Abstract
Background:
Obesity is associated with increased life-long morbidity and reduced lifespan, and is increasingly prevalent in the congenital heart disease population. Habitual exercise is an important aspect of a healthy lifestyle and primary prevention of obesity in the general population. The association between habitual activity and body mass index has not been studied in children with congenital heart disease.
Methods:
A cross-sectional analysis of two previously collected cohorts was performed, including subjects 8–18 years old with tetralogy of Fallot, transposition of the great arteries, and single ventricle heart disease after a Fontan operation. The association between body mass index and duration of habitual exercise (measured by questionnaire) was studied. Secondary analyses assessing the effect of other possible factors for body mass index were performed.
Results:
In total, 172 subjects were studied (45% tetralogy of Fallot, 12% transposition of the great arteries, and 43% Fontan). Median body mass index was 18.2, and 29% of the subjects were obese or overweight. Median habitual exercise was 5.9 hours/week. 38% of subjects reported having their activity restricted by their cardiologist. Increasing exercise duration was associated with lower body mass index (p=0.01) in univariate analysis. In secondary analyses, restriction to mild exertion and participation in low-intensity exercise were both associated with increased BMI.
Conclusion:
Increased habitual activity was associated with lower body mass index, emphasizing the potential role of recreational sport in the health of children with congenital heart disease.
Keywords: Hypoplastic left heart syndrome, Transposition of the great arteries, Pediatric cardiology, Exercise physiology, Outcomes research
BACKGROUND:
Obesity, defined as excessive body weight and adiposity for age, sex, and height, is a condition with alarming prevalence. Childhood obesity is associated with increased morbidity extending into adulthood and reduced lifespan1,2. Based on data from the National Health and Nutrition Examination Survey, 31% of children are overweight or obese3. Previous studies have found a similar prevalence amongst children with congenital heart disease(CHD)4–6. To our knowledge, there are no data about the associations between obesity, quality of life, and lifespan in the CHD population. However, the residual effects of cardiac surgery performed in infancy and childhood may magnify the health risks of obesity.
Though significant research has been invested in behavioral, pharmacologic, and surgical therapies for obese patients, primary prevention of obesity through lifestyle modification may be preferable. In otherwise healthy children, habitual activity has consistently been associated with lower body mass index (BMI) and lower degree of adiposity7–11. To our knowledge no studies have studied the association between habitual exercise and BMI in children with CHD. This population is at particular risk for a sedentary lifestyle, as many children with CHD are subject to exercise restriction or limits to participation.
To address these questions, we pooled data from two previous cross-sectional studies of habitual exercise in children with CHD, with the aim to study the association between habitual exercise and BMI in this population. We hypothesized that increased habitual activity would be associated with a lower BMI.
METHODS:
Study Population/Study Procedures:
Data for subjects in two previously collected cohorts were pooled for this analysis. These studies and subsequent analysis were approved by the Institutional Review Board of The Children’s Hospital of Philadelphia. Informed consent was obtained prior to participation in each of the two prior studies. In the first study, children with conotruncal anomalies (tetralogy of Fallot (TOF), interrupted aortic arch, and truncus arteriosus) of ages 8–18 years were recruited between 1/2005 and 5/2009 for a cross-sectional study assessing the relationship between genotype and outcome12–14. Subjects underwent contemporaneous clinical assessment, review of medical history and testing as described previously15. The exercise questionnaire has been validated16 and its application to children and adolescents has been described previously15,17. The second study included children and adolescents age 8–17.5 years with 1) transposition of the great arteries following an arterial switch operation (TGA) and 2) single ventricle heart disease who had undergone operative palliation culminating in a Fontan completion who were recruited prospectively between 3/1/2012–12/31/2013 at the time of a clinically indicated cardiopulmoanry exercise test18. This study included concomitant administration of the exercise questionnaire17, along with review of medical records and contemporaneous imaging data. Subjects with normal cardiac anatomy were recruited for the latter study but were not included in the current analysis..
Data from the previous two studies were combined directly to generate a single analytic cohort of subjects between 8–18 years. Inclusion was restricted to subjects with TOF, TGA, and Fontan subjects. Truncus arteriosus and interrupted aortic arch subjects were excluded because their individual numbers were prohibitively small. Normal cardiac anatomy subjects were excluded because the current study focused on the association of BMI and exercise in subjects with CHD. In addition, these three diagnoses were chosen for this study because they represent three strata of repaired/palliated heart disease with different expected clinical courses after their operations, while still being relatively homogenous within their sub-strata. Though the results of this analysis are less applicable to patients with relatively mild CHD (e.g. isolated ventricular or atrial septal defects) or those with severe dysfunction or multiple comorbidities, the chosen diagnoses provide a representative sample of patients with moderate to severe complexity and who are of interest to clinicians. The questionnaire used to measure habitual exercise habits has been described previously17. It measures duration (hours/week) of habitual exercise over the past 3 months, as well as the specific activities performed, the degree of exercise restriction, and separates the cohort into one of 4 exercise classes based on the aerobic intensity (calories or metabolic equivalents per hour) of those activities. As described previously, this methodology 1) focuses on the aerobic component of exercise at the expense of anabolic building components, 2) sacrifices detail about the intensity of activity, and 3) simplifies activities that involve combinations of different physical tasks into a single metric. This sacrifices precision and detail in exchange for a comparable statistic that, in previous studies17, provided a measure of habitual exercise.
