Abstract
Background
Recent evidence has demonstrated that interleukin 12p35 knockout (IL-12p35 KO) is involved in cardiac diseases by regulating the inflammatory response. The involvement of inflammatory cells has also been observed in doxorubicin (DOX)-induced cardiac injury. This study aimed to investigate whether IL-12p35 KO affects DOX-induced cardiac injury and the underlying mechanisms.
Methods
First, the effect of DOX treatment on cardiac IL-12p35 expression was assessed. In addition, to investigate the effect of IL-12p35 KO on DOX-induced cardiac injury, IL-12p35 KO mice were treated with DOX. Because IL-12p35 is the mutual subunit of IL-12 and IL-35, to determine the cytokine that mediates the effect of IL-12p35 KO on DOX-induced cardiac injury, mice were given phosphate-buffered saline (PBS), mouse recombinant IL-12 (rIL-12) or rIL-35 before treatment with DOX.
Results
DOX treatment significantly increased the level of cardiac IL-12p35 expression. In addition, IL-12p35 KO mice exhibited higher serum and heart lactate dehydrogenase levels, higher serum and heart creatine kinase myocardial bound levels, and greater cardiac dysfunction than DOX-treated mice. Furthermore, IL-12p35 KO further increased M1 macrophage and decreased M2 macrophage differentiation, aggravated the imbalance of oxidants and antioxidants, and further activated the mitochondrial apoptotic pathway and endoplasmic reticulum stress autophagy pathway. Both rIL-12 and rIL-35 protected against DOX-induced cardiac injury by alleviating the inflammatory response, oxidative stress, apoptosis and autophagy.
Conclusions
IL-12p35 KO aggravated DOX-induced cardiac injury by amplifying the levels of inflammation, oxidative stress, apoptosis and autophagy. (234 words).
Keywords: Doxorubicin, IL-12p35 knockout, Inflammation, Oxidative stress, Apoptosis, Autophagy
Abbreviations: IL-12, Interleukin 12; DOX, Doxorubicin; rIL-12, Recombinant IL-12; rIL-35, Recombinant IL-35; LDH, Lactate dehydrogenase; CK-MB, Creatine kinase myocardial bound; Mø, Macrophage; Mø1, M1 macrophage; Mø2, M2 macrophage; ILs, Interleukins; WT, Wild-type; KO, Knockout; i.p., Intraperitoneal; PBS, Phosphate-buffer saline; LV, Left ventricle; HR, Heart rate; LVEF, Left ventricular ejection fraction; FS, Fractional shortening; +dP/dt max, Maximal slope of the systolic pressure increment; −dP/dt max, Maximal slope of the diastolic pressure decrement; LVSP, Left ventricular systolic pressure; LVEDP, Left ventricular end-diastolic pressure; PVDF, Polyvinylidene fluoride; SDS, Sodium dodecyl sulfate; STAT, Signal transducer and activator of transcription; NO, Nitric oxide; iNOS, inducible NO synthase; Arg-1, Arginine 1; Nox, Nitrogen oxide; Nrf2, Nuclearfactor erythroid-2-related factor-2; HO-1, Hemeoxygenase-1; ER, Endoplasmic reticulu; PERK, Protein kinase R-like ER kinase; eIF2a, Eukaryotic inhibition factor 2a; ATF4, Activating transcription factor 4; CHOP, C/EBP homologous protein; Cle-cas, Cleaved-caspase; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; MDA, Malondialdehyde; SOD, Superoxide dismutase; Glu, Glutathione; KCl, Potassium chloride; HE, Hematoxylin and eosin; 4-HNE, 4-hydroxynonenal; TEM, Transmission electron microscopy; TUNEL, Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling; RT-qPCR, Quantitative polymerase chain reaction; TNF-α, Tumor necrosis factor-α; IFN-γ, Interferon-γ; MCP-1, Monocyte chemotactic protein-1; SD, Standard deviation; HPF, High-power field; Th, T helper cells; Treg, Regulatory T cells
Highlights
-
•
IL-12p35 KO aggravates DOX-induced cardiac injury and dysfunction.
-
•
IL-12p35 further increases the DOX-induced imbalance in inflammation, oxidative stress, apoptosis and autophagy.
-
•
Both exogenous rIL-12 and rIL-35 relieved cardiac injury mediated by DOX.
CD4+ T helper (Th) cells are closely related to cardiac injury; regulatory T cells (Tregs) are a new subset of Th cells, and IL-35 is the functional cytokine of Tregs. Cardiac injury mediated by DOX is the most serious complication during chemotherapy, and there are no good preventive measures. This study aimed to investigate whether IL-35 can reduce cardiac injury induced by DOX during chemotherapy. In addition to IL-35, IL-12p35 KO can cancel the biological effect of IL-12; therefore, we also determined whether IL-12 participates in DOX-induced cardiac injury and the underlying mechanisms.
Research in context.
CD4+ T helper (Th) cells are closely related to cardiac injury; regulatory T cells (Tregs) are a new subset of Th cells, and IL-35 is the functional cytokine of Tregs. Cardiac injury mediated by DOX is the most serious complication during chemotherapy, and there are no good preventive measures. This study aimed to investigate whether IL-35 can reduce cardiac injury induced by DOX during chemotherapy. In addition to IL-35, IL-12p35 KO can cancel the biological effect of IL-12; therefore, we also determined whether IL-12 participates in DOX-induced cardiac injury and the underlying mechanisms.
1. Introduction
Doxorubicin (DOX) is one of the most widely used chemotherapy drugs; DOX is used to treat leukemia, carcinoma, and soft tissue sarcoma [1]. However, its clinical use is limited because of the variety of clinical complications, the most serious of which is cardiac injury, which may even progress to heart failure [2, 3]. Because cardiac injury mediated by DOX is irreversible, there are no effective treatments available for cardiac injury in cancer patients following chemotherapy. Therefore, it is necessary to find effective targets to protect or relieve DOX-induced cardiac injury.
Although the specific mechanisms remain unknown, the inflammatory response has been demonstrated to be closely related to DOX-induced cardiac injury [4, 5]. Interleukins (ILs) are a type of cytokine closely related to the inflammatory response. In previous studies, IL-1β was reported to be increased in the strong inflammatory response to DOX treatment in mice [[6], [7], [8], [9]]. Other studies have demonstrated that ILs such as IL-10 and IL-33 could protect against DOX-induced cardiac injury [9, 10]. These findings suggest that ILs may be involved in the progression of DOX-induced cardiac injury.
Both IL-12 and IL-35 share the same subunit, the IL-12p35 subunit, and belong to the IL-12 family. Recent studies have also reported that IL-12 could play both anti-inflammatory and proinflammatory roles depending on the different inflammatory microenvironment [[11], [12], [13]]. In the cardiovascular system, IL-22 could aggravate atherosclerosis via amplifying inflammation [14]. IL-35 has been identified as a functional cytokine of regulatory T cells. IL-35 plays an anti-inflammatory role and ameliorates atherosclerosis [15], autoimmune disease [16], autoimmune diabetes [17], arthritis [18, 19], and explosive hepatitis [20]. The biological effects of IL-12 and IL-35 were abrogated when the IL-12p35 subunit was knocked out. Recent studies have reported that IL-12p35 knockout (KO) induces the inflammatory response and is involved in cardiac fibrosis and myocardial infarction [21, 22]. The evidence suggests that both IL-12 and IL-35 participate in the inflammatory response; however, whether IL-12 and IL-35 are involved in DOX-induced cardiac injury is still unknown. In this study, IL-12p35 KO mice and exogenous mouse recombinant IL-12 (rIL-12) and rIL-35 were used to explore the effect of IL-12 and IL-35 on DOX-induced cardiac injury.
