Abstract
Background: Antipsychotic-related weight gain is a common clinically relevant side effect when treating psychotic disorders in pediatric populations, yet few predictors and no moderators of antipsychotic-related weight gain are known.
Methods: The Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study randomized 119 youths (age 8–19 years) with schizophrenia or schizoaffective disorder to 8 weeks of antipsychotic treatment with molindone, risperidone, or olanzapine and assessed treatment response and side effects. In this secondary analysis, we used multivariable linear regression and receiver operating characteristic analysis to investigate predictors and moderators of weight change and percent weight change from baseline to week 8.
Results: Treatment assignment was the most discriminant predictor of weight change [F(2, 66) = 17.00, p < 0.001] and percent weight change [F(2, 66) = 16.85, p < 0.001]. Mean weight gain was 0.74 (standard deviation ±3.51) kg for molindone, 4.13 ± 3.79 kg for risperidone, and 7.29 ± 3.44 kg for olanzapine. After adjusting for treatment assignment, lower pretreatment hemoglobin A1C (HgbA1C) predicted more weight gain [F(1, 55) = 4.71, p = 0.03]. Diagnosis (schizoaffective vs. schizophrenia) moderated weight change [F(2, 63) = 6.02, p = 0.004] and percent weight change [F(2, 63) = 5.26, p = 0.008] such that schizoaffective diagnosis predicted larger weight gain for youths in the risperidone treatment arm. Age, sex, family income, baseline weight, and symptoms neither predicted nor moderated weight change or percent weight change.
Conclusion: We identified prognostic subgroups and novel risk factors for antipsychotic-related weight gain. We confirmed that antipsychotic choice is extremely important for predicting future weight gain. We also found that younger age did not predict greater weight gain, in contrast to prior studies. Our findings require replication in an independent sample because we did not adjust for multiple comparisons to minimize false negatives. ClinicalTrials.gov Identifier: NCT00053703
Keywords: : schizophrenia, children, clinical trial, antipsychotics, side effects, obesity
Introduction
Early-onset schizophrenia spectrum disorders are psychotic disorders with onset before the age of 18 years and are characterized by positive symptoms (e.g., hallucinations and delusions) and negative symptoms (e.g., avolition and reduced emotional expression). Antipsychotics are the first-line medications for treating psychotic symptoms in schizophrenia and schizoaffective disorder, and over half of youth prescribed antipsychotics will gain significant weight within a few months of treatment initiation (Correll et al. 2006). The Treatment of Early-Onset Schizophrenia Spectrum Disorders (TEOSS) study randomized 119 youths (age 8–19 years) with schizophrenia or schizoaffective disorder to 8 weeks of antipsychotic treatment with molindone, risperidone, or olanzapine and monitored treatment response and side effects. Antipsychotic randomization did not significantly affect response rates (50% response with molindone, 46% with risperidone, and 34% with olanzapine) (Sikich et al. 2008). However, there were significant differences in absolute weight gain. Weight gain in children and adolescents (referred to as “youths” henceforth) treated with olanzapine was so severe that randomization to olanzapine was stopped prematurely.
Weight gain is an important cardiovascular risk factor and particularly relevant to psychosis because the leading cause of mortality in adult patients with psychotic disorders is cardiovascular disease (e.g., strokes and myocardial infarctions) (Hennekens et al. 2005). Furthermore, childhood obesity is associated with type 2 diabetes mellitus, colorectal cancer, degenerative joint disease, sleep apnea, fatty liver disease, and higher healthcare costs in adulthood (Freedman et al. 1999; Park et al. 2012; Manu et al. 2015). In addition to the long-term medical implications, obesity is associated with low self-esteem and poor psychosocial functioning in adult patients with schizophrenia (De Hert et al. 2006; Manu et al. 2015). Reducing medication-induced weight gain would improve treatment satisfaction, quality of life, and medical outcomes in youth with psychotic disorders (Allison et al. 2003).
Predictors are pretreatment factors that affect outcome for all treatments in the same way, while moderators are treatment-specific predictors. Few studies have identified predictors of antipsychotic-related weight gain in youths with schizophrenia spectrum disorders, and no moderators have been identified. In terms of predictors of weight gain in adults and youth, the only definitive predictor is which particular antipsychotic is prescribed, with most studies finding that atypical antipsychotics (e.g., risperidone and olanzapine) cause more weight gain than first-generation antipsychotics (e.g., molindone) and that olanzapine is particularly obesogenic (Fraguas et al. 2011; Mostafavi et al. 2017; Patel et al. 2017).
Beyond antipsychotic choice, previous studies in youths with schizophrenia spectrum disorders have identified the following variables as potential predictors of greater antipsychotic-related weight gain: male sex (Ratzoni et al. 2002; Quintana et al. 2007; Schimmelmann et al. 2007), less concern about weight gain in females (Ratzoni et al. 2002), low baseline body mass index (BMI) (Ratzoni et al. 2002; Schimmelmann et al. 2007), and high paternal BMI (Ratzoni et al. 2002). To our knowledge, the largest study to identify potential predictors of weight gain in pediatric psychotic populations (beyond medication choice) was a naturalistic open-label study of 56 adolescents with schizophrenia spectrum disorders treated with quetiapine for 12 weeks, which found low baseline BMI (hazard ratio, HR 0.88, 95% confidence interval, CI 0.78–1.01, p = 0.06) and male sex (HR 1.95, 95% CI 0.92–4.15, p = 0.08) had a trend association with likelihood of gaining ≥7% baseline weight (Schimmelmann et al. 2007). The dearth of evidence regarding predictors and lack of identified moderators of antipsychotic-related weight gain in youth with schizophrenia spectrum disorders makes research investigating risk factors especially important.
Knowing which youths are likely to have the most severe weight gain may help clinicians identify youth who need especially rigorous monitoring and aggressive treatment of metabolic abnormalities (Klein et al. 2006). Preventing obesity and metabolic changes is important because treating these conditions after onset is often ineffective (Maayan and Correll 2010; Mitchell et al. 2013; Taylor et al. 2017). Furthermore, being able to identify patients unlikely to gain weight with particular antipsychotics would assist clinicians in potentially prescribing particular antipsychotics like olanzapine (which has clear data supporting increased efficacy and short-term tolerability in adult patients) and clozapine, despite the particularly high risk of weight gain and metabolic complications (Lieberman et al. 2005; Fraguas et al. 2011). Moreover, discovering predictors and moderators of weight gain may yield insights into potential mechanisms of antipsychotic-related metabolic changes, which are poorly understood (Teff et al. 2013; Manu et al. 2015).
We conducted a secondary analysis of the TEOSS acute trial data to identify risk factors for antipsychotic-related weight gain and identify prognostic subgroups. Using the TEOSS dataset was particularly advantageous because of its extensive baseline examination of metabolic, demographic, and psychiatric domains (McClellan et al. 2007). Moreover, given that molindone, risperidone, and olanzapine were chosen as random treatment arms in TEOSS due to putative differences in weight gain and possible differences in mechanisms for weight gain, TEOSS is an ideal study to examine whether risk factors for weight gain are drug-specific (i.e., moderator analyses). Furthermore, prior studies in youth looking at weight gain associated with antipsychotics have been limited by diagnostic heterogeneity (e.g., including disruptive disorders, mood disorders, and unspecified psychotic disorders) (Almandil et al. 2013; Martínez-Ortega et al. 2013). Antipsychotic-related weight gain findings in diagnostically heterogeneous youth may not be generalizable to youth with schizophrenia spectrum disorders because patients without schizophrenia spectrum disorders may differ in terms of weight gain and metabolism compared to patients with schizophrenia, even in the absence of antipsychotic exposure (Czobor et al. 2002; Stahl et al. 2009; Yarlagadda et al. 2011; Wu et al. 2013; Manu et al. 2015; Vancampfort et al. 2015).
