ABSTRACT
Horizontal gene transfer is known to occur in bacteria and archaea whereas higher organisms including mammals undergo vertical transfer. Our recent results demonstrate horizontal transfer of mitochondrial DNA (mtDNA) from normal host cells to tumor cells lacking mitochondrial DNA (mtDNA). This mtDNA migration results in recovery of respiration, restored tumor initiation, and metastasis.
KEYWORDS: intercellular DNA transfer, mitochondrial DNA deletion, metastasis, respiration, tumor growth
Genes are thought to remain the ‘property’ of somatic cells in higher organisms and are distributed between daughter cells during division. This textbook understanding is well established for nuclear genes and for the several hundred mitochondrial genomes within somatic cells that partition together with mitochondria during cell division, at least in mammalian systems.1
It was therefore highly unexpected to find that mouse tumor cell lines without mtDNA acquired a mitochondrial genome from host cells in their local microenvironment following injection into recipient mice.2 With highly metastatic B16 melanoma and 4T1 breast carcinoma cell lines in which mtDNA had been completely eliminated by low-dose ethidium bromide treatment (ρ° cells), tumors did not begin to form for 3–4 weeks, by which time these cells had acquired mtDNA from the recipient mouse. This was demonstrated by the presence of the mitochondrial cytochrome b gene, Cyt b, in cell lines derived from tumors that formed from B16ρ° and 4T1ρ° cells. The possibility that nuclear-encoded mitochondrial genes might explain these results was excluded because no signal was observed with ρ° cells whose nuclear genome remained intact. The evidence for genome acquisition from the host mouse was further strengthened when a mtDNA polymorphism was identified within the mitochondrial arginyl tRNA gene tRNAArg that matched the host’s mtDNA and differed from the respective parental tumor cells. Additionally, 2 further polymorphisms in the 16S rRNA gene, 16S rRNA, and the displacement loop, D-loop, loci were shown to be identical in cell lines from tumors that grew from 4T1ρ° cells and in the host mouse. This unequivocally demonstrates transfer of mtDNA from cells of the host mouse to the ρ° cells.
Surprisingly, cell lines derived from primary tumors that grew from 4T1ρ° cells, and similar lines that grew from circulating tumor cells and from lung metastases, were frozen in different states of respiration recovery, as confirmed by extensive characterization of morphological, biochemical, molecular, and respiratory properties of these cell lines and by detailed analysis of the appearance of the respirasome. These cell lines remained stable over time in culture. Therefore, it can be concluded that in this model, respiration recovery is a function of the local environment that the cells are exposed to, and that respiration remodeling does not occur without an ‘inductive’ microenvironment.2 In contrast, complete recovery was observed in cell lines derived from primary tumors grown from B16ρ° cells (unpublished data). Although we do not have a complete explanation for this difference, respiration of parental B16 cells is approximately 5-fold higher than that of 4T1 cells suggesting that they may be more ‘addicted’ to oxidative phosphorylation (OXPHOS) and require a certain threshold for tumor initiation and growth that might vary between different tumor types.
How can mtDNA from cells in the local microenvironment repopulate tumor cells lacking mtDNA? Because there are no known mechanisms for ‘naked’ mtDNA moving across mitochondrial double membrane bilayers, or across the plasma membranes, the most likely explanation for our results involves mitochondrial movement between cells. This could occur either by endocytosis of cell fragments or exosomes containing mitochondria, by exosome fusion with the plasma membrane or cell fusion, or by direct cell–cell contact or cytoplasmic bridging that has been shown to occur between cells in culture and facilitates transfer of mitochondria and other cellular organelles.3-5 The endocytic pathway is problematic because it normally involves degradation or recycling of endocytic vesicle contents, and encapsulated mitochondria would need to escape their membrane-bound package to appear free in the cytoplasm. In this context, a recent study showed that damaged mitochondria from the optic nerve head are packaged into specialized exosomes for uptake and degradation by astrocytes through the endocytic pathway.6
More plausible explanations of mitochondrial movement between cells involve exosome–cell fusion and intercellular cytoplasmic bridges or membrane nanotubes that we have observed during co-culture of 4T1 and 4T1ρ° cells, implicating a role for these structures in mitochondrial transfer (Fig. 1). Similar mitochondrial transfer has been observed in other co-culture studies.3,5 In our models mtDNA acquisition was a relatively rare event, since tumors were observed following subcutaneous injection of 105, but not 104, cells. However, we cannot exclude the possibility that consecutive mitochondrial transfer events may occur on a number of occasions, or that tumor cells with acquired mtDNA may themselves indulge in mitochondrial transfer to cells without mtDNA. These highly intriguing mechanisms and their molecular regulation are being addressed in ongoing studies.