2. Materials and Methods
2.1. Animals and Animal Models
Wild-type (WT) mice and IL-12p35 KO mice with a C57BL/6 background were purchased from HFK Bioscience (Beijing, China) and Jackson Laboratory (Bar Harbor, USA), respectively. All the mice were housed in the specific-pathogen-free mouse room of Renmin Hospital of Wuhan University and received water ad libitum from the Animal Care Facility Service. WT mice received an intraperitoneal (i.p.) injection of DOX (purity ≥ 98%, Novopharm, 15 mg/kg), and an equal volume of saline was administered to mice as a control. Then, cardiac IL-12p35 expression was evaluated (n = 4 in each group). In a separate study, male IL-12p35 KO mice and their WT (11–12 weeks old, 27–29 g) littermates were injected with DOX or saline (WT + saline = control, n = 12; IL-12p35 KO + saline = p35 KO, n = 12; DOX + saline = DOX, n = 12; DOX + IL-12p35 KO, n = 13). These mice were observed every 12 h and weighed every 24 h. In addition, C57BL/6 mice were treated (i.p.) with PBS (50 μl, n = 10), rIL-12 (5 μg, n = 10) [23] or rIL-35 (5 μg, n = 10) [23]. Three days later, all the mice were treated with DOX injections for five days. This study was reviewed and approved by the Institutional Animal Care and Use Committee at the Beijing AnZhen Hospital of Capital Medical University (Beijing, China), the People's Hospital of Guangxi Zhuang Autonomous Region (Nanning, China), and the Renmin Hospital of Wuhan University (Wuhan, China).
2.2. Echocardiography and Hemodynamics
Mice were anesthetized with 1.5% isoflurane, and echocardiography was performed using a MyLab 30CV ultrasound (Esaote SpA, Genoa, Italy) system with a 10-MHz linear array ultrasound transducer. M-mode images of the left ventricle at the papillary muscle level were recorded; then, the heart rate (HR), and left ventricular (LV) function, including the left ventricular ejection fraction (LVEF) and fractional shortening (FS), were measured. After a microtip catheter transducer (Millar, Inc., Houston, TX, USA) was inserted into the right carotid artery and advanced into the left ventricle, the signals were continuously recorded using a Millar PressureVolume system (Millar, Inc.), and the maximal slope of the systolic pressure increment (+dP/dt max) and diastolic pressure decrement (-dP/dt max), left ventricular systolic pressure (LVSP) and left ventricular end-diastolic pressure (LVEDP) were recorded on a beat-by-beat basis.
2.3. Western Blot
The LV tissue was lysed in RIPA lysis buffer containing protease inhibitors and phosphatase inhibitors. Then, the tissue was further fractured by ultrasound, and after centrifugation for 15 min at 3000g, total protein was collected. After detection with a BCA Protein Assay Kit (Thermo Fisher Scientific, MA, USA), approximately 40 μg of total protein was separated by electrophoresis on Laemmli sodium dodecyl sulfate (SDS) polyacrylamide gels. Then, the samples were transferred to Immobilon-FL polyvinylidene fluoride (PVDF) membranes (Millipore, USA). After blocking with 5% nonfat milk, the membranes were incubated with primary antibodies at 4 °C overnight. The membranes were then incubated with secondary antibodies at room temperature for 1 h. The blots were scanned using a two-colour infrared imaging system (Odyssey; LI-COR Biosciences, Lincoln, USA). The primary antibodies used in this study were as follows: anti-IL-12p35 (Abcam, Cambridge, England), anti-p-STAT3 (CST: Cell Signaling Technology, Boston, USA), anti-signal transducer and activator of transcription (STAT)3 (CST), anti-p-STAT4 (Abcam), anti-STAT4 (CST), anti-p-p65 (Bioworld Technology, Minnesota, USA), anti-p65 (CST), anti-inducible NO synthase (iNOS, CST), anti-arginine 1 (Arg-1, Abcam), anti-nitrogen oxide 2 (Nox2, Santa Cruz, Dallas, TX, USA), anti-Nox4 (Santa Cruz), anti-nuclear factor erythroid 2-related factor (Nrf2, GeneTex), anti-hemeoxygenase1 (HO-1, GeneTex), anti-Bax (CST), anti-Bcl2 (CST), anti-cytochrome C (Cyto C; CST), anti-cleaved-caspase3 (Cle-cas3; CST), anti-cleaved-caspase 9 (Cle-cas9; CST), anti-p-protein kinase R-like endoplasmic reticulum (ER) kinase (p-PERK, Abcam), anti-p-eukaryotic inhibition factor 2a (p-eIF2a, Santa Cruz), anti-activating transcription factor 4 (ATF4, Abcam), anti-C/EBP homologous protein (CHOP, CST), anti-cleaved-caspase 12 (Cle-cas12; CST), anti-beclin1 (CST), anti-LC3 (CST) and anti-glyceraldehyde-3-phosphate dehydrogenase (GADPH, CST).
2.4. Measurement of Enzymes and Cytokines
Blood samples were collected from each mouse and were centrifuged for 15 min at 4000g; then, serum was obtained from each sample. Total protein was collected from LV tissue, and lactate dehydrogenase (LDH) activity and the creatine kinase myocardial bound (CK-MB) levels in serum and LV tissue were measured. In addition, the levels of malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione (GSH) in the left ventricle were detected according to the manufacturer's instructions (all from Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.5. Histological Analysis
The mice were euthanized under anesthesia, and the hearts were immediately isolated and weighed. Then, the hearts were arrested in diastole with 10% potassium chloride (KCl). After being fixed with 4% neutral paraformaldehyde for 5 days, the hearts were embedded in paraffin, cut into approximately 4-mm sections and mounted onto slides. Hematoxylin and eosin (HE) staining was performed for histopathological analysis. For immunohistochemistry staining, the sections were subjected to a 5-minute high-pressure antigen retrieval process in citrate buffer at a pH of 6.0 and blocked with 10% bovine serum albumin for 1 h. Then, the sections were incubated overnight at 4 °C with the primary antibodies anti-IL-12p35 antibody (R&D Systems, Minneapolis, USA), anti-CD80 antibody (R&D Systems), anti-CD206 antibody (R&D Systems), anti-iNOS antibody (CST), and anti-Arg-1 antibody (Abcam). Binding was visualized with the appropriate peroxidase-conjugated secondary antibodies (horseradish peroxidase (HRP) AffiniPure goat anti-rabbit IgG) for 1 h at 37 °C. For 4-hydroxynonenal (4-HNE) analysis, the sections were deparaffinized and blocked with 8% goat serum in PBS, followed by incubation with anti-4-HNE antibody (Abcam) for 12 h at 4 °C. Subsequently, the sections were incubated with anti-rabbit HRP reagent (Gene Tech, Shanghai) for 1 h at room temperature and developed using a peroxide-based substrate DAB kit (Gene Tech). Finally, the sections were dehydrated in ethanol and cleared in xylene. Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining was performed using a commercially available kit according to the manufacturer's instructions (Millipore, USA) to detect apoptosis. In addition, parts of the freshly obtained LV tissue were fixed at room temperature in 2% glutaraldehyde (Servicebio, Wuhan). These samples were then analyzed by transmission electron microscopy (TEM).
2.6. Quantitative Polymerase Chain Reaction (RT-qPCR)
LV tissue was lysed by TRIzol reagent W, and total mRNA was collected. A 2-μg sample of total mRNA was used to synthesize cDNA using a reverse transcription kit according to the manufacturer's instructions (Roche Diagnostics GmbH, Mannheim, Germany). LightCycler 480 SYBR Green Master Mix (Roche) was used to perform the PCR amplifications. The relative mRNA expression levels of IL-1β, IL-6, IL-17, TNF-α, IFN-γ, monocyte chemotactic protein (MCP)-1, IL-4, IL-10 and IL-13 were investigated, and the results were normalized to the expression levels of GAPDH. The RT-qPCR primer sequences are shown in Table 1.
Table 1.
