Abstract
Glucocorticoids (GCs) are hormones secreted by the adrenal glands as an endocrine response to stress. Although the main purpose of GCs is to restore homeostasis when acutely elevated, animal studies indicate that chronic exposure to these hormones can cause damage to the hippocampus. This is indicated by reductions in hippocampal volume, and changes in neuronal morphology (i.e.., decreases in dendritic length and number of dendritic branch points) and ultrastructure (e.g., smaller synapse number). Smaller hippocampal volume has been also reported in humans diagnosed with major depressive disorder or Cushing's disorder, conditions in which GCs are endogenously and chronically elevated. Although a number of studies considered neuron loss as the major factor contributing to the volume reduction, recent findings indicated that this is not the case. Instead, alterations in dendritic, synaptic and glial processes have been reported. The focus of this paper is to review the GCs effects on the cell number, dendritic morphology and synapses in an effort to better understand how these changes may contribute to reductions in hippocampal volume. Taken together, the data from animal models suggests that hippocampal volumetric reductions represent volume loss in the neuropil, which, in turn, under represent much larger losses of dendrites and synapses.
Keywords: stereology, dentate gyrus, CA3, CA1, corticosterone, cortisol
I. Introduction
A primary endocrine response to stress is the secretion of glucocorticoids (corticosterone in rats and cortisol in humans). Although glucocorticoids are normally elevated as a neuroendocrine response to acute threats to maintain homeostasis [1, 2], they are chronically elevated in a number of neuropsychiatric disorders (e.g., depression, Cushing's syndrome) [3, 4] as well as during chronic stress [5, 6]. In addition to endogenous elevations, glucocorticoids are also elevated through the use of prescription drugs, which serve to reduce immune responses such as inflammation [7]. The variety of conditions in which glucocorticoids are chronically elevated raises concerns about the accumulating effects of the prolonged exposure to this hormone. Systemic effects of long-term glucocorticoid elevations include heart disease, osteoporosis, and muscle wasting [7]. The finding that glucocorticoid receptors are also expressed in the brain [8] raised the possibility that elevated GCs may affect brain structure and function.
Glucocorticoids act on the brain through two known receptor types, the mineralocorticoid (MR) and glucocorticoid (GR) receptors. The rodent hippocampus has a high density of both GR and MR, whereas in primates the hippocampus has a high density of MR [9, 10]. The hippocampus, a structure important for learning and memory [11], mediates the regulation of glucocorticoid release through its indirect action on the Hypothalamic-Pituitary-Adrenal axis activity [12, 13]. The hippocampus is not the sole structure providing negative feedback; additional structures have been involved in the regulation of the Hypothalamic-Pituitary-Adrenal axis activity [14, 15], the high expression glucocorticoid receptors in the hippocampus may render this brain structure a target of glucocorticoid elevations. For this reason, the effects of elevated glucocorticoids have been studied extensively in the hippocampus, and many investigators have questioned the effects of both acute and chronic glucocorticoid elevations on hippocampal structure and function. These studies have been performed by testing for relationships, correlational or causal, between glucocorticoids and hippocampal structure, and between glucocorticoids and behaviors dependent upon hippocampal integrity. This review focuses on the relationship between glucocorticoids and hippocampal structure.
The first studies to address whether high glucocorticoid levels damage the hippocampus were carried out on animals [16-18]. The initial findings of pyknosis or cell loss [16, 19-21] and dendritic atrophy [22-24] as a result of glucocorticoid elevations led to the assumption that these losses would be reflected by a reduction in hippocampal volume. Only a few studies in animals, which will be discussed below, have directly measured hippocampal volume. In contrast, brain imaging techniques allow for the study of hippocampal volume in clinical populations with disorders associated with elevated plasma glucocorticoids (e.g., Cushing's syndrome, depression). These studies have caused a surge of interest in the association between prolonged exposure to glucocorticoids and hippocampal volume. The focus of this review is primarily on the relationship between chronic glucocorticoid elevations and their effects on hippocampal volume, cell number and synapses, specifically exploring the relationship between these three variables in an effort to better inform the interpretation of volumetric differences reported in clinical conditions that include elevated glucocorticoids. Specifically, we will briefly review the reports of smaller hippocampal volume in major depressive disorder and Cushing's syndrome, and then discuss animal research that addresses the causal relationship between glucocorticoids and hippocampal volume. The latter studies also address glucocorticoid effects on neuron number, dendritic atrophy, synapse numbers and how well the glucocorticoid effects on volume reflect changes in these tissue constituents.
II. Human Hippocampal Volume: Relationship to Glucocorticoids
Effects on Hippocampal Volume
The use of brain imaging techniques has allowed investigators to explore whether hippocampal damage occurs in individuals who suffer from disorders associated with high plasma glucocorticoids, two of which are Cushing's syndrome (CS) and major depressive disorder. Patients with Cushing's syndrome (CS) are exposed to elevated levels of endogenous cortisol for months to years due to adrenal pathology or hypersecretion of pituitary adrenocorticotropin hormone (ACTH) (Cushing's disease). Because of the hypercortisolemia associated with this disorder, the study of CS patients provides valuable information on the effects of prolonged excessive elevations of glucocorticoids on the human hippocampus. Starkman et al. [4] reported a negative correlation between the hippocampal volume and plasma cortisol levels in this clinical population; higher levels of cortisol were associated with lower volumetric measurements of the hippocampus. The finding that tumor removal and the consequent reductions in plasma cortisol corresponded to increases in hippocampal volume [25] perhaps provides the best support obtained from human subjects for a direct causal relationship between glucocorticoid elevations and hippocampal volume. As expected, there are reports of cognitive deficits that correspond to the deficits expected from impaired hippocampal function [4, 26]
The relationship between prolonged glucocorticoid elevations and hippocampal volume is further supported by studies of patients with major depressive disorder. It is well established that a large proportion of people suffering from depression are characterized by hyperactivity of the Hypothalamic-Pituitary-Adrenal axis [27], resulting in plasma cortisol elevations [3, 28]. As a result, a large number of neuroimaging studies have explored whether atrophy of the hippocampus, one of several structures implicated in depression, is associated with depression. Like the hippocampus, the frontal lobe dysfunction has been implicated in depression [29-31] and the frontal lobe, like the hippocampus undergoes structural modification in response to glucocorticoids and stress [32-35]. For the sake of focus, the present review will limit the discussion to the relationship between depression, glucocoriticoids and hippocampal volume. Sheline [36] and her colleagues were the first to report smaller volume in both right and left hippocampi of women with a history of major depression. Since then, many investigators have reported bilateral reductions in depressed subjects [37-41]. A number of other studies report unilateral volume reductions [42-45]. These findings have been confirmed in drug free depressed patients [46, 47]. However, a significant number of studies failed to report smaller volume in patients with depression [48-52]
The lack of consensus regarding an association between depression and hippocampal volume may result from methodological differences among studies including differences in disease parameters (e.g., age of onset, duration of illness, number of depressive episodes, type of depression) or scanning protocols (e.g., MRI resolution and inclusion of the amygdala). The heterogeneity among patient groups may have prevented a clear conclusion from being drawn about a relationship between cortisol, depression and hippocampal volume in this disorder. Trying to explain the inconsistent findings, Sheline and colleagues suggested that reductions in hippocampal volume are usually seen in studies that used higher MRI resolution and tended to include subjects with early-onset unipolar depression, in contrast to late onset or bipolar forms of the disorder [53]. However, subsequent meta-analyses of studies that used MRI to assess the volume of the hippocampus in patients with major depression showed that differences in scanning resolution, age at onset of depression, severity of depression at the time of MRI scanning, and gender cannot account for the discrepancy [54-56]. On the contrary, factors such as the duration of depression and number of episodes or days of untreated depression are inversely correlated with hippocampal volume [37, 41, 57-59]. The duration of depression and the number of depressive episodes contribute to an 8%-10% reduction of hippocampal volume in patients with major depressive syndrome compared to controls [56, 60]. Taken together, these data highlight the critical interaction between course of illness, and hippocampal volume alterations in major depressive disorder.
