Abstract
Von Hippel-Landau (VHL) disease is characterized by malignant and benign tumors in multiple organs. Sunitinib, a tyrosine kinase inhibitor, has been clinically available for treating sporadic patients with recurrent or unresectable and metastatic clear renal cell carcinomas (cRCCs) and metastatic lesions of the lung, but its effect on VHL disease-associated tumors remains poorly understood. This retrospective case series examined the effect of sunitinib on RCC, hemangioblastomas, pheochromocytomas, and pancreatic neuroendocrine tumors in patients with confirmed VHL. Of note, three patients with VHL disease who were treated with sunitinib were identified from a review of their medical records. The efficacy of sunitinib was evaluated by comparing computed tomography (CT) or magnetic resonance imaging (MRI) scans conducted before and after treatment. Adverse side effects associated with sunitinib were assessed and recorded. All three patients with VHL disease exhibited clinical improvement after treatment with sunitinib. Patient 1 exhibited a decrease in the size of both their pheochromocytoma and RCC after 19 months of sunitinib treatment. RCCs in Patients 2 and 3 exhibited stable response to sunitinib for approximately 1 and 6 years, respectively. All the patients reported tolerable side effects. Therefore sunitinib treatment was associated with either partial response or stable control of VHL-related RCCs, pheochromocytomas and pancreatic neuroendocrine tumor (NET) with acceptable side effects. Further evaluation of sunitinib in patients with VHL disease in larger prospective studies is warranted.
Keywords: VHL disease, sunitinib, renal cell carcinoma, hemangioblastoma, pheochromocytoma
Introduction
Von Hippel-Lindau (VHL) disease, a rare autosomal dominant tumor syndrome caused by a germline mutation in the VHL gene, is characterized by the occurrence of multiple malignant and benign tumors in various organs, including retinal hemangiomas, hemangioblastomas of the central nervous system, pancreatic neuroendocrine tumors or cystadenomas, pheochromocytomas, and clear cell renal cell carcinomas (cRCCs)1, 2. The wild-type VHL protein exerts tumor-suppressive functions primarily by binding directly with hypoxia-inducible factors (HIF-1α and HIF-2α), which promotes their proteasomal degradation via ubiquitylation. The mutant VHL protein lacks this ubiquitin ligase activity, however, which ultimately results in increased levels of HIFs and the activation of their downstream pathways, including vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and transforming growth factor (TGF)-α related pathways3. Loss-of-function alterations in VHL therefore contribute to the carcinogenesis observed in both VHL-related and most sporadic cases of cRCC.
Hypoxia-induced activation of the VEGF pathway has been implicated in the pathogenesis of RCC. Molecular therapies that target components of the VEGF pathway have been developed to treat sporadic RCC. Sunitinib, an oral multi-kinase inhibitor that targets VEGF, PDGF, KIT, and FLT3 receptors4, is clinically available for treating sporadic patients with recurrent or unresectable and metastatic cRCCs and metastatic lesions of the lung5. In addition, sunitinib also has been used for the treatment of pancreatic neuroendocrine tumors, but has been shown to be less effective in treating lesions of the kidney6. To date, however, only a few large prospective studies have been conducted to test the efficacy of sunitinib in patients with VHL disease. It remains to be clarified, therefore, whether treatment with sunitinib is similarly effective for patients with VHL disease-associated tumors.
Results
Patients
Patient 1: A female was diagnosed with bilateral pheochromocytomas at age 29 when she presented with severe hypertension and two large masses (greater than 6 cm) in her bilateral adrenal glands. She underwent bilateral adrenal resections within one year of her diagnosis. At 38 years old, she underwent a resection for a mass found on her pancreas; this procedure was complicated by pancreatic leakage that persisted for more than 6 months to heal. Pathological analysis of the mass revealed a pancreatic neuroendocrine tumor. At 40 years old, a blood DNA analysis was given and identified a well-known pathogenic VHL mutation c.239G>T; p.(Ser80Ile), which is associated with a severe phenotype. At 42 years old, a follow-up CT scan revealed two heterogeneous contrast-enhanced masses, one on the right kidney (2.5 × 2.1 cm; Figure 1A) and one on the left adrenal gland (6.1 × 3.9 cm; Figure 1C), Given her surgical history, including last surgical complications, and multiple recurrent lesions, the patient was unsuitable for further surgery and started on oral administration of sunitinib. After 11 months, she experienced severe dizziness, headache, and emesis, and a head MRI scan revealed a 1.0 × 1.4 cm lesion in her right cerebellum (Figure 1E). The patient underwent a mass resection in her right cerebellum after 2 weeks sunitinib use was terminated. Subsequent pathological evaluation confirmed a hemangioblastoma. Sunitinib was prescribed again after one month after the operation. Follow-up duration for sunitinib administration lasted 2.5 years.
