Abstract
Detecting circulating tumor cells (CTCs) has proven valuable for evaluating the prognosis of cancer patients and for studying the mechanisms of treatment resistance. Owing to the lack of universal and specific tumor markers for neuroblastoma (NB), in this prospective study, we adopted an EpCAM-independent method to detect CTCs in the peripheral blood of NB patients.
We used an EpCAM-independent assay to delete leukocytes and to enrich the CTCs. CTCs were identified by immunostaining of CD45, DAPI and DNA fluorescence in situ hybridization (FISH) of the centromere of chromosome 8 probe (CEP8). Cells that were DAPI+/CD45-/CEP8 ≥ 3 were considered CTCs. We collected peripheral blood from 28 NB patients as well as clinical and follow-up data.
The number of CTCs among the different risk groups were significantly different (p = .0208, Kruskal–Wallis test). Patients with metastasis had more CTCs than those without metastasis (p < .0001, Mann–Whitney test). Patients with ≥3 CTCs per 4 ml of peripheral blood had an increased likelihood of having metastasis (sensitivity, 88.89%; specificity, 78.59%), and patients with ≥10 CTCs per 4 ml of peripheral blood had poorer overall survival.
The EpCAM-independent assay along with immunostaining-FISH (i-FISH) described here can detect CTCs in patients with NB at a high sensitivity and may have clinical value for prognosis evaluation and diagnosing metastasis when imaging data are ambiguous.
Keywords: Circulating tumor cells, Neuroblastoma, Prognosis, Metastasis
Research in context.
Evidence before this study
There were many studies on the CTC detection for cancer patients and CTC was proved valuable in disease surveillance, prognosis evaluation and heterogeneity study. We searched the Pubmed database and CNKI (Chinese database) we could only find few liquid biopsy for our young cancer patients. Most of the studies were about the cfDNA, mRNA and minimal residual diseases published mainly on the journal: Cancer Research, Clinical Cancer Research and Oncotarget. Articles published on the Cell about the CTC detection of breast cancer provided a potential biomarker for clinical evaluation and the CTC detected could be used for further study. We also wanted to detect the CTC from our neuroblastoma patients. For the lack of universal cell surface maker of neuroblastoma cells, we adopted the method according to the paper published on the J EXP CLIN CANC RES to detect the CTC for our young patients. The paper we researched mainly from 2002–20018, for the studies on the liquid biopsy for neuroblastoma developed slowly. The searched terms were as follow: ‘neuroblastoma’ AND ‘liquid biopsy’; ‘children’ AND ‘liquid biopsy’; or ‘CTC’
Added value of this study
It was firstly used the EpCAM-independent enrichment method combined with i-FISH method to detect CTCs in patients with NB. We found that CTC counts significantly correlated with prognosis. EpCAM-independent enrichment combined with i-FISH platform had high sensitivity for detection of CTCs in patients with NB patients.
Implications of all the available evidence
-
1.
Many children's medical centers around the world, such as in Mainland China, are not equipped with the appropriate instrumentation required to perform MIBG or PET scans. Repeated conventional enhanced CT scan makes children exposed to radiation and MRI sometimes are unable to detect small primary lesions and metastasis.
The cutoff value indicating the possible presence of metastasis was 3 CTCs per 4 ml of peripheral blood, which might help physicians verify whether a patient has metastatic disease when image examination is ambiguous.
-
2.
Moreover, this study shows that the number of CTCs significantly correlated with overall survival. Thus, the method adopted in the study could provide a new method for evaluation of the prognosis.
Alt-text: Unlabelled Box
1. Introduction
Neuroblastoma (NB) is one of the most common extracranial malignancies in children, with an incidence of 10.5 per million children younger than 14 years old. NB accounts for approximately 12–15% of all pediatric cancer-related deaths [1]. Given advances in molecular biology and individual treatment strategies, the 5-year overall survival (OS) rates of low- and intermediate-risk patients exceed 90%, although the OS of high-risk patients remains poor [2]. Fifty percent of NB patients present with metastasis at the initial diagnosis. To evaluate disease stage, the effects of treatment and prognosis, most medical centers have adopted meta-iodobenzylguanidine (MIBG) scanning, fluorodeoxyglucose positron emission tomography (FDG-PET) scanning, standard computed tomography (CT) and magnetic resonance imaging (MRI) to detect primary and metastatic lesions [3]. However, many children's medical centers around the world, including those in Mainland China, are not equipped with the appropriate instrumentation required to perform MIBG or PET scans. Repeated conventional enhanced CT or MRI scans are used. However, CT scans expose children to radiation, and MRI scans are sometimes unable to detect small primary lesions and metastases.
Circulating tumor cells (CTCs), which originate from the primary tumor, spread into the bloodstream and become the source of metastasis, contributing to disease progression and relapse [4]. Among the methods used to detect different types of CTCs, the standard EpCAM-based enrichment method can specifically detect CTCs in patients with epithelial cancers. A universal and specific cell-surface marker for NB cells is currently unavailable [5]. Therefore, we used an epithelial marker-independent enrichment method combined with immunostaining-fluorescence in situ hybridization (i-FISH) to detect CTCs from NB patients. This EpCAM-independent method has been described by Zhang et al. [6] in a study that quantified CTCs from patients with osteosarcoma, a mesenchymal tumor type that does not express epithelial markers. This method has also been used in the detection of CTCs in gastric [7], pancreatic [8] and lung cancer [9]. This method utilizes of the characterization of centromere of chromosome 8 probe (CEP8) to detect CTCs with hyperdiploidy of chromosome 8.
