caprae with 1 isolate each. The mecA gene was detected in 116 isolates (72.5%).27
Evidence has shown that food unsafe handling is a problem for immunocompromised and elderly patients and is not only widespread, but it is also expected to grow.28 Our results showed the presence of MRSE and MDR Staphylococcus spp. in the blender and mixer used in the hospital kitchen. Therefore, the meals prepared with these two pieces of equipment may potentially disseminate bacteria with important resistance mechanisms inside the hospital, especially because some of these foods do not follow a heat treatment afterward. The results of AST indicate high rates of antimicrobial resistance in S. epidermidis and Staphylococcus pasteuri isolates. Resistance to six antimicrobial agents were found in 50% (2/4) of S. epidermidis isolates, including cefoxitin, which is used to define resistance or susceptibility to oxacillin.
MDR is common in S. epidermidis, more than 70% of S. epidermidis isolates circulating in the hospital environment are resistant to oxacillin, and ∼60%, 40–55%, and 55% are resistant to gentamicin, clindamycin, and ciprofloxacin, respectively.29 Kitchen equipment contamination with S. epidermidis, carring the mecA gene, is an important epidemiological issue that must be highlighted. As previously mentioned, the food prepared using contaminated equipment by these bacteria can be a vehicle for the dissemination of resistance markers in the hospital environment. As a consequence, we can assume that patients ingesting these contaminated food can become colonized by these bacteria with the subsequent gene transfer to other pathogens or foster the multiresistant isolates emergence, which may lead to unsuccessful therapeutic options.
MRS have acquired and integrated SCCmec that carries the mecA gene and other antibiotic resistance determinants.8 Two S. epidermidis isolates were found carrying SCCmec types IV, both isolated from the mixer. Several studies have shown that SCCmec type IV is common among S. epidermidis.9,30 Other data show that SCCmec type IV also predominates among community-acquired (CA) MRSE.31 In another study, carriage of MRSE-SCCmec type IV was found to be common in patients at hospital admission, including those with no previous exposure to healthcare environments. MR-CoNS are probably disseminated in the community, notably in people with no previous exposure to healthcare environments. MRSE, the most prevalent species, may act as a reservoir of SCCmec type IV for CA-MRSA.8
In addition, resistance to five antimicrobials was observed in both strains of S. epidermidis, including resistance to erythromycin and gentamicin. In these two strains the erythromycin and aminoglycosides encoding resistance genes were found. MRS has the ability to display high rates of resistance to multiple antimicrobial drugs worldwide.29,32
The erm genes confer cross-resistance to macrolides, lincosamides, and streptogramin B and can be expressed constitutively or be inducible. According to Lenart-Boron et al.33 the PCR technique allows the detection of antibiotic resistance genes that may not be necessarily expressed. Therefore, the results of the molecular analyses may vary from the phenotypic results. Another important aspect of this study is that genes encoding resistance mechanisms to erythromycin were not found in isolates that were susceptible to AST and MIC techniques.33 A similar result was observed by Gatermann et al.34 who demonstrated that strains of Staphylococcus spp. that were phenotypically susceptible to erythromycin did not show any of the genes for resistance to this antimicrobial. In our study, several isolates were resistant to erythromycin but the gene coding for resistance were absent. The presence of ermA and ermB genes was evaluated in all strains of Staphylococcus spp. However, other genes may be responsible for erythromycin resistance, such as the ermC and msrA genes.32 It is believed that the observed phenotype is probably related to the presence of other genes that have not been investigated.
Resistance to aminoglycosides in CoNS isolates is more common than in S. aureus.35 CoNS have been identified as a reservoir for resistance determinants, including genes encoding aminoglycoside-modifying enzymes; moreover, the conjugal transfer of resistance determinants among S. epidermidis and S. aureus leads to the rapid spread of these determinants in the hospital environment.36
Seven strains of CoNS were found resistant in both AST and MIC testing to gentamicin, and all strains showed the aac(6′)-aph(2′′) gene. S. caprae and S. warneri were the most frequent species in our study and showed those characteristics. This result is relevant since S. caprae is considered a commensal and it becomes a human pathogen in many CA or/and hospital-acquired infections.37 Although S. warneri rarely causes disease in healthy people, there are reports of S warneri infection in immunocompromised patients with invasive treatments or medical device implants.38,39
In this study, the CoNS isolates showed resistance to a higher number of antimicrobial drugs than S. aureus isolates. The food prepared using the equipment contaminated by bacteria, showing the resistance profiles found, is a great concern since the food is often not heated after being processed in such equipment. Furthermore, the presence of pathogens or opportunistic bacteria in foods to be served to immunocompromised patients may be harmful.