Figure 1.

Computed tomography (CT) and magnetic resonance imaging (MRI) scans from Patient 1. The CT scans show the right renal cell carcinoma (RCC) and left pheochromocytoma before (A, C) and after (B, D) sunitinib treatment. The MRI scans show the hemangioblastoma in the right cerebellum before sunitinib treatment (E) and the CT scan shows the site of the lesion after the operation (F). Red arrows indicate the masses.
The patient has a notable family history. Her eldest brother had multiple RCCs in both kidneys, bilateral cerebellar hemangioblastomas, and pheochromocytoma in his left adrenal gland (see Patient 2). Her daughter also underwent cortex-sparing adrenalectomies for bilateral pheochromocytomas as well as hemangioblastoma resection in her left cerebellum at age of 19 years.
Patient 2: The brother of Patient 1 was referred to our hospital at 38 years old for bilateral hemangioblastomas in the cerebellum. In both 2001 and 2002, he underwent mass resections for these tumors. In 2009, an MRI scan revealed a 2.2 × 2.1 cm mass in his cerebellum, for which he underwent γ-knife treatment. In December 2013, a CT scan of his abdomen revealed uncountable masses in the whole left kidney and 1 mass in the left adrenal gland as well as 4 lesions in the right kidneys (Figure 2A-D). Pancreas appeared normal. The patient underwent the left nephrectomy and resection of the left adrenal mass. The pathological examinations confirmed cRCC in his left kidney and pheochromocytomas in his left adrenal gland. Sanger sequencing of the VHL gene revealed the same VHL mutation (c. 239G>T; p. Ser80Ile) that was identified in Patient 1. The masses in his right kidney were left and the patient started on oral administration of sunitinib in January 2017 and duation of sunitinib administration had lasted for almost one years.
Figure 2.
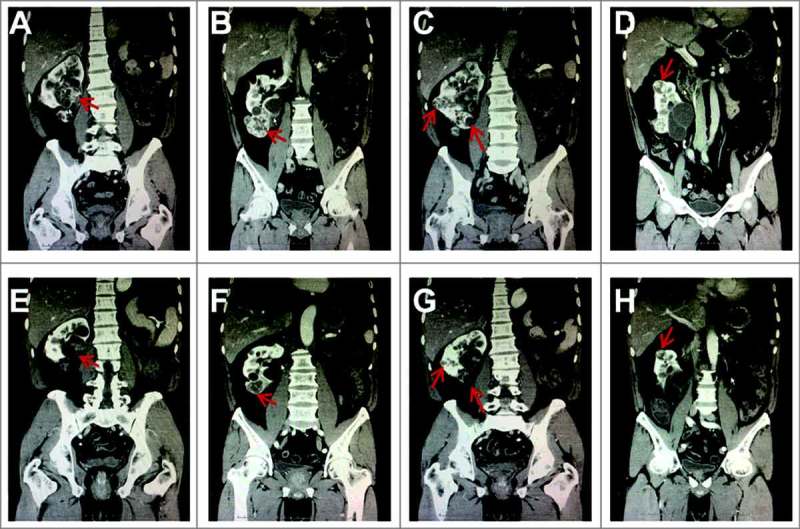
Computed tomography (CT) scans from Patient 2. The CT scans show multiple right renal cell carcinomas (RCCs) before and after sunitinib treatment, including the masses around the renal hilum (A and E), lower ventral (B and F), dorsal, lower dosal (C and G), superior pole (D and H). Red arrows indicate the masses.
