Abstract
Background
Circadian rhythms are fundamental to health and are particularly important for mental wellbeing. Disrupted rhythms of rest and activity are recognised as risk factors for major depressive disorder and bipolar disorder.
Methods
We conducted a genome-wide association study (GWAS) of low relative amplitude (RA), an objective measure of rest-activity cycles derived from the accelerometer data of 71,500 UK Biobank participants. Polygenic risk scores (PRS) for low RA were used to investigate potential associations with psychiatric phenotypes.
Outcomes
Two independent genetic loci were associated with low RA, within genomic regions for Neurofascin (NFASC) and Solute Carrier Family 25 Member 17 (SLC25A17). A secondary GWAS of RA as a continuous measure identified a locus within Meis Homeobox 1 (MEIS1). There were no significant genetic correlations between low RA and any of the psychiatric phenotypes assessed. However, PRS for low RA was significantly associated with mood instability across multiple PRS thresholds (at PRS threshold 0·05: OR = 1·02, 95% CI = 1·01–1·02, p = 9·6 × 10−5), and with major depressive disorder (at PRS threshold 0·1: OR = 1·03, 95% CI = 1·01–1·05, p = 0·025) and neuroticism (at PRS threshold 0·5: Beta = 0·02, 95% CI = 0·007–0·04, p = 0·021).
Interpretation
Overall, our findings contribute new knowledge on the complex genetic architecture of circadian rhythmicity and suggest a putative biological link between disrupted circadian function and mood disorder phenotypes, particularly mood instability, but also major depressive disorder and neuroticism.
Funding
Medical Research Council (MR/K501335/1).
Keywords: Circadian rhythmicity, Mood instability, Relative amplitude, Gwas, Polygenic risk score
Research in context.
Evidence before this study
Circadian rhythms are important for the maintenance of both physical and mental wellbeing, and disrupted rest/activity rhythms are recognised as core features of both bipolar disorder and major depressive disorder. The underlying genetic architecture of disrupted circadian rhythmicity is not yet fully understood. Genetic studies to date include candidate gene analyses of core “clock” gene variants and larger-scale genome-wide studies of subjective reports of sleep duration, insomnia and chronotype. We searched PubMed for human studies of the genetics of objectively-assessed circadian rhythmicity and mood disorders published in English before November 1, 2017. We used “accelerometry” or “actigraphy”, and “bipolar” or “depression”, and “circadian” and “amplitude”, and “gene” or “gwas” as search terms. We identified only 5 studies of interest. Some compared expression of circadian clock genes – as measures of circadian rhythmicity – between healthy controls and patients with mood disorders, or in response to different therapeutic drugs. Others applied genetic linkage analysis to actigraph (activity) data from both healthy individuals and from patients with mood disorders. Additional searches of “relative amplitude” (a common derived measure of circadian rest-activity rhythmicity) and “mood disorders” found that most studies reported lower amplitude in individuals with bipolar disorder or depression versus controls. More specifically, recently, low relative amplitude was reported to be associated with several mood disorder phenotypes within UK Biobank.
Added value of this study
To our knowledge, this is the first large scale genome-wide association study of relative amplitude, an objective measure of rest-activity cycles. We identified genes of interest with putative biological links to mood disorders. We also identified associations between polygenic risk of low relative amplitude and major depression, neuroticism and mood instability.
Implications of all the available evidence
Circadian rhythm disruption is a core feature of mood disorders and the genetic variants identified in this study may be involved in the pathophysiology of mood disorders. These findings could provide new insights into the biology of both circadian function and mood disorders, and may lead to new treatment targets for mood disorders.
Alt-text: Unlabelled Box
1. Introduction
Circadian rhythms are variations in physiology and behaviour that recur approximately every 24 h [1]. They include rhythms of body temperature, hormone release, activity, concentration, mood, eating and sleeping [2]. Circadian rhythmicity is coordinated centrally by the suprachiasmatic nucleus in the anterior hypothalamus [2] and plays a fundamental role in homeostasis and the maintenance of both physical and mental wellbeing [2, 3]. Disruption to circadian rhythmicity is associated with a range of adverse health outcomes, including cardiovascular disease, obesity, diabetes and some cancers [[4], [5], [6]], as well as increased risk for major depressive disorder (MDD) and bipolar disorder (BD) [[7], [8], [9]].
Circadian rhythmicity is regulated by both exogenous environmental stimuli (“zeitgebers”) and by genetic factors [10]. Several core circadian clock genes are involved in autoregulatory transcription/translational feedback loops that maintain cell-cycle function [11]. However, the control of circadian rhythms is likely to be polygenic, with regulatory genes and pathways still to be identified [3, 12].
To date, the most commonly used measure of circadian phenotypes has been subjectively-reported chronotype, defined as an individual's preference for morning or evening wakefulness and activity [13]. Evening chronotype is more likely to be associated with adverse health outcomes [3, [14], [15], [16]]. Recently, genome wide association studies (GWAS) of chronotype, self-reported sleep duration, and accelerometer-derived sleep traits have identified several independent genetic loci previously implicated in the regulation of circadian function (including PER2, PER3, RSG16, AK5, FBXL13), in addition to novel associated genetic loci [[17], [18], [19], [20], [21], [22]].
However, as a subjective measure, chronotype can be vulnerable to response bias. It may also have inconsistent associations with more objective measures of circadian rhythmicity [23]. In a recent study of over 91,488 individuals in the UK Biobank cohort, we derived objective measures of rest-activity rhythmicity from accelerometer data [24]. We found that low relative amplitude (RA), a measure of disrupted rest-activity rhythm (individuals with RA two standard deviations below the sample mean RA), was associated with several mood disorder phenotypes [24]. We now extend this work by conducting a GWAS of low RA in the largest sample known to date from UK Biobank. We also assess the degree of genetic correlation between low RA and several psychiatric phenotypes, including attention deficit hyperactivity disorder (ADHD), BD, MDD, mood instability, post-traumatic stress disorder (PTSD), schizophrenia, anxiety and insomnia. Further, within a group of UK Biobank participants who were not part of the primary GWAS study (up to 141,000 individuals), we test for association between increased polygenic risk score (PRS) for low RA and mood-related trait phenotypes (specifically BD, MDD, generalised anxiety disorder (GAD), mood instability and neuroticism).
2. Methods
2.1. Participants and Ethical Approval
Over 502,000 United Kingdom (UK) residents aged 37–73 years were recruited to the UK Biobank cohort from 2006 to 2010. At one of 22 assessment centres across the UK, participants completed a range of lifestyle, demographic, health, mood, cognitive and physical assessments and questionnaires [25]. Here, we used data from 91,448 participants who also provided accelerometer data that passed quality control (details below) and who had available data on all covariates included within fully and/or partially adjusted models for use in the GWAS. UK Biobank obtained informed consent from all participants and this study was conducted under generic approval from the NHS National Research Ethics Service (approval letter dated 13 May 2016, Ref 16/NW/0274) and under UK Biobank approvals for applications 12,761 (PI Cathy Wyse; accelerometer data for use in GWAS) and 6553 (PI Daniel Smith; correlations with psychiatric traits) (Supplementary Table 1).