Figure 1.
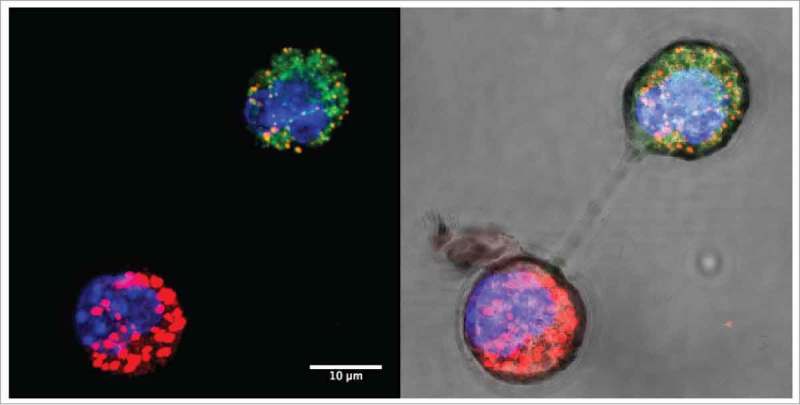
Intercellular mitochondrial transfer. Confocal images of mitochondrial transfer between MitoTracker-labeled 4T1 breast carcinoma cells (red) and 4T1ρ° cells (green) under dark field (left) and bright field (right) showing membrane nanotube connections. Cells were co-cultured for 24 h and nuclei were stained with NucBlue.
In summary, our results implicate intercellular mitochondrial transfer in a mouse tumor model, with functional consequences for tumor growth and metastasis.2 This has been shown in co-culture studies, where mitochondrial transfer rescued cancer cells suffering from OXPHOS insufficiency7 and those on the brink of apoptotic demise.6 Another report showed mitochondrial transfer and respiration recovery of injured lung epithelial cells in a mouse model that used exogenously administered mesenchymal stem cells.8 It is possible that the intercellular transfer of mitochondria that is implied from our results is a relatively frequent event in a number of tissues in a variety of physiological and pathophysiological conditions.
Besides cell-to-cell mitochondrial trafficking, our paper strongly supports OXPHOS addiction of cancer cells as an emergent property that updates the Warburg hypothesis, such that the glycolytic tumor cells need a respiration threshold to enable tumor formation. However, respiration seems to be essential not only for tumor growth, but also for tumor cells to intravasate into the circulatory system and extravasate at distal sites to give rise to metastases.2,9 While this may be good news for developing novel anticancer drugs targeting components of respiration, it may also invoke a paradigm shift in the underpinnings of cell biology.10
Funding Statement
MVB and CG were supported by the Malaghan Institute. JN was supported, in part, by funding from the Australian Research Council, the Czech Science Foundation (P301/10/1937), and the BIOCEV European Regional Development Fund (CZ.1.05/1.1.00/02.0109).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Wallace DC. Mitochondria and cancer. Nat Rev Cancer 2012; 12:685-98; PMID:23001348; https://doi.org/ 10.1038/nrc3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan AS, Baty JW, Dong LF, Bezawork-Geleta A, Endaya B, Goodwin J, Bajzikova M, Kovarova J, Peterka M, Yan B, et al.. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential in cancer cells without mitochondrial DNA. Cell Metabolism 2015; 21:81-94; PMID:25565207; https://doi.org/ 10.1016/j.cmet.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 3.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science 2004; 303:1007-10; PMID:14963329; https://doi.org/ 10.1126/science.1093133 [DOI] [PubMed] [Google Scholar]
- 4.Rogers RS, Bhattacharya J. When cells become organelle donors. Physiology 2013; 28:414-22; PMID:24186936; https://doi.org/ 10.1152/physiol.00032.2013 [DOI] [PubMed] [Google Scholar]
- 5.Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, Kinebuchi M, Phan S, Zhou Y, Bihlmeyer NA et al.. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci USA 2014; 111:9633-8; PMID:24979790; https://doi.org/ 10.1073/pnas.1404651111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Gerdes HH. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ 2015; 22:1181-91; PMID:25571977; https://doi.org/ 10.1038/cdd.2014.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA 2006; 103:1283-8; PMID:16432190; https://doi.org/ 10.1073/pnas.0510511103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 2012; 18:759-65; PMID:22504485; https://doi.org/ 10.1038/nm.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeBleu VS, O'Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, de Carvalho FM, Damascena A, Domingos Chinen LT, Rocha RM et al.. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol 2014; 16:992-1003; PMID:25241037; https://doi.org/ 10.1038/ncb3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; https://doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]


