Abstract
Neuroimaging studies suggest that schizophrenia is characterized by disturbances in oscillatory activity, although at present it remains unclear whether these neural abnormalities are driven by dimensions of symptomatology. Examining different subgroups of patients based on their symptomatology is thus very informative in understanding the role of neural oscillation patterns in schizophrenia. In the present study we examined whether neural oscillations in the delta, theta, alpha, beta and gamma bands correlate with positive and negative symptoms in individuals with schizophrenia (SZ) during rest. Resting-state brain activity of 39 SZ and 25 neurotypical controls was recorded using magnetoencephalography. Patients were categorized based on the severity of their positive and negative symptoms. Spectral analyses of beamformer data revealed that patients high in positive symptoms showed widespread low alpha power, and alpha power was negatively correlated with positive symptoms. In contrast, patients high in negative symptoms showed greater beta power in left hemisphere regions than those low in negative symptoms, and beta power was positively correlated with negative symptoms. We further discuss these findings and suggest that different neural mechanisms may underlie positive and negative symptoms in schizophrenia.
Keywords: Schizophrenia, Resting-state, MEG, Neural oscillations, Negative symptoms, Positive symptoms, Alpha band, Beta band
Highlights
-
•
Neural oscillations in schizophrenics' resting states were investigated using MEG
-
•
Schizophrenics high in positive symptoms showed widespread low alpha power
-
•
Alpha power was negatively correlated with positive symptoms
-
•
Schizophrenics high in negative symptoms showed stronger beta power in left regions
-
•
Beta power was positively correlated with negative symptoms
1. Introduction
While extensive research has attempted to identify specific brain regions related to the core symptoms of schizophrenia (e.g., Crow et al., 1989; Huang et al., 2010; Selemon et al., 1995) the neural mechanisms underlying the disorder remain largely unknown. Today it appears that any attempts to conceptualize schizophrenia by means of source localization alone are likely to prove deficient. Current theories emphasize the involvement of numerous brain regions in schizophrenia, assigning a crucial role to the patterns of synchronization (i.e., rhythmic fluctuations of activity) in or between these regions in understanding the psychopathology of the disorder (e.g., Friston, 1999; Phillips and Silverstein, 2003; Uhlhaas and Singer, 2010).
The mechanism through which brain regions synchronize is neural oscillations (Buzsáki, 2006). Recording changes in neural oscillations relies on fine-grained temporal resolution (which cannot be captured with fMRI) and provides new understanding of the functionalities of brain activity across multiple frequency bands (Buzsáki, 2006). In recent years a growing body of research in schizophrenia has relied on this emerging approach (for a review see, Uhlhaas et al., 2017) and has included the use of resting state magnetoencephalography (MEG; for a review see, Alamian et al., 2017). Findings from these studies suggest that the pathophysiology of schizophrenia can be largely explained by neural abnormalities in synchronized oscillatory activity (Uhlhaas and Singer, 2010). The most consistent finding across EEG studies on resting-state neural-oscillation patterns in individuals with schizophrenia is a pattern of increased slow waves (delta and theta), decreased alpha, and increased beta power (Boutros et al., 2008). However, many studies have been unable to replicate this pattern of results (e.g., Hinkley et al., 2011; Ikezawa et al., 2011; Narayanan et al., 2014; Sun et al., 2014). As suggested by Boutros and colleagues (Boutros et al., 2008), this heterogeneity across studies might be due to differences in spatial distribution, technique, or sample characteristics.
Another potential factor that might be contributing to the variability in previous studies' results is differences in patients' clinical presentation. In each of the above-cited studies individuals with schizophrenia were treated as a unitary group. We believe that separately examining different subgroups of patients based on their symptomatology would be very informative in understanding the role of neural oscillation patterns in schizophrenia. Separating individuals with schizophrenia according to the severity of their positive and negative symptoms may inform both research and treatment as those two subgroups have been shown to differ in many aspects including disease prognosis, cognitive, pharmacological, and psychophysiological features (e.g., Andreasen, 1985; Harvey et al., 2016). For example, the amplitude of the P300 event-related potential (ERP) component was found to be negatively correlated with negative symptoms but not with positive symptoms (Pfefferbaum, Ford, White, & Roth, 1989; Yefet, Goldstein, Rabany, & Levkovitz, 2015), whereas N400 anomalies (i.e., amplitude reductions) were shown to correlate with positive but not with negative symptoms (Kostova, Passerieux, Laurent, & Hardy-Baylé, 2005). Thus the focus of the current study was to investigate whether the oscillatory brain activity in individuals with schizophrenia is dependent on the severity of their positive and negative symptoms.
This study employed MEG. Unlike EEG, which has been used in most studies of neural oscillations, MEG records the magnetic fields around the head. Because these fields are less influenced by the conductivity of biological substances, such as the skull, they are relatively undistorted and thus reflect more accurately the original cortical brain activity. As a result, in addition to having high temporal resolution (and thus high frequency resolution), MEG enables us to localize the cortical source of neural oscillations much more precisely than EEG (Baillet, 2017) and this in itself is helpful for resolving the inconsistencies across previous SZ resting state studies.
Only a handful of studies have utilized MEG to link specific abnormalities in neural oscillations to positive and negative symptoms in schizophrenia. Fehr and colleagues used MEG to explore relationships between delta and theta resting-state activity and symptom severity (Fehr et al., 2003). It was found that individuals with schizophrenia had less delta activity and more theta activity in the temporal and parietal areas. Delta band activity (across all brain areas) was negatively correlated with positive symptoms. In another study that focused on the alpha band (Hinkley et al., 2011), negative correlations were found between positive symptoms and alpha coherence in the left inferior parietal lobe and the right anterior insula; and between negative symptoms and alpha coherence in the left prefrontal cortex. Nevertheless, when alpha power was examined no correlations with symptoms were found (Ikezawa et al., 2011). A more recent study investigating the default mode network found a positive correlation between positive symptoms and gamma power activity (Kim et al., 2014).