2.2. Accelerometry Data Collection and Pre-Processing
In 2013, 240,000 UK Biobank participants were invited to wear an accelerometer for seven days as part of a physical activity monitoring investigation [26]. Of these, 103,720 (43%) accepted and returned the accelerometer to UK Biobank after use. Participants received a wrist-worn Axivity AX3 triaxial accelerometer in the post and were asked to wear the device on their dominant wrist continuously for seven days, while continuing with their normal activities. At the end of the seven-day period, participants were instructed to return the accelerometer to UK Biobank using a prepaid envelope. Accelerometers were calibrated to local gravity. Devices recorded data at a sampling rate of 94-104 Hz, and data were resampled to 100 Hz offline. Periods where no data were recorded for >1 s were coded as missing, and machine noise was removed using a Butterworth low-pass filter (cut-off 20 Hz). Raw activity intensity data were combined into five second epochs. Further details on data pre-processing [26] are available from UK Biobank at http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=131600.
2.3. Circadian Rest-Activity Rhythmicity (Relative Amplitude)
From the summary five second epoch data, a measure of relative amplitude (RA) was calculated using Clocklab Version 6 (Actimetrics). This accelerometer-derived activity measure has demonstrated reliability and validity [27]. RA is used commonly as a non-parametric measure of rest-activity rhythm amplitude. It is defined as the relative difference between the most active continuous 10-h period (M10) and the least active continuous 5-h period (L5) in an average 24-h period, using the formula below: [28].
M10 is the mean activity during the continuous 10 h period containing maximum activity in each 24 h recording period (midnight to midnight). L5 is the mean activity for the corresponding 5 h period containing the minimum activity within the same recording period. For each individual, the RA data point was the mean RA value across all included 24-h periods (seven days). RA ranges from 0 to 1, with higher values indicating greater distinction between activity levels during the most and least active periods of the day.
Participants who provided accelerometer data for <72 h (excluded N = 3) or who did not provide data for all one-hour periods within the 24-h cycle were excluded from analyses (excluded N = 6860). Participants were also excluded if their data was identified by UK Biobank as having poor calibration; we excluded participants with data flagged by UK Biobank as unreliable (unexpectedly small or large size (excluded N = 32)) and participants whose wear-time overlapped with a daylight savings clock change (excluded N = 4500) [24]. In the current sample (N = 91,870), mean RA was 0·87 (SD = 0·06; range 0·121–0·997), similar to previously reported values in healthy populations [8], however the distribution of RA values observed in our sample was negatively skewed (Supplementary Fig. 1).
Several further metrics of circadian rhythm can be derived from accelerometry data, e.g., interdaily stability, intradaily variability and cosinor amplitude. However, these measure are more vulnerable than RA to noise or small numbers of data points (as used here), or assume data take sinusoidal form (cosinor amplitude) [29, 30], which often does not apply to rest-activity data.
2.4. Genotyping and Imputation
UK Biobank released genotypic data for over 500,000 participants using two genotyping arrays specifically designed for UK Biobank with 95% shared marker content [31]. Approximately 10% of these participants were genotyped using Applied Biosystems UK BiLEVE Axiom array by Affymetrix each containing over 800,000 markers (with 95% common content between arrays), with the remaining participants being genotyped using Applied Biosystems UK Biobank Axiom Array. Phasing on the autosomes was done using SHAPEIT3 using the 1000 Genomes Phase 3 dataset as a reference panel. Imputation of a further 90,000,000 single nucleotide polymorphism (SNP) genotypes was carried out using IMPUTE4; employing as reference both the merged UK10K and 1000 Genomes Phase 3 reference panel, as used for the UK Biobank interim genotype data release, and the HRC reference panel. Pre-imputation quality control, imputation and post-imputation cleaning were conducted centrally by UK Biobank, described in an open access document [31].
2.5. Primary GWAS of Low Relative Amplitude
Our primary GWAS was a study of cases of low RA defined as cases with a mean RA greater than two standard deviations below the overall mean RA, with the remaining participants as controls [24]. Before proceeding with genetic analyses, exclusions were applied to the data. Individuals were removed according to UK Biobank genomic analysis exclusions, failure of quality control, gender mismatch, sex chromosome aneuploidy, ethnicity (not Caucasian), lack of accelerometry data, plus other accelerometry exclusions, as noted above. For related individuals (first cousins or closer), a single individual was randomly selected from each pair of related individuals for inclusion in the analysis. After these (and the acclelerometry-based) exclusions, 71,500 individuals were available for GWAS. Data was further refined by removing SNPs with an imputation score of <0·8, minor allele frequency of <0·01 and Hardy-Weinberg equilibrium p < 1 × 10−6 resulting in 7,969,123 variants remaining.
The primary association analysis was conducted using logistic regression in PLINK [32]; an additive allelic effects model was used with sex, age, genotyping array, and the first eight genetic principal components as covariates. For the GWAS, genome-wide significance was less than p < 5 × 10−8.
2.6. Secondary GWAS of RA as a Continuous Trait
The BOLT-LMM method allows the inclusion of related individuals within GWAS. This method relaxes the assumptions of the standard GWAS, as used above, by using a mixture of two Gaussian priors and is a generalisation of a standard mixed model. This mixed model accounts for both relatedness and population stratification; this results in greater power when compared to principal component analysis [33]. As above, individuals were removed according to UK Biobank genomic analysis exclusions, failure of quality control, gender mismatch, sex chromosome aneuploidy, ethnicity (not Caucasian), and lack of accelerometry data. After these exclusions, 77,440 individuals were available for this GWAS. As above, genome-wide significance was less than p < 5 × 10−8. Note that due to the imbalance in sample size between cases and controls available for low RA, we were unable to use the BOLT-LMM approach for the primary GWAS [33].
2.7. Expression Quantitative Trait Locus (eQTL) Analysis
The lead SNP from each locus, identified by GWAS, was assessed for the possibility of expression quantitative trait loci (eQTLs). This genotype-specific gene expression was assessed using the GTEx portal. The portal was also used to investigate tissue-specific expression for the implicated genes [34].
2.8. Gene-Based Analysis
The summary statistics from both the primary and secondary GWAS analyses were uploaded to FUMA web application for gene-based analyses [35]. Gene-based analyses were carried out based on the MAGMA method using all genetic associations within the summary statistics [35, 36]. For these analyses genome-wide significance was set at p < 5 × 10−5.