Statistical analysis:
Descriptive statistics were calculated. Continuous variables are expressed as mean ± standard deviation or median (range: and interquartile range) as appropriate. For categorical variables percentages and counts are presented. Differences in the distribution of these baseline characteristics between the three different cardiac diagnoses were assessed using analysis of variance, Kruskal-Wallis, or Chi-square tests as appropriate.
The primary exposure was habitual exercise (measured as hours/week) over the 3 months prior the CPET, which were calculated as previously described. The primary outcome of interest was BMI. For categorization of subjects, BMI was also expressed as a percentile for age and sex (BMI%) based on normal values published by the United States Centers for Disease Control, and converted to categorical variable using the following standard strata: underweight (<5%), normal weight (5–85%), overweight (85–95%), obese (>95%). BMI percentile generates a statistic that accounts for differences in BMI by age and sex over childhood. The decision to use BMI instead of BMI percentile was based on both statistical and epidemiological concerns. Our proposed analytic framework was to use linear regression, which presumes a Gaussian distribution of the outcome. For this study population, the distribution of BMI was closer to a Gaussian distribution than that of BMI percentile (data not shown). Moreover, the transformation makes comparisons between extreme (high or low) and normal BMI more straightforward but has the potential to obscure differences in BMI along the normal continuum. This also makes BMI preferable as the primary outcome, since the goal of this study was to measure association of exercise duration to body composition (rather than with abnormal BMI such as obesity or underweight status). Population average BMI increases at puberty in boys and girls. For this to bias our analyses, age would also have to be associated with changes in activity level. To assess this, we performed an exploratory secondary analysis adjusting for age and sex in a multivariable model.
As an exploratory analysis, bivariable screening was used to assess whether other subject-level factors were associated with BMI. Factors included were sex, race, diagnosis, exercise class, and level of activity restriction. Exercise class and duration were strongly correlated with each other (Supplementary Table 1), so they were not included in the same model for fear of obscuring their effect. A multivariable model was constructed based on the bivariable screen. To avoid bias, a conservative threshold for inclusion was used (p-value<0.2 in the bivariable screen)19. No additional model refinement was performed19. A second model with exercise class as the primary exposure was calculated.
Three post-hoc analyses were performed. First, underweight subjects are more likely to have more severe disease, and their BMI may be more strongly linked to cachexia from heart failure or other chronic medical conditions. To address this, a sensitivity analysis restricted to patients with BMI percentile >5% for age and sex was performed. Second, we studied whether the extent of restriction from cardiologists was associated with the duration of habitual exercise and exercise class participation. Finally, post-hoc evaluations of the correlations between levels of restriction and duration (hours per week) and intensity of exercise (exercise class) were performed using the Kruskal-Wallis test.
This was a retrospective secondary analysis combining existing data from previously collected cohorts, and thus the size of the study population was fixed. However, before analysis a power calculation was applied for the primary outcome, over a range of potential Pearson’s correlation coefficients (r) with beta (0.2) and alpha (0.05) fixed. A study sample of 21 subjects were required for a minimally detectable difference of r=0.5. Similarly, the required sample size for r=0.3 was 64, and r=0.2 was 150.
The threshold for statistical significance was p<0.05. The primary analysis was identified before analyses. No compensation for multiple comparisons was made, and other analyses should be considered exploratory. There was minimal missing data, so case restriction was applied when necessary. All analyses were performed using Stata MP v13 (College Station TX, USA).