In pediatric cancers, the gain of chromosome 8 has also been reported in adrenocortical carcinoma and osteosarcoma [10]. NB is heterogeneous, and the most commonly reported observations of the variations of chromosome aberrations in NB include 6p22 in sporadic neuroblastoma, MYCN amplification (2p), 1p deletion, 11q deletion and unbalanced translocations of chromosome 17 [11]. One study has reported the gain of chromosome 8 in eight NB patients with stage IV disease and without MYCN amplification [12]. Because hyperdiploidy of chromosome 8 is widely observed in numerous cancer types, and CTC detection techniques based on FISH of CEP8 are common and fully developed, we conducted a prospective study to detect CTCs in NB patients by using CEP8 FISH to evaluate the clinical value of this method in NB.
2. Methods
2.1. Patients and samples
Children with a pathological diagnosis of NB who had not received any treatment were enrolled in the study between April 2015 and September 2016 at the Oncology Department of the Children's Hospital of Fudan University. We collected 4 ml of peripheral venous blood from each patient on the first day of hospitalization, and we stored the samples in acid citrate dextrose anticoagulant tubes (Becton Dickinson, NJ, USA). Blood samples were processed within 48 h of collection.
All patients received treatment according to the Children's Oncology Group (COG) NB risk classification. For high-risk NB patients, the treatment consists of induction chemotherapy, surgery, localized control, consolidation and maintenance therapy. For low-risk patients, surgery is typically curative, and chemotherapy is reserved for those with life- or organ-threatening situations. For intermediate-risk patients, treatment includes surgery and chemotherapy. The chemotherapy course depends on the biological features of the tumor, such as the pathological features and MYCN gene status. Patients with favorable features receive four cycles of chemotherapy, whereas those with unfavorable features require eight cycles.
We collected clinical data such as sex, age, disease stage (International Neuroblastoma Staging System (INSS stage)), metastatic sites, COG-risk classification, MYCN status, neuron-specific enolase (NSE) levels and 24-h urinary vanillymandelic acid (VMA) levels (Table 1). The Ethics Committee of the Children's Hospital of Fudan University approved this study, which was performed according to the principles of the Declaration of Helsinki.
Table 1.
Main characteristic of patients.
| Clinical features | Number |
|---|---|
| Gender (male/ female) | 22/6 |
| Age, median Years (range) | 3.18 (0.14–7) |
| ≥1 years old | 24 |
| <1 years old | 4 |
| VMA, Median (range), (mg/24,h)a | 16.7 (0.2–42.9) |
| NSE, Median (range), (ng/ml)a | 164.72 (17.63–370) |
| N-myc amplification/normalb | 4/23 |
| Metastasis/ Non-metastasis at diagnosis | 9/19 |
| Metastasis site | |
| Bone | 6 |
| Marrow | 5 |
| Liver | 1 |
| Distant lymph node | 2 |
| Skin | 1 |
| Lung | 2 |
| COG risk group | |
| High-risk | 12 |
| Intermedia-risk | 6 |
| Low-risk | 10 |
| INSS stage | |
| I | 10 |
| II | 0 |
| III | 9 |
| IV | 8 |
| IVs | 1 |
Normal 24-h urine VMA ranges are 0–13.6 mg/24 h, NSE 0–16.3 ng/ml.
Altogether 27 patients had the myc gene test.
2.2. CTC enrichment and identification
First, CTC enrichment was performed according to the instructions of the Cytelligen CTC Enrichment Kit (Cytelligen, San Diego, CA, USA). Blood samples were centrifuged, and the supernatant above the red blood cell layer was discarded. The remainder of the sample was mixed with 3 ml hCTC Separation Matrix and centrifuged. The white buffy was collected and mixed with 100 μl of immunomagnetic particles coated with anti-CD45 monoclonal antibodies. After being shaken, the mixture was subjected to magnetic separation. The bead-free solution was collected and washed, and the resulting cell pellet was mixed with 100 μl of fixative and used to coat CTC slides. The slides were dried at room temperature. CTC identification was performed according to the Human Tumor Cell Identification Kit (Cytelligen, USA). A mixture of 20 μl of pepsin and 180 μl of 1% formaldehyde was added to the slides, which were then washed twice and dehydrated in a series of increasing ethanol concentrations (70%, 80%, and 100%). A Centromere Probe (CEP 8) Spectrum Orange (Vysis, Abbott Laboratories, Abbott Park, IL, USA) was added to the slides, denatured at 73 °C for 10 min and hybridized at 37 °C for 4 h. The slides were incubated at room temperature for 1.5 h in the dark with antibody preparation solution, which contained the anti-CD45 antibody. After being washed twice, 5 μl of mounting medium containing 4′, 6-diamidino-2-phenylindole (DAPI, Vector Laboratories, CA, USA) was added, and the samples were subjected to fluorescence microscopy.