2.9. Genetic Correlations Between Low RA and Psychiatric Phenotypes
Linkage Disequilibrium Score Regression (LDSR) was applied to the GWAS summary statistics to provide an estimate of SNP heritability (h2SNP) [37, 38]. LDSR was also used to investigate genetic correlations between low RA and anxiety, ADHD, BD, MDD, mood instability, PTSD, schizophrenia and insomnia. The LD scores for these disorders were obtained using the summary statistics from the Psychiatric Genomics Consortium, CNCR-Complex Trait Genomics group, and UK Biobank. ADHD, BD, MDD, schizophrenia and insomnia were analysed using LD Hub [38].
2.10. Psychiatric Diagnoses, Neuroticism and Mood Instability
-
a)
Bipolar Disorder, Major Depressive Disorder and Generalised Anxiety Disorder
A mental health questionnaire (MHQ) was developed by a mental health research reference group to collect additional mental health phenotype data in UK Biobank and was administered during 2016–2017 [39]. The MHQ was used to obtain information about individuals' lifetime experiences of psychiatric disorders. The questions were based on a modified Composite International Diagnostic Interview Short Form (CIDI-SF). Lifetime depression (referred to here as ‘lifetime MDD’), ‘lifetime BD’ and lifetime generalised anxiety disorder (referred to as ‘lifetime GAD’) were evaluated based on answers provided by participants to the online MHQ. Therefore, these measurements represent a likelihood of diagnosis with the disorder of interest, rather than confirmed diagnoses [39]. Individuals who self-reported BD or MDD were excluded from the control groups. The ‘lifetime’ variables were also mutually exclusive. Designated cases for one variable were excluded from both the case and control groups for the other variables of interest. This approach was taken to minimise the confounding that comorbidity of psychiatric phenotypes could have on the analyses. Due to these exclusions, the number of observations for each association tested was different.
-
b)
Neuroticism
To define neuroticism a score was taken from the 12-item neuroticism scale of the Eysenck Personality Questionnaire-Revised Short Form (EPQ-R-S) [40, 41]. Individuals were given a score of 0 or 1 for a “no or yes” answer to each item, with total score from 0 to 12.
-
c)
Mood instability
A “mood instability” outcome measure was also obtained from the EPQ-R-S questionnaire: for one question participants were asked “Does your mood often go up and down?” and given the option to answer “yes”, “no”, “don't know” or “prefer not to answer” [40]. Individuals who selected “don't know” or “prefer not to answer” were coded as missing; this allowed the generation of a categorical mood instability variable where those who answered “yes” were designated as cases and participants who answered “no” were controls, those answering “don't know” and “prefer not to answer” were excluded [42].
2.11. Association Between PRS for Low RA and Affective Disorder Phenotypes
Associations between higher PRS for low RA and psychiatric diagnoses were examined in up to 76,018 individuals who had completed the MHQ and who were not included in the primary GWAS. Similarly, associations between low RA PRS and mood instability/neuroticism were examined in between 91,248 and 140,504 individuals (depending on the dependent variable) not included in the GWAS. PRS including SNPs at 6 different significance thresholds (p < 5 × 10−8, p < 5 × 10−5, p < 0·01, p < 0·05, p < 0·1, p < 0·5) were divided into quartiles, with the exception of p < 5 × 10−8 which was divided into tertiles as only three different PRS were generated for this threshold. The top and bottom quantiles were compared in logistic regression models that were adjusted for age, sex, Townsend deprivation index [43], genotype array and the first eight genetic principal components. False discovery rate (FDR) correction was applied [44, 45].
3. Results
3.1. GWAS of Low RA
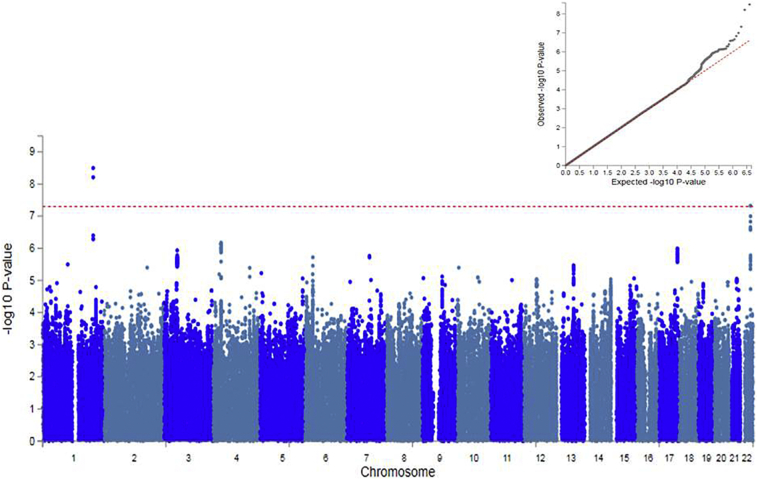
Our primary analysis was a case-control GWAS of low RA. The GWAS data showed only a slight deviation in test statistics compared to the null (λGC = 1·016, Fig. 1); this deviation may be due to the polygenic architecture of low relative amplitude. SNP heritability (h2SNP) accounted for <1% of the population variance (h2SNP = 0·0067, se = 0·0054). The Manhattan plot for low RA GWAS is presented in Fig. 1. Two independent genomic loci on chromosomes 1 and 22 were associated with low RA at genome-wide significance, including three SNPs (described in Supplementary Table 3). These SNPs highlighted two candidate gene loci: Neurofascin (NFASC) on chromosome 1 and Solute Carrier Family 25 Member 17 (SLC25A17) on chromosome 22 (Supplementary Fig. 3). As each of these SNPs is an intronic variant, the exact effect of each polymorphism is unclear.
Fig. 1.
SNP Manhattan plot and QQ plot (inset) of low RA GWAS (N = 2700 cases verses N = 68,300 controls).
Red line of Manhattan plot represents genome-wide significance (p < 5 × 10−8).
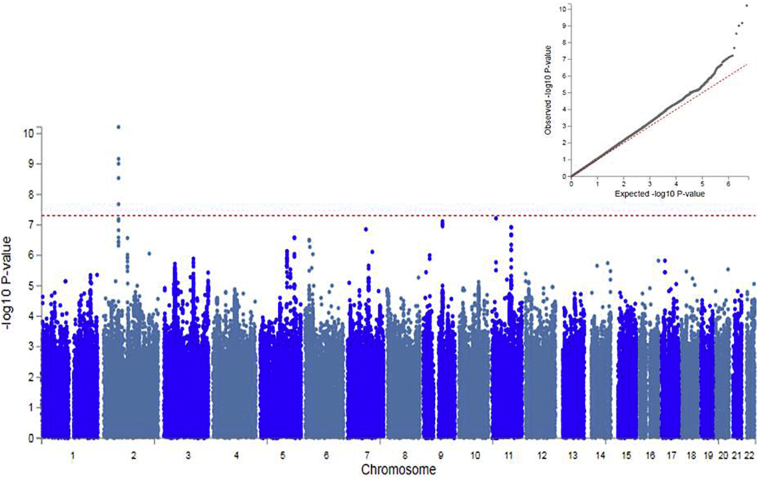
3.2. GWAS of Continuous RA
As a secondary analysis, we performed a GWAS of a continuous measure of RA using a BOLT-LMM model. The BOLT-LMM GWAS showed a slight deviation in the test statistics compared to the null (λGC = 1·054, Fig. 2), again consistent with a polygenic architecture for RA. The h2SNP for RA as a continuous measure accounted for >8% of the population variance (h2SNP = 0·085, se = 0·00035). Five SNPs, all localised to one locus on chromosome 2, were associated with continuous RA at genome-wide significance (described in Supplementary Table 3). These SNPs highlight the Meis Homeobox 1 (MEIS1) gene. Again, as noted above, these are intronic SNPs and their exact effects are not known.