RESULTS:
Study population:
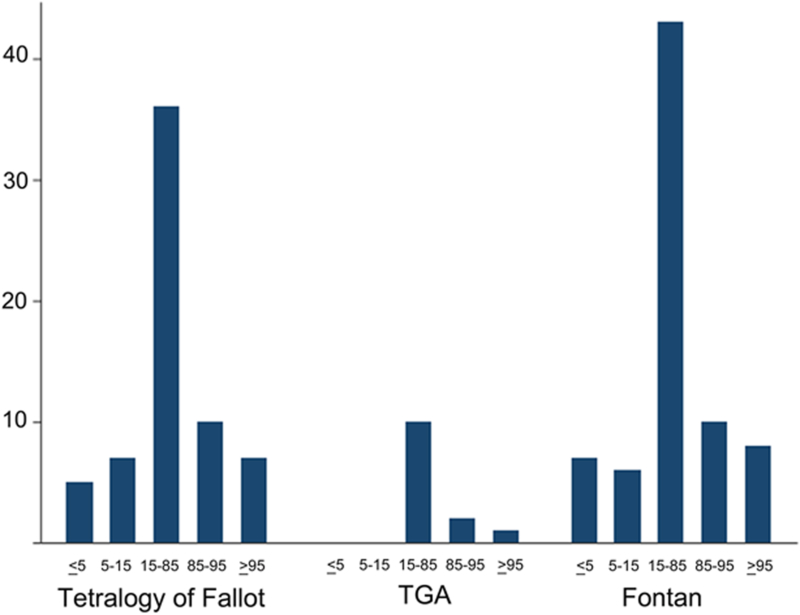
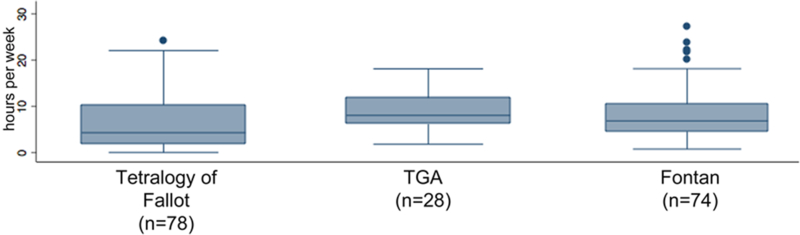
In total, 253 subjects were studied of which 78 (45%) had TOF, 20 (12%) TGA, and 74 (43%) a Fontan. The cohort had a mean age of 13.1±2.9, was 83% white, and 42% female. Baseline characteristics of the cohort are summarized in Table 1. The median BMI was 18.2 (IQR: 16.1–21.4). In terms of CDC defined categories for body composition based on BMI percentile for age and sex, 11% of the subjects were obese, 15% overweight, 59% normal weight, 7% underweight, and 8% severely underweight (Figure 1). The median habitual exercise for the cohort was 5.8 hours/week (IQR: 3–10.3) (Figure 2).
Table 1:
Characteristics of the study population
| Tetralogy of Fallot (n=78) | TGA (n=20) |
Fontan (n=74) |
p | |
|---|---|---|---|---|
| Age (years) | 12.6±3.4 | 12.5±2.6 | 12.5±2.8 | 0.97 |
| Male sex % (n) | 64% (50) | 60% (12) | 54% (40) | 0.45 |
| Race % (n) | ||||
| White | 88% (69) | 85% (17) | 76% (56) | 0.32 |
| Black or African-American | 5% (4) | 5% (1) | 12% (9) | |
| Other or chose not to answer | 6% (5) | 10% (2) | 12% (9) | |
| Height (cm) | 148±18 | 151±17 | 149±16 | 0.78 |
| Weight (kg) | 45.0±16.5 | 43.0±15.1 | 43.1±14.7 | 0.73 |
| Body mass index | 18.9 (IQR:16.3–22.5) | 17.2 (IQR: 15.9–19.4) | 18.2 (IQR: 16.1–20.2) | 0.31 |
| Body composition | ||||
| Obese (BMI>95%) | 12% (9) | 5% (1) | 11% (8) | 0.91 |
| Overweight (BMI 85–95%) | 17% (13) | 20% (4) | 14% (10) | |
| Normal weight (BMI 15–85%) | 55% (43) | 65% (13) | 58% (43) | |
| Underweight (BMI 5–15%) | 9% (7) | 0% (0) | 8% (6) | |
| Severely underweight (BMI <5%) | 8% (6) | 10% (2) | 9% (7) | |
| Habitual exercise (hours/week) | 4.3 (IQR: 1.9–10.3) | 8 (IQR: 6.1–11.8) | 6.7 (4.4–10.4) | 0.005 |
| Maximal exercise class % (n)* | ||||
| I | 5% (4) | 0% (0) | 0% (0) | 0.09 |
| II | 17% (13) | 10% (2) | 7% (5) | |
| III | 29% (23) | 20% (4) | 28% (21) | |
| IV | 49% (38) | 70% (14) | 65% (48) | |
| Restriction (by cardiologist) % (n) | ||||
| Unrestricted | 62% (48) | 55% (11) | 26% (19) | <0.001 |
| Moderately strenuous activity | 26% (20) | 35% (7) | 55% (41) | |
| Mildly strenuous activity | 10% (8) | 5% (1) | 19% (14) | |
| No activity | 1% (1) | 5% (1) | 0% (0) | |
| Missing/chose not to answer | 1% (1) | 0% (0) | 0% (0) |
Abbreviations: BMI body mass index, IQR interquartile range, TGA transposition of the great arteries
Figure 1: Distribution of body mass index in the study population.