Patient 3: In August 2011, a 50-year-old female was admitted to our hospital where a MRI scan revealed at least 3 lesions on her left kidney (Figure 3A and B) and 3 lesions in her pancreas (Figure 3C), which were most likely to be pancreatic NETs according to the characteristics on MRI scans. The size of the largest tumor on her kidney is about 3.1 × 2.4 cm. MRI scan on brain was negative and the abdominal CT scan discovered a mass with contrast enhancement on her back (Figure 3D) Five years ago before this admission, the patient had undergone a radical nephrectomy for the tumors on the right kidney. Given that the patient had solitary kidney and multiple tumors in her left kidney and pancreas, she was started on sunitinib to control the tumor. Since then, sunitinib had been administrated for total of 6 years. In 2012, A blood DNA sequencing analysis revealed a pathogenic VHL mutation c. 232A>T; p. Asn78Tyr in this patient. The same point mutation of VHL was identified in three family members including one sister, one brother and one nephew. Her sister was found with pancreatic NET and died of the hemorrhagic stroke at age of 40 years, and her brother and nephew refused for further examinations for VHL-related lesions. Her mother died of stroke at early of 50 years.
Figure 3.

Magnetic resonance imaging (MRI) and computed tomography (CT) scans from Patient 3. The MRI scans show multiple RCCs in the left kidney including RCC in the dorsal of the kidney (A and E), ventral of the kidney and beside the spine (B and F), as well as three pancreatic lesions (C and G) before and after sunitinib treatment. The CT scans show the lesion on her back before and after sunitinib treatment (D and H). Red arrows indicate the masses.
Clinical outcomes and therapeutic response
The clinical characteristics of the three patients are reported in Table 1. During the follow-up period, CT or MRI scans were conducted every 3–4 months to evaluate the responses of the tumors to sunitinib treatment. All three patients showed clinical improvement after treatment with sunitinib. In Patient 1, the representative CT scan after sunitinib treatment was performed in October 2016, which was 19 months after the start of sunitinib treatment. This scan revealed that the size of the RCC (Figure 1B) had decreased from 2.5 × 2.1 cm before sunitinib treatment to 1.1 × 0.9 cm after treatment. Similarly, the size of the left pheochromocytoma (Figure 1D) had decreased from 6.1 × 3.9 cm before sunitinib treatment to 3.3 × 2.4 cm after treatment (Table 2). More importantly, a reduction of iodine uptake was found in the tumors compared with baseline scans, suggesting necrosis of the tumor cells occurred after sunitinib treatment. Moreover, the size of the hemangioblastoma in her right cerebellum also seemed to decrease from 1.0 × 1.4 cm before sunitinib treatment to 1 × 1 cm during surgery, which was conducted after 11 months of sunitinib treatment. A post-operative head CT scan confirmed complete resection of the tumor (Figure 1F). In Patient 2, all the masses in his right kidney decreased in size and iodine uptake after 4 months of sunitinib treatment (Figure 2E-H), as reported in Table 2. In Patient 3, the sizes of the RCCs in her left kidney and pancreatic lesions decreased, evidenced by the representative MRI scan after sunitinib treatment on August 2017 (Figure 3E-G), and remained stable through 6 years of follow-up. Interestingly, the sizes of the lesions on her back also decreased due to the treatment of sunitinib (Figure 3H). All the changes of the tumor sizes were concluded in Table 2.
Table 1.
Characteristics of three patients with VHL disease
| Case No. | Age | Gender | VHL gene mutation | Phenotypes | Sunitinib | ||||
| RCC | Pheo | HB | Pancreatic NET | Initial date | Dosage | ||||
| 1 | 44 | Female | c. 239G>T; p. Ser80Ile | Right | Bilateral | Right cerebellum | Yes | May 2015 | 50 mg/day (4/2 scheme*) |
| 2 | 54 | Male | c. 239G>T; p. Ser80Ile | Bilateral | Left | Bilateral cerebellum | — | January 2017 | 50 mg/day (4/2 scheme) |
| 3 | 56 | Female | c. 232A>T; p. Asn78Tyr | Bilateral | — | — | Yes | August 2011 | 37.5 mg/day (4/2 scheme) |
*4/2 scheme: Patients took the indicated dosage once per day for 4 weeks followed by a 2-week break across repeated cycles; VHL: Von Hippel-Lindau, RCC: renal cell carcinoma, Pheo: pheochromocytoma, HB: hemangioblastoma, NET: neuroendocrine tumor.