In this study, we investigated demographics, treatment assignment (molindone, risperidone, or olanzapine), and baseline clinical, metabolic, and anthropometric characteristics as potential predictors and moderators of antipsychotic-related weight change in TEOSS. The variables identified as potential predictors or moderators of increased antipsychotic-related weight gain are based on (1) risk factors identified in naturalistic studies of schizophrenia spectrum youths—male sex and lower baseline BMI (Ratzoni et al. 2002; Schimmelmann et al. 2007), (2) a risk factor identified in diagnostically heterogeneous samples of youths treated with antipsychotics—younger age (Safer 2004; Fraguas et al. 2008; Martínez-Ortega et al. 2013), (3) risk factors identified in adults with first episode schizophrenia—lower BMI and undifferentiated schizophrenia subtype (Saddichha et al. 2008); therefore, we investigated BMI and schizophrenia vs. schizoaffective diagnosis. We investigated diagnosis instead of schizophrenia subtype because the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5; American Psychiatric Association, 2013) no longer utilizes schizophrenia subtypes due to limited diagnostic stability, (4) putative mechanisms of antipsychotic-related weight gain—specifically that poor diet and reduced physical activity due to negative symptoms (e.g., amotivation) play roles in antipsychotic-related weight gain. We therefore examined markers of diet and physical activity (cholesterol and blood glucose) and psychotic symptoms (both positive and negative) as potential predictors and moderators of antipsychotic-related weight gain (Zimmermann et al. 2003; Deng et al. 2010; Manu et al. 2015; Vancampfort et al. 2015), (5) studies identifying low socioeconomic status and African American race as risk factors for obesity in the general population (Paeratakul et al. 2002; Wang and Zhang 2006; Taylor et al. 2012; Taylor et al. 2017), and (6) data demonstrating that age, sex, and baseline weight affect weight gain during typical childhood development (Kuczmarski et al. 2000). We analyzed TEOSS data using multivariable regression to test our hypotheses. To complement regression analyses, we used receiver operating characteristics (ROC) to investigate predictors and moderators and identify prognostic subgroups for antipsychotic-related weight gain. To our knowledge, our study is the largest to investigate predictors and moderators of antipsychotic-related weight gain in youth with schizophrenia spectrum disorders.
Methods
TEOSS overview
Intervention
A brief overview of TEOSS is provided in this article as more detailed descriptions of the TEOSS intervention, participants, and assessments have been published previously (Frazier et al. 2007; McClellan et al. 2007; Sikich et al. 2008). TEOSS data are available through limited-access datasets of the National Institutes of Health. TEOSS was conducted at four academic sites in the United States—University of North Carolina at Chapel Hill, Harvard University, University of Washington, and Case Western Reserve University, and the study was reviewed and approved by the institutional review board at each site. TEOSS was a double-blind study that randomized 119 youths with schizophrenia or schizoaffective disorder to 8 weeks of treatment with molindone, risperidone, or olanzapine during the acute trial. If participants were on antipsychotic agents or medications to manage antipsychotic side effects like benztropine, those medications were cross-tapered to the TEOSS medications over 2 weeks: typically, antipsychotics were reduced to 67% of the initial dose on entry into the study on days 1–3, then 33% of the initial dose on days 4–6, and discontinued on day 7; for anticholinergic agents (e.g., benztropine), the dose was reduced to 67% on days 3–5, 33% of the initial dose on days 6–8, and then discontinued. A flexible dosing strategy (McClellan et al. 2007) was used to titrate antipsychotic dose, and endpoint mean doses at week 8 were molindone 59.9 ± 33.5 mg/day, risperidone 2.8 ± 1.4 mg/day, and olanzapine 11.4 ± 5.0 mg/day. Participants randomized to the molindone arm also received prophylactic oral benztropine 0.5 mg twice daily to minimize extrapyramidal symptoms and help protect the blind; participants in the risperidone and olanzapine arms received matching placebos.
TEOSS inclusion and exclusion criteria
All participants were aged 8–19 years and in good physical health, and had a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 2000) diagnosis of schizophrenia (n = 79) or schizoaffective (n = 40) disorder with symptom onset before the age of 18 years. At the time of enrollment, five youths were diagnosed with schizophreniform disorder (i.e., symptoms had lasted less than the 6-month duration required for schizophrenia or schizoaffective diagnosis), and by the end of the study, the ultimate diagnoses were schizoaffective for two of the youths and schizophrenia for the other three youths. Exclusion criteria were (1) history of an adequate trial of or intolerance to study medications, (2) medication changes within the month before enrollment, (3) a diagnosis of bipolar disorder, primary posttraumatic stress disorder, primary personality disorder, psychosis not otherwise specified, current major depressive episode, mental retardation, active substance abuse or dependence, and (4) pregnancy. Participants with pervasive developmental disorders were allowed to participate if they had pronounced hallucinations or delusions. Baseline mood stabilizer and antidepressant medications were continued during the trial if the doses were stable for at least 4 weeks before enrollment.
Baseline demographic characteristics
Sixty-six percent of participants were male, 62% were white and 31% were black, mean age was 13.8 ± 2.4 years, 66% were diagnosed with schizophrenia (34% schizoaffective), and mean Positive and Negative Syndrome Scale (PANSS) total score at baseline was 100.8 ± 19.9, corresponding to a severity between “markedly ill” and “severely ill” on the Clinical Global Impression Scale (Leucht et al. 2005; Frazier et al. 2007). At the time of randomization, 41% of youths with schizophrenia and 43% of youths with schizoaffective disorder were on an antipsychotic and required a 2-week cross-taper to the study drug.
Current secondary analysis of TEOSS data
Seventy youths completed 8 weeks of treatment with the first antipsychotic they were randomized to (these youths are referred to as “treatment completers” henceforth) (Sikich et al. 2008), and dropouts (n = 22) and re-randomized (n = 27) youths (youths who were randomized to another treatment arm due to side effects or lack of efficacy of the initial antipsychotic) were more likely to be African American and had higher parental reports of baseline youth aggression (Gabriel et al. 2017). Of the 70 treatment completers, 69 (98.5%) had baseline and week-8 weight assessments and were analyzed in this secondary analysis.
Outcome variables
For these regression analyses, the two outcomes were baseline to week 8 change in (1) weight measured in kilograms and (2) weight measured as percent of baseline weight, which is used by the United States Food and Drug Administration (FDA) (FDA, 2017) and has been used in previous studies (Reynolds et al. 2002; Maayan and Correll 2011) to quantify weight gain side effects of antipsychotics. ROC analyses require dichotomous outcomes, and for our 8-week dichotomous outcomes, we used the cutoffs: (1) >4 kg weight increase (i.e., averaging a weight increase of >0.5 kg/week) and (2) >7% increase from baseline weight, like the Food and Drug Administration (FDA, 2017) and previous studies (Reynolds et al. 2002; Saddichha et al. 2008; Correll et al. 2009; Maayan and Correll 2011).
Independent variables
We examined 16 baseline (i.e., pretreatment) variables as potential predictors and moderators in the following domains: (1) Demographic: age (years), sex, race (white vs. not white, black vs. not black), and total household income as a continuous variable (1 = <$20k, 2 = $20k–$40k, 3 = $40k–$60k, 4 = $60k–$80k, 5 = $80k–$100k, 6 = >$100k); (2) Psychopathology: schizophrenia versus schizoaffective diagnosis, PANSS Positive Scale, Negative Scale, and General Scale scores (Kay et al. 1987); (3) Physical health: baseline weight, BMI, hemoglobin A1C (a measure of average blood glucose level), and fasting lipid profile—low-density lipoprotein (LDL) cholesterol (“bad cholesterol”), high-density lipoprotein cholesterol (“good cholesterol”), and triglycerides (fat); and (4) Treatment group: molindone, risperidone, or olanzapine (reference group).