Distribution of the subjects’ body composition sorted by diagnosis. Body composition is defined by BMI percentile for age and weight and divided by United States Centers for Disease Control categories: severely underweight (<5%), underweight (5–15%), normal weight (5–85%), overweight (85–95%), and obese (>95%).
Figure 2: Habitual exercise in the study population.
Box and whiskers plot of study subject’s habitual exercise (expressed as hours/week) divided by diagnosis. The box plot demonstrates median, interquartile ranges, and outliers.
There were no significant differences in the distribution of demographic characteristics by cardiac diagnoses. Duration of weekly habitual exercise was greatest in TGA subjects, intermediate in Fontan subjects, and smallest in subjects with TOF (Figure 3, p=0.005). Restriction imposed by cardiologist also differed by diagnosis (p<0.001), with a higher percentage of Fontan subjects restricted to moderately (55%) and mildly (19%) strenuous activities.
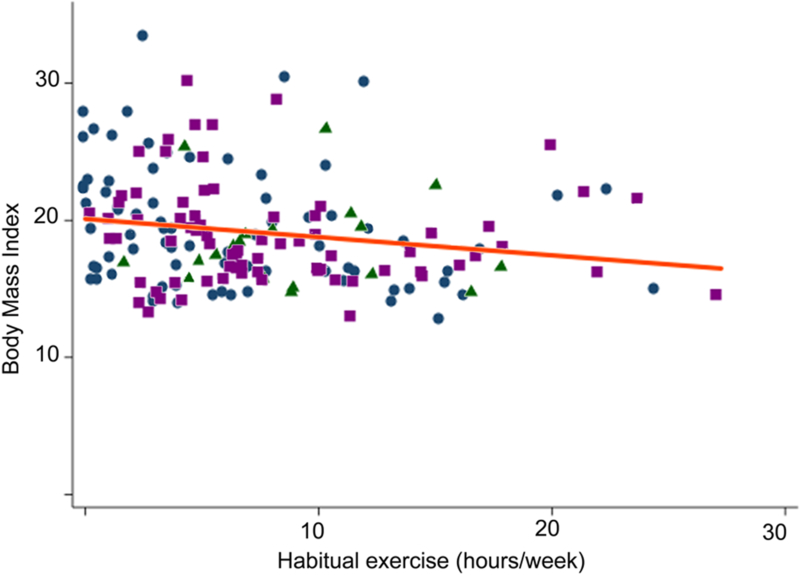
Figure 3: Distribution of BMI by hours of habitual exercise.
Scatterplot of subject habitual exercise (expressed in hours/week) and their BMI. Subject cardiac diagnoses are identified: tetralogy of Fallot (blue circles), TGA (green triangles), and Fontan subjects (purple squares). The line of best fit is also plotted for the entire population (orange line, beta=−0.13 95% CI: −0.24 to −0.03, p=0.01, r2=0.03).
Association of habitual exercise and BMI:
In the primary analysis, increased habitual exercise duration (hours per week) was associated with a lower BMI (Figure 3 beta=−0.13 95% CI: −0.24 to −0.03, p=0.01, r2=0.03). Other covariates were individually included in separate models. In these models, increasing age, parental BMI, participation in only low intensity (class I) exercise, and restriction to mild exercise were associated with increased BMI (Table 2).
Table 2:
Univariate and multivariate analyses of subject factors associated with BMI
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Beta (95% CI) | p | Beta (95% CI) | p | |
| Exercise duration (per hour/week) | −0.13 (−0.24 to −0.03) | 0.01 | −0.06 (−0.15 to 0.04) | 0.23 |
| Age (per year) | 0.61 (0.45 to 0.79) | <0.001 | 0.5 (0.3–0.7) | <0.001 |
| Sex | ||||
| Female | 1 | n/a | 1 | n/a |
| Male | −1.03 (−2.2 to 0.2) | 0.09 | −1.1 (−2.2 to 0.02) | 0.05 |
| Diagnosis | ||||
| Tetralogy of Fallot | 1 | n/a | 1 | n/a |
| TGA | −1.5 (−3.5 to 0.5) | 0.14 | −1.1 (−2.9 to 0.6) | 0.21 |
| Fontan | −1.0 (−2.3 to 0.3) | 0.13 | −0.9 (−2.1 to 0.3) | 0.15 |
| Race | ||||
| White | 1 | n/a | ||
| Black | −0.4 (−2.6 to 1.8) | 0.74 | ||
| Other | −0.2 (−2.6 to 1.9) | 0.87 | ||
| Level of restriction | ||||
| Unrestricted | 1 | n/a | 1 | n/a |
| Moderate | −0.5 (−1.8 to 0.7) | 0.42 | −0.3 (−1.5 to 0.9) | 0.61 |
| Mild | 2.0 (0.3 to 3.8) | 0.02 | 1.5 (−0.2 to 3.2) | 0.08 |
| No activity | 1.8 (−3.8 to 7.5) | 0.52 | 0.9 (−4.1 to 5.9) | 0.73 |
| Exercise class | ||||
| I (minimally strenuous) | 3.7 (0.6 to 6.8) | 0.02 | ||
| II | 1.7 (−0.1 to 3.5) | 0.07 | ||
| III | 0.1 (−1.2 to 1.5) | 0.85 | ||
| IV (very strenuous) | 1 | n/a | ||
The associated changes represented by beta for each covariate are measured in kgm−2.