Fig. 2.
SNP Manhattan plot and QQ plot (inset) of continuous RA GWAS (N = 77,440).
Red line of Manhattan plot represents genome-wide significance (p < 5 × 10−8).
3.3. Expression Quantitative Trait Loci (eQTL) Analysis
The lead SNPs from both GWAS were assessed for potential eQTLs. Only the lead SNP found on chromosome 22 (rs9611417) was associated with the expression of a nearby gene. Being heterozygous at rs9611417 was associated with lower expression of RANGAP1 gene in oesophageal mucosa in comparison to rs9611417 C allele homozygotes (Beta = −0·43, p = 7·2 × 10−5, Supplementary Fig. 6). Information on the influence of G homozygotes was not available.
3.4. Gene-Based Analysis of RA
Gene-based analyses of both low RA and continuous RA were undertaken. The gene-based analysis of low RA identified two genes significantly associated with low RA: Forkhead Box J1 (FOXJ1) on chromosome 17, and Zinc Finger FYVE-type Containing 21 (ZFYVE21) on chromosome 14 (Supplementary Fig. 4). The gene set analysis of continuous RA identified three genes: Copine 4 (CPNE4) and Chromosome 3 open reading frame 62 (C3orf62) on chromosome 3, and Renalase (RNLS) on chromosome 10 (Supplementary Fig. 5).
3.5. Genetic Correlation Between Low RA and Psychiatric Phenotypes
There was preliminary evidence of genetic correlation between low RA and insomnia (rg = 0·90, se = 0·42, p = 0·033), suggesting that the biology underlying low RA may be associated with the regulation of sleep (Table 1). However, on FDR correction this was not statistically significant. There was a negative genetic correlation between low RA and continuous RA (rg = −1·28, se = 0·38, p = 0·0008). As low RA is derived from the continuous measure of RA, a negative correlation was expected. There were no significant genetic correlations identified between low RA and ADHD, anxiety, BD, MDD, mood instability, PTSD and schizophrenia.
Table 1.
Genetic correlations between low relative amplitude and ADHD, anxiety, BD, MDD, mood instability, PTSD, schizophrenia and insomnia.
| Phenotype | rg | se | z | p | p FDR corrected |
|---|---|---|---|---|---|
| ADHD | 0·3476 | 0·4541 | 0·7656 | 0·444 | 0·932 |
| Anxiety | −0·0037 | 0·4224 | −0·0088 | 0·993 | 1·000 |
| BD | −0·0626 | 0·1842 | −0·3396 | 0·7341 | 1·000 |
| MDD | 0·0045 | 0·2527 | 0·0178 | 0·9858 | 1·000 |
| Mood Instability | −0·1587 | 0·2713 | -0·5847 | 0·5587 | 0·939 |
| PTSD | 0·7396 | 0·6201 | 1·1926 | 0·233 | 0·713 |
| Schizophrenia | 0·1541 | 0·1353 | 1·139 | 0·2547 | 0·713 |
| Insomnia | 0·8996 | 0·4222 | 2·1308 | 0·0331 | 0·278 |
Rg is the genetic correlation with low RA, se is the standard error of the correlation, z represents test statistic. p is the uncorrected p value and p FDR corrected has been adjusted for multiple testing.
3.6. Association Between PRS for Low RA and Affective Disorder Phenotypes
The findings of analyses assessing the association between low RA PRS and several mood disorder-related phenotypes are presented in Table 2. Positive associations were identified between increased PRS and mood instability at all PRS thresholds, with the exception of genome-wide significance (p < 5 × 10−8). For MDD, small positive associations were found for the low RA PRS at the top three significance thresholds (OR = 1·02–1·03), which remained significant after FDR correction (p = 0·025–0·05). A positive association with neuroticism was found for the highest threshold (p = 0·004, FDR adjusted p = 0·021). However, other associations between the remaining PRS thresholds and neuroticism score were not significant. These associations did not significantly differ when including individuals with more than one psychiatric phenotype (results not shown).
Table 2.
Associations between low RA PRS and psychiatric phenotypes.