Results from the above studies do not suggest a clear pattern regarding the relationship between positive and negative symptoms and neural oscillations. Our goal was to determine whether schizophrenia patients grouped according to their positive and negative symptoms can be dissociated at the level of resting-state neural oscillatory activity and to further localize this dissociation in the brain using MEG. Unlike previous studies, we took a broader approach instead of limiting the analysis to a specific frequency band or brain region/network.
2. Method
2.1. Participants
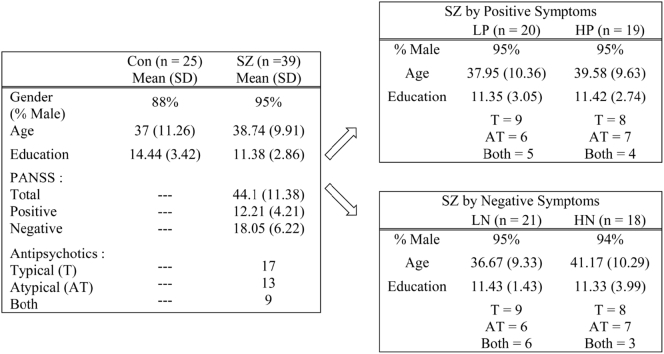
Forty-one subjects with schizophrenia (SZ) and 25 neurotypical controls (Con) were recruited into the study. All participants were right-handed, above the age of 18, capable and willing to provide informed consent, had normal or corrected to normal vision, no brain damage or history of seizures and no substance abuse/dependence in the past three months. Two patients were excluded from the analysis, one because of a dental implant causing excessive artifacts in his data, and another because of technical issues (data from reference coils were not recorded). Thus, the final sample consisted of 39 SZ and 25 Con.
Control participants were recruited from the community. They had no personal history of mental disorders and no first-degree relatives with schizophrenia. SZ outpatients and inpatients were recruited from Shalvata and Sha'ar Menashe Mental Health Centers in Israel. They met DSM-IV-TR criteria for the disorder (American Psychiatric Association, 2000) and were diagnosed with the SCID-I by trained psychiatrists who also evaluated the severity of their positive and negative symptoms using the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987). Each SZ subject was rated from 1 to 7 on 30 different symptoms and given a score on the PANSS Positive Scale and a separate score on the PANSS Negative Scale. Both scales consisted of 7 items each, with scores ranging from 7 to 49. Patients were identified as having either high or low positive symptoms according to the median of the overall severity of positive symptoms (Median = 11, Range = 7–32) where any participant scoring below or equal to the median was characterized as having low positive symptoms (LP) and any participant scoring above the median was characterized as having high positive symptoms (HP). Patients were also categorized as having low (LN) or high (HN) negative symptoms based on the median of the overall severity of negative symptoms (Median = 17, Range = 7–25), resulting in 20 LP and 19 HP participants or 21 LN and 18 HN participants. Positive and negative symptoms scores were not significantly correlated, r = −0.164, p = .319. In addition, all patients were medicated: 44% of the sample taking atypical antipsychotic medications, 33% taking typical antipsychotic medications, and 23% taking both types. There were no differences in medication distribution between the groups (χ2 = 0.221, p = .895, χ2 = 0.91, p = .634; when divided according to positive and negative symptoms respectively).
The research was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans, and was approved by the ethical boards of both Shalvata and Sha'ar Menashe Hospitals and Bar Ilan University. All participants received financial compensation for their participation in the study.
Con – Controls; SZ – Schizophrenia patients; LP – SZ with low levels of positive symptoms; HP – SZ with high levels of positive symptoms; LN – SZ with low levels of negative symptoms; HN – SZ with high levels of negative symptoms; T – Typical antipsychotic medications; AT – Atypical antipsychotic medications; BOTH – Typical and atypical antipsychotic medications.
2.2. Experimental procedure and recording
Spontaneous brain activity was measured using MEG while participants rested in supine position with open eyes for two minutes. Ongoing brain activity was recorded using a whole-head 248-channel magnetometer array (4-D Neuroimaging, Magnes 3600 WH) inside a magnetically shielded room. Data were sampled online at 1017.23 Hz with a bandpass of 0.1 to 400 Hz. Reference coils located approximately 30 cm above the head oriented by the x, y, and z axes were used to remove environmental noise. Five coils were attached to the participant's scalp for recording the head position relative to the 248 sensor-array. External noise (e.g., power-line, mechanical vibrations) and heart beat artifacts were removed from the data using a predesigned algorithm for that purpose (Tal & Abeles, 2013). Spectral analysis was performed using MATLAB R2012b (MathWorks, Natick, MA, USA) and the FieldTrip toolbox (Oostenveld et al., 2011). Data were segmented into 2000 ms epochs (with 1000 ms overlap between neighboring epochs). Trials containing muscle artifacts and signal jumps were rejected from further analysis by visual inspection, resulting in an average of 99.98 out of 120 trials in total (83.32%) accepted for final analysis for each participant. The number of trials rejected did not differ between groups (for SZ: M = 22.162, SD = 19.008; for Con: M = 17.074, SD = 11.868; t(62) = 1.226, p = .225). Data were then filtered in the 1–100 Hz range with 10 s padding. Finally, independent component analysis was applied in order to clean eye blinks, eye movements, and leftover heartbeats by visually identifying such components and reducing them from the data.
Slepian multitapers (Bell et al., 1993) were applied with a frequency smoothing of 1 Hz to each epoch of the 248-sensor data in order to calculate the Fast Fourier Transform in the frequency range of 1–70 Hz. We then calculated the averaged power across all sensors in five frequency bands: delta (2–4 Hz), theta (5–8 Hz), alpha (9–12 Hz), beta (13–30 Hz) and gamma (31–70 Hz). Then, source localization for the significant frequency bands (those differentiating between participants) was calculated by computing the cross-spectral density matrix between all MEG sensor pairs from the Fourier transforms of the tapered data epochs. Spatial filters were constructed for each grid location, based on the identified frequency bin, and the Fourier transforms of the tapered data epochs were projected through the spatial filters. To facilitate analysis at the source level, a single shell brain model was built for each participant based on a template brain (Montreal Neurological Institute). The template was modified to fit each participant's digitized head shape using SPM8 (Wellcome Department of Imaging Neuroscience University College London, www.fil.ion.ucl.ac.uk). The head shape was manually digitized (Polhemus Fastrak digitizer) and the participant's brain volume was then divided into a regular grid. The grid positions were obtained by a linear transformation of the grid positions in a canonical 1 cm grid. This procedure facilitates the group analysis because no spatial interpolation of the volumes of reconstructed activity is required. For each grid position, spatial filters (Gross et al., 2001) were reconstructed with the aim of optimally augmenting activity from the location of interest, while suppressing noise.