In addition to these factors, male sex met criteria for inclusion in a subsequent multivariable model (Table 2), in which there remained a significant association between age and BMI. Confidence intervals for all factors were less precise, but the direction of associations remained the same. The associations between BMI and other factors are not statistically significant in the multivariable model.
In a secondary analysis with exercise class as the primary exposure, there was no significant association between exercise class and BMI (Supplementary Table 2 and 3). In addition, no changes in the associations for covariates were seen from the previous model.
A sensitivity analysis excluding underweight subjects was performed. The observed association between exercise duration and BMI was preserved (beta= −0.14 95% CI: −0.24 to −0.03, p=0.01, r2 =0.04). Multivariable analysis was also performed with similar results (data not shown).
Exercise restriction and habitual activity duration and intensity:
The association between the degree of cardiologist imposed exercise restriction (reported by families) and exercise duration and intensity were assessed. The association between level of restriction and exercise duration was not significant (p=0.31, Table 3). The association between level of restriction and intensity of activity (as assessed with exercise class) was significant (p=0.02, Table 4), with 61% of subjects with no restrictions participating in high intensity activities, compared with 48% of subjects restricted to mildly strenuous activity participating in high intensity activities. In contrast, 10% of subjects with no exercise restriction participated in activities in the lowest two exercise classes compared to 26% of those restricted to mildly strenuous activity. Though exercise class and duration of habitual exercise measure different aspects of exercise there is an association between higher maximal exercise class and increasing habitual exercise duration (p=0.001, Supplementary Table 1).
Table 3:
Association of restriction imposed by the cardiologist and duration of habitual exercise
| Habitual exercise (hours per week) | p | |
|---|---|---|
| No restriction (n=87) | 6.1 (IQR: 2.6 to 10.3) | 0.31 |
| Restricted to moderately strenuous activity (n=70) | 6.2 (IQR: 4.0 to 10.4) | |
| Restricted to mildly strenuous activity (n=27) | 4.9 (IQR: 2.5 to 9.1) | |
| Restricted to no activity (n=2) | Range 0–7 |
Table 4:
Association of restriction imposed by the cardiologist and intensity of habitual exercise
| Exercise Class | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | p | |
| No restriction (n=87) | 2% (2) | 8% 7) | 29% (25) | 61% (53) | 0.02 |
| Restricted to moderately strenuous activity (n=70) | 1% (1) | 16% (11) | 29% (20) | 54% (38) | |
| Restricted to mildly strenuous activity (n=27) | 7% (2) | 19% (5) | 26% (7) | 48% (13) | |
| Restricted to no activity (n=2) | 50% (1) | 0% (0) | 0% (0) | 50% (1) | |
DISCUSSION:
In this cross-sectional study, there was a significant association between increasing duration of habitual exercise and lower BMI in children and adolescents with TGA, TOF, and Fontan palliation. Though less robust, participation limited to less intense activity (whether by report or by report of restriction) was also associated with increased BMI. Despite inherent limitations of this type of study, the observed findings reaffirms the association between an active lifestyle and reduced risk of obesity. This is especially true, as this study also demonstrated that the prevalence of obesity and overweight were similar to population averages and that the majority of the studied subjects were sedentary. Despite inherent limitations of a cross-sectional study of this type, these results suggest that effort in encouraging habitual exercise may have benefit in young people who have undergone congenital heart surgery.
Obesity and increased adiposity are prevalent in children and adolescents. Both are associated with increased lifelong morbidity and reduced life expectancy. Several observational studies have found that the prevalence of obesity in children with CHD matches that in the general pediatric population4. The risk of chronic obesity in children with CHD may be magnified by the fact that corrective and palliative surgery may result in chronic myocardial dysfunction and/or residual anatomic lesions, which may aggravate insidious damage from obesity. In addition, several palliative operations (e.g. the arterial switch operation and the Ross operation) require instrumentation and manipulation of the coronary arteries, which may pose increased risk of atherosclerotic disease. There is also recent evidence that obese school-age children and adolescents are at increased risk of adverse outcome following cardiac surgery20.