| PRS p threshold | Outcome (Cases/Controls) | OR (95% CI) | p uncorrected | p FDR corrected |
|---|---|---|---|---|
| p < 5 × 10−8 | BD (406/37,699) | 0·99 (0·92,1·06) | 0·748 | 0·785 |
| p < 5 × 10−5 | 1·05 (0·98, 1·12) | 0·206 | 0·746 | |
| p < 0·01 | 1·03 (0·96, 1·11) | 0·355 | 0·746 | |
| p < 0·05 | 1·04 (0·96, 1·12) | 0·309 | 0·746 | |
| p < 0·1 | 1·02 (0·94, 1·10) | 0·617 | 0·785 | |
| p < 0·5 | 1·02 (0·93, 1·11) | 0·754 | 0·785 | |
| p < 5 × 10−8 | MDD (9543/24,317) | 1·00 (0·99, 1·02) | 0·812 | 0·805 |
| p < 5 × 10−5 | 1·00 (0·98, 1·02) | 0·966 | 0·805 | |
| p < 0·01 | 1·01 (0·99, 1·03) | 0·395 | 0·494 | |
| p < 0·05 | 1·02 (1·00, 1·04) | 0·03 | 0·05 | |
| p < 0·1 | 1·03 (1·01, 1·05) | 0·005 | 0·025 | |
| p < 0·5 | 1·03 (1·00, 1·05) | 0·021 | 0·05 | |
| p < 5 × 10−8 | GAD (2587/23,564) | 0·97 (0·95, 1·00) | 0·092 | 0·3 |
| p < 5 × 10−5 | 0·98 (0·96, 1·01) | 0·274 | 0·548 | |
| p < 0·01 | 0·99 (0·97, 1·02) | 0·729 | 0·729 | |
| p < 0·05 | 1·01 (0·98, 1·04) | 0·475 | 0·713 | |
| p < 0·1 | 1·03 (0·99, 1·06) | 0·1 | 0·3 | |
| p < 0·5 | 1·01 (0·97, 1·04) | 0·699 | 0·729 | |
| p < 5 × 10−8 | Mood Instability (78,710/91,248) | 1·00 (0·99, 1·01) | 0·913 | 0·94 |
| p < 5 × 10−5 | 1·01 (1·00, 1·02) | 0·019 | 0·0096 | |
| p < 0·01 | 1·01 (1·01, 1·02) | 9·5 × 10−5 | 2·2 × 10−4 | |
| p < 0·05 | 1·02 (1·01, 1·02) | 3·6 × 10−6 | 9·6 × 10−5 | |
| p < 0·1 | 1·01 (1·01, 1·02) | 8·3 × 10−5 | 5·9 × 10−4 | |
| p < 0·5 | 1·02 (1·01, 1·03) | 1·2 × 10−6 | 1·5 × 10−5 | |
| PRS p threshold | Outcome | Beta (95% CI) | p uncorrected | p FDR corrected |
| p < 5 × 10−8 | Neuroticism score (140,504) | -0·004 (−0·02, 0·007) | 0·456 | 0·399 |
| p < 5 × 10−5 | 0·01 (−0·003, 0·02) | 0·124 | 0·134 | |
| p < 0·01 | 0·01 (−0·002, 0·02) | 0·098 | 0·134 | |
| p < 0·05 | 0·01 (−0·005, 0·03) | 0·059 | 0·134 | |
| p < 0·1 | 0·01 (−0·003, 0·02) | 0·128 | 0·134 | |
| p < 0·5 | 0·02 (0·007, 0·04) | 0·004 | 0·021 |
4. Discussion
We present the first large-scale genetic study of an objective measure of rest-activity cycles in humans, as well as the first study to examine how common risk SNPs for circadian disruption might be associated with mood disorder phenotypes. The primary GWAS of low RA identified three genome-wide significant SNPs within two independent loci; two of the SNPs highlighted were found to be in relatively high LD (r2 = 0·66–1·00) in many populations [46] and are potentially tagging a single underlying functional variant influencing low RA. The secondary GWAS of RA as a continuous measure also identified five genome-wide significant SNPs at a single locus, within the MEIS1 gene on chromosome 2. Again, the SNPs identified are in medium to high LD with each other (r2 = 0·39–1·00) in many populations [47].
On inspection of the Manhattan plots there were several peaks whose lead SNPs had p-values below the suggestive evidence for significance threshold (p < 5 × 10−5 to p < 1 × 10−7) (Supplementary Tables 4 and 5). There was no genetic overlap between the SNPs identified for low RA and those identified for continuous RA at these thresholds. This suggests there is a partial genetic distinction between the pathological low RA measure and the broader range of RA.
4.1. Genes of Interest
Of considerable interest is that one of the genes highlighted by GWAS was NFASC, a gene encoding the neurofascin protein [48]. Neurofascin is an L1 family immunoglobulin cell adhesion molecule that interacts with several proteins to anchor voltage-gated Na+ channels to the intracellular skeleton in neurons [48]. It is involved in neurite outgrowth and organization of axon initial segments (AIS) during early development [48]. These AIS complexes (comprising neurofascin, ankyrin G (encoded by ANK3), gliomedin and betaIV spectrin) are important for the generation of action potentials and for the maintenance of neuronal integrity [49]. Notably, polymorphisms in ANK3 are robustly associated with BD [50]. The direct binding of neurofascin to ankyrin G at the AIS therefore represents a potentially important biological link between circadian rhythmicity and BD. The NFASC SNPs identified by the GWAS were intronic variants and the NFASC transcript undergoes extensive alternative splicing with not all variants being functionally categorised [48]. The precise influence these variants have on the NFASC gene is currently unclear.
One of the genome-wide significant SNPs from the primary GWAS is located within SLC25A17. This gene encodes a peroxisomal solute carrier membrane protein that transports several cofactors from the cytosol to the peroxisomal matrix [51]. Variants of this gene have previously been associated with autism spectrum disorder and schizophrenia [52]. The SLC25A17 gene is also involved in adrenomyeloneuropathy [53], an inherited condition in which long chain fatty acids accumulate in the central nervous system (CNS) disrupting several brain functions [54]. The risk allele at the SNP identified in the GWAS of low RA (rs9611417) was associated with lower expression of RANGAP1, a GTPase activator protein involved in nuclear transport [55]. RANGAP1 is 439 kb downstream of rs9611417 and shows relatively high expression in the brain (Supplementary Fig. 7).
The GWAS of RA as a continuous measure highlighted SNPs within the MEIS1 gene. This gene encodes a homeobox transcription factor (TF) protein crucial for the normal development of several tissues, including the CNS [56, 57]. The MEIS1 gene is also associated with myeloid leukaemia [56] and restless leg syndrome 7 (a sleep-wake disorder) [56, 57].
4.2. Gene-Based Analyses
The gene-based analysis of low RA identified two genes: FOXJ1 on chromosome 17, and ZFYVE21 on chromosome 14. FOXJ1 encodes a forkhead TF protein which has a role in cell differentiation in respiratory, reproductive, immune, and CNS tissues. FOXJ1 is required for the formation of cilia [58]. In mouse models, FOXJ1 was reported to be important for neurogenesis within the forebrain and olfactory bulb [58]. The ZFYVE21 gene encodes the zinc-finger FYVE-type containing 21 protein, involved in cell migration and adhesion [59]. The potential involvement of this gene in the brain and circadian function is currently unclear.
For the continuous measure of RA, three genes were identified in the gene-based analysis: CPNE4 and C3orf62 (chromosome 3); and RNLS on (chromosome 10). The CPNE4 gene encodes a calcium-dependent phospholipid binding protein involved in membrane trafficking and may be involved in intracellular calcium-mediated processes [60]. Deletion of this gene has been associated with earlier age-of-onset of Alzheimer's disease [61]. Currently, the C3orf62 gene is an uncharacterised protein coding gene that has not been functionally annotated [62].
RNLS encodes a flavin adenine dinucleotide-dependent amine oxidase, known as Renalase, an enzyme hormone secreted from the kidney into the bloodstream [63]. Renalase is involved in mediating cardiac function and blood pressure by influencing heart rate and has been associated with hypertension, chronic kidney failure and type 1 diabetes prediction [[63], [64], [65], [66]]. It is worth noting that disrupted circadian rhythmicity has been associated with both diabetes and hypertension in several studies [4, 67, 68], and that RNLS has been associated with both treatment outcome and episode recurrence in BD [69].
