Abstract
Background
Resistance to chemotherapeutic treatment is a common phenomenon in cancers, especially in hepatocellular carcinoma (HCC). The Hippo signaling pathway has been demonstrated to play a role in tumor initiation, development, and progression. However, little is known about its roles in the HCC chemoresistance.
Methods
In this study, real-time PCR and western blotting were used to identify the expression profile of key components of Hippo signaling pathway between chemoresistant and chemosensitive HCC cell lines. In vitro and in vivo loss- and gain-of-function studies were performed to reveal the effects and related mechanism of microRNA-590-5p/YAP1 axis in the chemoresistant phenotype of HCC cells.
Findings
We identified yes-associated protein 1 (YAP1) as the major dysregulated molecules in adriamycin (ADR)-resistant HCC cells. YAP1 was profoundly implicated in the chemoresistant phenotype of HCC cells. Furthermore, microRNA-590-5p was revealed as a functional modulator of YAP1. Importantly, YAP1-mediated chemoresistant phenotype was closely related to increased expression of stemness markers and ATP-binding cassette transporters. HCC patients with poor response to transarterial chemoembolization (TACE) treatment had higher protein level of YAP1 than that in the responsive patients.
Interpretation
The microRNA-590-5p/YAP axis plays an important role in the chemotherapeutic resistance of HCC cells, suggesting new adjuvant chemotherapeutic directions in HCC.
Fund
National Natural Science Foundation of China, Zhejiang Province Medical and Health Care Key Project, Experimental Animal Science and Technology Projects of Zhejiang Province, Public Welfare Technology Application Research Project of Lishui, Chinese Medicine Science and Technology Projects of Zhejiang Province.
Keywords: microRNA-590-5p, YAP1, Liver cancer, Transarterial chemoembolization, Chemotherapy, Doxorubicin
Research in context.
Evidence Before this Study
Chemoresistance often results in treatment failure and death of the patient. Recently, many comprehensive studies reveal that the Hippo pathway is closely related to tumor initiation and progression. However, defining which components of Hippo signaling predominantly affects HCC chemoresistance is not well understood.
Added Value of this Study
Here, this is the first report of the microRNA-590-5p/YAP1 axis in HCC, in which YAP1 is regulated by microRNA-590-5p and is critical for HCC chemoresistance through regulating expression of stemness markers (Oct4, Sox2, Notch1, Nanog, and Nestin) and ATP-binding cassette transporters (ABCB1 and ABCC1).
Implications of all the Available Evidence
This finding has great potential for expanding our knowledge regarding the Hippo signaling pathway in cancer chemoresistant phenotype and indicate that the development of drugs targeting microRNA-590-5p/YAP1 axis via combinatorial therapy.
Alt-text: Unlabelled Box
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and the third leading cause of cancer-related death [1, 2]. Surgery is the main therapeutic strategy used to treat this disease; however, curative resection or transplantation applies to only approximately 30% of patients [3]. For the most advanced HCC patients, systemic chemotherapy is required [4, 5]. Currently, transarterial chemoembolization (TACE) is the most commonly selected treatment option for advanced HCC patients [6]. Adriamycin (ADR), also known as doxorubicin, is a first-line chemotherapy agent for TACE [7]. However, the prognosis of these HCC patients is still poor because of the intrinsic or acquired resistance to doxorubicin of HCC cells [8, 9]. Therefore, understanding the molecular mechanisms involved in the doxorubicin resistance of HCC may lead to improved clinical outcomes and develop suitable therapeutic target for HCC doxorubicin resistance.