2.3. Statistics
As a first step omnibus analysis, we performed a sensor-level analysis. This enabled us to narrow down the range of frequency bands for further cluster based regression and source localization analyses to the specific frequency bands proven to significantly differentiate between subgroups of SZ and Con. To this end, two separate mixed repeated measures ANOVAs were conducted on the averaged power across all sensors with group as the between subject variable and frequency band (delta, theta, alpha, beta, and gamma) as the within subject variable. One ANOVA was conducted when SZ participants were divided into two subgroups according to their positive symptom scores – LP and HP vs. Con. A second ANOVA was conducted when SZ participants were divided according to their negative symptom scores – LN and HN vs. Con. Significant interactions were decomposed using a series of one-way ANOVAs with group as a between subject variable for each frequency band. To follow up on significant group main effects, a pairwise comparison between groups was conducted with a Bonferroni correction (ps < 0.017). All sensor level analyses were conducted using SPSS (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Crop).
In order to demonstrate the importance of treating schizophrenia as a multi-dimensional phenomenon, we repeated the analysis without the division of SZ participants according to their symptoms. To this end, an additional separate mixed repeated measures ANOVA was conducted on the averaged power across all sensors with group (SZ vs. Con) as the between subject variable and frequency band (delta, theta, alpha, beta, and gamma) as the within subject variable.
Source statistics were implemented in the FieldTrip toolbox (Oostenveld et al., 2011) for the frequency bands found significant at the sensor level by applying a nonparametric cluster-based procedure (Maris and Oostenveld, 2007). This permutational approach takes into account the cross-participant variance, which is the basis for the width of the randomization distribution, does not make any assumptions about the underlying distribution, and is unaffected by partial dependence between neighboring pixels. We randomly assigned each participant to one of the three groups (Con, LP/LN or HP/HN). This was reiterated 1000 times to obtain the randomization distribution for the group-level statistic. For each randomization, only the maximal significant cluster-level test statistic across all clusters was retained and placed into a maximum cluster-level test statistic histogram. It is important to note that for a cluster to be considered significant all voxels within the cluster must have a p-value < .05. For each cluster from the observed data we then determined the fraction of the maximum cluster-level test statistic histogram that was greater than the cluster-level test statistic from the observed cluster. The fraction was retained and divided by 1000. The proportion of values in the randomization distribution exceeding the test statistic defines the Monte Carlo significance probability, also called a p-value (Nichols and Holmes, 2002; Maris and Oostenveld, 2007). This cluster-based procedure allowed us to obtain a correction for multiple comparisons in all sensor and source analyses. For the significant clusters, a Bonferroni post-hoc analysis was then conducted by retrieving the mean activity of each significant cluster from each participant.
Following the main effects found between groups, one-tailed cluster-based regression analyses were performed within the patient group between PANSS positive and negative scores and sensor and source activity using the FieldTrip toolbox (Oostenveld et al., 2011). This approach was aimed at identifying the specific sensors and brain areas correlated with symptom severity.
3. Results
3.1. Demographic characteristics
There were no significant differences in age (p = .518) or gender (p = .371) between the two groups (Table 1). As expected, Con participants had a significantly higher educational level than SZ participants, t(62) = −3.86, p < .001.
Table 1.
Demographic characteristics.
3.2. Sensor level results
3.2.1. Division of SZ according to positive symptoms
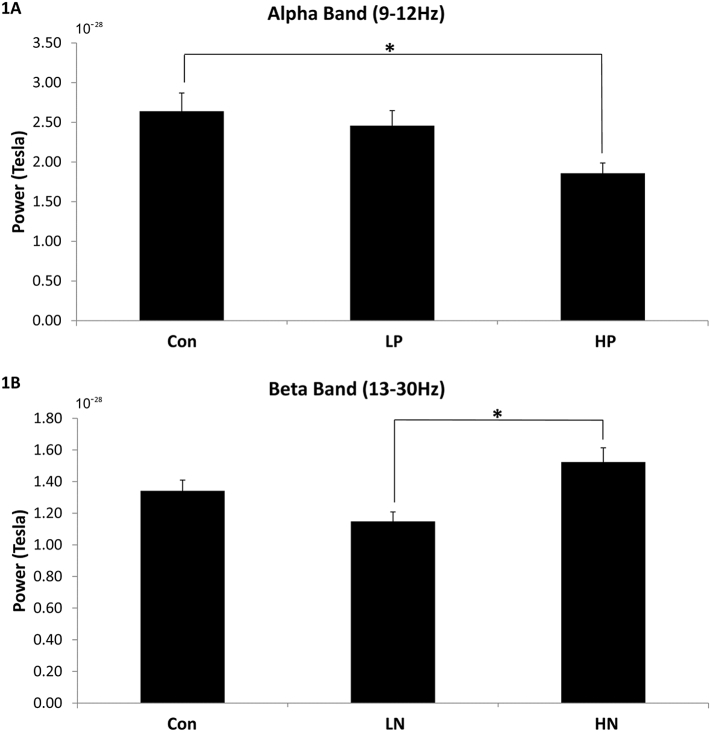
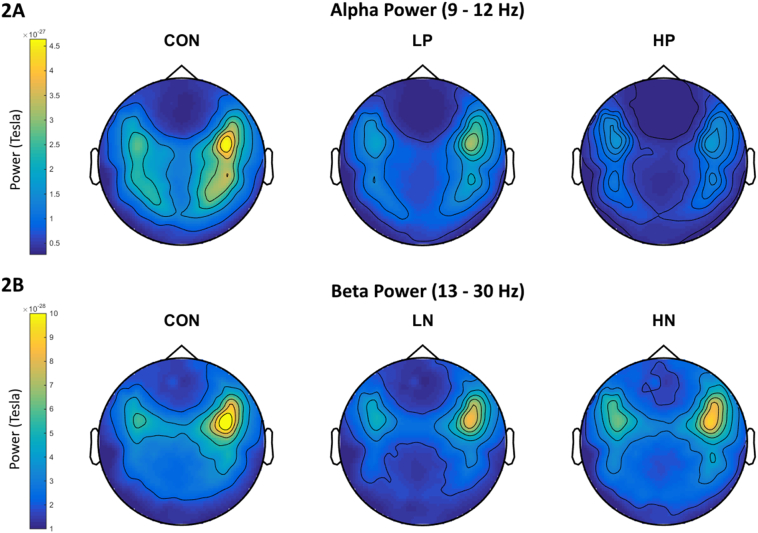
A main effect of frequency band was found, F(4, 63) = 125.977, p < .001, η2 = 0.674. More importantly, there was a significant interaction between group and frequency band, F(8, 63) = 1.992, p = .048, η2 = 0.061. Separate one-way ANOVAs revealed a significant main effect only for the alpha band, F(2, 63) = 4.102, p = .021, η2 = 0.119. Bonferroni post-hoc analysis (ps < 0.017) revealed that HP (M = 1.858*10−28, SD = 0.569*10−28) had lower levels of alpha power relative to Con (M = 2.639*10−28, SD = 1.152*10−28), t(42) = 2.708, p = .01, Cohen's d = 0.859, but not relative to LP (M = 2.458*10−28, SD = 0.850*10−28); see Fig. 1A for bar graph and Fig. 2A for topoplot image.