RT-PCR primers used.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| IL-1β | GGGCCTCAAAGGAAAGAATC | TACCAGTTGGGGAACTCTGC |
| IL-6 | AGTTGCCTTCTTGGGACTGA | TCCACGATTTCCCAGAGAAC |
| IL-17 | TCCAGAAGGCCCTCAGACTA | AGCATCTTCTCGACCCTGAA |
| TNF-α | CCCAGGGACCTCTCTCTAATC | ATGGGCTACAGGCTTGTCACT |
| IFN-γ | ACTGGCAAAAGGATGGTGAC | TGAGCTCATTGAATGCTTGG |
| MCP-1 | CTTCTGTGCCTGCTGCTCAT | CGGAGTTTGGGTTTGCTTGTC |
| IL-4 | ACGAGGTCACAGGAGAAGGGA | AGCCCTACAGACGAGCTCACTC |
| IL-10 | ATAACTGCACCCACTTCCCA | GGGCATCACTTCTACCAGGT |
| IL-13 | CGCAAGGCCCCCACTAC | TGGCGAAACAGTTGCTTTGT |
| GAPDH | AACTTTGGCATTGTGGAAGG | CACATTGGGGGTAGGAACAC |
2.7. Statistical Analysis
All data are expressed as the mean ± standard deviation (SD) and were analyzed by GraphPad Prism 6 software. The differences between two groups were compared with unpaired Student's t-test. A P value <0.05 was considered statistically significant.
3. Results
3.1. DOX Treatment Increased the Level of Cardiac IL-12p35 Expression
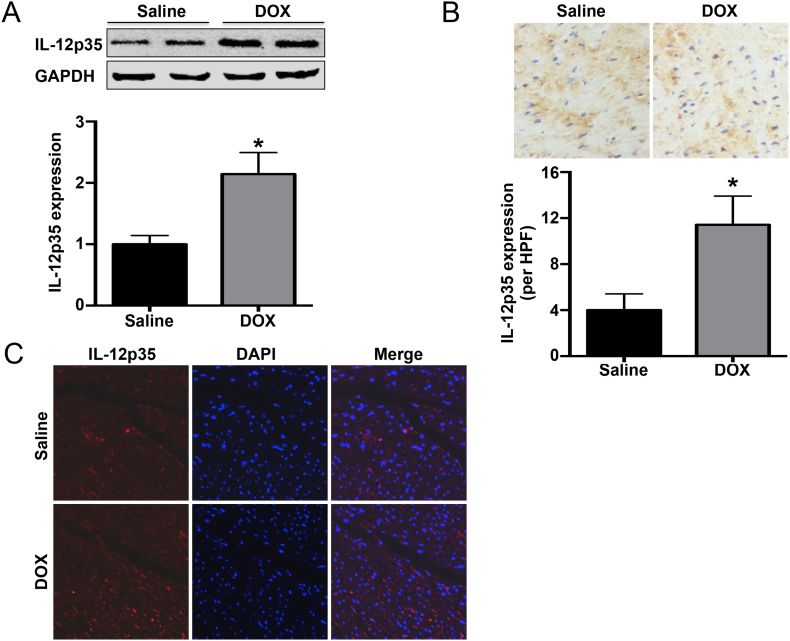
The IL-12p35 expression level in the left ventricle was determined by histological analysis and western blot. The results showed that the IL-12p35 levels were increased in DOX-treated mice when compared with baseline (Fig. 1A–C).
Fig. 1.
Cardiac IL-12p35 expression in DOX- or saline-treated mice. IL-12p35 expression levels in the saline group and DOX group were measured by western blotting (A), immunohistochemistry (brown granules, 400×) (B) and immunofluorescence (red granules, 400×) (C). HPF = high-power field; N = 4 in each group. *P < 0.05 vs. the saline group.
3.2. IL-12p35 KO Aggravated DOX-induced Injury
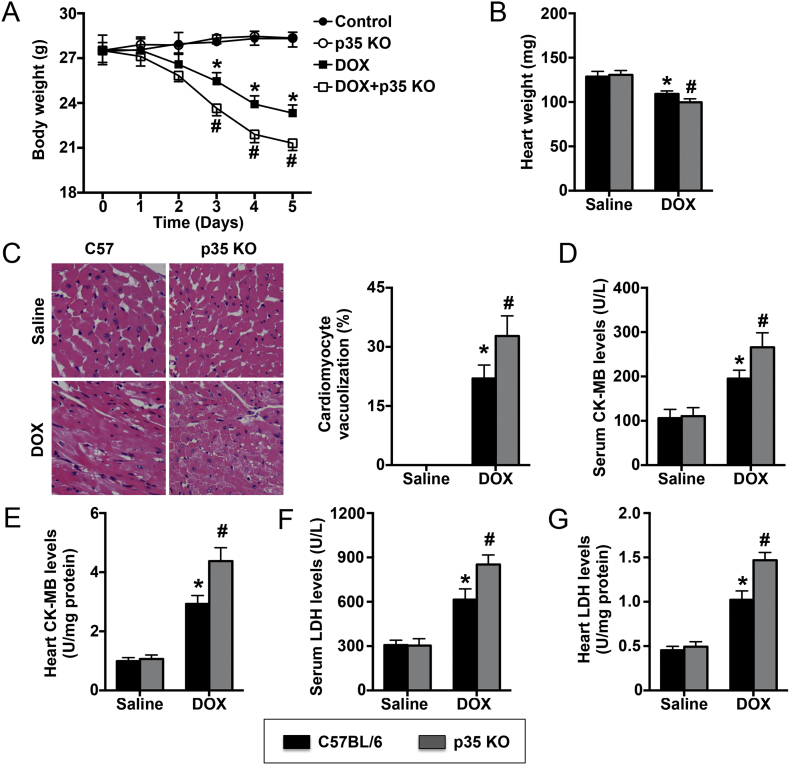
In the 5 days of DOX treatment, the body weights of both the DOX group and the DOX + p35 KO group gradually decreased. At the end of the fifth day, the body weight in the DOX group was reduced by approximately 4.2 g, while one mouse in the DOX + p35 KO group died, and the body weight in that group was reduced by approximately 6.2 g (Fig. 2A). The heart weight was significantly reduced in the DOX group and further reduced in the DOX + p35 KO group (Fig. 2B). In addition, the number of vacuolated cardiomyocytes was significantly increased in WT mice and further increased in IL-12p35 KO mice when treated with DOX for 5 days (Fig. 2C). Furthermore, the markers of cardiac injury, including LDH and CK-MB, in the serum and the heart exhibited a similar trend as the number of vacuolated cardiomyocytes (Fig. 2D–G).
Fig. 2.
Effect of IL-12p35 KO on DOX-induced cardiac injury. (A) Body weight was measured in each group at different times; N = 8 in each group. (B) Heart weight was measured at the end of the fifth day in the four groups; N = 8 in each group. (C) Heart sections were obtained and stained with HE. The vacuolated cardiomyocytes were quantified (400×); N = 6 in each group. Serum LDH (D) and CK-MB (F) levels were assessed; N = 8 in each group. Heart LDH (E) and CK-MB (G) levels were detected; N = 4 in each group. *P < 0.05 vs. the control group, and #P < 0.05 vs. the DOX group.
3.3. IL-12p35 KO Exacerbated DOX-induced Cardiac Dysfunction
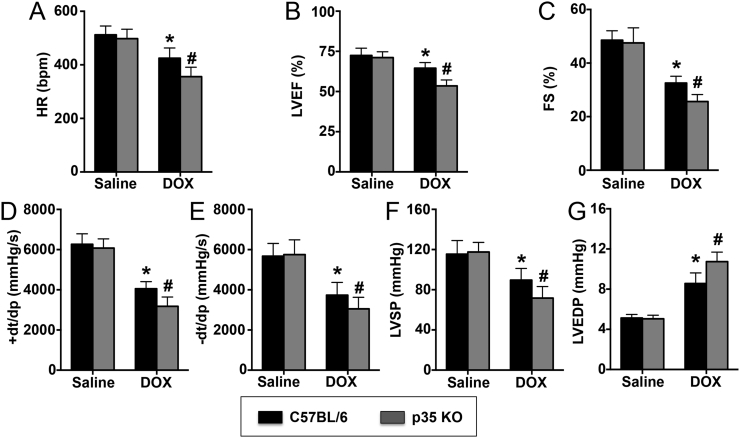
Echocardiography showed that the HR, LVEF and FS were significantly decreased in the DOX group compared with the control group after 5 days of DOX treatment and that IL-12p35 KO exacerbated the decrease in those parameters in DOX-treated mice (Fig. 3A–C). Similar results were obtained with invasive hemodynamic measurements. DOX-induced LV systolic (Fig. 3D and F) and diastolic (Fig. 3E and G) dysfunction was significantly increased by IL-12p35 KO.