4.3. Polygenic Risk for Low RA and Psychiatric Phenotypes
We found little evidence of genetic correlation between low RA and psychiatric phenotypes. This is perhaps surprising given the literature on circadian rhythmicity and mood disorders. Core circadian clock genes have been associated with both BD and depression, with altered circadian biology suggested to be a vulnerability marker for mood disorders [[70], [71], [72], [73], [74]]. It has been suggested that the treatment of disrupted circadian rhythmicity could be used in combination with current pharmaceutical therapies to develop more effective treatments for mood disorders [75]. Therefore, a more complete understanding of circadian rhythmicity in the context of mood disorders is required.
Further, as noted above, within the UK Biobank cohort we recently found that lower RA was associated with several adverse mental health outcomes [24]. In the current study, we found some evidence for association between greater polygenic risk for low RA and both MDD and neuroticism. Across several PRS thresholds, we identified a more robust association between increasing PRS for low RA and the phenotype of mood instability. Mood instability is a common symptom that cuts across several psychiatric disorders and, as such, may be a more useful phenotype than categorical diagnoses for understanding underlying biology [76]. The possibility of a direct link between genetic loading for circadian disruption and the experience of dysregulated or unstable mood is therefore of considerable interest and merits further investigation.
5. Limitations
Several limitations are acknowledged. UK Biobank may not be fully representative of the general UK population [77], with a possible under-representation of individuals with psychiatric disorders. The polygenic risk scores created using only the genome-wide significant SNPs did not show any associations with lifetime mood disorder diagnoses, mood instability or neuroticism outcomes tested. As only 3 genome-wide significant SNPs were included in these analyses, they may be underpowered. The PRSs (at other thresholds) showed relatively small effects overall on the traits investigated and appeared to explain only a small proportion of the variance within the traits.. The use of the LDSC package for estimating SNP heritability in low RA cases (1.2 million SNPs rather than 9 million SNPs assessed by the BOLT-LMM approach) could have resulted in an underestimation of trait heritability. Also, with the relatively small sample size of low RA cases, our analyses may be underpowered resulting in a lower estimate of SNP heritability than expected (for example, compared to chronotype) [18]. Previous investigations of accelerometer-based phenotypes using selected clinical samples (such as bipolar disorder) reported higher heritability estimates (h2 > 0·30) [78]. The use of a non-clinical, general population cohort with potential under-representation of psychiatric disorders, may also have resulted in a SNP heritability estimate lower than might be expected.
In terms of phenotyping, the mood instability phenotype was a self-reported subjective measure that may be influenced by response bias. Further, we were unable to identify a direction of causality between RA and the psychiatric phenotypes: approaches to causality such as Mendelian randomisation (MR) were not possible due to the small number of genome-wide significant SNPs identified by both GWAS analyses [79].
Circadian rhythms are subject to influence from both biological factors and environmental stimuli. It is a potential limitation of this investigation that the analyses were not adjusted for potential non-genetic confounders operating during the accelerometry data collection period, such as medical illness, medication status, chronic pain transmeridian air travel, obesity and irregular work patterns, all factors known to affect circadian rhythmicity. The accelerometers were worn for 7 days which may not accurately represent rhythmicity, particularly in working participants where weekend rhythms might differ substantially from weekday rhythms. Further studies are required to investigate the possible interaction between RA and environmental factors; future investigations may also benefit from the inclusion of other measures of RA variability to adjust for intra- and inter-individual activity levels.
6. Conclusions
Overall, our findings contribute new knowledge on the genetic architecture of rest-activity cycles and how this overlaps with some mood disorder phenotypes, such as mood instability. Several of the genetic variants identified are located within or close to genes which may have a role in the pathophysiology of mood disorders. It is hoped that these findings will act as stimulus for future work assessing the complex biological relationships between circadian function and psychiatric disorders.
Acknowledgments
Acknowledgements
We thank all participants in the UK Biobank study. UK Biobank was established by the Wellcome Trust, Medical Research Council, Department of Health, Scottish Government and Northwest Regional Development Agency. UK Biobank has also had funding from the Welsh Assembly Government and the British Heart Foundation. Data collection was funded by UK Biobank. RJS is supported by a UKRI Innovation-HDR-UK Fellowship (MR/S003061/1). JW is supported by the JMAS Sim Fellowship for depression research from the Royal College of Physicians of Edinburgh (173558). AF is supported by an MRC Doctoral Training Programme Studentship at the University of Glasgow (MR/K501335/1). KJAJ is supported by an MRC Doctoral Training Programme Studentship at the Universities of Glasgow and Edinburgh. DJS acknowledges the support of the Brain and Behaviour Research Foundation (Independent Investigator Award 1930) and a Lister Prize Fellowship (173096). JC acknowledges the support of The Sackler Trust and is part of the Wellcome Trust funded Neuroimmunology of Mood and Alzheimer's consortium that includes collaboration with GSK, Lundbeck, Pfizer and Janssen & Janssen. CLN is supported by a UKRI Innovation Fellowship (MR/R024774/1). AD acknowledges the support of the NIHR Biomedical Research Centre, Oxford; and the British Heart Foundation Centre of Research Excellence at Oxford (RE/13/1/30181). The funders had no role in the design or analysis of this study, decision to publish, or preparation of the manuscript.
Contributors
All authors contributed substantively to this work. DJS, MESB, JW, LML and AF were involved in study conception and design. CAW, JW, LML and AF were involved in data organization and statistical analyses. AF, LML, JW, RJS, BC, NG, CLN, KJAJ, CLN, AD, MESB, DML, CAW, DJS were involved in the interpretation of data. DJS, MESB, DML, JPP and CAW were involved in application to UK Biobank and data coordination. AF, LML and DJS drafted the report. All authors were involved in reviewing and editing of the manuscript and approved it. DJS, JW, LML and AF had full access to all the data in the study and take responsibility for the integrity and accuracy of analyses.
Declaration of Interests
No declaration of interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.08.004.
Contributor Information
Amy Ferguson, Email: a.ferguson.3@research.gla.ac.uk.
Daniel J. Smith, Email: Daniel.Smith@glasgow.ac.uk.