The human data are correlational. Without data on causation, it is difficult to fully exclude the possibility that smaller volume increases the vulnerability to multiple episodes of depression. One study of PTSD utilizing twins to explore the relationship between PTSD and hippocampal volume concluded that a smaller volume can predispose an individual to PTSD [61]. That raises the concern that individuals with smaller hippocampal volume may be predisposed to have a greater number of depression episodes and days untreated. Further raising concern that volume may precede rather than follow depression is a finding that variance in hippocampal volume in children is as great as in adulthood, and greater than the difference in volume reported between controls and patients with depression [62]. To further address this issue, a 3 year prospective study was conducted. Consistent with previous studies, individuals with depression had lower hippocampal volume than controls. Over the course of the three years, patients with incomplete remission and relapses had larger volume declines in the hippocampus, thereby supporting an active process of decline [63]. Because the population studies required in-patient treatment, the population studied had more severe depression than populations from other studies. Altogether, the severity of the disease appears to be correlated with the volume loss in the left hippocampus, and there is some support for progressive loss over the course of severe depression. The prospective study provides the strongest support for active volume reductions in the course of severe and recurrent depression. But these data do not directly address whether glucocorticoids elevations in depression cause such a reduction.
Although there is strong support for hippocampal volume reduction in depression, and some support for the reduction to result from an active process, there is little direct support for a glucocorticoid role in that process. There is a superficial correspondence between smaller volume and disorders with symptoms that include elevated plasma glucocorticoids. Few studies, however, have directly tested the relationship between glucocorticoid levels and hippocampal volume within samples. When the correlation between glucocorticoid concentrations and hippocampal volume was tested within samples with depression, neither study found a significant correlation [45, 51]. These results are surprising given the negative relationship between cortisol and hippocampal volume after tumor resection in Cushing's disease [25], and raise questions regarding whether the relationships are present only when plasma glucocorticoid concentrations are in extreme ranges. More generally, such studies raise the question of causality. Animal models offer the opportunity to directly test whether glucocorticoids can cause a reduction in hippocampal volume.
III. Glucocorticoid-induced Morphological Alterations in Hippocampus: Animal Models
The intriguing possibility that sustained cortisol elevations may decrease hippocampal volume is difficult to fully investigate in human subjects. The studies cited thus far have the advantage of measuring volume in humans at the time of a depression episode, and in some studies over time. The limitations, however, are the inability to investigate cells and ultrastructure at a time point close to volumetric measurement in order to understand how such changes contribute to volume reductions. Last, in many cases, it is difficult to control the duration of sustained cortisol, the magnitude of the elevations or the antidepressant naïve state to be certain that cortisol is the significant factor. Our understanding of the relations between glucocorticoids and hippocampal volume will be better understood by incorporating the use of animals models. These models provide the ability to test causal relations with the exclusion of confounding variables associated with disease states, treatments, variable environments and variable plasma glucocorticoid levels. They further provide the ability to carry out high resolution morphological analyses allowing for identification of the changes in dendritic, cellular and synapse changes related to volumetric reductions.
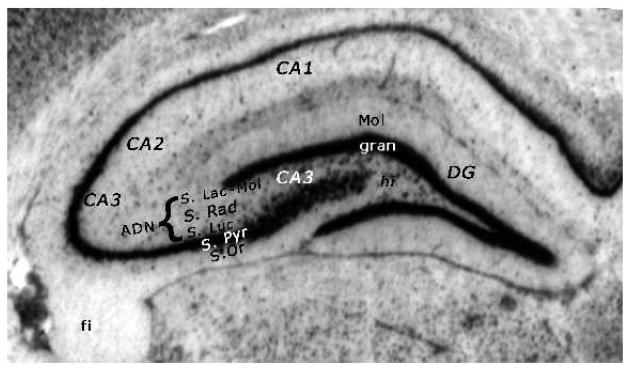
The incorporation of additional methods allow for higher resolution analysis of subregions, cells and ultrastructure. To date, subregional analysis in human subjects has been limited to analysis of the dorsal and ventral tails of hippocampus [64], whereas anatomical analysis in animals is typically divided into analysis of the dentate gyrus, CA3 and CA1 subregions, with further divisions into the layers such as the pyramidal cell layer, stratum oriens, and the neuropil containing the apical dendrites, which includes the stratum lucidum, radiatum and molecular (see Figure 1). The earliest focus was on whether or not glucocorticoid elevations affected hippocampal cell number. Subsequent studies focused on dendritic atrophy, synapse numbers, and overall volume.
Figure 1.
The hippocampus contains the dentate gyrus (DG) and Ammon's horn, which contains the CA3, CA2 and CA1 subregions. The dentate gyrus is the medial and inferior v-shaped structure that caps off the CA3 of Ammon's horn. The primary projection neurons of the DG are the granule cells, whose somata lie within a distinct layer (the granule cell layer: see gran on figure) and project dendrites into the molecular (MOL) layer. There are two blades of the dentate gyrus, the upper and lower blade, which together form the shape of a V. Within these blades lies another layer of the DG, the hilus (previously referred to as CA4), as well as a portion of the CA3. Extending laterally from DG is the remaining CA3 subregion. The CA3 region has distinct layers. The projection neurons are pyramidal cells whose cell bodies lie tightly packed in the Stratum Pyramidale. These cell bodies have basilar dendrites that project into the lower region, Stratum Oriens. The apical dendrites project upward through three layers, the through the Stratum Lucidum, Stratum Radiatrum and Stratum Lacunosum and Molecular. Since these distinct layers cannot be identified in Nissle stained or Golgi stained tissue, they have often been lumped together and referred to as the apical dendritic neuropil. Also unidentifiable in Nissl stained sections is the CA2 region, which shares many characteristics with the CA3 region except that cells in the CA3 region receive input from the DG granule cells in the form of mossy fiber projections forming contact in the Stratum Lucidum and this layer is missing in CA2. In Nissl stained sections it is possible to identify a thinning of the pyradmidal cell layer (S.Pyr) as a sign of the border between CA2 and CA1. CA1 has similar layers as those described for CA3, but without the S. Lucidum. For a thorough description of hippocampal anatomy see [113].