Table 2.
Changes of the masses in the Patients
| Patients | Kind and/or Location of the Mass | Before sunitinib treatment | After sunitinib treatment |
| Patient 1 | RCC | 2.50 × 2.10 cm | 1.10 × 0.90 cm |
| Pheo | 6.10 × 3.90 cm | 3.30 × 2.40 cm | |
| Patient 2 (RCC) | Renal hilus | 6.33 × 5.55 cm | 4.66 × 4.35 cm |
| Lower ventral | 5.67 × 3.89 cm | 3.48 × 2.56 cm | |
| Dorsal | 2.75 × 2.13 cm | 1.19 × 0.68 cm | |
| Lower dorsal | 3.64 × 3.12 cm | 2.91 × 2.03 cm | |
| Superior pole | 3.39 × 2.57 cm | 2.41 × 1.88 cm | |
| Patient 3 | Dorsal RCC | 2.84 × 2.40 cm | 1.11 × 1.00 cm |
| Ventral RCC | 2.24 × 2.05 cm | 0.96 × 0.95 cm | |
| RCC beside the spine | 3.47 × 3.21 cm | 0.90 × 0.84 cm | |
| Pancreas head | 3.09 × 2.4 cm | 1.79 × 1.70 cm | |
| Pancreas body | 3.25 × 2.24 cm | 1.84 × 1.05 cm | |
| Pancreas tail | 3.45 × 3.38 cm | 1.74 × 1.68 cm | |
| Mass on her back | 2.22 × 1.32 cm | 1.46 × 0.85 cm |
RCC: renal cell carcinoma, Pheo: pheochromocytoma.
Toxicity
All three patients reported adverse side effects to sunitinib treatment, including fatigue, diarrhea and mucositis (Table 3), but no severe toxic effects (i.e., grade 3–5) were observed in these patients. Discussion
Table 3.
Treatment-emergent adverse side effects
| Toxicity | Grades of the toxicity | ||
| Patient 1 | Patient 2 | Patient 3 | |
| Fatigue | 2 | 1 | 2 |
| Diarrhea | 1 | — | 1 |
| Mucositis | — | — | 2 (gastric) |
| Anemia | 2 | — | — |
| Hand/foot/skin reaction | 2 | 1 | 1 |
| Rash | — | — | — |
| Nausea | 1 | — | 1 |
| Neutropenia | 1 | — | 1 |
| Dysgeusia | 1 | — | — |
| Sensory neuropathy | — | — | — |
| Vomiting | — | — | 1 |
| Skin rash | 1 | — | — |
| Dyspnea | — | — | — |
| Edema (head and neck) | 1 | 1 | 1 |
| Hypertension | — | 1 | 2 |
| Pain | — | — | — |
| Headache | — | — | — |
| Anorexia | 1 | — | 1 |
| Hypophosphatemia | — | — | — |
| Epiphora | — | — | 1 |
| Transaminitis | — | — | — |
| Alopecia | 2 | 1 | 1 |
| Infection | — | — | — |
| Hyperbilirubinemia | — | — | 1 |
| Cough | — | — | — |
| Dysphagia | — | — | — |
| Confusion | 1 | — | 1 |
| Elevated creatinine | — | — | 2 |
| Epistaxis | — | — | — |
Our retrospective study identified three patients with confirmed VHL disease who exhibited positive clinical outcomes in response to sunitinib. These case studies indicate that sunitinib may be an effective treatment for VHL disease-related tumors.