Fig. 1.
Power level for alpha band (1A) and beta band (1B). Error bars show standard errors. Con – controls; LP – SZ with low positive symptoms; HP – SZ with high positive symptoms; LN – SZ with low negative symptoms; HN – SZ with high negative symptoms. * p < .017.
Fig. 2.
Topoplots of alpha power (2A) for Con, LP, and HP; and of beta power (2B) for Con, LN, and HN. Colors represent power levels.
3.2.2. Division of SZ according to negative symptoms
A main effect of frequency band was found, F(4, 63) = 126.245, p < .001, η2 = 0.674. There was also a significant interaction between group and frequency band, F(8, 63) = 1.987, p = .049, η2 = 0.061. Separate one-way ANOVAs revealed a significant main effect only for the beta band, F(2, 63) = 6.261, p = .003, η2 = 0.170. Bonferroni post-hoc analysis (ps < 0.017) revealed that HN (M = 1.524*10−28, SD = 0.381*10−28) had higher levels of beta power compared to LN (M = 1.152*10–28, SD = 0.267*10−28), t(37) = 3.62, p = .001, Cohen's d = 1.131, but not compared to Con (M = 1.346*10−28, SD = 0.346*10−28); see Fig. 1B for bar graph and Fig. 2B for topoplot image.
3.2.3. No division of SZ
A main effect of frequency band was found, F(4, 63) = 123.673, p < .001, η2 = 0.666. Bonferroni post-hoc analysis (ps < 0.005) revealed that participants had lower levels of theta power than delta power, and that participants had lower levels of beta and gamma powers than delta, theta and alpha powers. In addition, gamma power levels were found to be lower than beta power levels (for a full description of Bonferroni post-hoc analysis see Appendix A). More importantly, the main effect of group (p = .582) and group by frequency band interaction (p = .11) were not significant.
3.3. Source level results
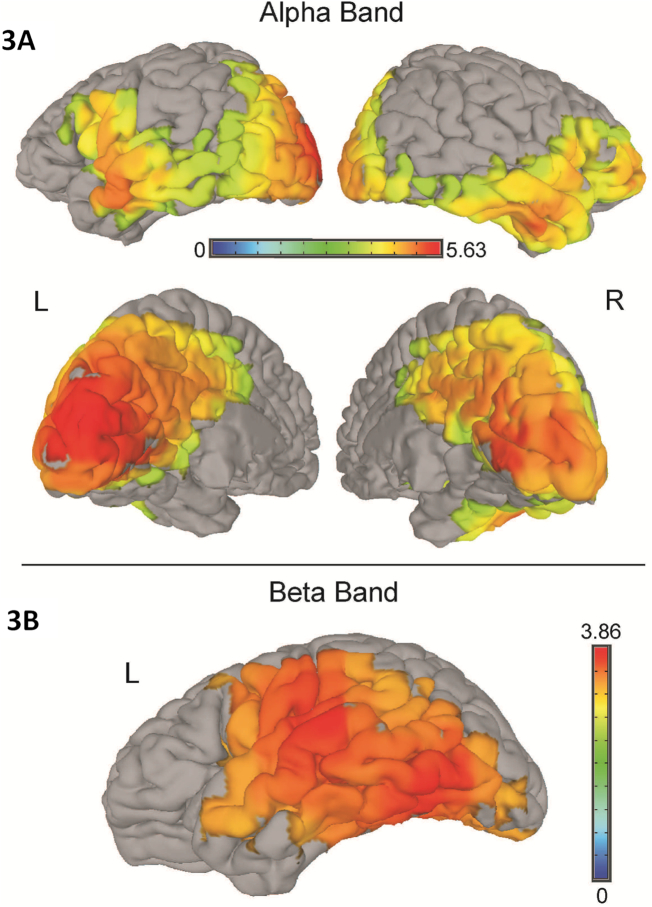
To follow up on the sensor-level results, two cluster-based analyses were conducted: one between Con, LP and HP in the alpha band and one between Con, LN and HN in the beta band. In the alpha band, a significant cluster of 571 voxels was found, F(2, 63) = 4.336, p = .017, η2 = 0.124, covering large areas across the brain (see Table 2 and Fig. 3A). In the beta band, a significant cluster of 225 voxels was found, F(2, 36) = 3.925, p = .025, η2 = 0.114, in the left hemisphere only (see Table 2 and Fig. 3B).
Table 2.