Effects on hippocampal cell number
Tissue volume reduction is typically assumed to reflect cell loss. Although cell loss in the hippocampus had been considered as a major factor contributing to the volume reduction [65], Czeh and Lucassen [66] have concluded that factors other than cell loss, such as alterations in dendritic, axonal, synaptic and glial processes are likely to explain the volume differences in the population of depressed patients. Studies of human post mortem tissue from people exposed to chronic elevations of glucocorticoids failed to support massive hippocampal pyramidal cell loss [67] or suppression of neurogenesis [68]. Hippocampal histological examination showed no loss of pyramidal cells in major-depressed patients [67, 69, 70]. Furthermore, the same patients exhibited no signs of apoptosis in CA3, as estimated by immunohistochemical labeling of a sensitive marker for cell death-associated DNA fragmentation [68].
In animals, the effects of elevated glucocorticoid levels on hippocampal neuronal number have been the focus of many researchers. The earliest research demonstrated that elevating cortisone increased pyknosis [16]. Subsequent investigators found a correlation between plasma adrenocorticoids and and astrocyte reactivity [18] and that controlling plastma adrenoscorticoids over age reduced cell loss [17]. Three month exposure of adult rats to corticosterone (5mg/day) caused a reduction in the neuronal density in the CA3 subfield of the hippocampus [19]. Similarly, CA3 neuronal loss was also reported after the same duration of corticosterone treatment [21] or one month of stress [20]. However, other researchers failed to detect neuronal loss in hippocampal areas after 3 months of corticosterone treatment [71]. All of these studies utilized traditional counting methods that rely on cell densities in single sections and are subject to bias from changes in cell size and volume of the brain. Such a bias is of particular concern because Sapolsky et al. [19] reported that corticosterone also reduced cell size. In the one study that controlled for cell size [71], cell loss was not reported. Thus, in the earlier studies, smaller cells may have been counted less often because they have a lower probability of lying within the section plane.
Subsequent investigators used unbiased counting methods and failed to find cell loss, despite using higher glucocorticoid doses. For example, no changes in the neuronal density in the dorsal CA3 hippocampal subfield have been reported after 56 days [72] of subcutaneous corticosterone injections of 27mg/kg. Even higher doses (40mg/kg) for one or three months failed to cause cell loss [73-75]. The absence of neuronal loss as a result of elevated glucocorticoids is further supported by the finding that corticosterone administration did not cause a reduction in the cell layer volume of the CA3 hippocampal area [72-74]. Consistent with the above findings, chronic cortisol administration (1 year) to nonhuman primates (macaques) [76] or exposure of tree shrews to psychosocial stress [77] failed to cause neuronal loss in the CA3 or CA1 hippocampal fields.
Although the parameters of treatment in the above studies vary, they have in common that they all used unbiased stereological methods. Each study using these methods has failed to support cell loss after prolonged glucocorticoid elevations. The only stereological estimates of cell loss to confirm fewer cells in CA3 have been reported in rats treated with corticosterone during the neonatal period [73]. The elevations occur during a critical period of development characterized by a “hypo-responsive” hypothalamic-pituitary-adrenal axis which serves to protect the developing brain from the catabolic actions of glucocorticoid elevations [78]. The mechanism of reduced cell number is likely to be through a suppression of neurogenesis [79]. Bypassing this protective mechanism through exogenous elevations demonstrates the susceptibility of the hippocampus during this developmental period. Elevation of glucocorticoids during this susceptible period may have permanent consequences. Similarly, in the original report of cell loss, the animals were older [19]. Thus, it seems possible that there are windows of vulnerability across the lifespan in which corticosterone could cause cell loss, but that it does not seem to do so in young adult animals. It has been proposed that repeated exposure to elevated glucocorticoids, producing dendritic retraction, could lead to greater vulnerability to cell loss from metabolic challenge or mechanical trauma [80].
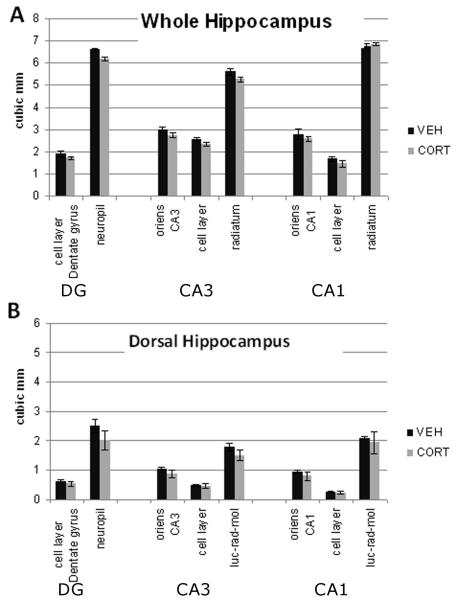
Altogether the data suggest that cell body loss is unlikely to contribute to volume differences in adult populations with disorders associated with elevated plasma glucocorticoids. Even if cells were to die, the cell bodies themselves contribute to less than 20% of the CA3 volume. More specifically, Sousa et al. [83] report that the cell body layer in DG, CA3 and CA1 is 17%, 22% and 14% of the overall volume, respectively. We have collected similar measures from the dorsal hippocampus (Tata, unpublished data, see Figure 2), making this layer alone unlikely to contribute significantly to a loss of overall volume. In contrast, the neuropil, which in hippocampus contains primarily dendrites, and axons and a very small proportion of glial processes, makes up 78-86% of the volume. A reduction of neuropil volume would be expected to contribute more to a loss of hippocampal volume. Thus, the general failure to support cell loss from glucocorticoid administration in rats does not preclude the possibility that glucocorticoids reduce volume. Instead, volume reduction may be more closely tied to a reduction in neuropil volume that could reflect dendritic atrophy and synapse loss.
Figure 2.
Volume of the hippocampal subregion layers (millimeter cubed) from the dorsal hippocampus, which corresponds to the region in which pyramidal cell dendritic lengths have been measured from whole hippocampus (A, as reported in [83] and dorsal hippocampus (B, Tata, unpublished observations). The layers containing the apical dendrites (labeled stratum radiatum, but including stratum lacunosum and molecular) makes up greater than 50% of the volume in the CA3 and CA1 subregions. Accordingly, neuronal process atrophy without cell loss is sufficient for a reduction in hippocampal volume.