Fig. 3.
Evaluation of LV function by echocardiography and hemodynamic data in each group. HR (A), LVEF (B), and FS (C) were evaluated by echocardiography in the control, p35 KO, DOX and DOX + p35 KO groups. Positive and negative dP/dt max (D, E), LVSP (F) and LVEDP (G) were assessed by hemodynamic data in the four groups; N = 8 in each group. *P < 0.05 vs. the control group, and #P < 0.05 vs. the DOX group.
3.4. IL-12p35 KO Reduced DOX-induced STAT3 Phosphorylation
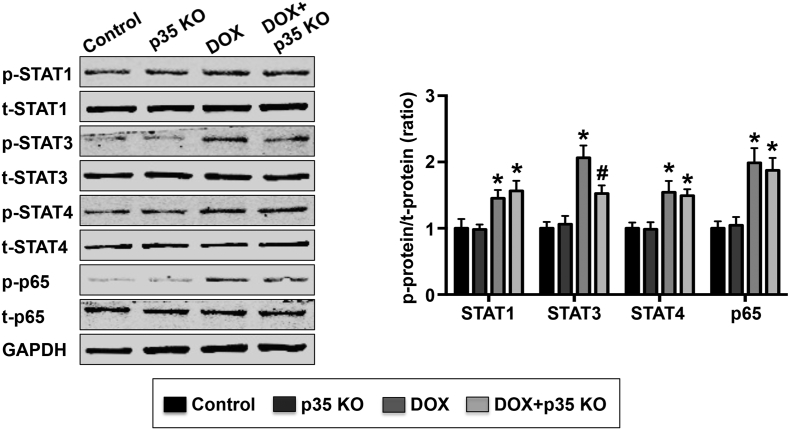
STAT1, STAT3, STAT4 and p65 phosphorylation was measured by western blot in LV tissues, and the results demonstrated that DOX treatment significantly increased STAT1, STAT3, STAT4 and p65 phosphorylation. STAT3 phosphorylation, but not STAT1, STAT4 or p65 phosphorylation, could be partly prevented by IL-12p35 KO (Fig. 4).
Fig. 4.
Effect of IL-12p35 KO on STAT3, STAT4 and p65 phosphorylation. Representative images and quantification of p-STAT1, t-STAT1, p-STAT3, t-STAT3, p-STAT4, t-STAT4, p-p65, t-p65 and GAPDH by western blot in the left ventricle of each group; N = 6 in each group. *P < 0.05 vs. the control group, and #P < 0.05 vs. the DOX group.
3.5. IL-12p35 KO Enhanced the DOX-induced Inflammatory Response
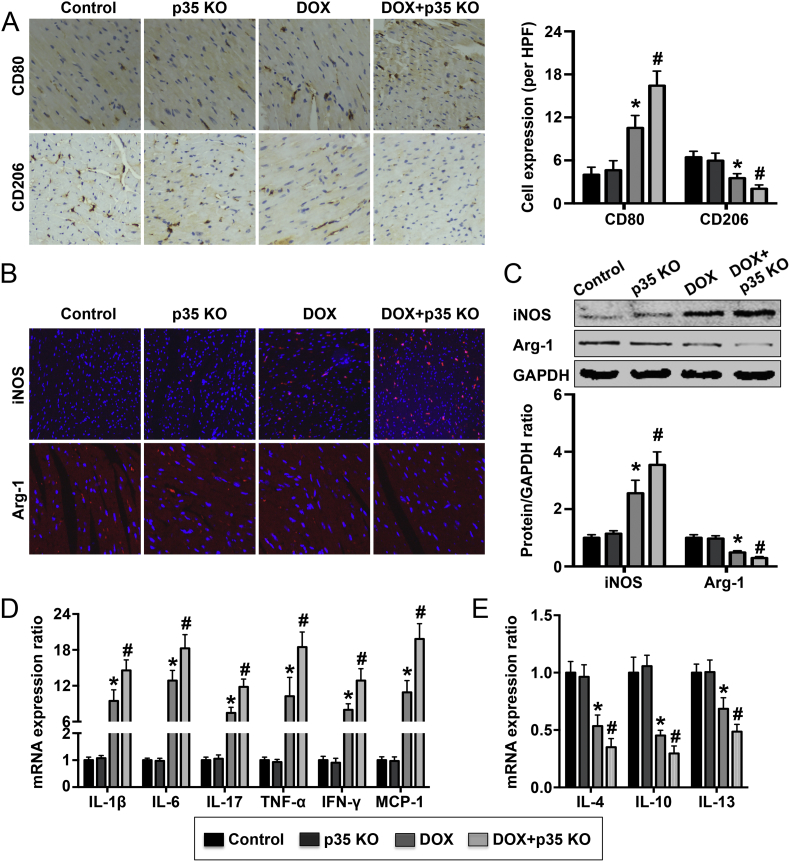
To examine the effect of IL-12p35 KO on the inflammatory response, we first detected M1 macrophage (Mø1) and M2 macrophage (Mø2) expression in the left ventricle. Both the surface markers and soluble mediators of Mø1 (CD80 and iNOS) and Mø2 (CD206 and Arg-1) were evaluated. The results of surface staining showed that treatment with DOX resulted in increased levels of CD80 expression in the heart and that IL-12p35 KO further increased CD80 levels in DOX-treated mice (Fig. 5A). Meanwhile, DOX induced a decrease in CD206 expression level in the heart, and IL-12p35 KO further reduced CD206 levels in DOX-treated mice (Fig. 5A). In addition, staining for the soluble mediators showed that the expression of iNOS and Arg-1 exhibited a similar trend as the expression of CD80 and CD206 in the heart, respectively (Fig. 5B). We also measured iNOS and Arg-1 expression levels by western blot, and higher iNOS levels and lower Arg-1 levels were observed in the DOX + p35 KO group than the DOX group (Fig. 5C). Inflammatory cytokine mRNA expression levels were also detected, and the results showed that IL-12p35 KO increased the DOX-induced increase in the expression levels of IL-1β, IL-6, IL-17, TNF-α, IFN-γ and MCP-1 mRNA but further reduced the DOX-induced decrease in the levels of IL-4, IL-10 and IL-13 mRNA expression (Fig. 5D).
Fig. 5.
Expression of macrophage markers and macrophage-related inflammatory cytokine mRNA in the control, p35 KO, DOX and DOX + p35 KO groups. (A). Surface markers of macrophages, including CD80 and CD206, in the left ventricle were detected by immunohistochemistry in the four groups (brown granules, 400×). (B). Soluble mediators of macrophages, including iNOS and Arg-1, in the left ventricle were detected by immunohistochemistry in each group (red granules, 400×). (C). Soluble mediators of macrophages, including iNOS and Arg-1, in the left ventricle were assessed by western blotting. (D). The mRNA expression levels of IL-1β, IL-6, IL-17, TNF-α, IFN-γ, MCP-1, IL-4, IL-10 and IL-13 were measured by RT-qPCR. HPF = high-power field; N = 6 in each group. *P < 0.05 vs. the control group, and #P < 0.05 vs. the DOX group.