An active lifestyle is not only an important strategy in both primary prevention and treatment of obesity20, but may also have other benefits. Both habitual recreational activity15 and regimented exercise programs21 have been associated with improved performance on exercise testing (specifically maximal oxygen consumption) in children with CHD. Though the association between exercise testing performance and health related quality of life22 and functional status23 have been inconsistent in prior studies, one might still hope that habitual exercise might plausibly improve both.
Despite the apparent benefits of habitual activity, previous studies have demonstrated that patients with CHD are more sedentary than cardiac normal peers24. These results are echoed in the current study. Fully two-thirds of subjects fail to meet World Health Organization recommendations of at least one hour of active play a day. It is important to note that inactivity in this population is not always attributable to disability. When measured carefully, the degree of activity or sedentary behavior was found to be independent of exercise capacity24, suggesting that the amount of habitual activity is a potentially modifiable factor for children with CHD.
The effect of physician-mandated exercise restriction on habitual activity is potentially important. The Bethesda Guidelines represent a concerted effort to minimize risk of worsening anatomic heart disease or sudden cardiac events by identifying high risk subgroups of the CHD population. . However, as noted in a recent American Heart Association Statement, the risk of high intensity training and competition as part of scholastic sports in adolescents and young adults may be different from those incurred during recreational play20. Given this uncertainty it is important to also consider the potential deleterious effects of exercise restriction on health. There are potentially mixed messages in activity restriction. Though a previous study demonstrated that exercise restricted subjects were at higher risk of obesity25, to our knowledge, no previous study has evaluated restriction, habitual activity and BMI.
In the current study, between 31% and 74% of subjects in the current study reported that their cardiologist restricted their exercise participation, depending on their diagnosis. Degree of exercise restriction did not have a significant association with duration of activity but was associated with lower levels of intensity in the activity performed. The effect of exercise restriction on patient behavior is complicated, and potentially deserves greater attention to maximize the health of vulnerable patients in this population. Because sudden cardiac death is thankfully rare, the evidence upon which to base recommendations for exercise restriction is limited. It is important to balance these risks against the potential benefit of exercise and play as part of a healthy, active lifestyle. It should be noted that, in this study, we asked families to report the level of restriction they felt was imposed by their cardiologist. It was beyond the scope of this study to determine the level of restriction intended by the cardiologist, and what the reasoning behind their recommendation. Research studying the interplay between cardiologist recommendations and family interpretations of these recommendations would be valuable, as would research on the impact of exercise restriction on health-related quality of life and other patient reported outcomes.
CHD patients who are underweight represent a distinct subpopulation that require further attention. In a multicenter cohort of children, adolescents, and young adults undergoing operation, underweight subjects have a higher prevalence of medical co-morbidities and undergo more complicated and lengthy operations, implying that they have a higher burden of cardiac co-morbidities26. Therefore, low body weight may be an indication of symptomatic cardiac dysfunction or the burden of other systemic medical conditions, both of which would also reduce their ability to participate in habitual exercise. This type of confounding would bias our sample away from the observed association between activity and BMI. To address this question, a sensitivity analysis excluding underweight patients was performed. In this model no change was seen from the previously reported results. This confirms that if present, confounding from inclusion of these underweight patients had minimal effects on the reported results.
There are limitations to this study. Habitual exercise was measured by focusing on the duration of activity, which does not measure the isometric component of individual exercise at the level of exposure (duration of exercise does not measure the muscular load placed by different forms of exercise). This is especially important as there is evidence that lean body mass and muscularity specifically may have clinical benefits in excess of traditional measures of aerobic fitness21,27,28. Unfortunately, measuring the isometric component of exercise was impossible in this study. The absence of a significant association between exercise class and BMI does not imply that the intensity of exercise is less important than duration. We could not measure the relative intensity of individual participants (i.e. how strenuously they exercised). Second, exercise class was not evenly distributed in our study sample and is a categorical variable, both of which reduce the statistical power to detect an association with BMI. Improved measurement of the aerobic intensity of recreational activities is an important area for future research. Another limitation is that BMI is an imperfect measure of adiposity. There are other more sensitive measures of obesity (such as abdominal girth or percent body fat), but these are not available in a retrospective study. BMI remains a straightforward statistic that is able to be calculated from readily available data. As noted in the section describing our study methods, the normal BMI of children and adolescents increases with age in both sexes during puberty. The persistent association between older age and increased BMI supports that this normal trend is seen in our study population and that adjustment for age and sex was successful.
The current study is a cross-sectional analyses and it must be emphasized that the observed associations do not imply causation. Observational or interventional trials with longitudinal follow-up are necessary to establish causality. Finally, this is a retrospective study with a fixed population size. Pre-analysis power calculations were made for a single comparison. Though the number of covariates included in the multivariable model follow conventions for linear regression, it is possible that the lack of a significant association in this model is due to insufficient statistical power.