Antimicrobial resistance aside, food outbreaks by Staphylococcus spp. are generally related to food contamination by food handlers or contaminated surfaces after the heat treatment. The importance of food contamination by Staphylococcus spp. relates to their ability to produce enterotoxins. In this study the presence of at least one EEG was observed in 83% (33/40) of the isolates studied. The seg, sei, sek, sel, sem, and sen enterotoxin genes were observed in 28%, 43%, 5%, 18%, 18%, and 78% of the isolates, respectively. The eight S. aureus isolates found had at least one enterotoxin gene, within the studied ones, whereas the enterotoxin genes studied were found in 25 isolates of the CoNS. Because prepared foods do not undergo heat treatment after use of the contaminated equipment, it may enable the proliferation of Staphylococcus spp. isolates in the food and consequent production of enterotoxin, since time between the preparation and distribution can be very long. Furthermore, it is known that staphylococcal enterotoxins have heat tolerance capacity.40
The present results confirmed the toxin profiles of S. aureus isolates, since all S. aureus isolates showed some staphylococcal enterotoxin searched. However, these findings also indicate that CoNS isolates have EEG. For a long time, S. aureus was considered the only pathogenic species of the genus, whereas the CoNS were classified only as contaminants. The reason for this is that the potential of enterotoxigenic in CoNS isolates had much controversy in the past. However, many studies have demonstrated that CoNS isolates not only have EEG but also produce clinically significant toxin concentrations.41,42
Significant contamination was found (colony counts above 2 × 10 CFU/cm2 in 70% of the equipment12) on the equipment used to prepare meals to hospitalized patients. These findings showed that good hygiene practices (GHP), especially handling the kitchen equipment, should be improved. A report of these results was sent to the Division of Nutrition of the University Hospital and GHP control measures were reviewed. Moreover, training was given to the food handlers, aiming to prevent cross-contamination within the kitchen environments and equipment.
The detection of Staphylococcus spp., isolates with high MIC testing to gentamicin, erythromycin, and oxacillin and the detection of ermA, ermB, aac (6′)-aph (2′′), and mecA genes turns meals into a potential vehicle to spread important resistance markers inside hospitals. Additionally, the isolation of coagulase positive Staphylococcus (CoPS) and CoNS isolates harboring EEG in equipment used to prepare meals is an additional complication in this scenario. Dissimilation and spread of potential harmful bacteria, such as Staphylococcus spp. in hospitals is an important clinical issue, especially hospital-associated bacteria containing genes coding for antimicrobial resistance and toxins, which are a potential threat to successful treatment in imunocompromised patients. Considering that there are few reports in the literature on the role of contamination of hospital diets with MDR staphylococcal strains and with genes for the production of enterotoxins, further studies are suggested to elucidate this subject.