3.6. IL-12p35 KO Aggravated DOX-induced Oxidative Stress
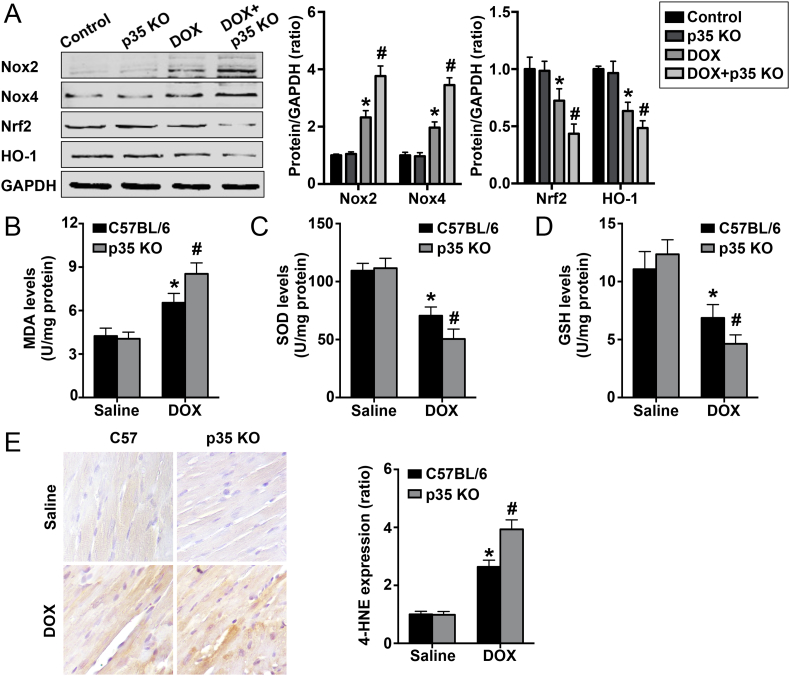
To investigate the effect of IL-12p35 KO on oxidative stress, we first detected oxidative stress and antioxidative stress signaling pathway expression. The results showed that DOX treatment activated the Nox2 and Nox4 pathways but suppressed the Nrf2 and HO-1 pathways; IL-12p35 KO further increased Nox2 and Nox4 activation but further reduced Nrf2 and HO-1 activation (Fig. 6A). In addition, we assessed MDA, SOD and GSH expression in the heart, and further increased MDA levels and further decreased SOD and GSH levels were observed in the DOX + p35 KO group compared with the DOX group (Fig. 6B–D). Furthermore, the 4-HNE expression level in each group was examined, and the results showed that treatment with DOX significantly increased 4-HNE levels, while IL-12p35 KO further increased the level of DOX-induced 4-HNE expression (Fig. 6E).
Fig. 6.
Regulation of IL-12p35 KO on DOX-induced oxidative stress in hearts. (A). Representative images and quantification of Nox2, Nox4, Nrf2, HO-1 and GAPDH by western blotting in the control, p35 KO, DOX and DOX + p35 KO groups. MDA (B), SOD (C) and GSH (D) levels in the left ventricle were measured in each group. (E). The immunohistochemical signal intensity was used to quantify the expression of 4-HNE in the four groups (400×); N = 6 in each group. *P < 0.05 vs. the control group, and #P < 0.05 vs. the DOX group.
3.7. IL-12p35 KO Aggravated DOX-induced Myocardial Apoptosis
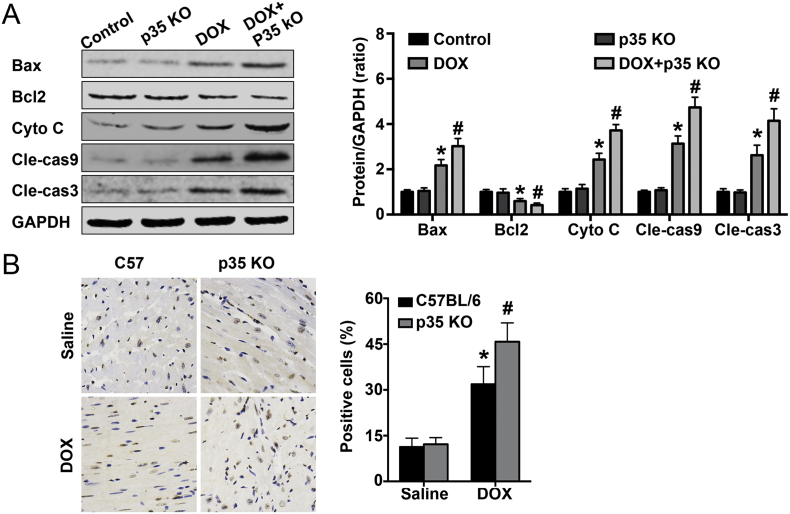
We first detected the activation of apoptosis-related signaling pathways, and the results showed that 5 days after DOX treatment, there were higher levels of Bax, Cyto C, Cle-cas9 and Cle-cas3 and lower levels of Bcl2 in the DOX group than in the control group. Meanwhile, IL-12p35 KO increased Bax, cytoplasmic Cyto C, Cle-cas9, Cle-cas3 levels and decreased Bcl2 levels in DOX-treated mice (Fig. 7A). In addition, after treatment with DOX for 5 days, an increase in the number of TUNEL-positive cells was observed in DOX-treated mice, and IL-12p35 KO could further increase the number of these TUNEL-positive cells (Fig. 7B).
Fig. 7.
Effect of IL-12p35 KO on DOX-induced myocardial apoptosis. (A). Representative images and quantification of Bax, Bcl2, Cyto C, Cle-cas9, Cle-cas3 and GAPDH by western blotting in the control, p35 KO, DOX and DOX + p35 KO groups. (B). Representative images of TUNEL staining and the quantitative results in each group (400×); N = 6 in each group. *P < 0.05 vs. the control group, and #P < 0.05 vs. the DOX group.
3.8. IL-12p35 KO Aggravated DOX-induced Endoplasmic Stress and Autophagy
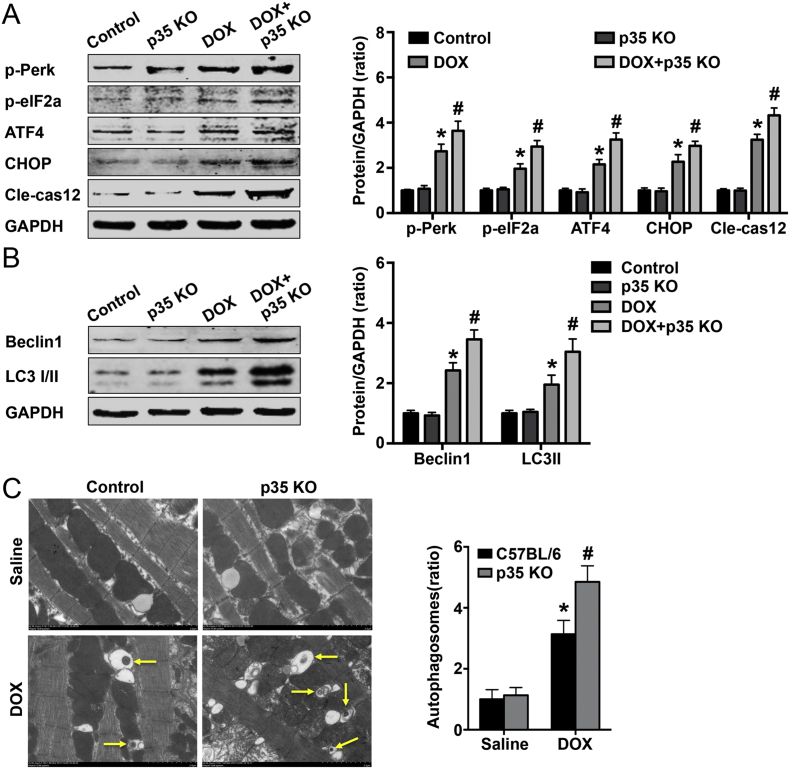
Next, we detected the activation of the endoplasmic stress-related pathway, and the results showed that DOX treatment could increase the expression levels of p-PERK, p-eIF2a, ATF4, CHOP and Cle-cas12 and that IL-12p35 KO could further increase the expression levels of these proteins (Fig. 8A). In addition, we examined the expression of autophagic proteins, and the results showed that IL-12p35 KO and treatment with DOX could result in higher beclin 1 and LC3-II protein expression levels than DOX treatment alone (Fig. 8B). Furthermore, to indicate the occurrence of autophagy, TEM analysis was performed, and the results showed that IL-12p35 KO increased the formation of autophagosomes in DOX-treated mice (Fig. 8C).
Fig. 8.
Effect of IL-12p35 KO on DOX-induced endoplasmic stress and autophagy. (A). Representative images and quantification of p-PERK, p-eIF2a, ATF4, CHOP, Cle-cas12 and GAPDH by western blotting in the control, p35 KO, DOX and DOX + p35 KO groups. (B). Representative images and quantification of beclin 1, LC3–1/II and GAPDH by western blotting in each group. (C). Representative transmission electron micrographs indicating the formation of an autophagosome (5000×); N = 6 in each group. *P < 0.05 vs. the control group, and #P < 0.05 vs. the DOX group.