Statistical analysis
We verified the normal distribution of the baseline weight data by visually inspecting the data and quantile-quantile plot and using the Shapiro–Wilk Test (W = 0.98, p = 0.29), all of which suggested normally distributed data. Mean and frequency of baseline patient characteristics of the analytic sample (69 treatment completers with weight assessments) were compared to youths who did not complete 8 weeks of treatment (49 dropouts and re-randomized youths) and treatment completers without weight assessments (1 treatment completer lacked weight assessment). In addition, analysis of variance (ANOVA) and the Tukey test were employed to compare mean weight change by treatment assignment. Next, generalized linear models were used for multivariable regression and analysis of covariance (ANCOVA) to investigate predictors and moderators of weight gain.
Specifically, multivariable linear regression and ANCOVA were applied to investigate the association between each potential predictor and weight change and percent weight change after adjusting for treatment assignment. Moderators were identified by investigating the interaction between the independent variable and treatment assignment. For each outcome variable, the regression model included treatment assignment, the independent variable, and the independent variable interaction with treatment assignment. If the interaction was not significant at the p < 0.05 threshold in ANCOVA analysis, the interaction term was removed from the ANCOVA and regression model. The significance threshold was two-tailed p < 0.05 for all analyses, and we did not correct for multiple comparisons to minimize Type II Error (false negatives), similar to prior studies (Taylor et al. 2015, 2018). We prioritized minimizing Type II Error so that if previously identified predictors were not significant in this analysis, our negative findings would have increased validity. Regression analysis was conducted using Statistical Analysis System (SAS version 9.4).
In addition, we used ROC analyses to identify prognostic subgroups based on likelihood of gaining >4 kg weight and having a >7% weight increase from baseline to week 8. The ROC analysis was performed using free software available online from Ruth O'Hara at Stanford University (www.stanford.edu/∼yesavage/ROC.html). ROC analysis (Yesavage et al. 2003) operates by recursive partitioning and was used as a nonparametric analytic method to complement regression analyses. ROC divides a population into subgroups based on probability of achieving a particular binary outcome (Kiernan et al. 2001; Yesavage et al. 2003). Across all potential predictor variables, ROC identifies the variable cutoff point that yields the best prediction, and the cutoff is then used to divide the total sample into two subsamples. The same procedure is repeated systematically in each subsample. Like other investigations using ROC, our methods were determined a priori (Taylor et al. 2015, 2018; Gabriel et al. 2017).
Results
Baseline demographic characteristics of analytic sample
Table 1 displays a list of pretreatment participant clinical variables and demographics for the 69 treatment completers with weight assessments. Sixty-eight percent of youths in the analytic sample were male, 68% were white and 23% were black, 65% were diagnosed with schizophrenia (35% schizoaffective), mean age was 14.15 ± 2.4 years, mean weight was 63.71 ± 18.08 kg, mean BMI was 23.53 ± 5.21 kg/m2, mean BMI Z-score was 0.84 ± 0.99, mean hemoglobin A1C was 5.20% ± 0.54%, and mean PANSS total score was 102.80 ± 20.84 at baseline, corresponding to a severity between “markedly ill” and “severely ill” on the Clinical Global Impression Scale (Leucht et al. 2005). Table 1 also compares the analytic sample (N = 69) to patients who were re-randomized, dropped out, or had insufficient weight data (N = 50), and the two samples significantly differed in that the analytic sample had higher baseline fasting triglycerides (p = 0.004) and were less likely to be black (p = 0.03).
Table 1.
Baseline Patient Characteristics
| Analytic sample | Dropouts, re-randomized, and insufficient weight data | ||||||
|---|---|---|---|---|---|---|---|
| Variable | N | Mean | Std. dev. | N | Mean | Std. dev. | p |
| Age (years) | 69 | 14.15 | 2.47 | 50 | 14.41 | 2.36 | 0.58 |
| Weight (kg) | 69 | 63.71 | 18.08 | 47 | 64.74 | 22.4 | 0.79 |
| Height (cm) | 69 | 163.4 | 14.62 | 47 | 163.2 | 12.71 | 0.92 |
| Body mass index (kg/m2) | 69 | 23.53 | 5.21 | 47 | 23.8 | 5.59 | 0.79 |
| Body mass index Z-score | 69 | 0.84 | 0.99 | 47 | 0.89 | 1 | 0.76 |
| PANSS positive subscale | 69 | 26.28 | 5.80 | 49 | 26.24 | 5.82 | 0.98 |
| PANSS negative subscale | 69 | 25.90 | 8.13 | 49 | 23.9 | 7.57 | 0.18 |
| PANSS general subscale | 69 | 50.59 | 11.24 | 49 | 48.22 | 9.97 | 0.24 |
| PANSS total score | 69 | 102.80 | 20.84 | 49 | 98.37 | 18.21 | 0.24 |
| Hemoglobin A1C (%) | 59 | 5.20 | 0.54 | 45 | 5.27 | 0.51 | 0.49 |
| Fasting low-density lipoprotein cholesterol (mg/dL) | 47 | 100.30 | 37.68 | 42 | 98.12 | 30.45 | 0.77 |
| Fasting high-denisty lipoprotein (mg/dL) | 48 | 49.86 | 13.83 | 42 | 54.78 | 13.55 | 0.09 |
| Fasting Triglycerides (mg/dL) | 49 | 112.80 | 58.84 | 42 | 81.95 | 39.58 | 0.004 |
| N | n | % | N | n | % | p | |
|---|---|---|---|---|---|---|---|
| Female | 69 | 22 | 31.88 | 50 | 19 | 38 | 0.49 |
| Random assignment | 69 | 49 | 0.23 | ||||
| Molindone | 25 | 36.23 | 16 | 32.65 | |||
| Risperidone | 27 | 39.13 | 19 | 38.78 | |||
| Olanzapine | 17 | 24.64 | 14 | 28.57 | |||
| Schizophrenia diagnosis | 69 | 45 | 65.22 | 49 | 33 | 67.35 | 0.81 |
| Race | 69 | 50 | |||||
| Black | 16 | 23.19 | 21 | 42 | 0.03 | ||
| White | 47 | 68.12 | 27 | 54 | 0.12 | ||
| Ethnicity | 69 | 50 | 0.4 | ||||
| Hispanic | 4 | 5.80 | 1 | 2 | |||
| Not Hispanic | 65 | 94.20 | 49 | 98 | |||
| Household income | 64 | 48 | 0.85 | ||||
| <$20,000 | 14 | 21.88 | 13 | 27.08 | |||
| $20,000–$40,000 | 15 | 23.44 | 12 | 25 | |||
| $40,000–$60,000 | 17 | 26.56 | 11 | 22.92 | |||
| $60,000–$80,000 | 8 | 12.50 | 6 | 12.5 | |||
| $80,000–$100,000 | 4 | 6.25 | 3 | 6.25 | |||
| >$100,000 | 6 | 9.38 | 3 | 6.25 |
Bold values in Table 1 correspond to significant (p < 0.05) differences between the analytic sample and trial participants excluded from analyses due to dropout, re-randomization, or insufficient weight data.
PANSS, Positive and Negative Syndrome Scale.
Weight change by treatment assignment
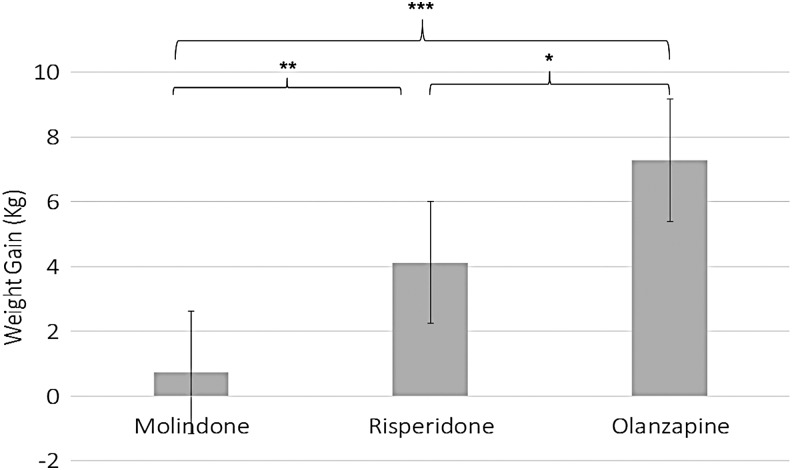
On average, weight increased by 3.68 ± 4.38 kg and 5.97% ±7.91%. ANOVA found that randomized treatment assignment significantly affected weight change [F(2, 66) = 17.00, p < 0.001] and percent weight change [F(2, 66) = 16.85, p < 0.001]. Mean weight increase was 0.74 ± 3.51 kg for molindone, 4.13 ± 3.79 kg for risperidone, and 7.29 ± 3.44 kg for olanzapine. Mean percent weight increase was 0.71% ± 5.67% for molindone, 6.70% ± 6.81% for risperidone, and 12.54% ± 7.25% for olanzapine. Tukey's studentized range test found that the weight and percent weight gained on olanzapine were significantly more than the gain on risperidone (p < 0.05), the gain on risperidone was more than on molindone (p < 0.01), and the gain on olanzapine was more than on molindone (p < 0.0001). Figure 1 depicts weight gain and Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/cap) depicts percent weight gain for each antipsychotic.