In conclusion, modern stereological methods have provided evidence that exposure to elevated glucocorticoids does not cause neuronal loss in the hippocampus of young and adult animals, although it can reduce cell number in neonates. Many of the earliest reports of loss more likely reflect undercounting small cells, or the use of aged rats with a more susceptible cell population. Overall, when unbiased counting methods were employed, the data from animal models fails to find glucocorticoid-induced cell loss in the hippocampus, and is consistent with conclusions from post-mortem studies, which failed to implicate hippocampal cell loss in the hippocampal volume reduction reported in depression.
Changes in hippocampal neuronal morphology and ultrastructure
Golgi staining of hippocampal cell dendritic fields revealed that 21 days of restraint stress, which elevates plasma glucocorticoids, decreased the number of dendritic branch points and the total dendritic length of CA3 apical dendrites in rats [23]. Dendritic alterations also have been reported after corticosterone treatment of the same or longer duration [22, 81] and after social or unpredictable stress in rats and tree shrews [24, 82]. These alterations correspond to a reduction in dendritic length in dentate gyrus and CA3, in terminal segments of CA3 and CA1 pyramidal cells and in branch point density of dentate gyrus granule cells. Although atrophy of dendrites could contribute to a volume reduction, it is also possible that atrophy would be compensated by glial hypertrophy, thereby canceling any effect on volume.
Evidence for glucocorticoid influence over hippocampal volume in animal models is mixed. Sousa et al. [73] showed that one or three months of corticosterone treatment (40mg/kg) during adulthood resulted in volumetric reduction in the nonsomatic layers of dentate gyrus (hilus) and CA3 (stratum radiatum-laconosum moleculare). The neuropil that contains apical dendrites makes up 66% of the CA3 subregion of the dorsal hippocampus (Tata, unpublished data, see Figure 1). If a 10% volume reduction in CA3 was selectively dependent upon volume loss in the neuropil that contains apical dendrites, that layer of neuropil would have to be 15% smaller. We found a non-significant 15% reduction in the apical dendritic neuropil in CA3 after daily s.c. injections of corticosterone over a two month period (Tata, unpublished observations). Sousa et al., [83] reported a 13% reduction in the CA3 neuropil layer with the same dose administered for only 30 days, which was statistically significant in five month old rats. Tata et al. (unpublished observations) and Sousa et al., [83] both found a non-significant 10% reduction after combining volume from all areas of the hippocampus. In younger rats, Sousa et al., [73] had non-significant reductions after 90 days, but no reduction after 30 days. Overall, there is support for the possibility that glucocorticoids can influence hippocampal volume via loss of the neuropil containing apical dendrites. The magnitude of glucocorticoid elevations, duration of plasma elevation, recovery and age may all be factors that influence these effects.
Within the neuropil containing the apical dendrites, neuronal processes (both dendrites and axons) make up ~93% of the volume [74]. Accordingly, while the whole CA3 apical dendritic neuropil would have to be reduced by 15% for a 10% volume reduction overall, dendrites in the neuropil would have to atrophy at a magnitude greater than 15% given that they make up less than 93% of the neuropil. The magnitudes of dendritic atrophy previously reported are consistent with these estimates. Woolley et al., [22] reported a 25% reduction in total length of apical dendrites after 21 days of corticosterone administration. Using a similar dose for 4 weeks, Sousa et al., [24] reported a 41% reduction in dendritic length in CA3 apical dendrites. Considering the data from these reports together, we can form the hypothesis that volume loss, which is reported to be around 10% overall, may be represented by an approximately 15% loss of apical dendrite containing neuropil volume, which is accounted by for a much larger atrophy of apical dendrites; thus a 10% volume reduction in major depressive disorder is likely to be the result of dendritic atrophy, and further is likely to grossly under-represent the magnitude of that atrophy.
Looking at corticosterone administration from postnatal day 0, Sousa et al. [73] reported that administration for the first 30 days decreased hippocampal volume with the reduction partially dependent upon decreases in the dentate gyrus molecular layer, CA3 and CA1 apical dendritic neuropil layers. Corticosterone administered later, from postnatal days 90-180, decreased the volume of the hippocampal formation, with reductions in the dentate gyrus molecular layer, but not reductions in the CA1 and CA3 apical dendrite containing neuropil. Corticosterone administered between days 150 and 180 was not sufficient to reduce overall volume or volume in any of these regions of neuropil that make up over half of the hippocampal volume. Sixty days of the same dose (40 mg/kg) of corticosterone also failed to significantly reduce the volume of the CA3 apical dendrite containing neuropil [74]. The dose used significantly elevates plasma corticosterone [84], and can have a dramatic effect on organ weights [74]. Therefore, the data suggest that corticosterone can affect hippocampal volume, but only does so when administered at an age equivalent to a prenatal period in humans, or for as long as 90 days [73]. Further work is needed to understand whether the plasma elevations serve as an appropriate representation of those seen in Cushing's disease and major depressive disorder [85, 86].
So far we have seen that glucocorticoids can cause a volume reduction [73] and dendritic atrophy [87] but these measures are not direct measures of synaptic contacts. Dendritic length indirectly reflects synaptic input since it decreases the dendritic receptive surface available for synaptic contacts, but does not directly represent synapse numbers, which could simply increase in density to compensate for atrophy. Direct measures of synapse numbers have been collected through the use of electron microscopy. One month of corticosterone treatment (40mg/kg) or unpredictable stress caused a significant decrease in the total number of mossy fiber-CA3 synapses as well as a reduction in the volume of the mossy fiber terminals and surface area of their plasmalemma [24]. Sandie et al. [88] found that 21 days of restraint stress caused a loss of total synapse number in the stratum lucidum. Restraint stress, which would elevate glucocorticoids, increased the density of vesicles in the mossy fiber terminals and caused a relocalization of vesicles near the active zones [89] suggesting that more vesicles are available for glutamate release. While the direction of synapse loss is consistent with the loss expected from reports of reduced neuropil volume, these effects do not fully explain a volume reduction, because the type of synapses measured in these studies are located in a relatively thin layer above the cell bodies, stratum lucidum, and contact only the most proximal dendrites, which could not be atrophied.
Measuring total synapse number in the neuropil that contains all apical dendrites (proximal and distal), Tata reported that 2-month administration of corticosterone (40mg/kg), influences the total number of synapses. In these studies all layers of apical neuropil were represented in proportion to their size. Since the stratum lucidum makes up only a small proportion of the apical dendritic neuropil, the synapses in this layer represent only a small proportion of the synapse population estimated. Synapse density in the proximal and middle CA3 were measured with unbiased stereological methods which allow direct counting of synapses irrespective of synapse size. The corticosterone-treated group had a 40% loss of synapses. The magnitude of loss is fairly large, but corresponds to the magnitude of dendritic atrophy reported (25-40%) [22, 24] for the dose used.