Appendix A. Supplementary data
Supplementary material
References
- 1.McClung C.A. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reppert S.M., Weaver D.R. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 3.Merikanto I. Circadian preference towards morningness is associated with lower slow sleep spindle amplitude and intensity in adolescents. Sci Rep. 2017;7:1–12. doi: 10.1038/s41598-017-13846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reutrakul S., Knutson K.L. Consequences of circadian disruption on cardiometabolic health. Sleep Med Clin. 2015;10:455–468. doi: 10.1016/j.jsmc.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wulff K., Gatti S., Wettstein J.G., Foster R.G. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- 6.Sigurdardottir L., Circadian Disription E.A. Sleep loss and prostate cancer risk: a systematic review of epidemiological studies. Cancer Epidemiol Biomarkers Prev. 2012;21:1002–1011. doi: 10.1158/1055-9965.EPI-12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton C. Activity monitoring in patients with depression: a systematic review. J Affect Disord. 2013;145:21–28. doi: 10.1016/j.jad.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Bullock B., Murray G. Reduced amplitude of the 24 hour activity rhythm: a biomarker of vulnerability to bipolar disorder? Clin Psychol Sci. 2014;2:86–96. [Google Scholar]
- 9.Ng T.H. Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: a systematic review and meta-analysis. Sleep Med Rev. 2015;20:46–58. doi: 10.1016/j.smrv.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Charrier A., Olliac B., Roubertoux P., Tordjman S. Clock genes and altered sleep – wake rhythms : their role in the development of psychiatric disorders. Int J Mol Sci. 2017;18:1–22. doi: 10.3390/ijms18050938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koike N. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals nobuya. Science. 2012;80(338):349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang E.E. A genome-wide RNAi screen for modifiers of the circadian clock in human cells. Cell. 2009;139:199–210. doi: 10.1016/j.cell.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alloy L.B., Ng T.H., Titone M.K., Boland E.M. Circadian rhythm dysregulation in bipolar Spectrum disorders. Curr Psychiatry Rep. 2017 doi: 10.1007/s11920-017-0772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corruble E. Morningness-eveningness and treatment response in major depressive disorder. Chronobiol Int. 2014;31:283–289. doi: 10.3109/07420528.2013.834924. [DOI] [PubMed] [Google Scholar]
- 15.Dmitrzak-Węglarz M. Chronotype and sleep quality as a subphenotype in association studies of clock genes in mood disorders. Acta Neurobiol Exp (Wars) 2016;76(32–42) doi: 10.21307/ane-2017-003. [DOI] [PubMed] [Google Scholar]
- 16.Goel N., Basner M., Rao H., Dinges D.F. Circadian rhythms, sleep deprivation, and human performance. Prog Mol Biol Transl Sci. 2014;119:155–190. doi: 10.1016/B978-0-12-396971-2.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu Y. GWAS of 89,283 individuals identifies genetic variants associated with self-reporting of being a morning person. Nat Commun. 2016;7:1–9. doi: 10.1038/ncomms10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones S.E. Genome-Wide association analyses in 128, 266 individuals identifies new morningness and sleep duration loci. PLoS Genet. 2016;12:1–19. doi: 10.1371/journal.pgen.1006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane J.M. Genome-wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK biobank. Nat Commun. 2016;7:1–10. doi: 10.1038/ncomms10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones S.E. Genetic studies of accelerometer-based sleep measures in 85,670 individuals yield new insights into human sleep behaviour. bioRxiv. 2018:303925. doi: 10.1038/s41467-019-09576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dashti H. GWAS in 446,118 European adults identifies 78 genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. bioRxiv. 2018;274977 doi: 10.1038/s41467-019-08917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones S.E. Genome-wide association analyses of chronotype in 697,828 individuals provides new insights into circadian rhythms in humans and links to disease. bioRxiv. 2018;303941 doi: 10.1038/s41467-018-08259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taillard J., Philip P., Coste O., Sagaspe P., Bioulac B. The circadian and homeostatic modulation of sleep pressure during wakefulness differs between morning and evening chronotypes. J Sleep Res. 2003;12:275–282. doi: 10.1046/j.0962-1105.2003.00369.x. [DOI] [PubMed] [Google Scholar]
- 24.Lyall L.M. Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: a cross-sectional study of 91 105 participants from the UK biobank. Lancet Psychiatr. 2018;0 doi: 10.1016/S2215-0366(18)30139-1. [DOI] [PubMed] [Google Scholar]
- 25.Sudlow C. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doherty A. Large scale population assessment of physical activity using wrist worn accelerometers: the UK biobank study. PLoS One. 2017;12:1–14. doi: 10.1371/journal.pone.0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259–267. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Van Someren E.J.W. Circadian rest-activity rhythm disturbances in Alzheimer's disease. Biol Psychiatry. 1996;40:259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 29.Gonçalves B.S.B., Cavalcanti P.R.A., Tavares G.R., Campos T.F., Araujo J.F. Nonparametric methods in actigraphy: An update. Sleep Sci (Sao Paulo, Brazil) 2014;7:158–164. doi: 10.1016/j.slsci.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zielinski T., Moore A.M., Troup E., Halliday K.J., Millar A.J. Strengths and limitations of period estimation methods for circadian data. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bycroft C. 2017. Genome-wide genetic data on ~ 500, 000 UK Biobank participants. [Google Scholar]
- 32.Purcell S. Vol. 81. 2007. REPORT PLINK: A tool set for whole-genome association and population-based linkage analyses; pp. 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loh P.-R. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat Genet. 2015;47:284–290. doi: 10.1038/ng.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.GTEx Consortium, T. Gte. The genotype-tissue expression (GTEx) project. Nat Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe K., Taskesen E., van Bochoven A., Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8:1826. doi: 10.1038/s41467-017-01261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Leeuw C.A., Mooij J.M., Heskes T., Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol. 2015;11 doi: 10.1371/journal.pcbi.1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bulik-Sullivan B. An atlas of genetic correlations across human diseases and traits. Nat Publ Gr. 2015;47:1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng J. LD hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics. 2017;33:272–279. doi: 10.1093/bioinformatics/btw613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis K.A.S. Mental health in UK Biobank: development, implementation and results from an online questionnaire completed by 157 366 participants *. BJ Psych Open. 2018;4:83–90. doi: 10.1192/bjo.2018.12. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Eysenck S.B.G., Eysenck H.J., Barrett P. A revised version of the psychoticism scale. Personal Individ Differ. 1985;6:21–29. [Google Scholar]
- 41.Smith D.J. 2015. Genome-wide association study of neuroticism in UK biobank. [Google Scholar]
- 42.Ward J. Genome-wide analysis in UK biobank identifies four loci associated with mood instability and genetic correlation with major depressive disorder, anxiety disorder and schizophrenia. Transl Psychiatry. 2017;7:1264. doi: 10.1038/s41398-017-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Townsend P. Deprivation J Soc Policy. 1987;16:125–146. [Google Scholar]
- 44.Pike N. 2011. Using false discovery rates for multiple comparisons in ecology and evolution; pp. 278–282. [Google Scholar]
- 45.Benjamini Y., Hochberg Y., Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 46.rs147964682 (SNP) - Linkage disequilibrium - Homo sapiens - ensembl genome browser 92. http://www.ensembl.org/Homo_sapiens/Variation/HighLD?db=core;r=1:204927176-204928176;v=rs147964682;vdb=variation;vf=32409503 Available at.