Size and location of significant clusters.
| Source Alpha (571 voxels) | Source Beta (225 voxels) | ||||
|---|---|---|---|---|---|
| % | Hem | Area | % | Hem | Area |
| 4.7 | L | Precuneus | 12.4 | L | Superior Temporal Gyrus |
| 4.6 | L | Middle Temporal Gyrus | 10.9 | L | Middle Temporal Gyrus |
| 3.7 | L | Cuneus | 5.5 | L | Parahippocampal Gyrus |
| 3.7 | R | Inferior Frontal Gyrus | 5.4 | L | Insula |
| 3.6 | R | Cuneus | 4.7 | L | Fusiform Gyrus |
| 3.4 | R | Precuneus | 4 | L | Precentral Gyrus |
| 3.3 | L | Cingulate Gyrus | 3.5 | L | Lingual Gyrus |
| 2.9 | L | Middle Occipital Gyrus | 3.2 | L | Inferior Temporal Gyrus |
| 2.6 | L | Lingual Gyrus | 3 | L | Culmen |
| 2.4 | R | Middle Temporal Gyrus | 2.6 | L | Postcentral Gyrus |
| 2.4 | R | Superior Temporal Gyrus | 2.4 | L | Declive |
| 2.4 | R | Middle Frontal Gyrus | 2.4 | L | Inferior Parietal Lobule |
| 2.2 | L | Superior Temporal Gyrus | 2.3 | L | Middle Occipital Gyrus |
| 2.1 | R | Lingual Gyrus | 1.6 | L | Inferior Frontal Gyrus |
| 1.8 | R | Middle Occipital Gyrus | |||
| 1.6 | L | Precentral Gyrus | |||
| 1.5 | R | Cingulate Gyrus | |||
| 1.3 | L | Posterior Cingulate | |||
| 1.3 | L | Inferior Frontal Gyrus | |||
| 1.2 | R | Inferior Temporal Gyrus | |||
| 1.1 | R | Fusiform Gyrus | |||
| 1.1 | L | Insula | |||
| 1.1 | L | Inferior Temporal Gyrus | |||
| 1 | R | Posterior Cingulate | |||
| 1 | R | Insula | |||
R – Right hemisphere; L – Left hemisphere. % - percentage from total number of voxels in cluster. Areas with <1% are not reported.
Fig. 3.
Significant clusters in the alpha band (2A) and beta band (2B). Colors represent F-values. L – left hemisphere; R – right hemisphere.
For the alpha band cluster, post-hoc analysis (ps < 0.017) revealed that Con participants (M = 43*10−12, SD = 117.89*10−12) had more brain activity relative to HP participants (M = 3.48*10−12, SD = 5.21*10−12), t(42) = 3.036, p = .004, Cohen's d = 0.474, but not relative to LP participants (M = 12.2*10−12, SD = 19.69*10−12). For the beta band cluster, post-hoc analysis revealed that HN participants (M = 5.07*10−12, SD = 6.04*10−12) had more brain activity than LN participants (M = 1.34*10−12, SD = 3.4*10−12), t(37) = 2.481, p = .017, Cohen's d = 0.762. A statistical trend was found between HN and Con (p = .075, corrected), showing that HN also had more brain activity than Con (M = 1.9*10−12, SD = 3.86*10−12).
3.4. Associations between symptoms and source activity
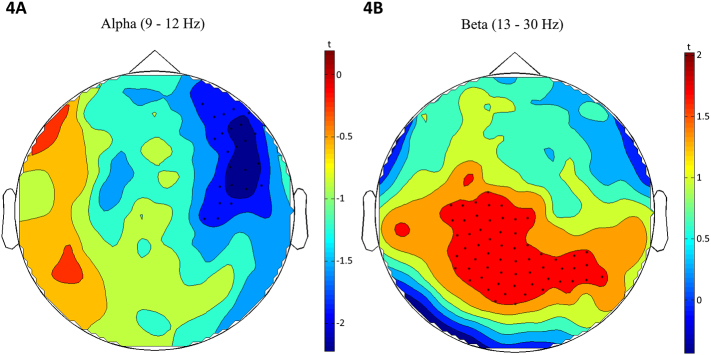
A significant negative cluster over the right frontal and medial sensors was found in the cluster-based regression analysis, t(37) = −1.788, p = .041, β = −0.282 (see Fig. 4A), indicating that in SZ, higher positive symptoms are related to lower levels of alpha power in these sensors. In addition, a significant positive cluster over posterior central sensors was found in the cluster-based regression analysis, t(37) = 1.967, p = .028, β = 0.308 (see Fig. 4B), indicating that in SZ, higher negative symptoms are related to higher levels of beta power in these sensors. Similarly, a significant positive cluster, t(37) = 2.115, p = .021, β = 0.328, was found in broad regions across the right and left temporal, frontal and parietal lobes (see Table 3 for a full list of locations).
Fig. 4.
Topoplots of cluster-based regression analysis results for alpha band (4A) and beta band (4B). Colors represent t-values.
Table 3.
Size and location of significant cluster.
| Source Beta (1127 voxels) | ||||||
|---|---|---|---|---|---|---|
| % | Hem | Area | % | Hem | Area | |
| 3 | L | Superior Temporal Gyrus | 1.3 | R | Insula | |
| 2.9 | L | Middle Temporal Gyrus | 1.3 | L | Insula | |
| 2.8 | L | Middle Frontal Gyrus | 1.3 | L | Cuneus | |
| 2.6 | R | Superior Temporal Gyrus | 1.3 | L | Lingual Gyrus | |
| 2.5 | L | Inferior Frontal Gyrus | 1.2 | R | Inferior Parietal Lobule | |
| 2.3 | R | Cingulate Gyrus | 1.2 | R | Lingual Gyrus | |
| 2.2 | L | Cingulate Gyrus | 1.2 | R | Postcentral Gyrus | |
| 2.1 | L | Precentral Gyrus | 1.2 | L | Middle Occipital Gyrus | |
| 1.9 | R | Precentral Gyrus | 1.2 | L | Parahippocampal Gyrus | |
| 1.8 | R | Middle Temporal Gyrus | 1.2 | R | Parahippocampal Gyrus | |
| 1.7 | L | Precuneus | 1.1 | L | Medial Frontal Gyrus | |
| 1.7 | L | Inferior Parietal Lobule | 1.1 | R | Middle Frontal Gyrus | |
| 1.7 | R | Inferior Frontal Gyrus | 1.1 | R | Medial Frontal Gyrus | |
| 1.5 | R | Precuneus | 1 | L | Superior Frontal Gyrus | |
| 1.4 | L | Postcentral Gyrus | 1 | L | Fusiform Gyrus | |
R – Right hemisphere; L – Left hemisphere. % - percentage from total number of voxels in cluster. Areas with <1% are not reported.