The current study supports the notion that increased activity is associated with decreased BMI in children with palliated CHD. Longitudinal studies of the effects of increased exercise on BMI, exercise performance, and clinical outcomes are necessary to determine whether there is an optimal amount of exercise in survivors of surgery for CHD.
Supplementary Material
ACKNOWLEDGEMENTS:
The authors acknowledge Elizabeth Ford, Shannon O’Malley, Sarah DiMaio, and Annie Linton who worked with the study team to recruit and enroll subjects. They also acknowledge Sharon Edman for her work as the database manager and study coordinator for the project.
FUNDING SOURCES:
The collection of data for the SCORR cohort was supported by grants from the National Institutes of Health [P50-HL74731] and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health [UL1-TR000003]. Fontan and TGA cohort data was collected with support from the National Institutes of Health [T32 HL007915] and the Entelligence Young Investigator Grant. Dr. O’Byrne currently receives research support from the National Institutes of Health and National Heart, Lung, and Blood Institute [K23-HL130420–01]. The content is solely the responsibility of the authors and does not necessarily represent the official view of the supporting agencies. They had no role in the design, conduct, interpretation, or decision to publish the data in this manuscript.
Footnotes
Conflicts of interest: The authors have no pertinent conflicts to disclose.
REFERENCE:
- 1.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. [DOI] [PubMed] [Google Scholar]
- 3.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of Overweight and Obesity Among US Children, Adolescents, and Adults, 1999–2002. JAMA. 2004;291:2847–2850. [DOI] [PubMed] [Google Scholar]
- 4.Pinto NM, Marino BS, Wernovsky G, de Ferranti SD, Walsh AZ, Laronde M, Hyland K, Dunn SO, Cohen MS. Obesity Is a Common Comorbidity in Children With Congenital and Acquired Heart Disease. PEDIATRICS. 2007;120:e1157–e1164. [DOI] [PubMed] [Google Scholar]
- 5.Pasquali SK, Marino BS, Pudusseri A, Wernovsky G, Paridon SM, Walker SA, Cohen MS. Risk factors and comorbidities associated with obesity in children and adolescents after the arterial switch operation and Ross procedure. Am Heart J. 2009;158:473–479. [DOI] [PubMed] [Google Scholar]
- 6.Shustak RJ, McGuire SB, October TW, Phoon CKL, Chun AJL. Prevalence of Obesity Among Patients With Congenital and Acquired Heart Disease. Pediatric Cardiology. 2011;33:8–14. [DOI] [PubMed] [Google Scholar]
- 7.Trost SG, Sirard JR, Dowda M, Pfeiffer KA, Pate RR. Physical activity in overweight and nonoverweight preschool children. Int J Obes Relat Metab Disord. 2003;27:834–839. [DOI] [PubMed] [Google Scholar]
- 8.Byrd-Williams C, Kelly LA, Davis JN, Spruijt-Metz D, Goran MI. Influence of gender, BMI and Hispanic ethnicity on physical activity in children. International Journal of Pediatric Obesity. 2007;2:159–166. [DOI] [PubMed] [Google Scholar]
- 9.Duncan EK, Scott Duncan J, Schofield G. Pedometer-determined physical activity and active transport in girls. Int J Behav Nutr Phys Act. 2008;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussey J, Bell C, Bennett K, O’Dwyer J, Gormley J. Relationship between the intensity of physical activity, inactivity, cardiorespiratory fitness and body composition in 7–10-year-old Dublin children. Br J Sports Med. 2007;41:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen LB, Harro M, Sardinha LB, Froberg K, Ekelund U, Brage S, Anderssen SA. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study). 2006;368:299–304. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0140673606690752 [DOI] [PubMed] [Google Scholar]
- 12.O’Byrne ML, Yang W, Mercer-Rosa L, Parnell AS, Oster ME, Levenbrown Y, Tanel RE, Goldmuntz E. 22q11.2 Deletion syndrome is associated with increased perioperative events and more complicated postoperative course in infants undergoing infant operative correction of truncus arteriosus communis or interrupted aortic arch. J Thorac Cardiovasc Surg. 2014;148:1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Byrne ML, Mercer-Rosa L, Zhao H, Zhang X, Yang W, Tanel RE, Marino BS, Cassedy A, Fogel MA, Rychik J, Paridon S, Goldmuntz E. Morbidity in Children and Adolescents After Surgical Correction of Interrupted Aortic Arch. Pediatric Cardiology. 2014;35:386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Byrne ML, Mercer-Rosa L, Zhao H, Zhang X, Yang W, Cassedy A, Fogel MA, Rychik J, Tanel RE, Marino BS, Paridon S, Goldmuntz E. Morbidity in children and adolescents after surgical correction of truncus arteriosus communis. Am Heart J. 2013;166:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Byrne ML, Mercer-Rosa L, Ingall E, McBride MG, Paridon S, Goldmuntz E. Habitual Exercise Correlates With Exercise Performance in Patients With Conotruncal Abnormalities. Pediatric Cardiology. 2012;34:853–860. [DOI] [PubMed] [Google Scholar]