The Hippo signaling is an highly conserved pathway that plays important roles in tumorigenesis, stem cell self-renewal and differentiation, organ size control, and many other cellular processes [[10], [11], [12], [13], [14]]. Dysregulation of Hippo pathway promotes tumorigenesis in diverse malignant human cancers, especially HCC [15]. The key components of Hippo signaling pathway include mammalian sterile 20-like kinases 1/2 (MST1/2), large tumor suppressor kinases 1/2 (LATS1/2), yes-associated protein 1 (YAP1), transcriptional co-activator with PDZ binding motif (TAZ), and transcriptional enhancer factor domain family members 1–4 (TEAD1–4) [13]. Under normal circumstance, MST1/2 combines with salvador family WW domain-containing protein 1 (SAV1) to form an activated complex that initiates LATS1/2 phosphorylation. Once Hippo signaling pathway is activated, LATS1/2 further phosphorylates YAP1 at Ser127 or TAZ at Ser89. Then phosphorylated YAP1 binds to 14–3-3 protein and remains in the cytoplasm for degradation. When the Hippo signaling pathway is inactivated, dephosphorylated YAP1 translocates into the nucleus and acts as a co-activator binding to the transcription factors TEAD1–4, which activates the expression of downstream targets to facilitate tumor progression [10, 13, 16]. Interestingly, the Hippo signaling pathway is involved in the chemoresistant phenotype of cancer cells [[17], [18], [19], [20], [21], [22], [23]]. In esophageal cancer, YAP1 mediated EGFR overexpression plays an important role in conferring chemotherapy resistance [20]. In breast cancer, loss of TAZ in tumor stem cells severely impairs metastatic colonization and chemoresistance [18]. In pancreatic cancer, miR-181c contributes to chemoresistance by targeting multiple components in Hippo signaling pathway including MST1, LATS2, MOB1 and SAV1 [19]. However, the role of Hippo signaling pathway in HCC doxorubicin resistance remains largely unknown.
MicroRNAs (miRNAs) are evolutionarily conserved small non-coding RNAs that regulate gene expression at the post-transcriptional level by binding to the 3′-untranslated region (3′UTR) of target mRNA [24, 25]. Dysregulated miRNAs have been reported in tumorigenesis, cancer diagnosis and prognosis, as well as predictions of outcomes and response to chemotherapy [26, 27]. Actually, miRNAs have become a research focus not only because their essential roles in human diseases, but also because synthetic miRNAs are similar to small-molecule inhibitors or activators [26]. Therefore, identification of key candidate miRNAs that regulate HCC chemoresistance may be helpful for improving treatment.
In this study, we showed that YAP1, a major component of Hippo signaling pathway, is responsible for the chemoresistant phenotype of HCC cells and patients. Moreover, we not only illustrated the role of miR-590-5p as a functional modulator of YAP1 but also demonstrated that miR-590-5p significantly improves the chemosensitivity of HCC to ADR in vitro and in vivo. Finally, we demonstrated that YAP1 contributes to chemotherapeutic resistance by inducing expression of stemness markers and ATP-binding cassette transporters.
2. Materials and methods
2.1. Tissue samples
The 20 patients received transarterial chemoembolization (TACE) in our hospital were enrolled and needle tissue samples were collected as reported previously [28]. All the process was performed in accordance with guidelines and regulations approved by the Fifth Affiliated Hospital of Wenzhou Medical University. Appropriate informed consent was obtained from each patient. All tissues were frozen in liquid nitrogen immediately and then store at −80 °C until use.
2.2. Cell culture
Human HCC cell lines HepG2 and Huh7 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The drug-resistant HepG2/ADR and Huh7/ADR cell lines were generated by our hospital. In brief, the HepG2 and Huh7 resistant sublines were selected based on a constant exposure of the WT parental cells to ADR in a stepwise dose incremental strategy. The two cell lines were treated with a sequential increase in dosage of the three drugs ranging from IC6.25, IC12.5, IC25 to IC50. The cells were grown in Dulbecco's modified Eagle's medium (Gibco, MD, USA) with 10% (v/v) fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin in a humidified incubator at 37 °C with 5% CO2. ADR (0.5 μmol/L) was added in the culture medium of HepG2/ADR and Huh7/ADR cells to maintain the ADR-resistant character. ADR-HCl and DMSO were purchased from Sigma-Aldrich (Shanghai, China). Verteporfin was purchased from Selleck (Shanghai, China).