In summary, growing evidence indicates that elevated plasma glucocorticoids concentrations after corticosterone treatment or stress alter hippocampal morphology and ultrastructure. Changes reported include a decrease in apical dendritic length and number of branch points. Consistent with dendritic atrophy, some studies report decreases in neuropil volume in CA3. Ultrastructural analyses yielded a loss of mossy fiber-CA3 synapses, as well as a loss of total synapse numbers in the CA3 apical dendritic neuropil. Considering the large proportion of volume contributed by the neuropil layers that contain the apical dendrites, the neuropil volume loss would have to be greater than 15% to produce a 10% overall volume reduction, with a much larger loss of dendritic processes, since they do not solely make up neuropil volume. The 25-40% loss of dendrites, and 40% loss of synapses reported after glucocorticoid administration support the hypothesis that volume loss can be accounted for by dendritic atrophy and synapse loss without cell loss. These calculations suggest that small volume reductions may represent much larger reductions in synaptic contacts.
Effects on hippocampal glial cells
Although most studies of the relationship between glucocorticoids and hippocampal cell morphology have focused on neurons, a subset of studies have focused on glucocorticoid effects on glial cells. Glial cells express both MR and GR [90, 91], although at lower levels than in neurons [92]. Accordingly, it is possible that glucocorticoids could act directly or indirectly on glial cells to influence their contribution to volume. Glial volume reductions could be expected to lead to an overall volume reduction without requiring a reduction in the neuronal population. Alternatively, neuronal atrophy could be compensated by increases in glial process volume to leave total volume unaffected. For example, in aging rats, astrocytic volume fraction increases in the dentate gyrus whereas thickness of the molecular layer does not change [93].
In animals, regulation of GFAP, the major intermediate filament protein in mature astrocytes [94], has received the most attention. Short or long-term administration of corticosterone decreased the concentration of GFAP in rat hippocampal astrocytes [95-97]. The regulation of GFAP by glucocorticoids is further supported by increases in GFAP levels after adrenalectomy, an effect that is reversed by corticosterone replacement [96-98]. In addition, short or long exposure of cerebral cortex astrocyte cultures to corticosterone increased GFAP gene expression [99, 100]. It is interesting to note that the corticosterone effects on GFAP are mediated by neuronal interactions [100]. Although GFAP is easy to label, and would seem to be an indirect measure of astrocyte number, it is not a valid indirect measure of volume, because the neurofilaments that contain GFAP are dense within the astrocyte body and primary processes and sparse within the thin processes that extend out to be interspersed among the neuronal processes [101, 102].
Glial cells are greater in number than neurons in the hippocampus [103], but their volume is significantly smaller than that of neurons [74], leaving them to make up only 8-20% of neuropil volume. The glial contribution to volume can be measured directly in electron micrographs [72, 102, 104, 105]. Glial cells in the CA3 region account for ~6.5% (Coburn-Litvak et al., 2004; Tata et al., 2006) of the neuropil volume in CA3, and 9.4% of volume in the dentate gyrus [104]. The glial volume fraction in these areas is far lower than in the CA1 region, where glial processes make up ~15% of the volume [105], which is closer to reports from the cerebellar molecular layer (~18%) [102]. Therefore, the potential for glial atrophy to contribute to volume loss depends upon the subregion of the hippocampus, but it is still relatively limited based on the small proportion of volume accounted for by glia. The small proportion of volume representing glial cell volume reduces the potential for glial volume loss to contribute to overall volume loss. Instead, glial cells have more potential for growth and partial compensation for neuronal process loss.
Studies of glucocorticoid effects on glial volume yield mixed results. Two-month corticosterone treatment (27 mg/kg,) had no effect on glial volume fraction [72]. Using a higher dose (40mg/kg) for the same period of time the effects were mixed. In one CA3 subregion, corticosterone administration produced a tendency toward increased glial volume that appeared to compensate for synapse loss so that volume appeared unchanged, whereas in another subregion, corticosterone produced no change in glial volume with synapse loss, therefore producing a tendency for a decrease in overall volume [74]. These data suggest that glial compensation varies by region and subregion.
Altogether, cell culture studies support the possibility that changes in GFAP reported in post-mortem samples from patients with depression could have been decreased by glucocorticoids, but the data from animal models suggest that corticosterone produces no effect on glial volume or produces tendencies toward glial process growth that compensates for synapse loss, leaving no change in overall volume in select regions [72, 74]. Overall, if there is any effect of glucocorticoids on glial cell volume, it may be one toward growth rather than atrophy. This is critical because it suggests that glial hypertrophy may compensate for neuronal volume loss, leaving volume to further under-represent neuronal atrophy.
IV. Association between Hippocampal Volume, Cell Number, Morphology and Ultrastructure: Concluding Remarks
By studying hippocampal volume differences in rats following treatment conditions that elevate glucocorticoids, we can address the causal relationships that may explain the differences in hippocampal volume reported in clinical populations without confounding factors such as differences in treatment parameters, age, duration and severity of illness. Volume reduction is often assumed to reflect a loss of cells without the understanding that packing density may increase, thus all cells may be intact with a higher density in a smaller volume. Experimental data fails to support the proposed cell loss following glucocorticoid elevations. Based on the experimental findings presented above we see clearly that volume loss is not dependent upon cell loss. Similarly, data from post mortem studies of humans exposed to high levels of glucocorticoids failed to support hippocampal cell loss [66]. Although previous investigators have also reported the appearance of pyknotic cells in primate CA3 after exposure to glucocorticoids [106-109], our qualitative observations in tissue handled to avoid artifacts with appearances similar to pyknoses yielded no indicators of dying cells [72]. The latter observations are in agreement with the failure to detect signs of apoptosis in CA3 post-mortem analysis of subjects with major depressive disorder [68].
Corticosterone administration (40 mg/kg) for at least 90 days in rats can decrease hippocampal volume [73], but does not decrease volume after 30 [75] or 60 days [74]. It is unclear how well the dose and duration in these studies represents the glucocorticoid elevations in depression.
The largest compartment contributing to hippocampal volume is the neuropil. Thus changes in the hippocampal volume may be attributed to changes in neuropil volume. Neuropil volume contains primarily dendrites, and axons and a very small proportion of glial processes. Sousa and colleagues [73] were the first to report a decrease in the volume of the nonsomatic layers of the CA3 area, a decrease that seems to be selective to the middle subregion of this area [74]. A number of studies have reported dendritic atrophy after exposure to stress [23, 110, 111] or corticosterone administration [22, 24, 112]. Few studies have measured both volume and dendritic atrophy to understand whether they co-occur.