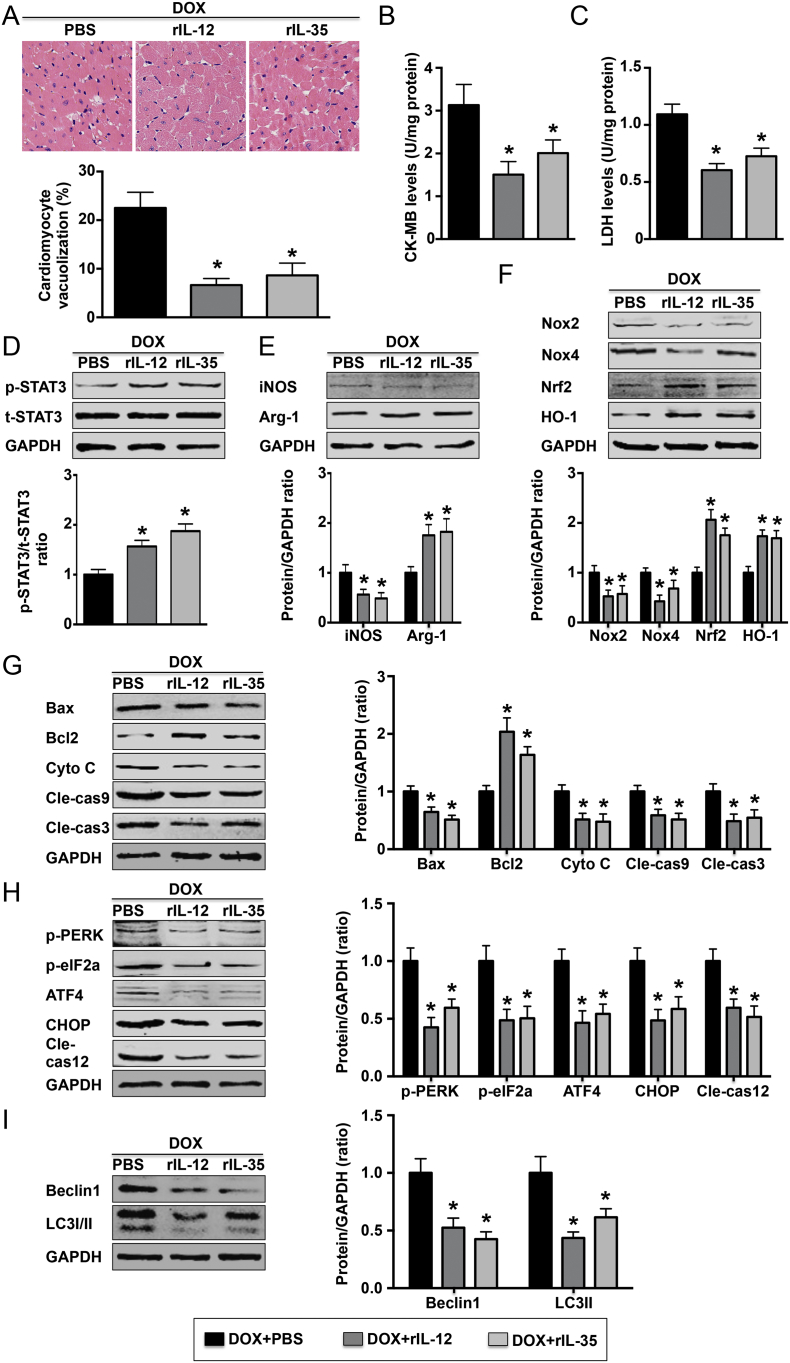
3.9. Both IL-12 and IL-35 Treatment Protected against DOX-induced Cardiac Injury
Pretreatment with exogenous rIL-12 and rIL-35 significantly reduced the number of vacuolated cardiomyocytes (Fig. 9A). Lower levels of cardiomyocyte injury markers, including LDH and CK-MB, were observed in the heart tissue (Fig. 9B and C). In addition, both rIL-12 and rIL-35 increased STAT3 pathway phosphorylation (Fig. 9D). Exogenous rIL-12 and rIL-35 also decreased Mø1 marker expression and prooxidative stress pathway activation but increased Mø2 marker expression and antioxidative stress pathway activation (Fig. 9E and F). In addition, lower levels of proteins in the apoptotic pathway and autophagy pathway, while higher Bcl2 levels were observed (Fig. 9G and H). Finally, decreased beclin 1 and LC3-II protein levels were observed in rIL-12- and rIL-35-treated mice (Fig. 9I).
Fig. 9.
Effect of rIL-12 and rIL-35 on DOX-induced cardiac injury. (A). Heart sections were obtained from the three indicated groups and stained with HE (400×). The number of vacuolated cardiomyocytes was quantified; N = 5 in each group. Heart LDH (B) and CK-MB (C) levels were assessed; N = 6 in each group. (D). Level of STAT3 phosphorylation in each group was measured; N = 5 in each group. The levels of iNOS and Arg-1 (E); Nox2, Nox4, Nrf2 and HO-1 (F); Bax, Bcl2, Cyto C, Cle-cas9 and Cle-cas3 (G); p-PERK, p-eIF2a, ATF4, CHOP and Cle-cas12 (H); and beclin 1 and LC3-I/II were detected by western blotting in each group; N = 5 in each group. *P < 0.05 vs. the DOX + PBS group.
4. Discussion
In the study, we demonstrated for the first time that DOX treatment for 5 days could increase the level of cardiac IL-12p35 expression. While IL-12p35 KO significantly aggravated DOX-induced cardiac injury and dysfunction in mice, more serious inflammatory responses, further imbalance of oxidative stress, and further deterioration of myocardial apoptosis and autophagy were the possible mechanisms. In addition, DOX-induced cardiac injury could be alleviated by both rIL-12 and rIL-35. Our present study thus provided a novel direction for the clinical treatment of DOX-induced cardiac injury.
In an earlier study, Yulin Li et al. reported that the cardiac IL-12p35 expression level was time dependently increased in angiotensin II-treated mice [19]. In a later article, Yulin Li et al. also reported that the level of cardiac IL-12p35 mRNA expression was increased in remote, infarction and border zones at 3 days after myocardial infarction [22]. In addition, IL-12p35 KO was found to enhance angiotensin II-induced cardiac fibrosis by promoting CD4 T cell-dependent Mø2 differentiation and to improve cardiac repair after myocardial infarction by promoting angiogenesis [21, 22]. This evidence supports that IL-12p35 may be closely related to cardiovascular diseases. Therefore, we first measured the level of IL-12p35 expression in DOX-treated mice, and the results showed that treatment with DOX for 5 days increased the cardiac IL-12p35 expression level by approximately 1.1-fold. These results suggest that IL-12p35 may participate in DOX-induced cardiac injury. To investigate the role of IL-12p35 in cardiac injury, IL-12p35 KO mice were treated with DOX, and compared with DOX-treated control mice, decreased body weight and heart weight, an increased number of vacuolated cardiomyocytes and an increased level of serum and heart myocardial injury marker expression were observed. In addition, IL-12p35 KO further deteriorated DOX-induced cardiac dysfunction in mice. This evidence demonstrated that IL-12p35 deletion further exacerbated DOX-induced myocardial injury.
