ABSTRACT
Extracellular signal-regulated kinase (ERK) plays a critical role in tissue homeostasis and tumorigenesis. By utilizing live imaging approaches, we recently uncovered ERK activity dynamics in the intestinal epithelium. Notably, we showed that ERK activity dynamics are defined by composite regulation from two distinct upstream receptors, and alteration of their functional balance underlies tumor cell-specific traits. Here, we discuss these findings.
Keywords: ERK MAP kinase, EGFR, ErbB2, the intestinal epithelium, tumorigenesis, in vivo imaging
The extracellular signal-regulated kinase (ERK) signaling pathway regulates various biological processes including cell proliferation, differentiation, and tumorigenesis1. Recent advances in bioimaging techniques have enabled to visualize ERK activity in real-time at the single-cell level2. Emerging evidence from such bioimaging approaches suggests unexpectedly complex spatio-temporal dynamics of ERK activity. For example, cultured cells and living mouse epidermis show spontaneous pulsatile ERK activation and its lateral propagation, both of which are correlated with cell proliferation3-5. Notably, ERK activity dynamics as well as its overall strength can be a critical determinant of cell proliferation. Despite their apparent significance, how ERK activity dynamics are regulated and how they affect the physiological processes remain largely unknown.
By using transgenic mice ubiquitously expressing a highly sensitive Förster resonance energy transfer (FRET) biosensor for ERK (EKAREV-NLS)6, we recently succeeded to visualize ERK activity in living intestinal epithelial cells (IECs)7. Our intravital imaging demonstrated that IECs at the crypts exhibit sporadic ERK activity pulses. The ERK activity pulses were generated by spontaneous firing in each cell or propagation from adjacent cells. Further detailed analyses revealed two properties of ERK activity in IECs: the sustained, basal activity and the pulse-like activity. We then generated and observed intestinal organoids8 expressing EKAREV-NLS to elucidate the nature of ERK activity dynamics. The spatio-temporal dynamics of ERK activity in vivo were well recapitulated in intestinal organoids: cells in the organoids often showed ERK activity pulses as observed in vivo, and propagation of ERK activity pulses was also observed in the absence of exogenous EGF. The ERK pulse propagation was diminished either by an epidermal growth factor receptor (EGFR) inhibitor, or by a matrix metalloproteinase (MMP) inhibitor, suggesting that propagation of ERK activity pulses requires shedding of intrinsic EGFR ligands and the resultant EGFR activation. Notably, EGFR inhibition also suppressed spontaneous firing of ERK activity pulses in each cell. Thus, EGFR signaling should be a major driver of ERK activity pulses. In contrast to this, the basal ERK activity was not significantly affected by EGFR inhibition. We found that inhibition of another growth factor receptor, erb-b2 receptor tyrosine kinase 2 (ErbB2), markedly decreased basal ERK activity in intestinal organoids. These results show that ERK activity pulses are generated by EGFR signaling whereas basal ERK activity is maintained by ErbB2 signaling in IECs (Figure 1). Importantly, ErbB2 inhibition, but not EGFR inhibition, strongly suppressed cell proliferation in intestinal organoids, suggesting that the ErbB2-dependent basal ERK activity plays a major role in promoting proliferation of IECs.
Figure 1.
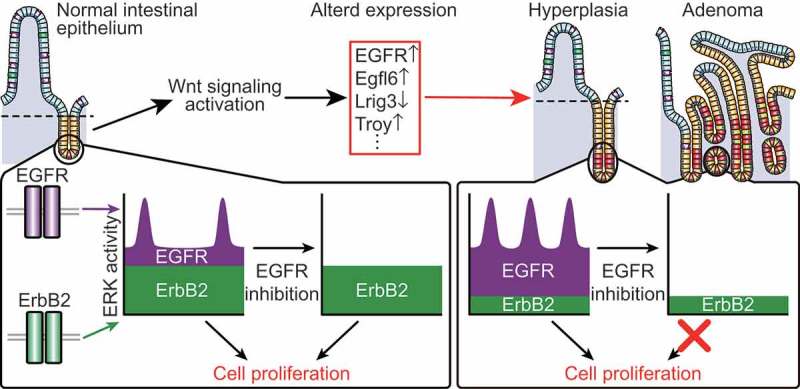
ERK activity dynamics generated by EGFR and ErbB2 signaling in the normal and tumor intestinal epithelium.
In normal intestinal epithelial cells (IECs), extracellular signal-regulated kinase (ERK) activity is defined by the erb-b2 receptor tyrosine kinase 2 (ErbB2)-dependent basal component and the epidermal growth factor receptor (EGFR)-dependent pulse-like component. During intestinal tumorigenesis, Wnt signaling activation alters expression of EGFR and its regulators, thereby augmenting EGFR signaling. As a result, the EGFR-dependent ERK activity pulses and contribution of EGFR signaling to the basal ERK activity are increased in tumor cells. Thus, inhibition of EGFR strongly suppresses both the pulsatile and basal ERK activities in tumor cells, but not in normal IECs, leading to selective suppression of tumor cell survival and proliferation.
Excessive activation of EGFR-ERK signaling has been implicated in colorectal cancers (CRC) and regimens containing anti-EGFR antibodies are suggested as initial therapy in non-operable CRCs without KRAS mutation. We thus investigated how ERK activity dynamics are altered during intestinal tumorigenesis. For this purpose, organoids were generated from adenomas developed in adenomatosis polyposis coli (Apc) mutant mice9, followed by observation of ERK activity dynamics. In stark contrast to normal organoids, the basal ERK activity, as well as the pulsatile activity, was dependent on EGFR signaling in adenoma organoids. The ERK activity pulses were also increased in an EGFR-dependent manner. Moreover, genetic and pharmacological activation of Wnt signaling caused similar changes in ERK activity dynamics and promoted cell proliferation in an EGFR-dependent manner. These results suggest that, during intestinal tumorigenesis, Wnt signaling activation alters ERK activity dynamics by augmenting EGFR signaling, which renders IECs sensitive to EGFR inhibition.