In addition, for each significant cluster a Spearman correlation coefficient table was produced between brain activity in the cluster and PANSS positive or negative symptom scores. The significant cluster in the alpha band in the sensor level was found to be correlated with the following positive items: P1 - delusions (r = −0.284, p = .04), P2 - conceptual disorganization (r = −0.306, p = .029), P4 - excitement (r = −0.361, p = .012), and P6 - suspiciousness/persecution (r = −0.284, p = .04). For the beta band, both in the sensor and source level, no correlations were found between brain activity in the clusters and specific items of the PANSS. None of these correlations survived correction for multiple comparisons (False Discovery Rate correction) and thus, we refrain from discussing them separately.
Additional analyses were conducted to rule out education and medication as the underlying cause for the various differences found. To rule out education as the underlying cause we ran the same analyses again with education as a covariate. No interaction between group and education reached significance. Furthermore, the pattern of results remained the same (i.e., LP had less alpha power than Con and HN had more beta power than LN, both in the sensor and source data). To rule out medication we compared alpha and beta powers in the sensor and source levels between patients taking typical, atypical or both typical and atypical antipsychotic drugs. No significant differences were found (ps > 0.155). In addition, no significant correlations were found between any of the main effects and illness duration (M = 17.87, SD = 10.33; ps > 0.12).
4. Discussion
Comparing the SZ to the CON group, we found a significant main effect of frequency band but no significant group differences or group by frequency band interaction. However, when the SZ group was divided into subgroups based on the severity of positive and negative symptoms, a different pattern of results emerged. More specifically, HP had less alpha power than Con, whereas HN had more beta power than LN. Although HP and LP did not differ in their alpha power, correlations within the patient group revealed that alpha power was negatively correlated with positive symptoms (at the sensor level), whereas beta power was positively correlated with negative symptoms (at the sensor and source level). The fact that the effects disappear when SZ is treated as a unitary phenomenon demonstrates most clearly the main premise of this study – the necessity to differentiate patients based on their symptomatology. Moreover, the dissociation found between alpha and beta bands in relation to positive and negative symptoms suggests that these two dimensions are related to distinct neural mechanisms.
A recent exhaustive review on MEG during rest (Alamian et al., 2017) found that only a few such studies have been conducted, which probably contributes to the inconsistencies between findings. For instance, some studies reported decreased alpha in SZ (Sponheim, Clementz, Iacono, & Beiser, 1994) while another reported increased alpha (Northoff and Duncan, 2016). Discrepancies can also be found in the beta band (for a review see, Boutros et al., 2008). There are theories about the roles of alpha rhythm (e.g., functional inhibition; Jensen and Mazaheri, 2010), beta rhythm (e.g., the maintenance of the current sensorimotor or cognitive state; Engel and Fries, 2010), and there are studies that have linked cognitive impairments in schizophrenia to beta band dysregulation (e.g., a reverse pattern of beta power activity during a salience task or reduced beta band phase synchrony during face detection; Liddle et al., 2016; Uhlhaas et al., 2006).
Given the scarcity of prior evidence on the functioning of alpha and beta rhythms in SZ, as well as the problem of generalizing from task- to rest-studies, we feel it would be premature at this stage to interpret the functional meaning of the rhythmic effects found. The fact we used MEG is important because of its advantage in localizing spectral activity (Baillet, 2017), a crucial issue because of the differential spatial distribution of power changes reported in EEG studies thus far (Maran, Grent-‘t-Jong, & Uhlhaas, 2016). Importantly, any single neuroimaging modality is imperfect and limited, and in that sense, comparing between research utilizing MEG, EEG and fMRI increases the richness of the collected information on both the signal of neural activity and brain structure, and improves our understanding of the underlying neural mechanisms in SZ. Our findings can also provide another outlook on the inconsistencies in the literature on schizophrenia and neural oscillations during rest (for a review see, Alamian et al., 2017; Boutros et al., 2008). Few studies have considered the two dimensions of symptomology (positive and negative) as a factor for characterizing neural oscillations in individuals with schizophrenia (Keil, Roa Romero, Balz, Henjes, & Senkowski, 2016), and none has done so with resting-state recordings. Thus the integration of symptomatology could be implemented in future studies in order to obtain a more consistent outlook on the neural oscillations underlying rest in SZ.
Despite the difficulties in interpreting the functional meaning of the rhythmic alterations observed in SZ rest studies, the findings do support the consistent observation of deficient oscillatory patterns in SZ during rest (Alamian et al., 2017). This can be found both in specific regions as well as between regions consisting of the frontal and temporo-parietal lobes, default mode network (DMN) and other resting-state networks. Perhaps one of the only MEG studies addressing DMN in SZ with frequency bands similar to those used here was conducted by Kim and colleagues, who reported increased activity in alpha (and other bands) in the PCC node of the DMN in SZ (Kim et al., 2014). It would not be straightforward to compare their results to the results from the present study, mainly because here we did not look specifically at DMN activity (nor did we test it compared to an active task), and because Kim and colleagues' findings did not apply the symptomatology approach used here. Likewise, Kim and colleagues' study relied on data collected from participants in a sitting rather than supine position (as was done here). Therefore, it is hard to compare results from those studies to ours, as there is evidence showing that brain resting-state patterns can differ as a function of the position participants are scanned in (Thibault, Lifshitz, & Raz, 2016). Altogether, our study is in line with the literature on deficient oscillatory patterns in SZ during rest, further contributes to the richness of data, and offers new approaches for interpreting it.