2.3. FISH study of chromosome abnormalities in tumor tissue
A series of 5-μm tumor tissue sections was prepared from each patients before chemotherapy. One section was stained with H&E in order to define FISH areas. Section was deparaffinized by dimethylbenzene, dehydrated in 100% ethanol, and air dried. And the section was soaked in pretreatment reagent for 20 min at 80 °C and in distilled water 3 minus. The slides were digested in Pepsin solution at 37 °C for 15 min and were soaked in distilled water and air dried. The slides were dehydrated in 70%, 85% and 100% ethanol. The 10 μL CEP8 Spectrumorange DNA Probe (Abbott, USA) was applied to the target area on the slide. Slides were denatured at 75 °C for 5 min and hybridized at 37 °C for 16 h. The slides were immersed in posthybridization wash buffer at 72 °C for 2 min, dehydrated with ethanol, and air dried in the dark. DAPI I solution (10 μL; Abbott) was then applied to the hybridized area at −20 °C for 30 min.
The hybridization signal was counted for each single cells in a view with a 100× oil immersion objective (Olympus, Japan) and we count 50 cells for each case. According to CEP8 Spectrumorange DNA Probe kit (Abbott, USA), cells in one case with >2.2% tri-signaled nuclei were considered to have abnormal trisomy 8 clones. The counting criteria of positive FISH spots was as following: cells without high background signals had clearly FISH signals, and the FISH signals were homogeneous light and size. Besides, cells boundary were clear under the microscope. Only those cells that met the above criteria were counted (Supplementary Fig. 1; https://www.molecular.abbott/int/zh/vysis-fish-knowledge-center/enumeration-guidelines).
Supplementary Fig. 1.
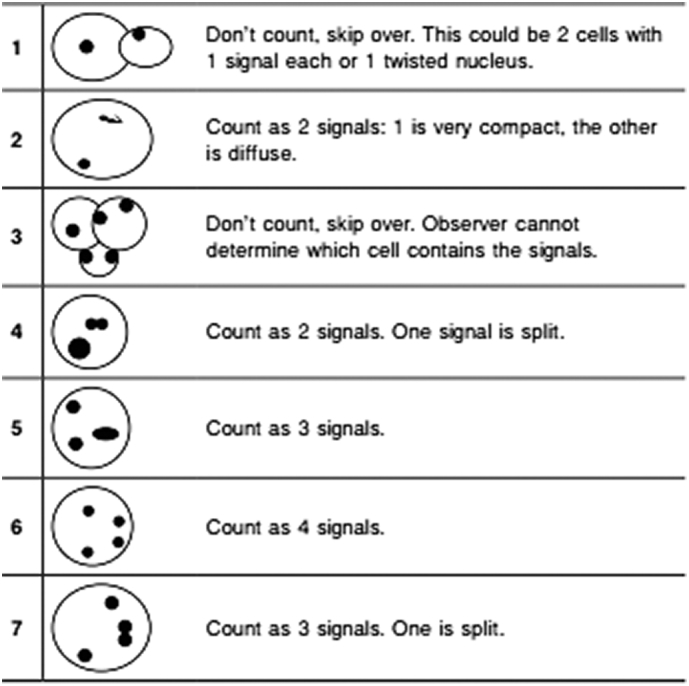
The CEP8 signal counting guide. The counting guide was from Abbott (https://www.molecular.abbott/int/zh/vysis-fish-knowledge-center/enumeration-guidelines).
2.4. Data and statistical analysis
Data not normally distributed are described by the median, 25th and 75th percentile. To compare the amounts of CTCs among the different clinical groups, Mann-Whitney, Wilcoxon, and Kruskal-Wallis tests were used. Pairwise comparisons among groups was done with Dunn's multiple comparisons test. Correlation analyses were performed using Spearman's test. Kaplan-Meier analysis and log-rank test were used to compare OS. Univariate and multivariate logistic and Cox regression analyses were performed to identify independent risk factors associated with metastasis and OS. Receiver operating characteristic (ROC) curves were used to analyze the predictive value of CTCs for metastasis and OS. All statistical tests were performed in Stata 13.0 and were two sided. P values <.05 were considered statistically significant.
3. Results
3.1. Identification of CTCs in NB patients
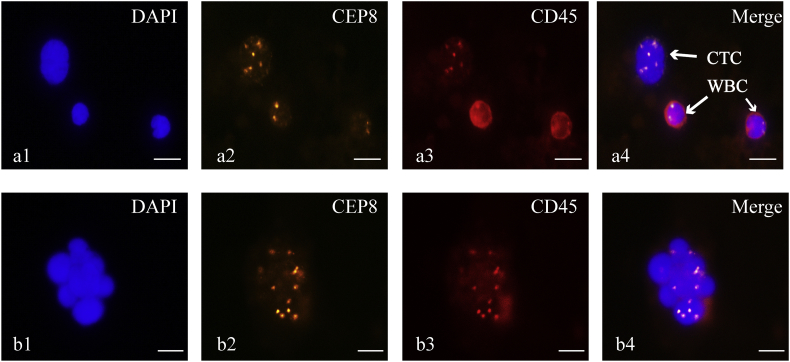
CTCs were defined as hyperdiploid cells without detectable CD45 expression. Specifically, the CTCs in this study were defined as CD45-/DAPI+/CEP8 ≥ 3 spots. A total of 374 CTCs in 28 NB blood samples were detected. A CTC cluster was defined as having more than two adhered CTCs. During counting, cells within a cluster were not included in the analysis. We found only one CTC cluster from a COG-defined high-risk patient with distant lymph node metastasis (Fig. 1).
Fig. 1.