2.3. RNA isolation and real-time quantitative PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, USA) according to the manufacturer's protocol. The mRNA and miRNA reverse transcription were performed using a 5 × All-In-One RT MasterMix kit (Applied Biological Materials Inc., Richmond, BC, Canada) and RT-PCR miRcute miRNA First-Strand cDNA Synthesis Kit (Tiangen Biotech, Beijing, China), respectively. Quantitative RT-PCR was performed through the SYBR green assay (Invitrogen, USA) with the Applied Biosystems 7500. MiR-590-5p expression was detected by qRT-PCR using a miRcute miRNA qPCR Detection kit (Tiangen Biotech), and the relative expression of miR-590-5p was normalized to U6 expression. All PCR assays were performed in triplicate. Sequences of specific primers are listed in Table S1.
2.4. Western blotting
Lysates of cultured cells were washed with PBS and prepared with RIPA lysis buffer (Millipore, Billerica, MA) supplemented with Complete EDTA-free protease inhibitor cocktail (Roche, Indianapolis, IN), phosphatase inhibitor cocktails 1 and 2 (Sigma-Aldrich, St. Louis, MO). Protein concentration was quantified using the Bio-Rad DC protein assay kit (Bio-Rad, USA). Samples of total cell lysate (10–15 μg) were separated on 10% odium dodecyl sulfate polyacrylamide gel electrophoresis before electrotransfer onto the polyvinylidene difluoride (PVDF) membrane. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline with 1% Tween at room temperature for 1 h. After blocking, the membranes were washed and incubated with specific primary antibodies against the selected proteins at 4 °C overnight. Then membranes were incubated with the species-matched peroxidase-conjugated secondary antibody. Specific reactive proteins were detected by enhanced chemiluminescence (Pierce, USA). The detailed information of primary antibodies used in this study was shown in Table S2.
2.5. Immunohistochemistry and immunofluorescence
Formalin fixed paraffin embedded needle tissue samples from HCC patients were cut into 5 μm for immunohistochemical (IHC) analysis. IHC analysis was carried out routinely and the intensity of YAP1 staining was classified according to a two-level scale: low, weak or partial staining to cytoplasm and nucleus of cancer cell; high, strong and diffuse staining to cytoplasm and nucleus. All specimens were evaluated by two independent investigators. For evaluating Ki67 and cleaved caspase-3 (CCS3) staining, the following primary antibodies were used: anti-Ki67 (diluted at 1:400, Cell Signaling Technology, #9449) and anti-CCS3 (diluted at 1:200, Cell Signaling Technology, #9661). For immunofluorescence, primary antibodies against YAP1 (diluted at 1:200, Cell Signaling Technology, #4912) were incubated at 4 °C overnight, followed by secondary antibodies donkey anti-mouse Alexa Fluor 488 (1:400, Jackson ImmunoResearch, #715–545-150) at room temperature for 1 h. Nuclei were counterstained with DAPI and digital images were acquired with confocal microscopes equipped with a digital camera (Nikon).
2.6. Plasmid transfection
The full-length YAP1 sequence was synthesized from Sangon (Shanghai, China). The YAP fragments containing the S127A and the S94A mutations were inserted into the vector pLNCX2 to construct the recombinant retroviral plasmid. For generation of YAP stable knockdown and overexpression HCC cells, 293 T cells were transfected with a DNA mix containing the plasmid of interest, pVPR and pVSVG using Lipofectamine 2000 (Invitrogen, USA) with Opti-MEM in low serum medium. Virus was collected after incubation for 72 h, passed through a 0.45 μm filter and stored at −80 °C. Then the stably transduced HepG2 and Huh7 cells were selected with 2 μg/mL Puromycin and MHCC-97H cells were selected with 250 μg/mL G418. For miRNA study, synthetic pre-miR-590-5p, anti-miR-590-5p and scrambled negative control RNA (pre-scramble and anti-scramble) were purchased from GenePharma (Shanghai, China).