Dendrites receive the large majority of synaptic contacts onto a cell. Consistent with their role in receiving input, and the loss of receptive surface area from atrophy [24, 87], there are reports of glucocorticoid-induced synapse loss [24, 74](Sousa et al., 2000; Tata et al., 2006). So we see that volume reductions and synapse loss can both occur after corticosterone treatment, but it is important to note that in studies with a direct comparison, the magnitude of the synapse loss exceeds the magnitude of the volume reduction [74]. In direct comparisons within studies, glial hypertrophy may partially compensate for synapse loss in some subregions of the CA3 [74]. These data raise the possibility that volume reductions in clinical disorders with elevated glucocorticoids could grossly under-represent the loss of dendrites and synapses.
To date, animal models support the potential for elevated glucocorticoids to affect hippocampal volume, dendritic atrophy and synapse loss. The largest proportion of volume in the hippocampus is constituted by the neuropil layers that contain the large neuronal processes, along with axons, and terminals, and to a far lesser extent glial processes. Thus for volume loss, the loss must be occurring in the neuropil, and is not dependent upon cell loss. When unbiased counting methods are used, there is no evidence that glucocorticoids cause cell loss. Glial processes may hypertrophy to partially compensate for dendritic loss. The data leave open the possibility that volume reductions under represent the loss of neuronal process volume. Future studies are needed to further our understanding of the relationship between dose and duration of glucocorticoid administration and hippocampal anatomical change. The animal studies reporting anatomical effects of glucocorticoids to date have used the same dose (40 mg/kg). It is unclear whether this dose is representative of the elevations in glucocorticoids seen in disorders or whether lower doses need to be tested.
Acknowledgments
This work was supported by MH62075
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Jama. 1992;267(9):1244–52. [PubMed] [Google Scholar]
- 2.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 3.Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression. II. Discrimination of depressed from nondepressed patients. Arch Gen Psychiatry. 1976;33(9):1051–8. doi: 10.1001/archpsyc.1976.01770090041003. [DOI] [PubMed] [Google Scholar]
- 4.Starkman MN, et al. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing's syndrome. Biol Psychiatry. 1992;32(9):756–65. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 5.Williams GW, McGinnis MY, Lumia AR. The effects of olfactory bulbectomy and chronic psychosocial stress on serum glucocorticoids and sexual behavior in female rats. Physiol Behav. 1992;52(4):755–60. doi: 10.1016/0031-9384(92)90410-4. [DOI] [PubMed] [Google Scholar]
- 6.Kant GJ, et al. Effects of chronic stress on plasma corticosterone, ACTH and prolactin. Physiol Behav. 1987;40(6):775–9. doi: 10.1016/0031-9384(87)90282-4. [DOI] [PubMed] [Google Scholar]
- 7.Porter NM, Herman JP, Landfield PW, Goodman BSMHM, editors. Copying the environment: Neural and endocrine mechanisms. IV. Oxford Press, American Physiological Society; Oxford: 2000. Mechanisms of glucocorticoid actions in stress and brain aging. Handbook of Physiology: A critical comprehensive presentation of physiological knowledge and concepts; pp. 293–309. Section 7. [Google Scholar]
- 8.McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220(5170):911–2. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- 9.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117(6):2505–11. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 10.De Kloet ER, et al. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19(3):269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 11.Squire LR, Amaral DG, Press GA. Magnetic resonance imaging of the hippocampal formation and mammillary nuclei distinguish medial temporal lobe and diencephalic amnesia. J Neurosci. 1990;10(9):3106–17. doi: 10.1523/JNEUROSCI.10-09-03106.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci U S A. 1984;81(19):6174–7. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herman JP, et al. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci. 1989;9(9):3072–82. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobrakovova M, et al. Changes of plasma and adrenal catecholamines and corticosterone in stressed rats with septal lesions. Physiol Behav. 1982;29(1):41–5. doi: 10.1016/0031-9384(82)90363-8. [DOI] [PubMed] [Google Scholar]
- 15.Magarinos AM, Somoza G, De Nicola AF. Glucocorticoid negative feedback and glucocorticoid receptors after hippocampectomy in rats. Horm Metab Res. 1987;19(3):105–9. doi: 10.1055/s-2007-1011753. [DOI] [PubMed] [Google Scholar]
- 16.Aus der Muhlen K, Ockenfels H. Morphologische veranderungen'im diencephalon und telencephalon nach storungen des regelkreises adenohypophyse-nebennierenrinde. III. Ergebnisse beim meerschweinchen nach verabreichung von cortison und hydrocortison. Z. Zellforsch. 1969;93:126–141. [PubMed] [Google Scholar]
- 17.Landfield PW, Baskin RK, Pitler TA. Brain aging correlates: retardation by hormonal-pharmacological treatments. Science. 1981;214(4520):581–4. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- 18.Landfield PW, Waymire JC, Lynch G. Hippocampal aging and adrenocorticoids: quantitative correlations. Science. 1978;202(4372):1098–102. doi: 10.1126/science.715460. [DOI] [PubMed] [Google Scholar]
- 19.Sapolsky RM. A mechanism for glucocorticoid toxicity in the hippocampus: increased neuronal vulnerability to metabolic insults. J Neurosci. 1985;5(5):1228–32. doi: 10.1523/JNEUROSCI.05-05-01228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizoguchi K, et al. Stress induces neuronal death in the hippocampus of castrated rats. Neurosci Lett. 1992;138(1):157–60. doi: 10.1016/0304-3940(92)90495-s. [DOI] [PubMed] [Google Scholar]
- 21.Clark AS, Mitre MC, Brinck-Johnsen T. Anabolic-androgenic steroid and adrenal steroid effects on hippocampal plasticity. Brain Res. 1995;679(1):64–71. doi: 10.1016/0006-8993(95)00202-2. [DOI] [PubMed] [Google Scholar]
- 22.Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531(1-2):225–31. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research. 1992;588(2):341–5. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 24.Sousa N, et al. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97(2):253–66. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 25.Starkman MN, et al. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing's disease. Biol Psychiatry. 1999;46(12):1595–602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- 26.Starkman MN, et al. Improvement in learning associated with increase in hippocampal formation volume. Biol Psychiatry. 2003;53(3):233–8. doi: 10.1016/s0006-3223(02)01750-x. [DOI] [PubMed] [Google Scholar]
- 27.Young EA, et al. Loss of glucocorticoid fast feedback in depression. Arch Gen Psychiatry. 1991;48(8):693–9. doi: 10.1001/archpsyc.1991.01810320017003. [DOI] [PubMed] [Google Scholar]
- 28.Checkley S. The neuroendocrinology of depression and chronic stress. British Medical Bulletin. 1996;52(3):597–617. doi: 10.1093/oxfordjournals.bmb.a011570. [DOI] [PubMed] [Google Scholar]