Identification of CTCs and CTC clusters from NB patients.
a1, b1) Nuclei were stained with DAPI (blue fluorescence). a2, b2) Chromatin was stained with CEP8 (orange fluorescence). a3, b3) White blood cells (WBC) were stained with antibody to CD45 (red circle around the cell surface). a4) Identification of a CTC (DAPI+/CD45-/CEP8 ≥ 3 pots), and identification of WBCs (DAPI+/CD45+/CEP8 = 2 spots). b4) CTC cluster, defined as more than two CTCs adhered together. (Scale bar = 10 μm).
3.2. CTC detected across COG risk classification groups
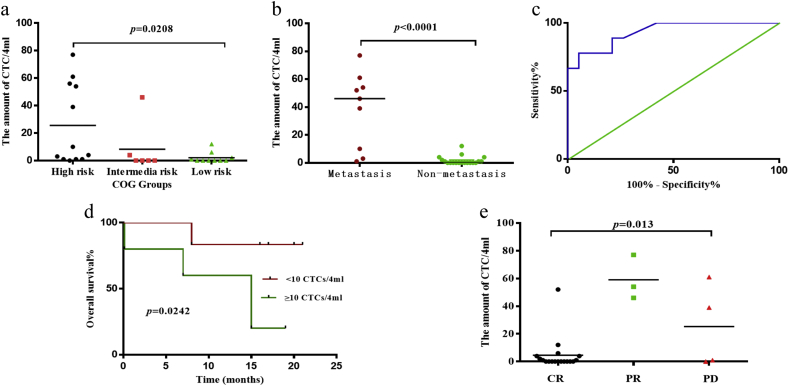
According to the COG risk classification scheme, there were 12 high-, 6 intermediate-, and 10 low-risk patients included in the study. A Kruskal-Wallis test revealed that the difference in the amounts of CTCs these three groups was statistically significant (p = .0208, Fig. 2a). In addition, pair-wise comparisons using Dunn's multiple comparisons test showed a significant difference between the amounts of CTCs in only the high-risk and the low-risk groups (mean rank difference = 8.583).
Fig. 2.
Correlation between the amounts of CTCs and clinical data.
a) The number of CTCs in these three groups were statistically significant (p = .0208, Kruskal-Wallis test). b) The comparison of CTCs between patients with and without metastasis (p < .0001, Mann–Whitney test). c) The CTC ROC curve for discriminating between patients with and without metastases. The area under the ROC curve was 0.93. The cutoff value was three CTCs per 4 ml of peripheral blood. d) The OS curves between patients with CTC counts ≥10 per 4 ml of blood and those with CTC counts <10 per 4 ml of peripheral blood were significantly different (p = .0242, log-rank test). e) The median numbers of CTCs in the three treatment response groups were: CR 0 (0, 4), PR 54 (46, 77) and PD 20 (0.25, 55.5). (p = .013, Kruskal–Wallis test).
3.3. Correlations among CTCs, VMA levels, NSE levels and MYCN amplification
We collected 24 h urine VMA test samples from 15 of the 28 patients. The Spearman test revealed no statistically-significant correlation between VMA levels and the amount of CTCs (p = .16). All 28 patients had NSE test samples collected at the initial diagnosis. There was a positive correlation between the amount of CTCs and NSE levels (p = .0131, r = 0.463, 95% CI (0.097, 0.718), Spearman test). Of the 27 patients for whom tumor tissue measurements of MYCN aberration status were conducted, only four were found to have MYCN amplification. The number of CTCs was not significantly different between the patients with MYCN amplification and those without (p = .6133, Spearman test).
3.4. CTCs and metastasis
Only nine patients had distant metastases according to CT or MRI scans, whole-body nuclear bone scans and bone marrow aspirations performed at the initial diagnosis. The median CTC count for patients with metastases was 46 (6.5, 7.5), whereas the median CTC count for patients without metastases was 0 (0, 2.0). There was a significant difference between the number of CTCs between these two groups (p < .0001, Mann–Whitney test) (Fig. 2b).
To evaluate the ability of CTC detection to discriminate between patients with and without metastases, ROC curves were utilized. The area under the ROC curve was 0.93 (95% CI (0.838, 1.00)) (Fig. 2c). We defined cutoff value as three CTCs per 4 ml of blood on the basis of the greatest sensitivity (88.85%) and specificity (78.59%). To further validate our cutoff value (≥3 CTCs/4 ml blood), CTC enumeration, NSE levels and age were included in a univariate logistic regression analysis to evaluate the association with metastasis. Only the variables that were significantly associated with metastasis were subjected to a multivariate logistic regression analysis. The univariate logistic regression results showed that CTC and NSE levels were associated with metastasis. However, further multivariate logistic regression analysis showed that the cutoff value for CTCs was not an independent risk factor (Table 2).
Table 2.
Univariate and multivariate logistic and cox regression analyses.
| Parameter | P value | RR or HR | 95% CI |
|---|---|---|---|
| Univariate Logistic regression analysis | RR | ||
| CTC ≥ 3/4 ml | 0.009⁎ | 13.125 | 1.924–89.515 |
| NSE (ng/ml) | 0.002⁎ | 1.016 | 1.006–1.027 |
| Age (year) | 0.665 | 1.047 | |
| Multivariate Logistic regression analysis | |||
| CTC ≥ 3/4 ml | 0.149 | 7.909 | |
| NSE (ng/ml) | 0.008⁎ | 1.015 | 1.004–1.027 |
| Univariate Cox regression analysis | HR | ||
| CTC ≥ 10/4 ml | 0.034⁎ | 6.279 | 1.143–34.487 |
| NSE (ng/ml) | 0.021⁎ | 1.010 | 1.001–1.019 |
| Age (year) | 0.632 | 0.937 | |
| INSS stage | 0.039⁎ | 8.788 | 1.115–69.268 |
| Multivariate Cox regression analysis# | |||
| CTC ≥ 10/4 ml | 0.000 | 0.389 | |
| NSE (ng/ml) | 0.563 | 0.984 | |
| INSS stage | 0.809 | 1.419 | |
The interaction item among the CTC, NSE and INSS stage in multivariate Cox regression was statistically significant (p = .005).