FIG. 1.
Weight gain (kilograms). Error bars are standard error. *p < 0.05, **p < 0.01, ***p < 0.001.
Weight change (kilograms) regression results
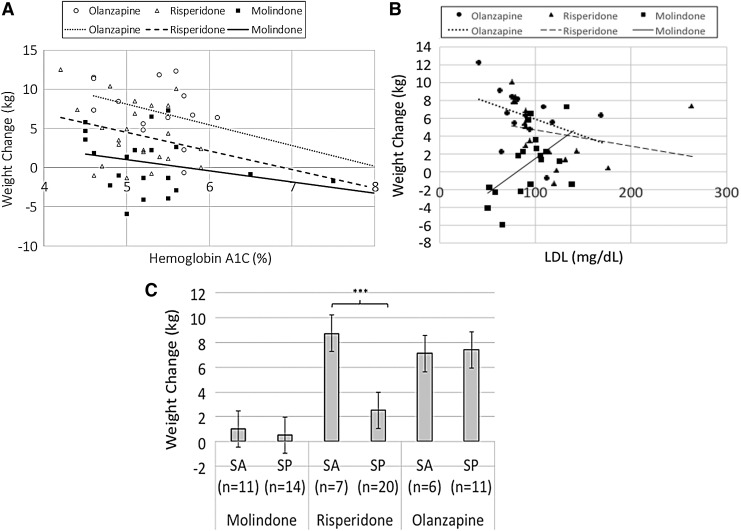
When treatment assignment was the only variable in regression analyses, molindone (parameter estimate [PE] = −6.55, standard error [SE] = 1.13, t = −5.77, p < 0.001) and risperidone (PE = −3.16, SE = 1.12, t = −2.83, p = 0.006) predicted lower weight increase relative to olanzapine, and treatment assignment accounted for 34% of the variance in weight change (r-squared = 0.34). Table 2 displays individual predictors and moderators of weight change after adjusting for treatment assignment. Lower pretreatment hemoglobin A1C predicted more weight gain (PE = −1.91, SE = 0.88, t = −2.17, p = 0.03) (Figure 2A), and the regression model with treatment assignment and hemoglobin A1C explained 38% of the variance in weight change. Second, pretreatment LDL moderated weight change [F(2, 41) = 4.68, p = 0.01] such that youths with lower LDL tended to gain more weight if assigned to risperidone or olanzapine, but the reverse was true for molindone (Figure 2B). The regression model with treatment assignment, LDL, and the interaction explained 44% of the variance in weight change. Third, diagnosis moderated weight change [F(2, 63) = 6.02, p = 0.004] such that relative to schizophrenia, schizoaffective diagnosis predicted more weight gain for youths randomized to risperidone (mean for schizoaffective 8.73 ± 2.51 kg, schizophrenia 2.52 ± 2.67 kg; t(25) = 5.36, p < 0.0001), but not molindone (mean for schizoaffective 1.02 ± 3.78 kg, schizophrenia 0.52 ± 3.41 kg; t(23) = 0.35, p = 0.73), or olanzapine (mean for schizoaffective 7.09 ± 4.88 kg, schizophrenia 7.39 ± 2.65 kg; t(15) = −0.17, p = 0.89) (Figure 2C). The regression model with treatment assignment, diagnosis, and the interaction explained 50% of the variance in weight change. Schizoaffective diagnosis moderated weight change in the same way, whether we used final diagnosis (schizophrenia or schizoaffective) or entering diagnosis (schizophrenia, schizoaffective, or schizophreniform). Other independent variables investigated neither predicted nor moderated weight change. As a sensitivity analysis, we conducted predictor and moderator regression analyses after adjusting for treatment assignment, age, sex, baseline weight, concurrent antipsychotic prescription at the time of randomization (coded as continuous variable: 0 = none, 1 = aripiprazole, 2 = risperidone, 3 = quetiapine, 4 = olanzapine), and study antipsychotic dose at week 8 (antipsychotic milligrams per kilogram of youth weight), and these additional adjustments did not significantly alter results.
Table 2.
Regression Results: Predictors and Moderators of Weight Change and Percent Weight Change
| Weight gain | Percent weight gain | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline variables | Parameter estimate | Standard error | T | p | Baseline variables | Parameter estimate | Standard error | T | p |
| Age (years) | 0.18 | 0.18 | 1.03 | 0.31 | Age (years) | 0.07 | 0.33 | 0.23 | 0.82 |
| R-squared | 0.35 | R-squared | 0.34 | ||||||
| Male | −0.06 | 1.00 | −0.06 | 0.96 | Male | 0.36 | 1.81 | 0.20 | 0.85 |
| R-squared | 0.34 | R-squared | 0.34 | ||||||
| White | 0.27 | 0.94 | 0.29 | 0.77 | White | 0.99 | 1.70 | 0.58 | 0.56 |
| R-squared | 0.34 | R-squared | 0.34 | ||||||
| Black | −1.05 | 1.05 | −1.00 | 0.32 | Black | −2.35 | 1.90 | −1.23 | 0.22 |
| R-squared | 0.35 | R-squared | 0.35 | ||||||
| Income | 0.22 | 0.29 | 0.74 | 0.46 | Income | 0.35 | 0.54 | 0.65 | 0.52 |
| R-squared | 0.39 | R-squared | 0.37 | ||||||
| Weight | 0.03 | 0.02 | 1.12 | 0.27 | Weight | −0.03 | 0.04 | −0.58 | 0.56 |
| R-squared | 0.35 | R-squared | 0.34 | ||||||
| Body mass index | 0.05 | 0.09 | 0.53 | 0.60 | Body mass index | −0.75 | 0.38 | −1.96 | 0.05 |
| R-squared | 0.34 | X MOLINDONE | 1.09 | 0.46 | 2.38 | 0.02 | |||
| X RISPERIDONE | 0.51 | 0.44 | 1.16 | 0.25 | |||||
| X OLANZAPINE | reference | ||||||||
| R-squared | 0.40 | ||||||||
| Hemoglobin A1C (%) | −1.91 | 0.88 | −2.17 | 0.03 | Hemoglobin A1C (%) | −2.76 | 1.55 | −1.79 | 0.08 |
| R-squared | 0.38 | R-squared | 0.35 | ||||||
| Fasting low-density lipoprotein (mg/dL) | −0.04 | 0.03 | −1.31 | 0.20 | Fasting low-density lipoprotein (mg/dL) | −0.09 | 0.05 | −1.59 | 0.12 |
| X MOLINDONE | 0.12 | 0.04 | 2.76 | 0.009 | X MOLINDONE | 0.20 | 0.08 | 2.61 | 0.01 |
| X RISPERIDONE | 0.02 | 0.03 | 0.58 | 0.56 | X RISPERIDONE | 0.05 | 0.06 | 0.77 | 0.45 |
| X OLANZAPINE | Reference | X OLANZAPINE | reference | ||||||
| R-squared | 0.44 | R-squared | 0.44 | ||||||
| Fasting high-density lipoprotein (mg/dL) | −0.05 | 0.04 | −1.38 | 0.18 | Fasting high-density lipoprotein (mg/dL) | −0.06 | 0.07 | −0.85 | 0.40 |
| R-squared | 0.33 | R-squared | 0.33 | ||||||
| Fasting triglycerides (mg/dL) | −0.01 | 0.01 | −0.66 | 0.51 | Fasting triglycerides (mg/dL) | −0.01 | 0.02 | −0.39 | 0.70 |
| R-squared | 0.30 | R-squared | 0.32 | ||||||
| Schizoaffective diagnosis | −0.30 | 1.64 | −0.19 | 0.85 | Schizoaffective diagnosis | −0.83 | 3.05 | −0.27 | 0.79 |
| X MOLINDONE | 0.80 | 2.09 | 0.38 | 0.70 | X MOLINDONE | 0.90 | 3.89 | 0.23 | 0.82 |
| X RISPERIDONE | 6.52 | 2.17 | 3.01 | 0.004 | X RISPERIDONE | 11.06 | 4.03 | 2.74 | 0.008 |
| X OLANZAPINE | Reference | X OLANZAPINE | reference | ||||||
| R-squared | 0.50 | R-squared | 0.47 | ||||||
| PANSS positive subscale | 0.08 | 0.08 | 0.97 | 0.33 | PANSS positive subscale | 0.15 | 0.14 | 1.08 | 0.28 |
| R-squared | 0.35 | R-squared | 0.35 | ||||||
| PANSS negative subscale | 0.01 | 0.06 | 0.16 | 0.87 | PANSS negative subscale | 0.01 | 0.10 | 0.09 | 0.93 |
| R-squared | 0.34 | R-squared | 0.34 | ||||||
| PANSS general subscale | 0.01 | 0.04 | 0.20 | 0.85 | PANSS general subscale | −0.02 | 0.07 | −0.30 | 0.76 |
| R-squared | 0.34 | R-squared | 0.34 | ||||||
Bold values in Table 2 correspond to variables that significantly (p < 0.05) predicted or moderated weight change or percent weight change.