DOX-induced cardiac injury and subsequent cardiac dysfunction are common clinical challenges in tumor patients after chemotherapy. Although the specific mechanisms of DOX-induced cardiac injury and dysfunction are unclear, increasing evidence has demonstrated that inflammation and oxidative stress play an important role in the progression of DOX-induced cardiac injury and dysfunction, as an intense inflammatory response and imbalance of oxidative stress could mediate cardiomyocyte apoptosis [2, 5, 24]. Macrophages are the most important and most studied cells in inflammation and the immune response. Activated macrophages can differentiate into Mø1 and Mø2, which play proinflammatory and anti-inflammatory roles and are involved in tissue injury and repair [25]. An imbalance between Mø1 and Mø2 may underlie many inflammatory diseases, including aortic dissection, atherosclerosis, and coronary artery disease [[26], [27], [28]]. An effect of IL-12p35 KO on the regulation of Mø2 differentiation has been reported previously [21]. Therefore, to investigate whether IL-12p35 KO affects DOX-induced cardiac injury by regulating the macrophage-induced inflammatory response, we first detected cardiac Mø1 and Mø2 expression and the mRNA expression levels of their inflammatory cytokines. Our data indicate that IL-12p35 KO promoted Mø1 differentiation and Mø1-related inflammatory cytokine expression but inhibited Mø2 differentiation and Mø2-related inflammatory cytokine expression. We also found that DOX treatment inhibited Mø2 differentiation, which is consistent with a recent study [4]. However, the present study found that IL-12p35 KO further reduced Mø2 differentiation, which is in contrast to the findings of a previous article [21], potentially due to the use of different experimental models. Nevertheless, our results suggest that further imbalance of the inflammatory response was one of the mechanisms by which IL-12p35 KO aggravated DOX-induced cardiac injury. The literature overwhelmingly highlights the unique vulnerability of the heart to DOX-induced cardiac injury [29]. The induction of free radical production, in addition to overwhelming the enzymatic defenses of cardiomyocytes, is the best-described major mechanism through which DOX injures the myocardium [30, 31]. To define whether IL-12p35 KO affects the regulation of DOX-induced oxidative stress, we measured the oxidative stress level, and the results showed that IL-12p35 KO resulted in more severe oxidative stress. Our data suggest that inducing a higher oxidative stress level is another mechanism by which IL-12p35 KO aggravates DOX-induced cardiac injury.
STAT3 is an important signaling pathway in the regulation of cell apoptosis caused by various factors, including myocardial infarction and tumors. The basis of DOX-induced cardiac toxicity is the apoptosis of cardiomyocytes [5, 32, 33]. Another important mechanism is outer mitochondrial membrane permeabilization and the release of Cyto C, which could lead to mitochondrial dysfunction [34]. There is also evidence showing that STAT3 activation could alleviate DOX-induced cardiac injury and cardiac dysfunction by reducing cardiomyocyte apoptosis [35, 36]. In the present study, we found that IL-12p35 KO inhibited DOX-induced activation of the STAT3 pathway, further activated mitochondria-associated apoptotic signaling pathways and increased the number of apoptotic cells, and our results are consistent with those of previous studies.
ER stress is an important signal in the regulation of cell survival and death; ER stress is one of the most important mediators of cardiac cell apoptosis [36]. Interestingly, the effect of ER stress on the regulation of apoptosis has a dual nature; mild autophagy partly protects cells from harmful conditions and promotes cell survival, whereas severe or rapid autophagy will induce programmed cell death, known as autophagic cell death [[37], [38], [39], [40], [41]]. To investigate whether autophagy participates in apoptosis, we detected ER stress-related pathways and beclin 1 and LC3-II expression; we found that the ER stress-related pathways were further activated and that the autophagy-associated protein expression level was higher in the DOX + p35 KO group than in the DOX-only group. To define whether higher autophagy levels were related to the higher apoptotic rate in the DOX + p35 KO group, we also measured the level of Cle-cas12, which was localized to the ER and is specifically activated by ER stress. DOX-treated IL-12p35 KO mice exhibited increased expression levels of Cle-cas12, suggesting that ER stress activates the apoptotic pathways and may contribute to cardiac cell apoptosis but is not a protective mechanism against cardiac cell apoptosis. Consistent with this hypothesis, autophagosomes were observed by electron microscopy in DOX-treated mice, and these changes were magnified in IL-12p35 KO mice. These results suggest that IL-12p35 KO may aggravate autophagy and thereby increase apoptosis in DOX-treated mice as another mechanism of aggravating cardiac injury.
In summary, the results of the present study are the first to demonstrate that IL-12p35 KO aggravates DOX-induced cardiac injury and cardiac dysfunction. Although the exact mechanisms of the IL-12p35 in DOX-induced cardiac injury have not been fully clarified, upregulation of the inflammatory response, oxidative stress, apoptosis and autophagy may be underlying mechanisms. In addition, we found that autophagy may participate in the progression of DOX-induced cardiac injury, which has not been reported in previous studies. Consequently, we may have found a new therapeutic option in the clinical prevention and treatment of DOX-induced cardiac injury. (3612 words).
Funding Sources
This work was supported by the National Natural Science Foundation of China (No. 81770472, 81460081, 81460061 and 81760051).
Conflicts of Interest
None.
Author Contributions
Jing Ye, Ying Huang, Bin Que., Chao Chang, Wenjing Liu, Haiying Hu, Ling Liu, Ying Shi, Yuan Wang, Menglong Wang and Tao Zeng performed research; Jing Ye, Wang Zhen, Yao Xu, Lei Shi, Jianfang Liu, Huimin Jiang and Di Ye analyzed data; Jing Ye, Yingzhong Lin, Jun Wan and Qingwei Ji conceived the study and participated in its design and coordination and drafting the manuscript. All authors have read and approved the final manuscript.
Contributor Information
Yingzhong Lin, Email: yingzhonglin@126.com.
Jun Wan, Email: wanjun@whu.edu.cn.
Qingwei Ji, Email: jqw124@163.com.
References
- 1.Duggan S.T., Keating G.M. Pegylated liposomal doxorubicin: A review of its use in metastatic breast cancer, ovarian cancer, multiple myeloma and AIDS-related Kaposi's sarcoma. Drugs. 2011;71(18):2531–2558. doi: 10.2165/11207510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Octavia Y., Tocchetti C.G., Gabrielson K.L., Janssens S., Crijns H.J., Moens A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52(6):1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Takemura G., Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49(5):330–352. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Li M., Sala V., De Santis M.C., Cimino J., Cappello P., Pianca N. Circulation. 2018 [Google Scholar]
- 5.Yuan Y.P., Ma Z.G., Zhang X., Xu S.C., Zeng X.F., Yang Z. CTRP3 protected against doxorubicin-induced cardiac dysfunction, inflammation and cell death via activation of Sirt1. J Mol Cell Cardiol. 2018;114:38–47. doi: 10.1016/j.yjmcc.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Van Tassell B.W., Toldo S., Mezzaroma E., Abbate A. Targeting interleukin-1 in heart disease. Circulation. 2013;128(17):1910–1923. doi: 10.1161/CIRCULATIONAHA.113.003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J., Zhang J., Xiang D., Zhang Z., Zhang L., Wu M. Recombinant human interleukin-1 receptor antagonist protects mice against acute doxorubicin-induced cardiotoxicity. Eur J Pharmacol. 2010;643(2–3):247–253. doi: 10.1016/j.ejphar.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Wang L., Zhang T.P., Zhang Y., Bi H.L., Guan X.M., Wang H.X. Protection against doxorubicin-induced myocardial dysfunction in mice by cardiac-specific expression of carboxyl terminus of hsp70-interacting protein. Sci Rep. 2016;6:28399. doi: 10.1038/srep28399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi M., Usui F., Karasawa T., Kawashima A., Kimura H., Mizushina Y. NLRP3 deficiency reduces macrophage Interleukin-10 production and enhances the susceptibility to doxorubicin-induced cardiotoxicity. Sci Rep. 2016;6:26489. doi: 10.1038/srep26489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao Y., Chen R., Ying C., Zhang G., Rui T., Tao A. Interleukin-33 attenuates doxorubicin-induced cardiomyocyte apoptosis through suppression of ASK1/JNK signaling pathway. Biochem Biophys Res Commun. 2017;493(3):1288–1295. doi: 10.1016/j.bbrc.2017.09.153. [DOI] [PubMed] [Google Scholar]