p value<0.05 indicate the statistical difference
3.5. CTCs and OS
The median follow-up time for the patients was 14.5 (12, 16) months. Therefore, we set the death within the 16 months after initial diagnosis as the outcome event, and the amount of CTC as the variable to identify the cutoff value to predict prognosis. An ROC curve was constructed, and the area under the ROC curve was 0.81 (95% CI (0.57, 1.00)). The cutoff value for this analysis was identified to be 10 CTCs per 4 ml of peripheral blood, on the basis of the greatest sensitivity (66.67%) and specificity (90.91%). Patients were categorized according to whether the CTC levels measured in their blood were greater or less than the cutoff value. A log-rank test was used to compare OS Kaplan-Meier curves between the two groups, and the results showed that the OS curves were significantly different (p = .0242, (Fig. 2d).). Specifically, the 16-month OS rate for patients with ≥10 CTCs/4 ml of peripheral blood and for patients with <10 CTCs/4 ml of peripheral blood was 37.5% and 89.4%, respectively.
CTC enumeration (≥ or < 10 CTCs/4 ml blood), NSE levels, INSS stage and age were included in a univariate Cox proportion hazards regression analysis to evaluate the association with OS. Only the variables that were significantly associated with metastasis were included in a multivariate Cox regression analysis. The univariate Cox regression results showed that CTC enumeration, NSE levels and INSS stage were associated with OS, and the three variables interacted with one and other (p = .005). A multivariate Cox regression analysis showed that the cutoff value for CTCs was not an independent risk factor (Table 2).
3.6. CTC and treatment effect
The 28 patients were divided into groups according to treatment response (complete regression (CR), partial regression (PR) and progressive disease (PD)). The median CTC numbers per 4 ml of blood were 0 (0, 4) for CR, 54 (46, 77) for PR and 20 (0.25, 55.5) for PD. The Kruskal-Wallis test revealed that the differences in the number of CTCs between the three groups were statistically significant (p = .013). Furthermore, pairwise comparisons with Dunn's multiple comparisons test revealed that only the number of CTCs in the CR and PR groups were significantly different (mean difference = −12.28) (Fig. 2e).
3.7. FISH of chromosome 8 in tumor tissue
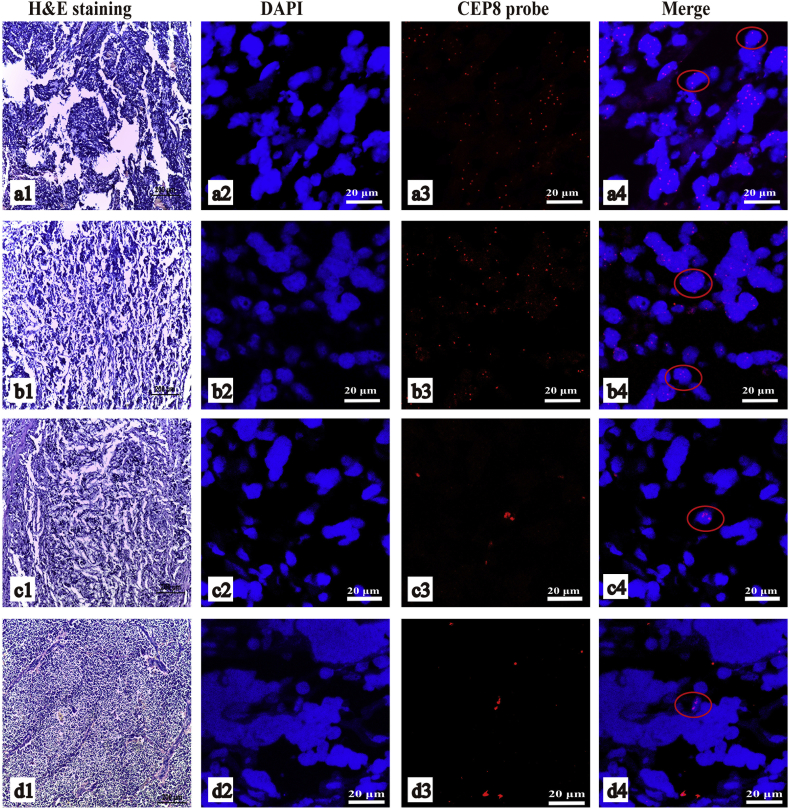
Based on the result of CTC enumeration, we choose four patients' paraffin-embedded NB tissue in order to study the chromosome 8 abnormalities. Two patients were classified as COG high-risk and had distant metastases, and they had 61 and 54 CTCs/4 ml of blood. The remaining two patients who were categorized as COG low- and intermediate-risk did not have distant metastases or CTCs in their blood samples. In the tumor section of the two COG high-risk patients, we could find 20/50 (40%) and 7/50 (14%) chromosome 8 hyperdiploid cells (Fig. 3a-b). According to the instruction of the CEP8, >2.2% tri-signaled nuclei are considered as an abnormal trisomy 8 clone for clinical specimen. So these two patients' tumor tissues were considered as abnormal trisomy 8. But there was no chromosome 8 hyperdiploid cells in a view of the rest two patients' tumor tissue section (Fig. 3c-d).