To date, no VHL-targeted drugs have been approved by the FDA for the treatment of VHL-related tumors. Several treatments are available, however, that target molecules downstream of VHL. Several of these treatments, including VEGF monoclonal antibodies, tyrosine kinase inhibitors (TKI), and mechanistic target of rapamycin (mTOR) inhibitors, have been shown to be effective for the treatment of VHL-related tumors7. Of note, TKIs are currently used to treat advanced renal cancer, and recently have been used to treat pancreatic neuroendocrine tumors. In clinical studies of patients with advanced cRCC, 40% of cases exhibited a partial response and up to 90% of cases exhibited stable disease on TKIs8. Previous studies have reported that the effectiveness of VEGF-targeted agents may be associated with the patient's VHL inactivation status, as patients with loss-of-function mutations (i.e., frameshift, nonsense, splice, and in-frame deletions/insertions) had a better response rate than those with wild-type VHL 9, 10. Our three patients had mutations associated with an inactivated VHL protein, and all three patients, especially Patient 1, responded positively to sunitinib treatment.
Several previous pilot and small retrospective studies have indicated that sunitinib, which is a common TKI in China, may be effective for the treatment of VHL disease-related tumors, which is consistent with the findings from our small retrospective case study. A Phase-II trial by Jonasch et al. that evaluated the effect of sunitinib on 18 RCCs and 21 hemangioblastomas among 15 patients with VHL disease reported partial response in six (33%) of the RCCs but none of the hemangioblastomas, and disease control (i.e., partial response + stable disease) in 90% of the RCCs and 91% of the hemangioblastomas8. Kim et al. reported that VHL-related metastatic RCC is highly responsive to VEGF TKI for a prolonged period of time, and suggested that benign cysts or tumors associated with VHL disease also could be controlled with VEGF TKIs7. A multicenter, Phase-II, open-label study of sunitinib from the PREDIR VHL network has shown that all five enrolled patients with VHL disease exhibited stable disease as the best response at 6 months. This study also showed that sunitinib was more effective for VHL-related RCCs than for other VHL-related lesions11. More recently, a retrospective study by Roma et al. showed that 9 of 14 (64%) patients with VHL who received sunitinib treatment as a first-line therapy exhibited radiological response not only in renal and hepatic lesions but also in pancreatic nodules12. In addition, a case study reported that sunitinib may be effective in treating a patient with primitive neuroectodermal tumors that express a copy number loss of the VHL gene13.
According to these previous studies, however, sunitinib may not be equally effective across all VHL disease-associated lesions. In 2009, Jimenez et al. reported that a carrier of a VHL germline mutation with an aggressive malignant pheochromocytoma and several RCCs and pancreatic neuroendocrine tumors exhibited decreases in the sizes of most tumors after 6 months of treatment with sunitinib14. Furthermore, several other studies7, 8,11,15 have shown that sunitinib may be more effective in cRCCs arising from patients with VHL disease compared to sporadic non-VHL-related RCCs. Previous studies are equivocal, however, with respect to the effects of sunitinib on other VHL-related lesions. For example, Kim et al., but not other studies, found that sunitinib may control the growth of lesions in the pancreas and cerebellum. Knickelbein et al. found that treatment with sunitinib improved hemangioblastoma-related retinal edema in patients with VHL-related retinal hemangioblastomas, but did not improve their visual acuity or reduce the size of the tumor16. In contrast, Roma et al. recorded radiological responses to sunitinib in renal, pancreatic, adrenal, hepatic, pulmonary, and subcutaneous tumors but not in hemangioblastomas12. A recent study reported that sunitinib was effective in pancreatic neuroendocrine tumors associated with VHL17. In our three patients, sunitinib treatment was associated with decreases in RCC size and may also have effects on VHL-related pheochromocytomas and hemangioblastomas, as well as pancreatic NET. These discrepancies across studies may be due to differences in individuals, mutations, and/or tissues, which in turn may be associated with differential expressions of VEGF and therefore diverse responses to sunitinib.
Other agents that target molecules downstream from VHL have been used for the treatment of VHL-related tumors. In the study by Kim et al., for example, patients responded to treatment with the mTOR inhibitor, everolimus, after not responding to a VEGF TKI; moreover, one patient exhibited a partial response to sorafenib after her disease progressed on sunitinib and everolimus7. Choi et al. also reported on two patients with VHL disease who were treated effectively with sorafenib18. Furthermore, two small non-controlled prospective clinical trials investigating the effects of pegaptanib and ranibizumab on the treatment of VHL-associated retinal capillary hemangioblastomas have shown that these drugs might reduce exudation without any evidence for tumor repression19, 20. In addition, Metelo et al. have demonstrated that the HIF2a inhibitor, lead compound 76, can inhibit zebrafish orthologs of human HIF2a and ameliorate phenotypic abnormalities in vhl−/− embryos, indicating that HIF2a inhibitors may be effective for treating VHL disease-related abnormalities21. Further studies are needed to determine whether these agents are suitable for treatment of patients with VHL disease.