2.7. Measurement of cell viability and caspase-3/7 activity
Cell viability of HCC cell was assayed by staining with the cell counting kit (CCK-8, DojinDo, Japan) according to the manufacturer's instructions. Briefly, transfected cells were seeded in 96-well plates at densities of 5 × 103 cells per well. At indicted time points, the culture medium was replaced by 100 μL new medium with 10% CCK-8. After incubation for 1–2 h, the absorbance was measured using an activation wavelength of 450 nm. In addition, cell apoptosis was determined by measuring caspase-3/7 activity, which was detected by a commercial available Kit according to the manufacturer's instructions (Promega, G7790 USA). Each experiment was repeated three times, independently.
2.8. Luciferase activity assay
The 3′-UTR of human YAP1 containing putative binding sites was cloned into the pmirGLO vector (Promega, Madison, WI, USA), and efficient insertion was confirmed by sequencing. To test the binding specificity, the sequences in human YAP 3′-UTR that interact with miR-590-5p seed sequence were mutated. For reporter assays, HepG2 and Huh7 cells were co-transfected with luciferase reporter plasmid, and miR-590-5p mimics, inhibitors or negative controls. The Renilla luciferase plasmid was used as a transfection control. After transfection for 48 h, cells were harvested and analyzed using luciferase activity in a Dual-Glo Luciferase Assay kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. Each assay was performed in triplicate. For MCAT-Luc YAP1/TEAD responsive reporter assay, detained protocol was reported previously [29].
2.9. HCC xenograft model
Animal experiments were approved by the animal care and usage committee of the Fifth Affiliated Hospital of Wenzhou Medical University. The athymic male nu/nu mice ages 6 to 8 weeks were used to evaluate the effects of miR-590-5p/YAP axis on tumor growth of HCC cells. A total of 2 × 106 indicated HCC cells in 100 μL of PBS were subcutaneously inoculated. ADR was administered every three days at a dose of 10 mg/kg after the tumors reached about 150 mm3 in volume. For the in vivo study of miR-590-5p, mice were also injected with the control agomir and the miR-590-5p agomir (150 μL, 200 nM) or Verteporfin (5 mg/Kg). The mice were sacrificed two weeks later. Tumor growth was measured every three days and assessed in three dimensions using calipers, and tumor volume was calculated using the formula: volume = length × width2/2.
2.10. Statistical analysis
The statistical analysis was performed with GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA) and SAS version 9.1 for Windows (SAS Institute Inc., Cary, NC). All experiments were performed at least three times. Differences between two groups using the Student's t-test. One way analysis of variance (ANOVA) was used to determine the statistical significance of differences between two groups and among more than three groups. Frequency counts were compared between groups using the two-tailed Fisher's exact test. P-values <.05 were considered statistically significant (*, P < .05; **, P < .01; ***, P < .001).
3. Results
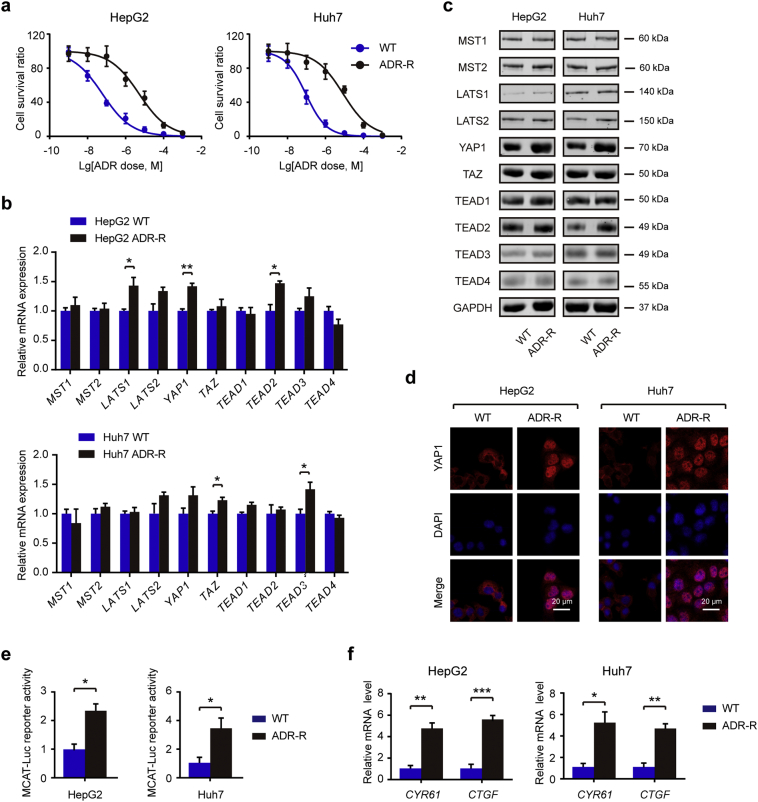
3.1. YAP1 protein expression is significantly increased in ADR-resistant HCC cells