PANSS, Positive and Negative Syndrome Scale; “X,” denotes interaction.
FIG. 2.
(A) Relationship between weight change and hemoglobin A1C. (B) Relationship between weight change and fasting low-density lipoprotein cholesterol. (C) Weight change by diagnosis. Error bars are standard error. SA, schizoaffective; SP, schizophrenia. ***p < 0.001.
Weight change (kilograms) ROC results
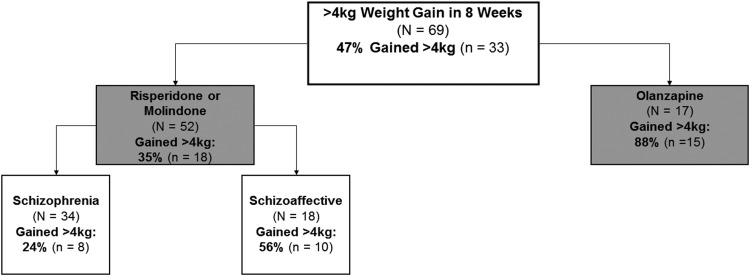
Overall, 47% of youths gained >4 kg of weight. The most discriminative predictor of weight gain was being prescribed olanzapine (88% gained >4 kg) versus risperidone (48% gained >4 kg) or molindone (20% gained >4 kg) (χ2 = 14.76, p < 0.001). Among youths who received risperidone or molindone, the most discriminative predictor of >4 kg weight gain was diagnosis of schizoaffective (56% gained >4 kg) instead of schizophrenia (24% gained >4 kg) (χ2 = 5.33, p < 0.05). Figure 3 depicts hierarchical prognostic subgroups based on likelihood of >4 kg weight gain.
FIG. 3.
Prognostic subgroups for weight gain (kg). Gray: p < 0.001, white: p < 0.05.
Percent weight change regression results
When treatment assignment was the only variable in regression analyses, molindone (PE = −11.83, SE = 2.05, t = −5.76, p < 0.001) and risperidone (PE = −5.84, SE = 2.02, t = −2.88, p = 0.005) predicted lower percent weight increase relative to olanzapine, and treatment assignment accounted for 34% of the variance. Table 2 displays predictor and moderator results for percent weight change. After adjusting for randomized antipsychotic assignment, there were no significant predictors. Pretreatment BMI [F(2, 63) = 3.20, p = 0.04], LDL [F(2, 41) = 3.88, p = 0.03], and diagnosis [F(2, 63) = 5.26, p = 0.008] moderated percent weight change such that youths with lower BMI tended to have greater increases in percent weight gain with olanzapine but not risperidone or molindone (Supplementary Figure 2A), youths with lower LDL tended to gain more weight if assigned to olanzapine or risperidone, but the reverse was true for molindone (Supplementary Figure 2B), and schizoaffective diagnosis predicted greater weight gain if randomized to risperidone (mean for schizoaffective 14.28 ± 6.25%, schizophrenia 4.05 ± 4.75%; t(25) = 4.52, p = 0.0001), but not molindone (mean for schizoaffective 0.75 ± 6.31%, schizophrenia 0.68 ± 5.36%; t(23) = 0.03, p = 0.98) or olanzapine (mean for schizoaffective 12.00 ± 8.77%, schizophrenia 12.83 ± 6.74%, t(15) = −0.22, p = 0.83) (Supplementary Figure 2C). The regression models with treatment assignment and interaction in addition to BMI, LDL, or diagnosis explained 40%, 44%, and 47% of the variation in percent weight change, respectively. In sensitivity analyses, adjusting for treatment assignment, sex, age, baseline weight, concurrent antipsychotic prescription at randomization (coded as continuous variable: 0 = none, 1 = aripiprazole, 2 = risperidone, 3 = quetiapine, 4 = olanzapine), and study antipsychotic dose at week 8 (milligrams antipsychotic per kilogram youth weight), findings did not significantly change. Schizoaffective diagnosis moderated percent weight change in the same way, whether we used final diagnosis (schizophrenia or schizoaffective) or entering diagnosis (schizophrenia, schizoaffective, or schizophreniform).
Percent weight change ROC results
Overall, 42% of youth increased >7% from baseline weight. The most discriminative predictor of >7% weight gain was randomization to olanzapine (88% increased >7%) versus risperidone (37% increased >7%) or molindone (16% increased >7%) (χ2 = 19.77, p < 0.001). Among youths who received risperidone or molindone, the most discriminative predictor of >7% weight increase was diagnosis of schizoaffective (44% increased >7%) instead of schizophrenia (18% increased >7%) (χ2 = 4.30, p < 0.05). Supplementary Figure S3 depicts hierarchical prognostic subgroups based on likelihood of >7% increase from baseline weight.
Discussion
In this study, we investigated predictors and moderators of antipsychotic-related weight gain during the 8 weeks of randomized antipsychotic treatment in TEOSS and identified prognostic subgroups based on weight gain. We found olanzapine was associated with severe weight gain, risperidone with moderate weight gain, and molindone with minimal weight gain, consistent with prior findings (Sikich et al. 2008; Fraguas et al. 2011). Furthermore, we extend previous TEOSS reports by finding that 88% of youths randomized to olanzapine, 37% of youths randomized to risperidone, and 16% of youths randomized to molindone had severe weight gain as defined by >7% weight gain, a cutoff used by the FDA (FDA) and previous studies (Reynolds et al. 2002; Saddichha et al. 2008; Correll et al. 2009; Maayan and Correll 2011). In addition, our study identified two novel risk factors for antipsychotic-related weight gain: low pretreatment hemoglobin A1C when treated with molindone, risperidone, or olanzapine, and schizoaffective diagnosis when treated with risperidone. Moreover, while previous studies have suggested younger age is associated with antipsychotic-related weight gain when prescribed for youths with heterogeneous diagnoses (e.g., mood disorders, psychotic disorders, or various behavioral disturbances) (Safer 2004; Fraguas et al. 2008; Martínez-Ortega et al. 2013), we did not find an association or even a trend between younger age and greater weight gain in this sample of youths with schizophrenia spectrum disorders. To our knowledge, our study is the largest to identify a predictor beyond antipsychotic choice and the first to identify moderators of antipsychotic-related weight gain in youths with schizophrenia spectrum disorders.