Although our study and previous studies are limited by small numbers of patients, together their results suggest that sunitinib may be an effective treatment in patients with VHL disease. Further evaluation of sunitinib in patients with VHL disease is warranted. It also will be important to examine the role of the VHL/HIF pathway in sporadic RCCs, pheochromocytomas, and hemangioblastomas, and to examine whether we can better select drugs that target these tumor types.
Materials and methods
Study design
We performed a retrospective analysis of clinical and radiological data from approximately 20 patients with VHL disease from five families. Three of these patients with mutiple RCCs as well as Pheos or paragangliomas were treated with sunitinib. We retrospectively compared changes in their VHL disease-associated tumors before and after sunitinib treatment.
Compliance with ethical standards
All procedures involving human participants were carried out in accordance with the 1964 Declaration of Helsinki. The Daping Hospital of the Third Military Medical University waived institutional review board approval for the study; however, written informed consent for the use of medical records and related images was obtained from each patient.
Treatment
According to all the patients’ disease status, sunitinib was prescribed to these patients by the professional urologist. All three patients received oral sunitinib once per day for 4 weeks, followed by 2 weeks with no treatment (4/2 scheme). The starting dose of sunitinib was 50 mg/day. If side effects were severe, however, the daily dose was reduced to 37.5 mg. After one cycle, the treatment was continued unless the side effects became unacceptable or the progression of the disease occurred.
Clinical evaluation of responses to sunitinib
A computed tomography (CT) or magnetic resonance imaging (MRI) scan was extracted from each medical record prior to treatment with sunitinib to evaluate the VHL disease-associated tumors. Scans taken after sunitinib treatment also were extracted from the medical record to evaluate changes in the tumors. The adverse side effects associated with sunitinib also were assessed according to NCI-CTC v3.0 criteria.
References
- 1.Gossage L, Eisen T: Alterations in VHL as potential biomarkers in renal-cell carcinoma. Nature reviews Clinical oncology 2010, 7(5):277–288. [DOI] [PubMed] [Google Scholar]
- 2.Shuin T, Yamasaki I, Tamura K, Okuda H, Furihata M, Ashida S: Von Hippel-Lindau disease: molecular pathological basis, clinical criteria, genetic testing, clinical features of tumors and treatment. Japanese journal of clinical oncology 2006, 36(6):337–343. [DOI] [PubMed] [Google Scholar]
- 3.Gossage L, Eisen T, Maher ER: VHL, the story of a tumour suppressor gene. Nature reviews Cancer 2015, 15(1):55–64. [DOI] [PubMed] [Google Scholar]
- 4.Faivre S, Demetri G, Sargent W, Raymond E: Molecular basis for sunitinib efficacy and future clinical development. Nature reviews Drug discovery 2007, 6(9):734–745. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. : Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. The New England journal of medicine 2007, 356(2):115–124. [DOI] [PubMed] [Google Scholar]
- 6.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, et al. : Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. The New England journal of medicine 2011, 364(6):501–513. [DOI] [PubMed] [Google Scholar]
- 7.Kim HC, Lee JS, Kim SH, So HS, Woo CY, Lee JL: Sunitinib treatment for metastatic renal cell carcinoma in patients with von hippel-lindau disease. Cancer research and treatment : official journal of Korean Cancer Association 2013, 45(4):349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonasch E, McCutcheon IE, Waguespack SG, Wen S, Davis DW, Smith LA, Tannir NM, Gombos DS, Fuller GN, Matin SF: Pilot trial of sunitinib therapy in patients with von Hippel-Lindau disease. Annals of oncology : official journal of the European Society for Medical Oncology 2011, 22(12):2661–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choueiri TK, Vaziri SA, Jaeger E, Elson P, Wood L, Bhalla IP, Small EJ, Weinberg V, Sein N, Simko J, et al. : von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. The Journal of urology 2008, 180(3):860–865; discussion 865–866. [DOI] [PubMed] [Google Scholar]