Supplementary Material
Acknowledgments
We would like to thank Dr. Sergio Eduardo Longo Fracalanzza from Instituto de Microbiologia Paulo de Góes (IMPG/UFRJ) for performing the mass spectrometry analysis (MALDI-TOF) identification. This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Oludare A.O., Ogundipe A., Odunjo A., Komolafe J., and Olatunji I. 2016. Knowledge and food handling practices of nurses in a tertiary health care hospital in Nigeria. J. Environ. Health 78:32–38 [PubMed] [Google Scholar]
- 2.Lee M.B., and Greig J.D. 2013. A review of nosocomial Salmonella outbreaks: infection control interventions found effective. Public Health 127:199–206 [DOI] [PubMed] [Google Scholar]
- 3.El Derea H., Salem E., Fawzi M., and Azeem M.A. 2008. Safety of patient meals in 2 hospitals in Alexandria, Egypt before and after training of food handlers. East Mediter. Health J. 14:941–952 [PubMed] [Google Scholar]
- 4.Borges L.J., Campos M.R.H., Cardoso J.L., M.C.André D.P.B., and Serafini A.B. 2010. Molecular epidemiology of microorganisms isolated from food workers and enteral feeding of public hospitals. J. Food Sci. 75:449–454 [DOI] [PubMed] [Google Scholar]
- 5.Köck R., Werner P., Friedrich A.W., Fegeler C., and Becker K. 2016. Persistence of nasal colonization with human pathogenic bacteria and associated antimicrobial resistance in the German general population. New Microbes New Infect. 9:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dancer S.J. 2014. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin. Microbiol. Rev. 27:665–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolan A., Bartlett M., McEntee B., Creamer E., and Humphreys H. 2011. Evaluation of different methods to recover meticillin-resistant Staphylococcus aureus from hospital environmental surfaces. J. Hosp. Infect. 79:227–230 [DOI] [PubMed] [Google Scholar]
- 8.Murugesan S., Perumal N., Mahalingam S.P., Dilliappan S.K., and Krishnan P. 2015. Analysis of antibiotic resistance genes and its associated sccmec types among nasal carriage of methicillin resistant coagulase negative staphylococci from community settings, Chennai, southern India. J. Clin. Diagn. Res. 9:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rolo J., Lencastre H., and Miragaia M. 2012. Strategies of adaptation of Staphylococcus epidermidis to hospital and community: amplification and diversification of SCCmec. J. Antimicrob. Chemother. 67:1333–1341 [DOI] [PubMed] [Google Scholar]
- 10.Zhao C., Sun H., Wang H., Liu Y., Hu B., Yu Y., Sun Z., Chuf Y., Caog B., Liaoh K., Leii J., Hu Z., Zhang L., Zhang X., Xu Y., Wang Z., and Chen M. 2012. Antimicrobial resistance trends among 5608 clinical Gram-positive isolates in China: results from the Gram-Positive Cocci Resistance Surveillance program (2005–2010). Diagn. Microbiol. Infect. Dis. 73:174–181 [DOI] [PubMed] [Google Scholar]
- 11.Hennekinne J.A., De Buyser M.L., and Dragacci S. 2012. Staphylococcus aureus and its food poisoning toxins: characterization and outbreak investigation. FEMS Microbiol. Rev. 36:815–836 [DOI] [PubMed] [Google Scholar]
- 12.Miyahira R.F., E.Santos F.A.S., Freitas-Almeida A.C., and Queiroz M.L.P. 2013. Occurrence and antimicrobial resistance of Salmonella spp. and other enterobacteria recovered from kitchen equipment of a university hospital in Rio de Janeiro, Brazil. Inter. J. Microbiol. Res. 5:467–471 [Google Scholar]
- 13.Bruker Guide to MALDI Sample Preparation–Instructions for Use. 2015. Revision E. Available at www.bruker.com/fileadmin/user_upload/8-PDF-Docs/Separations_MassSpectrometry/InstructionForUse/8702557_IFU_Bruker_Guide_MALDI_Sample_Preparation_Revision_E.pdf