- 11.Chou F.C., Chen H.Y., Chen H.H., Lin G.J., Lin S.H. Sytwu HK6. Differential modulation of IL-12 family cytokines in autoimmune islet graft failure in mice. Diabetologia. 2017;60(12):2409–2417. doi: 10.1007/s00125-017-4418-9. [DOI] [PubMed] [Google Scholar]
- 12.Schönfelder T., Brandt M., Kossmann S., Knopp T., Münzel T., Walter U. Lack of T-bet reduces monocytic interleukin-12 formation and accelerates thrombus resolution in deep vein thrombosis. Sci Rep. 2018;8(1):3013. doi: 10.1038/s41598-018-21273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Issaranggun Na Ayuthaya B., Everts V., Pavasant P. The immunopathogenic and immunomodulatory effects of interleukin-12 in periodontal disease. Eur J Oral Sci. 2018;126(2):75–83. doi: 10.1111/eos.12405. [DOI] [PubMed] [Google Scholar]
- 14.Uyemura K., Demer L.L., Castle S.C., Jullien D., Berliner J.A., Gately M.K. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97(9):2130–2138. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X., Shao Y., Sha X., Fang P., Kuo Y.M., Andrews A.J. IL-35 (Interleukin-35) suppresses endothelial cell activation by inhibiting mitochondrial reactive oxygen species-mediated site-specific acetylation of H3K14 (histone 3 lysine 14) Arterioscler Thromb Vasc Biol. 2018;38(3):599–609. doi: 10.1161/ATVBAHA.117.310626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dambuza I.M., He C., Choi J.K., Yu C.R., Wang R., Mattapallil M.J. IL-12p35 induces expansion of IL-10 and IL-35-expressing regulatory B cells and ameliorates autoimmune disease. Nat Commun. 2017;8(1):719. doi: 10.1038/s41467-017-00838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bettini M., Castellaw A.H., Lennon G.P., Burton A.R., Vignali D.A. Prevention of autoimmune diabetes by ectopic pancreatic b-cell expression of Interleukin-35. Diabetes. 2012;61(16):1519–1526. doi: 10.2337/db11-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niedbala W., Wei X.Q., Cai B., Hueber A.J., Leung B.P., Mcinnes I.B. IL-35 is anovel cytokine with therapeutic effects against collagen-induced arthritis throughthe expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol. 2007;37(11):3021–3029. doi: 10.1002/eji.200737810. [DOI] [PubMed] [Google Scholar]
- 19.Kochetkova I., Golden S., Holderness K., Callis G., Pascual D.W. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen IIinduced arthritis via the production of IL-10. J Immunol. 2010;184(12):7144–7153. doi: 10.4049/jimmunol.0902739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W., Guo H., Li H., Yan Y., Wu C., Wang X. Interleukin-35 gene-modified mesenchymal stem cells protect concanavalin A-induced fulminant hepatitis by decreasing the interferon gamma level. Hum Gene Ther. 2018;29(2):234–241. doi: 10.1089/hum.2017.171. [DOI] [PubMed] [Google Scholar]
- 21.Li Y., Zhang C., Wu Y., Han Y., Cui W., Jia L. Interleukin-12p35 deletion promotes CD4 T-cell-dependent macrophage differentiation and enhances angiotensin II-Induced cardiac fibrosis. Arterioscler Thromb Vasc Biol. 2012;32(7):1662–1674. doi: 10.1161/ATVBAHA.112.249706. [DOI] [PubMed] [Google Scholar]
- 22.Kan X., Wu Y., Ma Y., Zhang C., Li P., Wu L. Deficiency of IL-12p35 improves cardiac repair after myocardial infarction by promoting angiogenesis. Cardiovasc Res. 2016;109(2):249–259. doi: 10.1093/cvr/cvv255. [DOI] [PubMed] [Google Scholar]
- 23.Zaharoff David A., Hoffman Benjamin S., Brooks Hooper H., Benjamin Compton J., Jr., Khurana Kiranpreet K., Hance Kenneth W. Intravesical immunotherapy of superficial bladder cancer with chitosan/interleukin-12. Cancer Res. 2009;69(15):6192–6199. doi: 10.1158/0008-5472.CAN-09-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akolkar G., da Silva Dias D., Ayyappan P., Bagchi A.K., Jassal D.S., Salemi V.M.C. Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2017;313(4):H795–H809. doi: 10.1152/ajpheart.00253.2017. [DOI] [PubMed] [Google Scholar]
- 25.Thomas A.W., Kevin M.V. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Son B.K., Sawaki D., Tomida S., Fujita D., Aizawa K., Aoki H. Granulocyte macrophage colony-stimulating factor is required for aortic dissection/intramural haematoma. Nat Commun. 2015;6:6994. doi: 10.1038/ncomms7994. [DOI] [PubMed] [Google Scholar]
- 27.Vozenilek A.E., Navratil A.R., Green J.M., Coleman D.T., Blackburn C.M.R., Finney A.C. Macrophage-associated Lipin-1 enzymatic activity contributes to modified low-density lipoprotein-induced proinflammatory signaling and atherosclerosis. Arterioscler Thromb Vasc Biol. 2018;28(2):324–334. doi: 10.1161/ATVBAHA.117.310455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yahagi K., Kolodgie F.D., Lutter C., Mori H., Romero M.E., Finn A.V. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2017;37(2):191–204. doi: 10.1161/ATVBAHA.116.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu M.F., Tang P.L., Qian Z.M. M Ashraf. Effects by doxorubicin on the myocardium are mediated by oxygen free radicals. Life Sci. 2001;68(8):889–901. doi: 10.1016/s0024-3205(00)00990-5. [DOI] [PubMed] [Google Scholar]
- 30.Simůnek T., Stérba M., Popelová O., Adamcová M., Hrdina R., Gersl V. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep. 2009;61(1):154–171. doi: 10.1016/s1734-1140(09)70018-0. [DOI] [PubMed] [Google Scholar]
- 31.Horenstein M.S., Vander Heide R.S., L'Ecuyer T.J. Molecular basis of anthracycline-induced cardiotoxicity and its prevention. Mol Genet Metab. 2000;71(1–2):436–444. doi: 10.1006/mgme.2000.3043. [DOI] [PubMed] [Google Scholar]
- 32.Li K., Sung R.Y., Huang W.Z., Yang M., Pong N.H., Lee S.M. Thrombopoietin protects against in vitro and in vivo cardiotoxicity induced by doxorubicin. Circulation. 2006;113(18):2211–2220. doi: 10.1161/CIRCULATIONAHA.105.560250. [DOI] [PubMed] [Google Scholar]
- 33.Zhu S.G., Kukreja R.C., Das A., Chen Q., Lesnefsky E.J., Xi L. Dietary nitrate supplementation protects against Doxorubicin-induced cardiomyopathy by improving mitochondrial function. J Am Coll Cardiol. 2011;57(2):2181–2189. doi: 10.1016/j.jacc.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timmins J.M., Ozcan L., Seimon T.A., Li G., Malagelada C., Backs J. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119(10):2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu W., Zhang W., Shou W., Field L.J. P53 inhibition exacerbates late-stage anthracycline cardiotoxicity. Cardiovasc Res. 2014;103(1):81–89. doi: 10.1093/cvr/cvu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J., Guo W., Lin S.Z., Wang Z.J., Kan J.T., Chen S.Y. Gp130-mediated STAT3 activation by S-propargyl-cysteine, an endogenous hydrogen sulfide initiator, prevents doxorubicin-induced cardiotoxicity. Cell Death Dis. 2016;7(8) doi: 10.1038/cddis.2016.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schröder M., Sutcliffe L. Consequences of stress in the secretory pathway: The ER stress response and its role in the metabolic syndrome. Methods Mol Biol. 2016;648:43–62. doi: 10.1007/978-1-60761-756-3_3. [DOI] [PubMed] [Google Scholar]
- 38.Ryter S.W., Mizumura K., Choi A.M. The impact of autophagy on cell death modalities. Int. J. Cell Biol. 2014;2014:502676. doi: 10.1155/2014/502676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jing K., Lim K. Why is autophagy important in human diseases? Exp Mol Med. 2012;44(2):69–72. doi: 10.3858/emm.2012.44.2.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gump J.M., Thorburn A. Autophagy and apoptosis-what is the connection? Trends Cell Biol. 2011;21(7):387–392. doi: 10.1016/j.tcb.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su M., Mei Y., Sinha S. Role of the crosstalk between autophagy and apoptosis in cancer. J Oncol. 2013;2013:102735. doi: 10.1155/2013/102735. [DOI] [PMC free article] [PubMed] [Google Scholar]