- 47.rs113851554 (SNP) - Linkage disequilibrium - Homo sapiens - ensembl genome browser 92. http://www.ensembl.org/Homo_sapiens/Variation/HighLD?db=core;r=2:66522932-66523932;v=rs113851554;vdb=variation;vf=21204591;second_variant_name=rs142412330
- 48.Taylor A.M., Saifetiarova J., Bhat M.A. Postnatal loss of neuronal and glial Neurofascins differentially affects node of Ranvier maintenance and myelinated axon function. Front Cell Neurosci. 2017;11 doi: 10.3389/fncel.2017.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thaxton C. In vivo deletion of immunoglobulin domains 5 and 6 in Neurofascin (Nfasc) reveals domain-specific requirements in myelinated axons. J Neurosci. 2010;30:4868–4876. doi: 10.1523/JNEUROSCI.5951-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferreira M.A.R. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agrimi G., Russo A., Scarcia P., Palmieri F. The human gene SLC25A17 encodes a peroxisomal transporter of coenzyme A, FAD and NAD + Biochem J. 2012;443:241–247. doi: 10.1042/BJ20111420. [DOI] [PubMed] [Google Scholar]
- 52.The Autism Spectrum Disorders Working Group of The Psychiatric Genomics Consortium. Meta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophreniaMol Autism. 2017;8(21) doi: 10.1186/s13229-017-0137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Veldhoven P.P. Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J Lipid Res. 2010;51:2863–2895. doi: 10.1194/jlr.R005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moser A.B. Plasma very long chain fatty acids in 3,000 peroxisome disease patients and 29,000 controls. Ann Neurol. 1999;45:100–110. doi: 10.1002/1531-8249(199901)45:1<100::aid-art16>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 55.Bischoff F.R., Krebber H., Kempf T., Hermes I., Ponstingl H. Human RanGTPase-activating protein RanGAP1 is a homologue of yeast Rna1p involved in mRNA processing and transport. Proc Natl Acad Sci U S A. 1995;92:1749–1753. doi: 10.1073/pnas.92.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Q. Regulation of MEIS1 by distal enhancer elements in acute leukemia. Leukemia. 2014;28:138–146. doi: 10.1038/leu.2013.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiong L. MEIS1 intronic risk haplotype associated with restless legs syndrome affects its mRNA and protein expression levels. Hum Mol Genet. 2009;18:1065–1074. doi: 10.1093/hmg/ddn443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacquet B.V. Specification of a Foxj1-dependent lineage in the forebrain is required for embryonic-to-postnatal transition of neurogenesis in the olfactory bulb. J Neurosci. 2011;31:9368–9382. doi: 10.1523/JNEUROSCI.0171-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagano M. ZF21 protein, a regulator of the disassembly of focal adhesions and cancer metastasis, contains a novel noncanonical pleckstrin homology domain. J Biol Chem. 2011;286:31598–31609. doi: 10.1074/jbc.M110.199430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tomsig J.L., Creutz C.E. Copines: a ubiquitous family of Ca(2+)-dependent phospholipid-binding proteins. Cell Mol Life Sci. 2002;59:1467–1477. doi: 10.1007/s00018-002-8522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szigeti K. Genome-wide scan for copy number variation association with age at onset of Alzheimer's disease. J Alzheimers Dis. 2013;33:517–523. doi: 10.3233/JAD-2012-121285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gene: C3orf62 (ENSG00000188315) - Summary - Homo sapiens - ensembl genome browser 92. http://www.ensembl.org/Homo_sapiens/Gene/Summary?g=ENSG00000188315;r=3:49268602-49277909 Available at.
- 63.Xu J. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. 2005;115:1275–1280. doi: 10.1172/JCI24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lv Y.-B. Association of renalase SNPs rs2296545 and rs2576178 with the risk of hypertension: a meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frohnert B.I. Prediction of type 1 diabetes using a genetic risk model in the diabetes autoimmunity study in the young. Pediatr Diabetes. 2018;19:277–283. doi: 10.1111/pedi.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Desir G.V. Renalase deficiency in chronic kidney disease, and its contribution to hypertension and cardiovascular disease. Curr Opin Nephrol Hypertens. 2008;17:181–185. doi: 10.1097/MNH.0b013e3282f521ba. [DOI] [PubMed] [Google Scholar]
- 67.Merikanto I. Evening types are prone to depression. Chronobiol Int. 2013;30:719–725. doi: 10.3109/07420528.2013.784770. [DOI] [PubMed] [Google Scholar]
- 68.Merikanto I., Suvisaari J., Lahti T., Partonen T. Eveningness relates to burnout and seasonal sleep and mood problems among young adults. Nord J Psychiatry. 2016;70:72–80. doi: 10.3109/08039488.2015.1053519. [DOI] [PubMed] [Google Scholar]
- 69.Fabbri C., Serretti A. Genetics of long-term treatment outcome in bipolar disorder. Prog Neuro-Psychopharmacol Biol Psychiatr. 2016;65:17–24. doi: 10.1016/j.pnpbp.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 70.Partonen T. Clock gene variants in mood and anxiety disorders. J Neural Transm. 2012;119:1133–1145. doi: 10.1007/s00702-012-0810-2. [DOI] [PubMed] [Google Scholar]
- 71.Etain B., Milhiet V., Bellivier F., Leboyer M. Genetics of circadian rhythms and mood spectrum disorders. Eur Neuropsychopharmacol. 2011;21:S676–S682. doi: 10.1016/j.euroneuro.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 72.Liberman A.R., Halitjaha L., Ay A., Ingram K.K. Modeling strengthens molecular link between circadian polymorphisms and major mood disorders. J Biol Rhythms. 2018;33:318–336. doi: 10.1177/0748730418764540. [DOI] [PubMed] [Google Scholar]
- 73.Geoffroy P.A. Sleep in remitted bipolar disorder: a naturalistic case-control study using actigraphy. J Affect Disord. 2014;158:1–7. doi: 10.1016/j.jad.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 74.McCarthy M.J. Genetic and clinical factors predict lithium's effects on PER2 gene expression rhythms in cells from bipolar disorder patients. Transl Psychiatry. 2013;3 doi: 10.1038/tp.2013.90. e318–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bellivier F., Geoffroy P.-A., Etain B., Scott J. Sleep- and circadian rhythm–associated pathways as therapeutic targets in bipolar disorder. Expert Opin Ther Targets. 2015;19:747–763. doi: 10.1517/14728222.2015.1018822. [DOI] [PubMed] [Google Scholar]
- 76.Broome M.R., Saunders K.E.A., Harrison P.J., Marwaha S. Mood instability: significance, definition and measurement. Br J Psychiatry. 2015;207:283–285. doi: 10.1192/bjp.bp.114.158543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fry A. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pagani L. Genetic contributions to circadian activity rhythm and sleep pattern phenotypes in pedigrees segregating for severe bipolar disorder. Proc Natl Acad Sci U S A. 2016;113:E754–E761. doi: 10.1073/pnas.1513525113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davey Smith G., Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material