- 14.Becker K., and von Eiff C. 2011. Staphylococcus, micrococcus, and other catalase-positive cocci. In Versalovic J., Carroll K.C., Funke G., Jorgensen J.H., Landry M.L., and Warnock D.W. (eds.), Manual of Clinical Microbiology. ASM Press, Washington, DC, pp. 308–330 [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. 2015. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-fifth Informational Supplement. M100-S25. CLSI, Wayne, PA [Google Scholar]
- 16.Stucliffe J., Grebe T., Tait-Kamradt A., and Wondrack L. 1996. Detection of erythromicin-resistant determinants by PCR. Antimicrob. Agents Chemother. 40:2562–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martineau F., Picard F.J., Grenier L., Roy P.H., Quellette M., and Bergeron M. 2000. Multiplex PCR assays for the detection of clinically relavant antibiotic resistance gene in staphylococci isolated from patients infected after cardiac surgery. J. Antimicrob. Chemother. 46:527–533 [DOI] [PubMed] [Google Scholar]
- 18.York M.K., Gibbs L., Chehab F., and Brooks G.F. 1996. Comparison of PCR detection of mecA with standard susceptibility testing methods to determine methicillin resistance in coagulase-negative staphylococci. J. Clin. Microbiol. 34:249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carneiro L.A.M., Queiroz M.L.P., and V.L.C. Merquior. 2004. Antimicrobial-resistance and enterotoxin-encoding genes among staphylococci isolated from expressed human breast milk. J. Med. Microbiol. 53:761–768 [DOI] [PubMed] [Google Scholar]
- 20.Boye K., Bartels M.D., Andersen I.S., Møller J.A., and Westh H. 2007. A new multiplex PCR for easy screening of methicillin-resistant Staphylococcus aureus SCCmec types I–V. Clin. Microbiol. Infect. 13:725–727 [DOI] [PubMed] [Google Scholar]
- 21.Chiang Y.C., Liao W.W., Fan C.M., Pai W.Y., Chiou C.S., and Tsen H.Y. 2008. PCR detection of Staphylococcal enterotoxins (SES) N, O, P, Q, R, U, and survey of SE types in Staphylococcus aureus isolates from food-poising case in Taiwan. Inter. J. Food Microbiol. 121:66–73 [DOI] [PubMed] [Google Scholar]
- 22.Bania J., Dabrowska A., Bystron J., Korzekwa K., Chrzanowska J., and Molenda J. 2006. Distribution of newly described enterotoxin-like genes in Staphylococcus aureus from food. Int. J. Food Microbiol. 108:36–41 [DOI] [PubMed] [Google Scholar]
- 23.Monday S.R., and Bohachm G.A. 1999. Use of multiplex PCR to detect classical and newly described pyrogenic toxin genes in staphylococcal isolates. J. Clin. Microbiol. 37:3411–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silk B.J., McCoy M.H., Iwamoto M., and Griffin P.M. 2014. Foodborne listeriosis acquired in hospitals. Clin. Infect. Dis. 59:532–540 [DOI] [PubMed] [Google Scholar]
- 25.Gales A.C., Sader H.S., Ribeiro J., Zoccoli C., Barth A., and Pignatari A.C. 2009. Antimicrobial susceptibility of gram-positive bacteria isolated in Brazilian hospitals participating in the SENTRY program (2005–2008). Braz. J. Infect. Dis. 13:90–98 [DOI] [PubMed] [Google Scholar]
- 26.Oliveira C.F., Cavanagh J.P., Fredheim E.G.A., Reiter K.C., Rieger A., Klingenberg C., d'Azevedo P.A., and Sollid J.E. 2016. Coagulase-negative staphylococci in Southern Brazil: looking toward its high diversity. Rev. Soc. Bras. Med. Trop. 49:292–299 [DOI] [PubMed] [Google Scholar]
- 27.Martins A., Riboli D.F.M., Camargo C.H., Pereira V.C., Sampaio R.A., and Cunha M.L.R.S. 2013. Antimicrobial resistance and persistence of Staphylococcus epidermidis clones in a Brazilian university hospital. Diagn. Microbiol. Infect. Dis.77:164–168 [DOI] [PubMed] [Google Scholar]
- 28.Pedroso S.H.S.P., Sandes S.H.C., Luiz K.C.M., Dias R.S., Filho R.A.T., Serufo J.C., Farias L.M., Carvalho M.A.R., Bomfim M.R.Q., and Santos S.G. 2016. Biofilm and toxin profile: a phenotypic and genotypic characterization of coagulase-negative staphylococci isolated from human bloodstream infections. Microb. Pathog. 100:312–318 [DOI] [PubMed] [Google Scholar]
- 29.Leclercq R. (2009). Epidemiological and resistance issues in multidrug-resistant staphylococci and enterococci. Clin. Microbiol. Infect. 15:224–231 [DOI] [PubMed] [Google Scholar]