Mean weight gain was 0.74 ± 3.51 kg for molindone, 4.13 ± 3.79 kg for risperidone, and 7.29 ± 3.44 kg for olanzapine. Our estimates for antipsychotic-related weight gain in TEOSS were larger than findings described previously: 0.3 ± 2.9 kg for molindone, 3.6 ± 4.0 kg for risperidone, and 6.1 ± 3.6 kg for olanzapine (Sikich et al. 2008). The difference is likely due to our analytic exclusion of youths who dropped out or were re-randomized to another antipsychotic, whom we excluded because these youths did not receive the full 8-week regimen of the initial antipsychotic (Sikich et al. 2008). In contrast, previously reported estimates of weight gain in TEOSS used the intent-to-treat approach using the last observation carried forward and included youths who were exposed to fewer than 8 weeks of the initial antipsychotic.
Despite knowledge that antipsychotics frequently cause weight gain, the mechanisms are poorly understood (Manu et al. 2015). There are several theories regarding the mechanisms of antipsychotic-related weight gain. The simplest mechanism is that antipsychotics result in an increase in caloric intake (Deng et al. 2010), which may be related to activation of orexigenic hypothalamic structures, particularly for olanzapine (Manu et al. 2015). Reduced energy expenditure due to negative symptoms is also thought to contribute to antipsychotic-related weight gain (Vancampfort et al. 2015). Moreover, resting energy expenditure seems to decrease with exposure to antipsychotics. Specifically, a study examining a diagnostically heterogeneous group of 46 adolescents found atypical antipsychotics resulted in a hypometabolic state—that is, reduced resting energy expenditure per kilogram of body mass (Cuerda et al. 2011).
Furthermore, a possible explanation for the severe weight gain associated with olanzapine (even relative to other atypical antipsychotics) is that olanzapine has an especially high affinity for and antagonism of the H1 histaminergic receptors, and antipsychotic H1 receptor antagonism correlates with weight gain (Kroeze et al. 2003; Deng et al. 2010). Finally, a 2016 pharmacogenetic meta-analysis of 72 studies with various sample ages and diagnoses found that Adrenoceptor Alpha-2A, Dopamine Receptor D2, 5HT2C Receptor, and Melanocortin-4 Receptor genes associated with increased weight gain; however, only 3 of the 72 studies were in youths and none was in youths with schizophrenia spectrum disorders (Zhang et al. 2016). While the precise mechanisms of antipsychotic-related weight gain remain elusive, our predictor and moderator findings can be explained by some of the hypothesized mechanisms.
For instance, we found that lower hemoglobin A1C predicted greater weight gain in TEOSS. Similarly, LDL and BMI moderated weight change such that lower LDL youths had greater weight gain and percent weight gain in the olanzapine and risperidone arms and lower BMI youths had greater percent weight gain in the olanzapine arm. Previous studies have found a relationship between lower BMI and clinically significant antipsychotic-related weight gain in adults and youths with psychotic disorders (Schimmelmann et al. 2007; Saddichha et al. 2008). A possible explanation for the tendency for lower BMI, LDL, and hemoglobin A1C youths to have greater weight gain or percent weight gain with some antipsychotics is that lower BMI, LDL, and hemoglobin A1C may be indicative of healthier diet, higher levels of physical activity, and/or higher metabolic rates pretreatment. Youth with healthier baseline habits or higher metabolisms may in turn be particularly vulnerable to antipsychotic-induced appetite increases, reduced physical activity due to sedation and antipsychotic-induced worsening of negative symptoms (Vancampfort et al. 2015), and hypometabolic changes related to antipsychotics (Cuerda et al. 2011). In contrast, if a youth already has a poor diet, low level of physical activity, and low resting metabolic rate at baseline, antipsychotic-related increases in appetite and decreases in physical activity level and metabolism may have muted effects on weight because there may be a floor for how low resting metabolism and how poor diets and physical activity levels can be. Interrogating this hypothesis would require future studies to rigorously collect data on food intake, physical activity, and metabolism at baseline and change over time. Furthermore, findings of distinct relationships between BMI and LDL and weight change based on the particular antipsychotic highlights the complexity of identifying risk factors for antipsychotic-related weight gain.
In addition, we found that diagnosis moderated antipsychotic-related weight change in that schizoaffective diagnosis predicted increased weight gain relative to schizophrenia for youths in the risperidone treatment arm. In fact, in ROC analyses, diagnosis was the most discriminative predictor of weight gain after accounting for randomization to olanzapine. Affective symptoms are associated with changes in appetite and weight (e.g., depression is classically associated with reduced appetite and weight loss). In schizoaffective disorder, it may be that risperidone and olanzapine improve mood regulation and thereby increase appetite and weight (Sachs et al. 2002); however, the relative increase in appetite and weight due to mood stabilization may only be apparent in risperidone because the severe increase in weight due to potential olanzapine-specific mechanisms (Kroeze et al. 2003; Deng et al. 2010) may overshadow any change due to mood stabilization. The lack of significant difference in weight gain by diagnosis in the molindone arm may be due to the fact that atypical antipsychotics are generally more effective at treating mood disorders relative to first-generation antipsychotics like molindone (Sachs et al. 2002).
Our study also had several pertinent negative findings, and we did not adjust for multiple comparisons to maximize the validity of our negative findings. First, we did not find an association between younger age and increased weight gain in contrast to prior studies (Safer 2004; Martínez-Ortega et al. 2013). The evidence regarding younger age increasing antipsychotic-related weight gain is primarily extrapolated from analyses that have pooled data from multiple studies and compared results in youth studies (most of which have diagnostically heterogeneous samples) to adults studies (most of which have samples predominated by patients with schizophrenia spectrum disorders) (Safer 2004; Martínez-Ortega et al. 2013). Given our findings of no association and the limitations of previous studies suggesting younger age is associated with greater antipsychotic-related weight gain, further research regarding the relationship between antipsychotic-related weight gain and age in youth with schizophrenia spectrum disorders is warranted.
Another pertinent negative finding was the lack of relationship between male sex and increased weight gain, in contrast to a previous study (Schimmelmann et al. 2007). A possible explanation for our lack of sex findings is that this 8-week study was shorter in duration than the Schimmelmann et al. (2007) 12-week study, which found a trend association between male sex and >7% weight gain. The differences based on length of study may be due to the fact that during typical childhood development, adolescent boys gain weight at a faster rate than girls (Kuczmarski et al. 2000), and thus the longer a study on adolescent weight gain is, antipsychotic-related or not, the larger the difference between weight gain in boys and girls should become. Finally, we found that family income, baseline weight, and PANSS symptoms did not predict or moderate antipsychotic-related weight gain.
Our study should be interpreted in the context of its limitations. First, while we did not find associations between race and weight gain or baseline triglycerides and weight gain, because we found that dropouts, re-randomized youths, and those without weight assessments were more likely to be black and have lower baseline triglycerides relative to our analytic sample, we cannot draw decisive conclusions about how race and triglycerides predict or moderate antipsychotic-related weight gain. Second, only eight youths were concurrently taking antidepressants and only four were taking mood stabilizers, and so due to small sample size, we were unable to investigate whether these medications with demonstrated effects on weight gain (Zimmermann et al. 2003) affected antipsychotic-related weight gain. Third, we did not adjust for multiple comparisons, which strengthen the validity of our negative findings, but it also makes our study more vulnerable to false positives (Type I Error). Therefore, our findings regarding hemoglobin A1C, LDL, BMI, and schizoaffective diagnosis require replication in an independent sample. Fourth, data regarding family history of obesity were unavailable in TEOSS, and previous research suggests that high paternal BMI may predict antipsychotic-related weight gain (Ratzoni et al. 2002). Still, the strengths of our study stem from TEOSS's randomized design with three antipsychotics enabling moderator analyses and our sample consisting exclusively of youths with schizophrenia spectrum disorders, addressing limitations of the extant literature on antipsychotic-related weight gain in youth: diagnostic heterogeneity and confounding by indication (Martínez-Ortega et al. 2013).