Fig. 3.
FISH of CEP8 from NB tumor tissue.
a and b were the two COG high-risk patients with metastasis. c and d were the two patients without distant metastases. a1, b1, c1, d1) HE stain of the area of tumor tissue performing FISH study. a2, b2, c2, d2) the DAPI stain. a3, b3, c3, d3) the FISH of centromeric probe of chromosome 8 (CEP8 probe). a4, b4, c4, d4) the merge images. In a4 and b4, the cells in red circle were hyperdiploidy cells, and in c4 and d4 were the diploid cells.
4. Discussion
Numerous studies have provided evidence supporting the clinical use of CTC measurements to monitor treatment response and for evaluating the prognosis of breast, pancreatic and lung cancer patients [4, 13, 14]. Most of the methods published rely on cancer cell EpCAM expression. However, to our knowledge, there is not a specific or universal antigen marker expressed by NB cells, because even the expression of GD-2 on NB cells varies between studies. Consequently, the EpCAM-dependent CTC identification method cannot be used to detect CTCs in NB patients.
Although hyperdiploidy of chromosome 8 is common in adult cancers, it has been rarely reported in NB. To evaluate hyperdiploidy in NB tumor tissue, we performed FISH of the CEP8 probe in tumor sections from four patients. It reveal that patients with more CTCs had trisomy chromosome 8 in tumor tissue. We speculate that NB tumors with abnormal trisomy chromosome 8 clones might correlate with advanced disease stage, and patients may be more likely to have metastases. Patients without trisomy chromosome 8 clones may have a lower-risk disease. We were unable to detect CTCs in the peripheral blood on the basis of CEP8 FISH in these patients.
Studies of liquid biopsies from NB patients have mainly focused on circulating free DNA (cfDNA). Previous studies have reported that cfDNA reflects the ALK and MYCN gene status of the primary tumor [15, 16]. Studies have also demonstrated the feasibility of copy number profiling of cfDNA in NB patients [17] and have revealed that cfDNA can serve as a surrogate for primary tumor genomic profiles, a useful measure when primary tumor tissue samples are unavailable. However, studies have not explored the relationship between cfDNA and clinical data such as prognosis. The total level of circulating miRNAs detected in the peripheral blood in mouse models with a high risk of NB development is higher than those from low-risk groups [18], thus suggesting that circulating miRNAs might potentially be used as a prognostic biomarker. cfDNA and circulating miRNAs are released from necrotic and apoptotic tumor cells. The biological characterization of CTCs from NB patients may provide more information on the biological mechanisms of metastasis and resistance to therapy. Moreover, CTCs can be transplanted in vivo and cultured in vitro to aid in identification of the mechanisms of drug resistance and metastasis [19, 20].
In the present study, the amounts of CTCs in the peripheral blood of NB patients differed significantly among the different COG risk groups. Further analysis revealed that the number of CTCs in the high- and low-risk groups were significantly different. However, the number of CTCs in the high- and intermediate-risk groups were not significantly different, a result that might be explained by the small sample size.
An increase in NSE levels suggests disease progression [21, 22]. We found a positive correlation between NSE levels and the number of CTCs in NB patients, thus suggesting that CTCs are associated with disease stage. However, we did not find a significant difference in the number of CTCs between patients with and without MYCN amplifications.
The primary methods used in the clinic to identify metastatic lesions involve CT, MRI and MIBG scans. However, these methodologies are often very cumbersome and ultimately exposes patients to radiation.
CTCs have been associated with metastasis [4]. In the present study, patients with metastases had a greater number of CTCs in their peripheral blood than did subjects without metastases (p < .0001). The area under the ROC curve was 0.93, and we determined that three or more CTCs per 4 ml of peripheral blood was a highly sensitive and specific cutoff value. NB patients with three or more CTCs per 4 ml of peripheral blood were therefore considered to have a high likelihood of distant metastases, thus suggesting that when the CT or MRI does not determine whether a lesion is metastasis or just normal tissue, the detection of CTCs may therefore help predict metastases.
We defined a CTC cutoff based on the ROC curve (10 CTCs per 4 ml of peripheral blood) to evaluate prognosis in this study. We found that the OS of those with CTC values above the cutoff was shorter. These results support the conclusion that a patient with a high number of CTCs may have an adverse prognosis and may require aggressive evaluation and therapy. However, the specific CTC cutoff value for prognosis in the peripheral blood of patients with the same tumor type probably differs depending on the method of detection.
Although the multivariate logistic and Cox regression analysis demonstrated that the CTC cutoff values were not an independent risk factor for metastasis and OS. In the present study, the number of CTCs was significantly correlated with NSE levels and INSS stage, and because the sample size was small, this correlation was shared with clinical outcome in a multivariate analyses and typically was not seen as an independent risk factor.
Furthermore, we found a significant difference in the numbers of CTCs among the different treatment response groups (p = .013, Kruskal-Wallis test). Although the median number of CTCs in the PD group was less than that of the PR group, this difference was not statistically significant. The CTC values used for the comparison among CR, PR and PD patients were those determined at their first hospitalization, thus suggesting that dynamic detection of CTC is preferable to evaluate treatment effects.