- 29.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201(2):239–43. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koenigs M, et al. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci. 2008;28(47):12341–8. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konarski JZ, et al. Volumetric neuroimaging investigations in mood disorders: bipolar disorder versus major depressive disorder. Bipolar Disord. 2008;10(1):1–37. doi: 10.1111/j.1399-5618.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- 32.Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15(11):1714–22. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- 33.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60(2):236–48. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 34.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49(3):245–53. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 35.Dias-Ferreira E, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325(5940):621–5. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 36.Sheline YI, et al. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93(9):3908–13. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacQueen GM, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100(3):1387–92. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lloyd AJ, et al. Hippocampal volume change in depression: late- and early-onset illness compared. Br J Psychiatry. 2004;184:488–95. doi: 10.1192/bjp.184.6.488. [DOI] [PubMed] [Google Scholar]
- 39.Hickie I, et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- 40.Weniger G, Lange C, Irle E. Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. J Affect Disord. 2006;94(1-3):219–29. doi: 10.1016/j.jad.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 41.MacMaster FP, et al. Amygdala and hippocampal volumes in familial early onset major depressive disorder. Biol Psychiatry. 2008;63(4):385–90. doi: 10.1016/j.biopsych.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bremner JD, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115–8. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 43.Mervaala E, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30(1):117–25. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- 44.Steffens DC, et al. Hippocampal volume and incident dementia in geriatric depression. Am J Geriatr Psychiatry. 2002;10(1):62–71. [PubMed] [Google Scholar]
- 45.O'Brien JT, et al. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161(11):2081–90. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 46.Saylam C, et al. Reduced hippocampal volume in drug-free depressed patients. Surg Radiol Anat. 2006;28(1):82–7. doi: 10.1007/s00276-005-0050-3. [DOI] [PubMed] [Google Scholar]
- 47.Neumeister A, et al. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry. 2005;57(8):935–7. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Vakili K, et al. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry. 2000;47(12):1087–90. doi: 10.1016/s0006-3223(99)00296-6. [DOI] [PubMed] [Google Scholar]
- 49.Ashtari M, et al. Hippocampal/amygdala volumes in geriatric depression. Psychol Med. 1999;29(3):629–38. doi: 10.1017/s0033291799008405. [DOI] [PubMed] [Google Scholar]
- 50.Posener JA, et al. High-dimensional mapping of the hippocampus in depression. Am J Psychiatry. 2003;160(1):83–9. doi: 10.1176/appi.ajp.160.1.83. [DOI] [PubMed] [Google Scholar]
- 51.Vythilingam M, et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 2004;56(2):101–12. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Keller J, et al. Hippocampal and amygdalar volumes in psychotic and nonpsychotic unipolar depression. Am J Psychiatry. 2008;165(7):872–80. doi: 10.1176/appi.ajp.2008.07081257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry. 2000;48(8):791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- 54.Campbell S, et al. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161(4):598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 55.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11):1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 56.McKinnon MC, et al. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- 57.Sheline YI, et al. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19(12):5034–43. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516–8. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 59.Caetano SC, et al. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res. 2004;132(2):141–7. doi: 10.1016/j.pscychresns.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29(6):417–26. [PMC free article] [PubMed] [Google Scholar]
- 61.Gilbertson MW, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lupien SJ, et al. Hippocampal volume is as variable in young as in older adults: implications for the notion of hippocampal atrophy in humans. Neuroimage. 2007;34(2):479–85. doi: 10.1016/j.neuroimage.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 63.Frodl T, et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33(5):423–30. [PMC free article] [PubMed] [Google Scholar]
- 64.Maller JJ, Daskalakis ZJ, Fitzgerald PB. Hippocampal volumetrics in depression: the importance of the posterior tail. Hippocampus. 2007;17(11):1023–7. doi: 10.1002/hipo.20339. [DOI] [PubMed] [Google Scholar]
- 65.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57(10):925–35. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 66.Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257(5):250–60. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- 67.Muller MB, et al. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci. 2001;14(10):1603–12. doi: 10.1046/j.0953-816x.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- 68.Lucassen PJ, et al. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol. 2001;158(2):453–68. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O'Brien J, et al. Cognitive impairment in depression is not associated with neuropathologic evidence of increased vascular or Alzheimer-type pathology. Biol Psychiatry. 2001;49(2):130–6. doi: 10.1016/s0006-3223(00)00944-6. [DOI] [PubMed] [Google Scholar]
- 70.Stockmeier CA, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56(9):640–50. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bodnoff SR, et al. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15(1 Pt 1):61–9. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coburn-Litvak PS, et al. Chronic corticosterone affects brain weight, and mitochondrial, but not glial volume fraction in hippocampal area CA3. Neuroscience. 2004;124(2):429–38. doi: 10.1016/j.neuroscience.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 73.Sousa N, Madeira MD, Paula-Barbosa MM. Effects of corticosterone treatment and rehabilitation on the hippocampal formation of neonatal and adult rats. An unbiased stereological study. Brain Res. 1998;794(2):199–210. doi: 10.1016/s0006-8993(98)00218-2. [DOI] [PubMed] [Google Scholar]
- 74.Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: relationship to neuropil volume and cell number in hippocampal area CA3. J Comp Neurol. 2006;498(3):363–74. doi: 10.1002/cne.21071. [DOI] [PubMed] [Google Scholar]
- 75.Sousa N, Madeira MD, Paula-Barbosa MM. Corticosterone replacement restores normal morphological features to the hippocampal dendrites, axons and synapses of adrenalectomized rats. J Neurocytol. 1999;28(7):541–58. doi: 10.1023/a:1007015321767. [DOI] [PubMed] [Google Scholar]