To investigate the molecular mechanisms of Wnt signaling-dependent augmentation of EGFR signaling, we performed gene expression profiling of intestinal organoids in which Wnt signaling was pharmacologically activated by a glycogen synthase kinase 3 (GSK3) inhibitor. We identified four EGFR regulators whose expression levels were upregulated (EGF-like-domain, multiple 6 (Egfl6), filamin, alpha (Flna), and tumor necrosis factor receptor superfamily, member 19 (Tnfrsf19) (a.k.a. Troy)) and downregulated (leucine-rich repeats and immunoglobulin-like domains 3 (Lrig3)) by Wnt signaling activation. The knockdown of either Egfl6 or Troy, or exogenous expression of Lrig3 partially restored normal ERK activity dynamics in adenoma organoids. In addition to the altered expression of EGFR regulators, expression of EGFR itself was also increased in adenomas compared to the normal epithelium. These results suggest that altered expression of multiple EGFR regulators, together with enhanced EGFR expression, coordinately promotes EGFR-ERK signaling in adenoma cells (Figure 1).
By visualizing ERK activity in the intestinal epithelium at the single-cell level, we unveiled two distinct modes of ERK activity dynamics driven by EGFR and ErbB2 signaling. The distinct ERK activity dynamics might be attributed to different regulatory mechanisms and characteristics of the two receptors. For example, ligand-induced activation causes rapid internalization of EGFR, while ErbB2 has no known ligands and is assumed to transmit more sustained signals. In addition, ERK activation has been shown to trigger negative feedback regulation of the upstream molecules including the growth factor receptors, which should play a key role in shaping the pulsatile ERK activation pattern at different levels of the signaling cascade10. An important question regarding ERK activity dynamics is whether the pulsatile and sustained ERK activities lead to distinct outputs at the molecular level. Given that, upon Wnt signaling activation, increased ERK activity pulses promote proliferation of IECs, it would be interesting to examine whether the pulsatile nature of the ERK activity per se has some meaning in intestinal tumorigenesis. We also found that Wnt signaling activation increased the contribution of EGFR signaling to ERK activity dynamics, thereby rendering cells addicted to EGFR signaling. These findings provide a rationale for why pharmacological inhibition of EGFR can specifically target colorectal cancers in clinical practice without causing severe damage to the normal tissues (Figure 1). Altogether, our findings highlight the importance of elucidating signaling activity dynamics at the single-cell level. Live imaging with FRET biosensors can be a powerful tool to examine activity of biomolecules in the living tissues and to gain a better understanding of the mechanisms involved in many physiological and pathological processes.
Funding Statement
M.I. was supported by the Takeda Science Foundation and Japan Society for the Promotion of Science (JSPS) (Grant-in-Aid for Scientific Research (KAKENHI) on Innovative Areas, “Integrated analysis and regulation of cellular diversity” (18H05100) and for Scientific Research (C) (18K06929)). M.M. was supported by the Nakatani Foundation, CREST JPMJCR1654 and JSPS KAKENHI Grant Numbers 15H02397, 15H05949 “Resonance Bio”, and 16H06280 “ABiS”.
Acknowledgments
We are grateful to the Medical Research Support Center of Kyoto University for technical assistance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Shaul YD, Seger R.. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Komatsu N, Aoki K, Yamada M, Yukinaga H, Fujita Y, Kamioka Y, Matsuda M Development of an optimized backbone of FRET biosensors for kinases and GTPases. Mol Biol Cell. 2011;22:4647–4656. doi: 10.1091/mbc.E11-01-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albeck JG, Mills GB, Brugge JS. Frequency-modulated pulses of ERK activity transmit quantitative proliferation signals. Mol Cell. 2013;49:249–261. doi: 10.1016/j.molcel.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoki K, Kumagai Y, Sakurai A, Komatsu N, Fujita Y, Shionyu C, Matsuda M Stochastic ERK activation induced by noise and cell-to-cell propagation regulates cell density-dependent proliferation. Mol Cell. 2013;52:529–540. doi: 10.1016/j.molcel.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Hiratsuka T, Fujita Y, Naoki H, Aoki K, Kamioka Y, Matsuda M Intercellular propagation of extracellular signal-regulated kinase activation revealed by in vivo imaging of mouse skin. Elife. 2015;4:e05178. doi: 10.7554/eLife.06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamioka Y, Sumiyama K, Mizuno R, Sakai Y, Hirata E, Kiyokawa E, Matsuda M Live imaging of protein kinase activities in transgenic mice expressing FRET biosensors. Cell Struct Funct. 2012;37:65–73. [DOI] [PubMed] [Google Scholar]
- 7.Muta Y, Fujita Y, Sumiyama K, Sakurai A, Taketo MM, Chiba T, Seno H, Aoki K, Matsuda M, Imajo M. Composite regulation of ERK activity dynamics underlying tumour-specific traits in the intestine. Nat Commun. 2018;9:2174. doi: 10.1038/s41467-018-04527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ , et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 9.Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc Natl Acad Sci U S A. 1995;92:4482–4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avraham R, Yarden Y. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat Rev Mol Cell Biol. 2011;12:104–117. doi: 10.1038/nrm3048. [DOI] [PubMed] [Google Scholar]