The EpCAM-independent CTC detection method used in this study has been used to detect CTCs in patients with many different cancer types [23]. Ramirez et al. have used an EpCAM-independent method combined with cytokeratin-19 protein staining to detect CTCs with secretion function. The blood sample volumes were 7.5 ml, and 59% of the metastatic breast cancer patients in the study had detectable CTCs [24]. Density gradient centrifugation has also been used in several studies, in which the blood sample volumes were between 10 and 20 ml, and the recovery rates were 70–90% [25, 26]. Janice Lu et al. have used a collagen adhesion matrix assay to detect CTCs in breast cancer patients. This assay was able to detect CTCs from 5 to 20 ml of blood from all 10 patients with metastasis, with an average of 126 CTCs/ml per patient [27]. In the present study, we used a protocol requiring only 4 ml of peripheral blood, instead of 7 ml or more, which was better for our young patients. According to our previous retrieval experiments, the numbers of CTCs ranged from eight to 236 CTCs per 4 ml of peripheral blood, and the retrieval rate was maintained at 70.6–83.1%. We detected 374 CTCs in total from our cohort of 28 patients, and the average number of CTCs detected in patients with distant metastases was 38 CTCs/4 ml of blood. All patients with metastases had detectable CTCs.
To our knowledge, this is the first study to use an EpCAM-independent enrichment method combined with FISH to detect CTCs in NB patients. We found that CTC counts significantly correlated with prognosis. However, there are several limitations to this study. First, the sample size was too small to accurately evaluate the diagnostic value of CTC counts. We did not have follow-up information for children, thus resulting in the lack of dynamic monitoring of CTC counts during each cycle of chemotherapy. In future studies, we will enroll higher numbers of patients and obtain sufficient follow-up information to verify our present conclusions and provide a basis to promote the use of CTC detection in NB patients.
The EpCAM-independent enrichment method combined with immunostaining-FISH used in this study had a high sensitivity for CTC detection in NB patients. The cutoff value indicating potential metastasis was three CTCs per 4 ml of peripheral blood. This cutoff value may help physicians verify a patient's risk for metastatic disease when imaging results are ambiguous. Moreover, this study demonstrates that the number of CTCs is significantly correlated with OS. Thus, the method used in this study may provide a novel prognostic approach.
The following are the supplementary data related to this article.
Funding
This study received financial support from Shanghai Key Disciplines (no. 2017ZZ02022), the National Natural Science Foundation of China (81572324), the Shanghai Rising-Star Program (A type, no. 15QA1400800), the Science Foundation of Shanghai Excellent Youth Scholars (no. 2017YQ042), the Science Foundation of Shanghai (nos. 15ZR1404200 and 17411960600) and the National High Technology Research and Development Program of China (2015AA020104).
Declaration of interests
All the authors had nothing to disclose.
Author contributions
Xiangqi Liu: literature search, lab, figures, data analysis, writing.
Zhenzhen Zhang: study design, figures, data interpretation.
Binbin Zhang: data collection, lab.
Yijie Zheng: literature search, data analysis, technical support.
Chao Zheng: data collection.
Baihui Liu: figure, data analysis.
Shan Zheng: data interpretation.
Kuiran Dong: study design, data interpretation.
Rui Dong: study design, data interpretation.
Contributor Information
Kuiran Dong, Email: kuirand@fudan.edu.cn.
Rui Dong, Email: rdong@fudan.edu.cn.
References
- 1.Whittle S.B., Smith V., Doherty E., Zhao S.B., McCarty S., Zage P.E. Overview and recent advances in the treatment of neuroblastoma. Expert Rev Anticanc. 2017;17(4):369–386. doi: 10.1080/14737140.2017.1285230. [DOI] [PubMed] [Google Scholar]
- 2.Park J.R., Bagatell R., London W.B., Maris J.M., Cohn S.L., Mattay K.M. Children's oncology Group's 2013 blueprint for research: neuroblastoma. Pediatr Blood Cancer. 2013;60(6):985–993. doi: 10.1002/pbc.24433. [DOI] [PubMed] [Google Scholar]
- 3.Uslu L., Donig J., Link M., Rosenberg J., Quon A., Daldrup-Link H.E. Value of 18F-FDG PET and PET/CT for evaluation of pediatric malignancies. J Nucl Med. 2015;56(2):274–286. doi: 10.2967/jnumed.114.146290. [DOI] [PubMed] [Google Scholar]
- 4.Alix-Panabieres C., Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14(9):623–631. doi: 10.1038/nrc3820. [DOI] [PubMed] [Google Scholar]