A major limitation of this study is the psychometric approach we employed to compare different subgroups of schizophrenia. By categorizing patients based on medians on the PANSS, we could only examine each clinical dimension independently. Thus, there is a possibility that, for example, a high level of positive symptoms in predominantly positive symptom patients may result in different resting-state brain patterns than in patients exhibiting both positive and negative symptoms. Relatedly, some individuals with schizophrenia display an unstable clinical picture – switching from one set of symptoms to another (i.e., positive or negative) along the time course of the disease (Andreasen, Berrios, Bogerts, & Brenner, 2012). This raises the question of whether the relationship between different symptoms and resting-state brain patterns is the same regardless of whether the clinical presentation is stable or not. Another limitation to this study is that due to the lack of information regarding medication dosages we could not equivalent the medication dosages. As a result, it might be that the differences found are due to medication rather than symptomology. These limitations are empirical questions that should be addressed in future research.
Lastly, we believe that the current findings have the potential to inform future improvements in therapeutic interventions for schizophrenia. These interventions should be frequency band dependent and specifically targeted at positive or negative symptoms. For example, using transcranial alternating current stimulation (tACS) it is possible to directly modulate specific ongoing rhythmic brain activity (Zaehle, Rach, Herrmann, Schurmann, & Marshall, 2010). Studies investigating the efficacy of tACS in treating individuals with schizophrenia have shown promising results (e.g., Hoy et al., 2016; Kallel et al., 2016; Pinault and Didier, 2017). In this respect, our results open the possibility for a tailor-made use of tACS targeting positive or negative symptoms.
Acknowledgments
The work was supported by the German-Israeli Foundation for scientific research and development (grant 1071).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.09.007.
Appendix A. Supplementary data
Supplementary material
References
- Alamian G., Hincapié A.S., Pascarella A., Thiery T., Combrisson E., Saive A.L., Jerbi K. Measuring alterations in oscillatory brain networks in schizophrenia with resting-state MEG: State-of-the-art and methodological challenges. Clin. Neurophysiol. 2017;128(9):1719–1736. doi: 10.1016/j.clinph.2017.06.246. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington DC: 2000. Diagnostic and statistical manual of mental disorders: DSM-IV-TR (4th Editio) [Google Scholar]
- Andreasen N.C. Positive vs. Negative Schizophrenia: A Critical Evaluation. Schizophr. Bull. 1985;11(3):380–389. doi: 10.1093/schbul/11.3.380. [DOI] [PubMed] [Google Scholar]
- Andreasen, N., Berrios, G., Bogerts, B., & Brenner, H. (2012). Negative versus positive schizophrenia. Retrieved from https://www.google.com/books?hl=en&lr=&id=Bf_sCAAAQBAJ&oi=fnd&pg=PA1&dq=Negative+Versus+Positive+Schizophrenia&ots=YP5JBckOG6&sig=YI8ovGqOEj4aC1YJfFy2OZwepCA
- Baillet S. Magnetoencephalography for brain electrophysiology and imaging. Nat. Neurosci. 2017;20(3):327–339. doi: 10.1038/nn.4504. [DOI] [PubMed] [Google Scholar]
- Bell B., Percival D.B., Walden A.T. Calculating Thomson's spectral multitapers by inverse iteration. J. Comput. Graph. Stat. 1993;2(1):119–130. [Google Scholar]
- Boutros N.N., Arfken C., Galderisi S., Warrick J., Pratt G., Iacono W. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr. Res. 2008;99(1–3):225–237. doi: 10.1016/j.schres.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Oxford University Press; 2006. Rhythms of the brain. [Google Scholar]
- Crow T.J., Ball J., Bloom S.R., Brown R., Bruton C.J., Colter N.…Roberts G.W. Schizophrenia as an anomaly of development of cerebral asymmetry: a postmortem study and a proposal concerning the genetic basis of the disease. Arch. Gen. Psychiatr. 1989;46(12):1145–1150. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- Engel A.K., Fries P. Beta-band oscillations-signalling the status quo? Curr. Opin. Neurobiol. 2010;20(2):156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Fehr, T., Kissler, J., Wienbruch, C., Moratti, S., Elbert, T., Watzl, H., & Rockstroh, B. (2003). Source distribution of neuromagnetic slow-wave activity in schizophrenic patients—effects of activation. Schizophr. Res., 63(1–2), 63–71. 10.1016/S0920-9964(02)00213-X. [DOI] [PubMed]
- Friston, K. J. (1999). Schizophrenia and the disconnection hypothesis. Acta Psychiatr. Scand. Supplementum, 395, 68–79. (Retrieved from) http://www.ncbi.nlm.nih.gov/pubmed/10225335 [DOI] [PubMed]
- Gross J., Kujala J., Hämäläinen M., Timmermann L., Schnitzler A., Salmelin R. Tomographic mapping of functional connectivities from MEG recordings. Neuroimage. 2001;6(13):136. [Google Scholar]
- Harvey R.C., James A.C., Shields G.E. A Systematic Review and Network Meta-Analysis to Assess the Relative Efficacy of Antipsychotics for the Treatment of Positive and Negative Symptoms in Early-Onset Schizophrenia. CNS Drugs. 2016;30(1):27–39. doi: 10.1007/s40263-015-0308-1. [DOI] [PubMed] [Google Scholar]
- Hinkley L.B.N., Vinogradov S., Guggisberg A.G., Fisher M., Findlay A.M., Nagarajan S.S. Clinical Symptoms and Alpha Band Resting-State Functional Connectivity Imaging in Patients With Schizophrenia: Implications for Novel Approaches to Treatment. Biol. Psychiatry. 2011;70(12):1134–1142. doi: 10.1016/j.biopsych.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy K.E., Whitty D., Bailey N., Fitzgerald P.B. Preliminary investigation of the effects of γ-tACS on working memory in schizophrenia. J. Neural Transm. 2016;123(10):1205–1212. doi: 10.1007/s00702-016-1554-1. [DOI] [PubMed] [Google Scholar]
- Huang X.Q., Lui S., Deng W., Chan R.C., Wu Q.Z., Jiang L.J.…Chen L. Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. Neuroimage. 2010;49(4):2901–2906. doi: 10.1016/j.neuroimage.2009.11.072. [DOI] [PubMed] [Google Scholar]