- 16.Kriska AM, Sandler RB, Cauley JA. The assessment of historical physical activity and its relation to adult bone parameters. AmJEpidemiol. 1988; [DOI] [PubMed] [Google Scholar]
- 17.O’Byrne ML, Desai S, Lane M, McBride M, Paridon S, Goldmuntz E. Relationship Between Habitual Exercise and Performance on Cardiopulmonary Exercise Testing Differs Between Children With Single and Biventricular Circulations. Pediatric Cardiology. 2017;38:472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wernovsky G, Rome JJ, Tabbutt S, Rychik J, Cohen MS, Paridon SM, Webb G, Dodds KM, Gallagher MA, Fleck DA, Spray TL, Vetter VL, Gleason MM. Guidelines for the outpatient management of complex congenital heart disease. Congenit Heart Dis [Internet]. 2006;1:10–26. Available from: http://doi.wiley.com/10.1111/j.1747-0803.2006.00002.x [DOI] [PubMed] [Google Scholar]
- 19.Sun G-W, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. Journal of Clinical Epidemiology. 1996;49:907–916. [DOI] [PubMed] [Google Scholar]
- 20.Longmuir PE, Brothers JA, de Ferranti SD, Hayman LL, Van Hare GF, Matherne GP, Davis CK, Joy EA, McCrindle BW, American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Promotion of physical activity for children and adults with congenital heart disease: a scientific statement from the American Heart Association. 2013. p. 2147–2159. [DOI] [PubMed] [Google Scholar]
- 21.Duppen N, Etnel JR, Spaans L, Takken T, van den Berg-Emons RJ, Boersma E, Schokking M, Dulfer K, Utens EM, Helbing W, Hopman MT. Does exercise training improve cardiopulmonary fitness and daily physical activity in children and young adults with corrected tetralogy of Fallot or Fontan circulation? A randomized controlled trial. Am Heart J. 2015;170:606–614. [DOI] [PubMed] [Google Scholar]
- 22.d’Udekem Y, Cheung MMH, Setyapranata S, Iyengar AJ, Kelly P, Buckland N, Grigg LE, Weintraub RG, Vance A, Brizard CP, Penny DJ. How good is a good Fontan? Quality of life and exercise capacity of Fontans without arrhythmias. Ann Thoracic Surg. 2009;88:1961–1969. [DOI] [PubMed] [Google Scholar]
- 23.McCrindle BW, Zak V, Sleeper LA, Paridon SM, Colan SD, Geva T, Mahony L, Li JS, Breitbart RE, Margossian R, Williams RV, Gersony WM, Atz AM, for the Pediatric Heart Network Investigators. Laboratory Measures of Exercise Capacity and Ventricular Characteristics and Function Are Weakly Associated With Functional Health Status After Fontan Procedure. Circulation. 2010;121:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCrindle BW, Williams RV, Mital S, Clark BJ, Russell JL, Klein G, Eisenmann JC. Physical activity levels in children and adolescents are reduced after the Fontan procedure, independent of exercise capacity, and are associated with lower perceived general health. Arch Dis Child [Internet]. 2007;92:509–514. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=17307794&retmode=ref&cmd=prlinks [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefan MA, Hopman WM, Smythe JF. Effect of activity restriction owing to heart disease on obesity. Arch Pediatr Adolesc Med. 2005;159:477–481. [DOI] [PubMed] [Google Scholar]
- 26.O’Byrne ML, Kim S, Hornik CP, Yerokun BA, Matsouaka RA, Jacobs JP, Jacobs ML, Jonas RA. Effect of Obesity and Underweight Status on Perioperative Outcomes of Congenital Heart Operations in Children, Adolescents, and Young Adults: An Analysis of Data from the Society of Thoracic Surgeons Database. Circulation. 2017;epub ahead of print:CIRCULATIONAHA.116.026778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg DJ, Avitabile CM, McBride MG, Paridon SM. Exercise capacity in the Fontan circulation. Cardiology in the Young. 2014;23:824–830. [DOI] [PubMed] [Google Scholar]
- 28.Duppen N, Kapusta L, de Rijke YB, Snoeren M, Kuipers IM, Koopman LP, Blank AC, Blom NA, Dulfer K, Utens EMWJ, Hopman MTE, Helbing WA. The effect of exercise training on cardiac remodelling in children and young adults with corrected tetralogy of Fallot or Fontan circulation: a randomized controlled trial. Int J Cardiol. 2015;179:97–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.