- 76.Leverenz JB, et al. Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primates. J Neurosci. 1999;19(6):2356–61. doi: 10.1523/JNEUROSCI.19-06-02356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vollmann-Honsdorf GK, Flugge G, Fuchs E. Chronic psychosocial stress does not affect the number of pyramidal neurons in tree shrew hippocampus. Neuroscience Letters. 1997;233(2-3):121–4. doi: 10.1016/s0304-3940(97)00647-2. [DOI] [PubMed] [Google Scholar]
- 78.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396(1):64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 79.Gould E, Woolley CS, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J Comp Neurol. 1991;313(3):479–85. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- 80.Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19(6):395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Magarinos AM, Orchinik M, McEwen BS. Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration: a paradox. Brain Res. 1998;809(2):314–8. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- 82.Magarinos AM, et al. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16(10):3534–40. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sousa N, Paula-Barbosa MM, Almeida OF. Ligand and subfield specificity of corticoid-induced neuronal loss in the rat hippocampal formation. Neuroscience. 1999;89(4):1079–87. doi: 10.1016/s0306-4522(98)00311-x. [DOI] [PubMed] [Google Scholar]
- 84.Angelucci L. The glucocorticoid hormone: from pedestal to dust and back. Eur J Pharmacol. 2000;405(1-3):139–47. doi: 10.1016/s0014-2999(00)00547-1. [DOI] [PubMed] [Google Scholar]
- 85.den Hartog HM, et al. Salivary cortisol patterns and cognitive speed in major depression: a comparison with allergic rhinitis and healthy control subjects. Biol Psychol. 2003;63(1):1–14. doi: 10.1016/s0301-0511(03)00050-4. [DOI] [PubMed] [Google Scholar]
- 86.Deuschle M, et al. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab. 1997;82(1):234–8. doi: 10.1210/jcem.82.1.3689. [DOI] [PubMed] [Google Scholar]
- 87.Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531(1-2):225–31. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 88.Sandi C, et al. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. European Journal of Neuroscience. 2003;17(11):2447–56. doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- 89.Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997;94(25):14002–8. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bohn MC, et al. Glial cells express both mineralocorticoid and glucocorticoid receptors. J. Steroid Biochem. Mol. Biol. 1991;40(1-3):105–11. doi: 10.1016/0960-0760(91)90173-3. [DOI] [PubMed] [Google Scholar]
- 91.Cintra A, et al. Mapping and computer assisted morphometry and microdensitometry of glucocorticoid receptor immunoreactive neurons and glial cells in the rat central nervous system. Neuroscience. 1994;62(3):843–97. doi: 10.1016/0306-4522(94)90481-2. [DOI] [PubMed] [Google Scholar]
- 92.Bohn MC, et al. In vitro studies of glucocorticoid effects on neurons and astrocytes. Ann N Y Acad Sci. 1994;746:243–58. doi: 10.1111/j.1749-6632.1994.tb39241.x. discussion 258-9. [DOI] [PubMed] [Google Scholar]
- 93.Geinisman Y, Bondareff W, Dodge JT. Dendritic atrophy in the dentate gyrus of the senescent rat. Am J Anat. 1978;152(3):321–9. doi: 10.1002/aja.1001520305. [DOI] [PubMed] [Google Scholar]
- 94.Pixley SK, de Vellis J. Transition between immature radial glia and mature astrocytes studied with a monoclonal antibody to vimentin. Brain Research. 1984;317(2):201–9. doi: 10.1016/0165-3806(84)90097-x. [DOI] [PubMed] [Google Scholar]
- 95.Nichols NR. Glial responses to steroids as markers of brain aging. J Neurobiol. 1999;40(4):585–601. [PubMed] [Google Scholar]
- 96.O'Callaghan JP, Brinton RE, McEwen BS. Glucocorticoids regulate the concentration of glial fibrillary acidic protein throughout the brain. Brain Res. 1989;494:159–161. doi: 10.1016/0006-8993(89)90156-x. [DOI] [PubMed] [Google Scholar]
- 97.O'Callaghan JP, Brinton R, McEwen BS. Glucocorticoids regulate the synthesis of glial fibrillary acidic protein in intact and adrenalectomized rats but do not affect its expression following brain injury. J Neurochem. 1991;57(3):860–9. doi: 10.1111/j.1471-4159.1991.tb08230.x. [DOI] [PubMed] [Google Scholar]
- 98.Bye N, Nichols NR. Adrenalectomy-induced apoptosis and glial responsiveness during ageing. Neuroreport. 1998;9(6):1179–84. doi: 10.1097/00001756-199804200-00040. [DOI] [PubMed] [Google Scholar]
- 99.Melcangi RC, et al. Corticosteroid effects on gene expression of myelin basic protein in oligodendrocytes and of glial fibrillary acidic protein in type 1 astrocytes. J Neuroendocrinol. 1997;9(10):729–33. doi: 10.1046/j.1365-2826.1997.00621.x. [DOI] [PubMed] [Google Scholar]
- 100.Rozovsky I, et al. Transcriptional regulation of glial fibrillary acidic protein by corticosterone in rat astrocytes in vitro is influenced by the duration of time in culture and by astrocyte-neuron interactions. Endocrinology. 1995;136(5):2066–73. doi: 10.1210/endo.136.5.7720656. [DOI] [PubMed] [Google Scholar]
- 101.Peters A, Palay SL, Webster H. The fine structure of the nervous system. The neurons and supporting cells. Saunders; Philadelphia: 1976. [Google Scholar]
- 102.Anderson BJ, et al. Glial hypertrophy is associated with synaptogenesis following motor- skill learning, but not with angiogenesis following exercise. Glia. 1994;11(1):73–80. doi: 10.1002/glia.440110110. [DOI] [PubMed] [Google Scholar]
- 103.Joelving FC, et al. Hippocampal neuron and glial cell numbers in Parkinson's disease--a stereological study. Hippocampus. 2006;16(10):826–33. doi: 10.1002/hipo.20212. [DOI] [PubMed] [Google Scholar]
- 104.Geinisman Y, Bondareff W, Dodge JT. Hypertrophy of astroglial processes in the dentate gyrus of the senescent rat. Am J Anat. 1978;153(4):537–43. doi: 10.1002/aja.1001530405. [DOI] [PubMed] [Google Scholar]
- 105.Klintsova A, Levy WB, Desmond NL. Astrocytic volume fluctuates in the hippocampal CA1 region across the estrous cycle. Brain Res. 1995;690(2):269–74. doi: 10.1016/0006-8993(95)00642-4. [DOI] [PubMed] [Google Scholar]
- 106.Uno H, et al. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9(5):1705–11. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Endo Y, et al. Long-term glucocorticoid treatments decrease local cerebral blood flow in the rat hippocampus, in association with histological damage. Neuroscience. 1997;79(3):745–52. doi: 10.1016/s0306-4522(97)00044-4. [DOI] [PubMed] [Google Scholar]
- 108.Sapolsky RM. Stress in the wild. Sci Am. 1990;262(1):116–23. doi: 10.1038/scientificamerican0190-116. [DOI] [PubMed] [Google Scholar]
- 109.Uno H, et al. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9(5):1705–11. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luine V, et al. Restraint stress reversibly enhances spatial memory performance. Physiol Behav. 1996;59(1):27–32. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- 111.Conrad CD, et al. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behavioral Neuroscience. 1999;113(5):902–13. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 112.Magarinos AM, Orchinik M, McEwen BS. Morphological changes in the hippocampal CA3 region induced by non-invasive glucocorticoid administration: a paradox. Brain Research. 1998;809(2):314–8. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- 113.Bayer SA, Paxinos G, editors. The Rat Nervous System. Vol. 1. Academic Press; San Diego, CA: 1985. Hippocampal Region; pp. 335–352. [Google Scholar]