Conclusions
Previous analyses of TEOSS data found that while antipsychotic choice did not affect response rate, it profoundly affected weight gain. We confirmed that antipsychotic choice is extremely important for predicting weight gain. Additionally, our study is the largest to identify a predictor of weight gain beyond antipsychotic choice (lower hemoglobin A1C predicted increased weight gain) and the first to identify moderators of antipsychotic-related weight gain (LDL and schizoaffective diagnosis) and percent weight gain (BMI, LDL, and schizoaffective diagnosis) in youth with schizophrenia spectrum disorders.
Clinical Significance
Our finding that schizoaffective diagnosis (relative to schizophrenia) was a risk factor for antipsychotic-related weight gain in the risperidone treatment arm highlights the importance of diagnostic specificity in studies investigating antipsychotic-related weight gain (Martínez-Ortega et al. 2013). Furthermore, our finding that lower hemoglobin A1C is a potential risk factor for antipsychotic-related weight gain underscores the importance of rigorous monitoring of weight gain for all youth prescribed atypical antipsychotics, even when the youth does not have pretreatment clinical characteristics typically associated with metabolic dysfunction.
Supplementary Material
Acknowledgments
Data used in the preparation of this article were obtained from the limited access datasets distributed from the NIH-supported “Treatment of Early Onset Schizophrenia Spectrum Disorders Study” (TEOSS). This is a multisite, clinical trial of youth with schizophrenia spectrum disorders comparing the effectiveness of long-term randomly assigned medication treatment. The study was supported by NIMH Contracts U01 MH62726, DDTR B2-NDS, R01 MH61528, R01 MH61355, R01 MH62726, and R01 MH61464 to the University of North Carolina, Chapel Hill. The ClinicalTrials.gov identifier is NCT00053703. This article reflects the views of the authors and may not reflect the opinions or views of the TEOSS Study Investigators or the NIH.
The authors acknowledge the support of the American Academy of Child and Adolescent Psychiatry Pilot Research Award (J.H.T.); Substance Abuse and Mental Health Services Administration-American Psychiatric Association Minority Fellowship Program (J.H.T.); National Institute of Mental Health 5T32MH18268, 5T32MH19961, and 5T32MH019112 (J.H.T.); and UL1 RR024139 from the National Center for Research Resources, a component of the National Institutes of Health; and NIH roadmap for Medical Research (M.H.B.).
Disclosures
M.H.B. received research support from Biohaven Pharmaceuticals and Therapix Biosciences, neither of which provided funds for this study. J.H.T., E.J., and D.G., do not have relevant disclosures or any conflicts of interest.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, text revision: DSM-IV-TR. Washington, DC: American Psychiatric Association, 2000 [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fifth edition. Arlington, VA: American Psychiatric Publishing, 2013 [Google Scholar]
- Allison DB, Mackell JA, McDonnell DD: The impact of weight gain on quality of life among persons with schizophrenia. Psychiatr Serv 54:565–567, 2003 [DOI] [PubMed] [Google Scholar]
- Almandil NB, Liu Y, Murray ML, Besag FM, Aitchison KJ, Wong IC: Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: A systematic review and meta-analysis. Pediatric Drugs 15:139–150, 2013 [DOI] [PubMed] [Google Scholar]
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK: Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302:1765–1773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Penzner JB, Parikh UH, Mughal T, Javed T, Carbon M, Malhotra AK: Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am 15:177–206, 2006 [DOI] [PubMed] [Google Scholar]
- Cuerda C, Merchan-Naranjo J, Velasco C, Gutierrez A, Leiva M, de Castro MJ, Parellada M, Giráldez M, Bretón I, Camblor M: Influence of resting energy expenditure on weight gain in adolescents taking second-generation antipsychotics. Clin Nutr 30:616–623, 2011 [DOI] [PubMed] [Google Scholar]
- Czobor P, Volavka J, Sheitman B, Lindenmayer J-P, Citrome L, McEvoy J, Cooper TB, Chakos M, Lieberman JA: Antipsychotic-induced weight gain and therapeutic response: A differential association. J Clin Psychopharmacol 22:244–251, 2002 [DOI] [PubMed] [Google Scholar]
- De Hert M, Peuskens B, van Winkel R, Kalnicka D, Hanssens L, Van Eyck D, Wyckaert S, Peuskens J: Body weight and self-esteem in patients with schizophrenia evaluated with B-WISE®. Schizophr Res 88:222–226, 2006 [DOI] [PubMed] [Google Scholar]
- Deng C, Weston-Green K, Huang X-F: The role of histaminergic H1 and H3 receptors in food intake: A mechanism for atypical antipsychotic-induced weight gain? Prog Neuropsychopharmacol Biol Psychiatry 34:1–4, 2010 [DOI] [PubMed] [Google Scholar]
- FDA: United states food and drug administration. Zyprexa and risperdal label. www.Fda.Gov Accessed July1, 2017
- Fraguas D, Correll CU, Merchán-Naranjo J, Rapado-Castro M, Parellada M, Moreno C, Arango C: Efficacy and safety of second-generation antipsychotics in children and adolescents with psychotic and bipolar spectrum disorders: Comprehensive review of prospective head-to-head and placebo-controlled comparisons. Eur Neuropsychopharmacol 21:621–645, 2011 [DOI] [PubMed] [Google Scholar]
- Fraguas D, Merchán-Naranjo J, Laita P, Parellada M, Moreno D, Ruiz-Sancho A, Cifuentes A, Giráldez M, Arango C: Metabolic and hormonal side effects in children and adolescents treated with second-generation antipsychotics. The J Clin Psychiatry 69:1166–1175, 2008 [DOI] [PubMed] [Google Scholar]
- Frazier JA, McClellan J, Findling RL, Vitiello B, Anderson R, Zablotsky B, Williams E, McNamara NK, Jackson JA, Ritz L: Treatment of early-onset schizophrenia spectrum disorders (TEOSS): Demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry 46:979–988, 2007 [DOI] [PubMed] [Google Scholar]
- Freedman DS, Dietz WH, Srinivasan SR, Berenson GS: The relation of overweight to cardiovascular risk factors among children and adolescents: The Bogalusa Heart Study. Pediatrics 103:1175–1182, 1999 [DOI] [PubMed] [Google Scholar]
- Gabriel D, Jakubovski E, Taylor JH, Artukoglu BB, Bloch MH: Predictors of treatment response and drop out in the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Psychiatry Res 255:248–255, 2017 [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE: Schizophrenia and increased risks of cardiovascular disease. Am Heart J 150:1115–1121, 2005 [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opfer LA: The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261, 1987 [DOI] [PubMed] [Google Scholar]
- Kiernan M, Kraemer HC, Winkleby MA, King AC, Taylor CB: Do logistic regression and signal detection identify different subgroups at risk? Implications for the design of tailored interventions. Psychol Methods 6:35–48, 2001 [DOI] [PubMed] [Google Scholar]
- Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA: A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry 163:2072–2079, 2006 [DOI] [PubMed] [Google Scholar]
- Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL: H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 28:519–526, 2003 [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL: CDC growth charts: United states. Adv Data 1–27, 2000 [PubMed] [Google Scholar]
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR: What does the PANSS mean? Schizophr Res 79:231–238, 2005 [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223, 2005 [DOI] [PubMed] [Google Scholar]