- 5.Stutterheim J., Gerritsen A., Zappeij-Kannegieter L., Kleijn I., Dee R., Hooft L. PHOX2B is a novel and specific marker for minimal residual disease testing in neuroblastoma. J Clin Oncol. 2008;26(33):5443–5449. doi: 10.1200/JCO.2007.13.6531. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H.Q., Gao P., Xiao X., Heger M., Geng L., Fan B. A liquid biopsy-based method for the detection and quantification of circulating tumor cells in surgical osteosarcoma patients. Int J Oncol. 2017;50(4):1075–1086. doi: 10.3892/ijo.2017.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Zhang X., Ge S., Gao J., Gong J., Lu M. Clinical significance of phenotyping and karyotyping of circulating tumor cells in patients with advanced gastric cancer. Oncotarget. 2014;5(16):6594–6602. doi: 10.18632/oncotarget.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ning N., Zhan T., Zhang Y.J., Chen Q., Feng F.Z., Yang Z. Improvement of specific detection of circulating tumor cells using combined CD45 staining and fluorescence in situ hybridization. Clin Chim Acta. 2014;433:69–75. doi: 10.1016/j.cca.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Gao Y., Zhu Y.Y., Zhang Z.Z., Zhang C., Huang X.Y., Yuan Z. Clinical significance of pancreatic circulating tumor cells using combined negative enrichment and immunostaining-fluorescence in situ hybridization. J Exp Clin Cancer Res. 2016;35 doi: 10.1186/s13046-016-0340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grobner S.N., Worst B.C., Weischenfeldt J., Buchhalter I., Kleinheinz K., Rudneva V.A. The landscape of genomic alterations across childhood cancers. Nature. 2018;555(7696) doi: 10.1038/nature25480. 321. [DOI] [PubMed] [Google Scholar]
- 11.Pugh T.J., Morozova O., Attiyeh E.F., Asgharzadeh S., Wei J.S., Auclair D. The genetic landscape of high-risk neuroblastoma. Nat Genet. 2013;45(3):279–284. doi: 10.1038/ng.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilke S., Chen Q.R., Wei J.S., Khan J. Whole chromosome alterations predict survival in high-risk neuroblastoma without MYCN amplification. Clin Cancer Res. 2008;14(17):5540–5547. doi: 10.1158/1078-0432.CCR-07-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bidard F.C., Huguet F., Louvet C., Mineur L., Bouche O., Chibaudel B. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol. 2013;24(8):2057–2061. doi: 10.1093/annonc/mdt176. [DOI] [PubMed] [Google Scholar]
- 14.Aceto N., Bardia A., Miyamoto D.T., Donaldson M.C., Wittner B.S., Spencer J.A. Circulating tumor cell clusters are Oligoclonal precursors of breast Cancer metastasis. Cell. 2014;158(5):1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Combaret V., Audoynaud C., Iacono I., Favrot M.C., Schell M., Bergeron C. Circulating MYCN DNA as a tumor-specific marker in neuroblastoma patients. Cancer Res. 2002;62(13):3646–3648. [PubMed] [Google Scholar]
- 16.Combaret V., Iacono I., Bellini A., Brejon S., Bernard V., Marabelle A. Detection of tumor ALK status in neuroblastoma patients using peripheral blood. Cancer Med-Us. 2015;4(4):540–550. doi: 10.1002/cam4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chicard M., Boyault S., Daage L.C., Richer W., Gentien D., Pierron G. Genomic copy number profiling using circulating free tumor DNA highlights heterogeneity in neuroblastoma. Clin Cancer Res. 2016;22(22):5564–5573. doi: 10.1158/1078-0432.CCR-16-0500. [DOI] [PubMed] [Google Scholar]
- 18.Ramraj S.K., Aravindan S., Somasundaram D.B., Herman T.S., Natarajan M., Aravindan N. Serum-circulating miRNAs predict neuroblastoma progression in mouse model of high-risk metastatic disease. Oncotarget. 2016;7(14):18605–18619. doi: 10.18632/oncotarget.7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu M., Bardia A., Aceto N., Bersani F., Madden M.W., Donaldson M.C. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345(6193):216–220. doi: 10.1126/science.1253533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan N.V., Bardia A., Wittner B.S., Benes C., Ligorio M., Zheng Y. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537(7618):102–106. doi: 10.1038/nature19328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang Q.M., Li K., Ma L.J., Sun R.P. Clinical research on neuroblastoma based on serum lactate dehydrogenase. J Biol Reg Homeos Ag. 2015;29(1):131–134. [PubMed] [Google Scholar]
- 22.Isgro M.A., Bottoni P., Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:125–143. doi: 10.1007/978-94-017-7215-0_9. [DOI] [PubMed] [Google Scholar]
- 23.Gabriel M.T., Calleja L.R., Chalopin A., Ory B., Heymann D. Circulating tumor cells: a review of non-EpCAM-based approaches for cell enrichment and isolation. Clin Chem. 2016;62(4):571–581. doi: 10.1373/clinchem.2015.249706. [DOI] [PubMed] [Google Scholar]
- 24.Schneck H., Gierke B., Uppenkamp F., Behrens B., Niederacher D., Stoecklein N.H. EpCAM-independent enrichment of circulating tumor cells in metastatic breast cancer. Plos One. 2015;10(12) doi: 10.1371/journal.pone.0144535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg R., Gertler R., Friederichs J., Fuehrer K., Dahm M., Phelps R. Comparison of two density gradient centrifugation systems for the enrichment of disseminated tumor cells in blood. Cytometry. 2002;49(4):150–158. doi: 10.1002/cyto.10161. [DOI] [PubMed] [Google Scholar]
- 26.Gertler R., Rosenberg R., Fuehrer K., Dahm M., Nekarda H., Siewert J.R. Detection of circulating tumor cells in blood using an optimized density gradient centrifugation. Recent Res Cancer. 2003;162:149–155. doi: 10.1007/978-3-642-59349-9_13. [DOI] [PubMed] [Google Scholar]
- 27.Lu J., Fan T., Zhao Q., Zeng W., Zaslavsky E., Chen J.J. Isolation of circulating epithelial and tumor progenitor cells with an invasive phenotype from breast cancer patients. Int J Cancer. 2010;126(3):669–683. doi: 10.1002/ijc.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]