- 10.Rini BI, Jaeger E, Weinberg V, Sein N, Chew K, Fong K, Simko J, Small EJ, Waldman FM: Clinical response to therapy targeted at vascular endothelial growth factor in metastatic renal cell carcinoma: impact of patient characteristics and Von Hippel-Lindau gene status. BJU international 2006, 98(4):756–762. [DOI] [PubMed] [Google Scholar]
- 11.Oudard S, Elaidi R, Brizard M, Le Rest C Caillet V, Deveaux S, Benoit G, Correas JM, Benoudiba F, David P, et al. : Sunitinib for the treatment of benign and malignant neoplasms from von Hippel-Lindau disease: A single-arm, prospective phase II clinical study from the PREDIR group. Oncotarget 2016, 7(51):85306–85317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roma A, Maruzzo M, Basso U, Brunello A, Zamarchi R, Bezzon E, Pomerri F, Zovato S, Opocher G, Zagonel V: First-Line sunitinib in patients with renal cell carcinoma (RCC) in von Hippel-Lindau (VHL) disease: clinical outcome and patterns of radiological response. Familial cancer 2015, 14(2):309–316. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Zhang J, Wang G, Xu J, Li Y, Guo Q, Zheng T, Zhang Y: Benefit of Sunitinib in the treatment of pulmonary primitive neuroectodermal tumors: a case report and literature review. Oncotarget 2016, 7(52):87543–87551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jimenez C, Cabanillas ME, Santarpia L, Jonasch E, Kyle KL, Lano EA, Matin SF, Nunez RF, Perrier ND, Phan A, et al. : Use of the tyrosine kinase inhibitor sunitinib in a patient with von Hippel-Lindau disease: targeting angiogenic factors in pheochromocytoma and other von Hippel-Lindau disease-related tumors. The Journal of clinical endocrinology and metabolism 2009, 94(2):386–391. [DOI] [PubMed] [Google Scholar]
- 15.Tsimafeyeu I: Sunitinib treatment of metastatic renal cell carcinoma in von Hippel-Lindau disease. Journal of cancer research and therapeutics 2015, 11(4):920–922. [DOI] [PubMed] [Google Scholar]
- 16.Knickelbein JE, Jacobs-El N, Wong WT, Wiley HE, Cukras CA, Meyerle CB, Chew EY: Systemic Sunitinib Malate Treatment for Advanced Juxtapapillary Retinal Hemangioblastomas Associated with von Hippel-Lindau Disease. Ophthalmology retina 2017, 1(3):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali T, Kandil D, Piperdi B: Long-term disease control with sunitinib in a patient with metastatic pancreatic neuroendocrine tumor (NET) associated with Von Hippel-Lindau syndrome (VHL). Pancreas 2012, 41(3):492–493. [DOI] [PubMed] [Google Scholar]
- 18.Choi KH, Yu YD, Kang MH, Park DS: Sorafenib treatment for recurrent stage T1 bilateral renal cell carcinoma in patients with Von Hippel- Lindau disease: A case report and literature review. Canadian Urological Association journal = Journal de l'Association des urologues du Canada 2015, 9(9-10):E651–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahr SS, Cusick M, Rodriguez-Coleman H, Srivastava SK, Thompson DJ, Linehan WM, Ferris FL, 3rd Chew EY: Intravitreal anti-vascular endothelial growth factor therapy with pegaptanib for advanced von Hippel-Lindau disease of the retina. Retina 2007, 27(2):150–158. [DOI] [PubMed] [Google Scholar]
- 20.Wong WT, Liang KJ, Hammel K, Coleman HR, Chew EY: Intravitreal ranibizumab therapy for retinal capillary hemangioblastoma related to von Hippel-Lindau disease. Ophthalmology 2008, 115(11):1957–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metelo AM, Noonan H, Iliopoulos O: HIF2a inhibitors for the treatment of VHL disease. Oncotarget 2015, 6(27):23036–23037. [DOI] [PMC free article] [PubMed] [Google Scholar]