- Ikezawa K., Ishii R., Iwase M., Kurimoto R., Canuet L., Takahashi H., Takeda M. Decreased alpha event-related synchronization in the left posterior temporal cortex in schizophrenia: A magnetoencephalography-beamformer study. Neurosci. Res. 2011;71(3):235–243. doi: 10.1016/j.neures.2011.07.1819. [DOI] [PubMed] [Google Scholar]
- Jensen O., Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 2010;4(November):186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallel L., Mondino M., Brunelin J. Effects of theta-rhythm transcranial alternating current stimulation (4.5 Hz-tACS) in patients with clozapine-resistant negative symptoms of schizophrenia: a case series. J. Neural Transm. 2016;123(10):1213–1217. doi: 10.1007/s00702-016-1574-x. [DOI] [PubMed] [Google Scholar]
- Kay, S. R., Fiszbein, A., & Opler, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull., 13(2), 261–276. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3616518 [DOI] [PubMed]
- Keil J., Roa Romero Y., Balz J., Henjes M., Senkowski D. Positive and Negative Symptoms in Schizophrenia Relate to Distinct Oscillatory Signatures of Sensory Gating. Front. Hum. Neurosci. 2016;10(104) doi: 10.3389/fnhum.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.S., Shin K.S., Jung W.H., Kim S.N., Kwon J.S., Chung C.K. Power spectral aspects of the default mode network in schizophrenia: an MEG study. BMC Neurosci. 2014;15:104. doi: 10.1186/1471-2202-15-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostova M., Passerieux C., Laurent J.P., Hardy-Baylé M.C. N400 anomalies in schizophrenia are correlated with the severity of formal thought disorder. Schizophr. Res. 2005;78(2–3):285–291. doi: 10.1016/j.schres.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Liddle E.B., Price D., Palaniyappan L., Brookes M.J., Robson S.E., Hall E.L., Liddle …., F P. Abnormal salience signaling in schizophrenia: The role of integrative beta oscillations. Hum. Brain Mapp. 2016;37(4):1361–1374. doi: 10.1002/hbm.23107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maran M., Grent-‘T-Jong, T., & Uhlhaas, P. J. Electrophysiological insights into connectivity anomalies in schizophrenia: a systematic review. Neuropsychiatric Electrophysiology. 2016 [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Narayanan B., O'Neil K., Berwise C., Stevens M.C., Calhoun V.D., Clementz B.A., Pearlson …., D G. Resting state electroencephalogram oscillatory abnormalities in schizophrenia and psychotic bipolar patients and their relatives from the bipolar and schizophrenia network on intermediate phenotypes study. Biol. Psychiatry. 2014;76(6):456–465. doi: 10.1016/j.biopsych.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Duncan N.W. How do abnormalities in the brain's spontaneous activity translate into symptoms in schizophrenia? From an overview of resting state activity findings to a proposed spatiotemporal psychopathology. Prog. Neurobiol. 2016 doi: 10.1016/j.pneurobio.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011:1. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A., Ford J.M., White P.M., Roth W.T. P3 in Schizophrenia Is Affected by Stimulus Modality, Response Requirements, Medication Status, and Negative Symptoms. Arch. Gen. Psychiatry. 1989;46(11):1035. doi: 10.1001/archpsyc.1989.01810110077011. [DOI] [PubMed] [Google Scholar]
- Phillips W.A., Silverstein S.M. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav. Brain Sci. 2003;26(01):65–82. doi: 10.1017/s0140525x03000025. [DOI] [PubMed] [Google Scholar]
- Pinault, D., & Didier. (2017). A Neurophysiological Perspective on a Preventive Treatment against Schizophrenia Using Transcranial Electric Stimulation of the Corticothalamic Pathway. Brain Sciences, 7(4), 34. 10.3390/brainsci7040034 [DOI] [PMC free article] [PubMed]
- Selemon L.D., Rajkowska G., Goldman-Rakic P.S. Abnormally high neuronal density in the schizophrenic cortex: a morphometric analysis of prefrontal area 9 and occipital area 17. Arch. Gen. Psychiatr. 1995;52(10):805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Sponheim S.R., Clementz B.A., Iacono W.G., Beiser M. Resting EEG in first-episode and chronic schizophrenia. Psychophysiology. 1994;31(1):37–43. doi: 10.1111/j.1469-8986.1994.tb01023.x. Retrieved from. [DOI] [PubMed] [Google Scholar]
- Sun J., Tang Y., Lim K.O., Wang J., Tong S., Li H., He B. Abnormal dynamics of EEG oscillations in schizophrenia patients on multiple time scales. IEEE Trans. Biomed. Eng. 2014;61(6):1756–1764. doi: 10.1109/TBME.2014.2306424. [DOI] [PubMed] [Google Scholar]
- Thibault R.T., Lifshitz M., Raz A. Body position alters human resting-state: Insights from multi-postural magnetoencephalography. Brain Imaging and Behavior. 2016;10(3):772–780. doi: 10.1007/s11682-015-9447-8. [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews. Neuroscience. 2010;11(2):100–113. doi: 10.1038/nrn2774. Retrieved from. [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J., Linden D.E.J., Singer W., Haenschel C., Lindner M., Maurer K., Rodriguez E. Neurobiology of Disease Dysfunctional Long-Range Coordination of Neural Activity during Gestalt Perception in Schizophrenia. J. Neurosci. 2006;26(31) doi: 10.1523/JNEUROSCI.2002-06.2006. (8168–7175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Liddle P., Linden D.E.J., Nobre A.C., Singh K.D., Gross J. Magnetoencephalography as a Tool in Psychiatric Research: Current Status and Perspective. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2017;2(3):235–244. doi: 10.1016/j.bpsc.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yefet K., Goldstein A., Rabany L., Levkovitz Y. Impairments of event-related magnetic fields in schizophrenia patients with predominant negative symptoms. Psychiatry Res. Neuroimaging. 2015;231(3):325–332. doi: 10.1016/j.pscychresns.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Zaehle T., Rach S., Herrmann C.S., Schurmann M., Marshall L. Transcranial Alternating Current Stimulation Enhances Individual Alpha Activity in Human EEG. PLoS One. 2010;5(11) doi: 10.1371/journal.pone.0013766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material