- Maayan L, Correll CU: Management of antipsychotic-related weight gain. Expert Rev Neurother 10:1175–1200, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan L, Correll CU: Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol 21:517–535, 2011 [DOI] [PubMed] [Google Scholar]
- Manu P, Dima L, Shulman M, Vancampfort D, De Hert M, Correll C: Weight gain and obesity in schizophrenia: Epidemiology, pathobiology, and management. Acta Psychiatr Scand 132:97–108, 2015 [DOI] [PubMed] [Google Scholar]
- Martínez-Ortega JM, Funes-Godoy S, Díaz-Atienza F, Gutiérrez-Rojas L, Pérez-Costillas L, Gurpegui M: Weight gain and increase of body mass index among children and adolescents treated with antipsychotics: A critical review. Eur Child Adolesc Psychiatry 22:457–479, 2013 [DOI] [PubMed] [Google Scholar]
- McClellan J, Sikich L, Findling RL, Frazier JA, Vitiello B, Hlastala SA, Williams E, Ambler D, Hunt-Harrison T, Maloney AE: Treatment of early-onset schizophrenia spectrum disorders (TEOSS): Rationale, design, and methods. J Am Acad Child Adolesc Psychiatry 46:969–978, 2007 [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Vancampfort D, De Herdt A, Yu W, De Hert M: Is the prevalence of metabolic syndrome and metabolic abnormalities increased in early schizophrenia? A comparative meta-analysis of first episode, untreated and treated patients. Schizophr Bull 39:295–305, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi S-A, Solhi M, Mohammadi M-R, Akhondzadeh S: Melatonin for reducing weight gain following administration of atypical antipsychotic olanzapine for adolescents with bipolar disorder: A randomized, double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol 27:440–444, 2017 [DOI] [PubMed] [Google Scholar]
- Paeratakul S, Lovejoy J, Ryan D, Bray G: The relation of gender, race and socioeconomic status to obesity and obesity comorbidities in a sample of us adults. Int J Obes 26:1205, 2002 [DOI] [PubMed] [Google Scholar]
- Park M, Falconer C, Viner R, Kinra S: The impact of childhood obesity on morbidity and mortality in adulthood: A systematic review. Obes Rev 13:985–1000, 2012 [DOI] [PubMed] [Google Scholar]
- Patel A, Chan W, Aparasu RR, Ochoa-Perez M, Sherer JT, Medhekar R, Chen H: Effect of psychopharmacotherapy on body mass index among children and adolescents with bipolar disorders. J Child Adolesc Psychopharmacol 27:349–358, 2017 [DOI] [PubMed] [Google Scholar]
- Quintana H, Wilson MS, 2nd, Purnell W, Layman AK, Mercante D: An open-label study of olanzapine in children and adolescents with schizophrenia. J Psychiatr Pract 13:86–96, 2007 [DOI] [PubMed] [Google Scholar]
- Ratzoni G, Gothelf D, Brand-Gothelf A, Reidman J, Kikinzon L, Gal G, Phillip M, Apter A, Weizman R: Weight gain associated with olanzapine and risperidone in adolescent patients: A comparative prospective study. J Am Acad Child Adolesc Psychiatry 41:337–343, 2002 [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Zhang Z-J, Zhang X-B: Association of antipsychotic drug-induced weight gain with a 5-HT2C receptor gene polymorphism. Lancet 359:2086–2087, 2002 [DOI] [PubMed] [Google Scholar]
- Sachs GS, Grossman F, Ghaemi SN, Okamoto A, Bowden CL: Combination of a mood stabilizer with risperidone or haloperidol for treatment of acute mania: A double-blind, placebo-controlled comparison of efficacy and safety. Am J Psychiatry 159:1146–1154, 2002 [DOI] [PubMed] [Google Scholar]
- Saddichha S, Ameen S, Akhtar S: Predictors of antipsychotic-induced weight gain in first-episode psychosis: Conclusions from a randomized, double-blind, controlled prospective study of olanzapine, risperidone, and haloperidol. J Clin Psychopharmacol 28:27–31, 2008 [DOI] [PubMed] [Google Scholar]
- Safer DJ: A comparison of risperidone-induced weight gain across the age span. J Clin Psychopharmacol 24:429–436, 2004 [DOI] [PubMed] [Google Scholar]
- Schimmelmann BG, Mehler-Wex C, Lambert M, Schulze-zur-Wiesch C, Koch E, Flechtner HH, Gierow B, Maier J, Meyer E, Schulte-Markwort M: A prospective 12-week study of quetiapine in adolescents with schizophrenia spectrum disorders. J Child Adolesc Psychopharmacol 17:768–778, 2007 [DOI] [PubMed] [Google Scholar]
- Sikich L, Frazier JA, McClellan J, Findling RL, Vitiello B, Louise Ritz M, Ambler D, Puglia M, Maloney AE, Michael E: Double-blind comparison of first-and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: Findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Am J Psychiatry 165:1420–1431, 2008 [DOI] [PubMed] [Google Scholar]
- Stahl S, Mignon L, Meyer J: Which comes first: Atypical antipsychotic treatment or cardiometabolic risk? Acta Psychiatr Scand 119:171–179, 2009 [DOI] [PubMed] [Google Scholar]
- Taylor J, Xu Y, Li F, Shaw M, Dziura J, Caprio S, Tamborlane W, Nowicka P, Savoye M: Psychosocial predictors and moderators of weight management programme outcomes in ethnically diverse obese youth. Pediatr Obes 12:453–461, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Jakubovski E, Bloch MH: Predictors of anxiety recurrence in the coordinated anxiety learning and management (CALM) trial. J Psychiatr Res 65:154–165, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Lebowitz ER, Jakubovski E, Coughlin CG, Silverman WK, Bloch MH: Monotherapy insufficient in severe anxiety? Predictors and moderators in the child/adolescent anxiety multimodal study. J Clin Child Adolesc Psychol 47:266–281, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Jr, Belay B, Park S, Onufrak S, Dietz W: Association of church-sponsored activity participation and prevalence of overweight and obesity in African American Protestants, National Survey of American Life, 2001–2003. Ethn Dis 23:322–328, 2012 [PMC free article] [PubMed] [Google Scholar]
- Teff KL, Rickels MR, Grudziak J, Fuller C, Nguyen H-L, Rickels K: Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes 62:3232–3240, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D, De Hert M, Stubbs B, Ward PB, Rosenbaum S, Soundy A, Probst M: Negative symptoms are associated with lower autonomous motivation towards physical activity in people with schizophrenia. Compr Psychiatry 56:128–132, 2015 [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang Q: Are American children and adolescents of low socioeconomic status at increased risk of obesity? Changes in the association between overweight and family income between 1971 and 2002. Am J Clin Nutr 84:707–716, 2006 [DOI] [PubMed] [Google Scholar]
- Wu X, Huang Z, Wu R, Zhong Z, Wei Q, Wang H, Diao F, Wang J, Zheng L, Zhao J: The comparison of glycometabolism parameters and lipid profiles between drug-naive, first-episode schizophrenia patients and healthy controls. Schizophr Res 150:157–162, 2013 [DOI] [PubMed] [Google Scholar]
- Yarlagadda A, Taylor JH, Jr, Hampe CS, Alfson E, Clayton AH: Gad65 antibodies, chronic psychosis, and type 2 diabetes mellitus. Innov Clin Neurosci 8:34, 2011 [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Hoblyn J, Sheikh J, Tinklenberg JR, Noda A, O'Hara R, Fenn C, Mumenthaler MS, Friedman L, Kraemer HC: Age and disease severity predict choice of atypical neuroleptic: A signal detection approach to physicians' prescribing decisions. J Psychiatr Res 37:535–538, 2003 [DOI] [PubMed] [Google Scholar]
- Zhang J-P, Lencz T, Zhang RX, Nitta M, Maayan L, John M, Robinson DG, Fleischhacker WW, Kahn RS, Ophoff RA: Pharmacogenetic associations of antipsychotic drug-related weight gain: A systematic review and meta-analysis. Schizophr Bull 42:1418–1437, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U, Kraus T, Himmerich H, Schuld A, Pollmächer T: Epidemiology, implications and mechanisms underlying drug-induced weight gain in psychiatric patients. J Psychiatr Res 37:193–220, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.