The Hippo pathway is a developmental pathway that controls organ size by regulating cell proliferation, apoptosis, and stem cell self renewal [12, 14]. Dysregulation of the Hippo pathway is profoundly implicated in HCC development and progression [13, 30, 31]. However, the potential roles of Hippo signaling pathway in the chemoresistant phenotype of HCC remain largely unexplored. In this study, we first generated two cell models with acquired ADR resistance, named HepG2/ADR and Huh7/ADR, by continuous exposure to gradually increased concentrations of ADR. Compared with WT cells, ADR-resistant cells had a remarkable higher IC50 value (Fig. 1a). There is no significant difference in the growth rate between ADR-resistant cells and its parental WT cells (Fig. S1). To investigate the potential difference of Hippo pathway between WT and ADR-R cells, we detected the expression changes of several core components of Hippo pathway at both mRNA and protein level. As shown in Fig. 1b, HepG2/ADR-R cells had a higher mRNA level of LATS1, YAP1, and TEAD2 compared with HepG2/WT cells; Huh7/ADR-R cells had a higher mRNA level of TAZ and TEAD3 compared with Huh7/WT cells. However, those results at mRNA level were not commonly noticed in both HepG2/ADR-R and Huh7/ADR-R cells except for LATS2, which was increased in both ADR-R cells but without significant difference. Next, we determined their expression at protein level by western blotting. As a result, we found that HepG2/ADR-R and Huh7/ADR-R cells displayed significantly increased YAP1 protein expression in comparison with the WT cells (Fig. 1c). No significant difference was found in other components of Hippo signaling pathway. Meanwhile, there was more nuclear YAP1 protein level in ADR-R cells compared with the WT cells (Fig. 1d). To further confirm the activation of YAP1 in ADR cells, we inserted several YAP1/TEAD1 occupied putative enhancer regions into reporter plasmids. As a result, a significant enhanced luciferase reporter activity was observed in ADR-R HCC cells compared with the WT cells (Fig. 1e). Moreover, the downstream targets of YAP, cysteine rich angiogenic inducer 61 (CYR61) and connective tissue growth factor (CTGF), were also up-regulated in the ADR-R cells compared with the WT cells (Fig. 1f). Taken together, these findings suggest that YAP1 is the major dysregulated component of Hippo signaling in ADR-R cells and might be contribute to the chemoresistant phenotype of HCC cells.
Fig. 1.
YAP1 protein expression is significantly increased in ADR-resistant HCC cells. (a) Indicated cells were treated with indicated concentration of ADR and CCK-8 assays were performed 48 h after treatment to detect chemosensitivity (n = 3). (b) Key components of Hippo signaling pathway in ADR-R HCC cells and its parental WT cells were analyzed by qRT-PCR (n = 3). (c) Key components of Hippo signaling pathway in ADR-R HCC cells and its parental WT cells were analyzed by Western blotting. (d) The protein expression of YAP1 in ADR-R HCC cells and its parental WT cells were analyzed by immunofluorescence. Scale bar: 20 μm. (e) Luciferase reporter assay of YAP1/TEAD binding sites containing single or double TEAD motifs in WT and ADR-R HCC cells (n = 3). (f) qRT-PCR analysis of the relative mRNA expression of CYR61 and CTGF in WT and ADR-R HCC cells (n = 3). Statistical analysis was performed by Student's t-test. Data were shown as the means ± SD. *, P < .05; **, P < .01; ***, P < .001.