- 30.Lebeaux D., Barbier F., Angebault C., Benmahdi L., Ruppé E., Felix B., Gaillard K., Djossou F., Epelboin L., Dupont C., Renard M., Peroz G., Vandenesch F., Wolff M., Andremont A., and Ruimy R. 2012. Evolution of nasal carriage of methicillin-resistant coagulase- negative staphylococci in a remote population. Antimicrob. Agents Chemother. 56:315–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamaluddin T.Z.M.T., Kuwahara-Arai K., Hisata K., Terasawa M., Cui L., Baba T., Sotozono C., Kinoshita S., Ito T., and Hiramatsu K. 2008. Extreme genetic diversity of methicillin-resistant Staphylococcus epidermidis strains disseminated among healthy japanese children. J. Clin. Microbiol. 46:3778–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouchami O., Achour W., Mekni M.A., Rolo J., and Hassen A.B. 2011. Antibiotic resistance and molecular characterization of clinical isolates of methicillin-resistant coagulase-negative staphylococci isolated from bacteremic patients in oncohematology. Folia Microbiol. 56:122–130 [DOI] [PubMed] [Google Scholar]
- 33.Lenart-Boron A., Wolny-Koładka K., Stec J., and Kasprowic A. 2016. Phenotypic and molecular antibiotic resistance determination of airborne coagulase negative Staphylococcus spp. strains from healthcare facilities in southern Poland. Microb. Drug Resist. 22:515–522 [DOI] [PubMed] [Google Scholar]
- 34.Gatermann S.H., Koschinski T., and Friedrich S. 2007. Distribution and expression of macrolide resistance genes in coagulase-negative staphylococci. Clin. Microbiol. Infect. 13:777–781 [DOI] [PubMed] [Google Scholar]
- 35.Hope R., Livermore D.M., Brick G., Lillie M., and Reynolds R. (2008). Non-susceptibility trends among staphylococci from bacteraemias in the UK and Ireland, 2001–2006. J. Antimicrob. Chemother. 62:65–74 [DOI] [PubMed] [Google Scholar]
- 36.Liakopoulos A., Foka A., Vourli S., Zerva L., Tsiapara F., Protonotariou E., Dailiana Z., Economou M., Papoutsidou E., Koutsia-Carouzou C., Anastassiou E.D., Diza E., Zintzaras E., Spiliopoulou I., and Petinaki E. 2011. Aminoglycoside-resistant staphylococci in Greece: prevalence and resistance mechanisms. Eur. J. Clin. Microbiol. Infect. Dis. 30:701–705 [DOI] [PubMed] [Google Scholar]
- 37.Seng P., Barbe M., Pinelli P.O., Gouriet F., Drancourt M., Minebois A., Cellier N., Lechiche C., Asencio G., Lavigne J.P., Sotto A., and Stein A. 2014. Staphylococcus caprae bone and joint infections: a re-emerging infection? Clin. Microbiol. Infect. 20:O1052–O1058 [DOI] [PubMed] [Google Scholar]
- 38.Bhardwaj B., Bhatnagar U.B., and Conaway D.G. 2016. An unusual presentation of native valve endocarditis caused by Staphylococcus warneri. Rev Cardiovasc. Med. 17:140–143 [DOI] [PubMed] [Google Scholar]
- 39.Kini G.D., Patel K., Parris A.R., and Tang J.S. 2010. An unusual presentation of endocarditis caused by Staphylococcus warneri. Open Microbiol. J. 4:103–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadariya J., Smith T.C., and Thapaliya D. 2014. Staphylococcus aureus and Staphylococcal food-borne disease: an ongoing challenge in public health. Biomed. Res. Int. 2014:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rall V.L.M., Sforcin J.M., Deus M.F.R., de Sousa D.C., Camargo C.H., Godinho N.C., Galindo L.A., Soares T.C.S., and Araújo Jr J.P. 2010. Polymerase chain reaction detection of enterotoxin genes in coagulase-negative Staphylococci isolated from brazilian minas cheese. Foodborne Pathog. Dis. 7:1121–1123 [DOI] [PubMed] [Google Scholar]
- 42.Zell C., Resch M., Rosenstein R., Albrecht T., Hertel C., and Götz F. 2008. Characterization of toxin production of coagulase-negative staphylococci isolated from food and starter cultures. Int. J. Food Microbiol. 127:246–251 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.