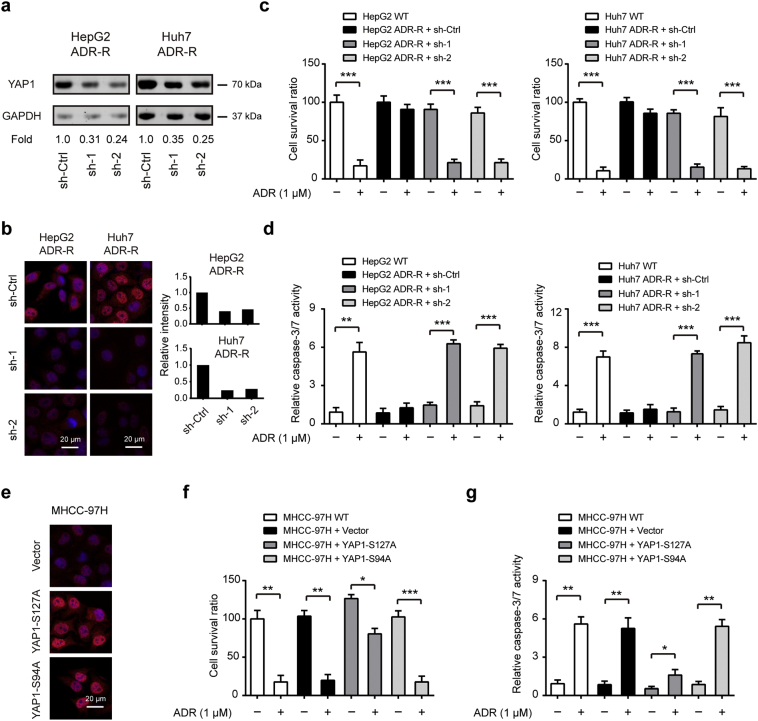
3.2. YAP1 mediates the chemoresistant phenotype of HCC cells in vitro
Next, to demonstrate whether YAP1 is responsible for ADR resistant in HCC cells, we silenced YAP1 in HepG2/ADR-R and Huh7/ADR-R cells. Two specific shRNAs targeting YAP led to pronounced reduction of YAP1 protein level in both two cell lines as revealed by western blotting (Fig. 2a) and immunofluorescence (Fig. 2b), respectively. By CCK-8 cell viability assay, we found that YAP1 knockdown only showed faint influence at the cell viability of ADR-R cells. Interestingly, ADR-R cells failed to respond to ADR treatment but displayed significantly diminished cell viability in the presence of YAP1 knockdown (Fig. 2c). Meanwhile, YAP1 knockdown also contributed to increased cell apoptosis of ADR-R cells upon ADR treatment as demonstrated by measuring caspase-3/7 activity (Fig. 2d). Moreover, to identify the role of activated YAP1 signaling in ADR resistant, we determined the effects of YAP1 activation in ADR-sensitive cells. To address this issue, we established stable MHCC-97H clones that overexpress YAP1-S127A (constitutively active YAP that cannot be phosphorylated by LATS kinases), YAP-S94A (TEAD-binding deficient YAP protein), and an empty vector control by lentiviral infection. The overexpression efficiency was shown in Fig. 2e. As a result, overexpression of YAP-S127A but not YAP-S94A contributed to increased cell proliferation and decreased cell apoptosis (Fig. 2f-g). Notably, overexpression of YAP-S127A can significantly reduce the chemosensitivity of MHCC-97H cells to ADR as revealed by increased cell viability and reduced cell apoptosis (Fig. 2f-g). Collectively, these data above suggest that YAP1 is critically involved in the chemoresistant phenotype of HCC cells.
Fig. 2.
YAP1 mediates the chemoresistant phenotype of HCC cells in vitro. The interference efficiency of YAP1 in HepG2/ADR-R and Huh7/ADR-R cells was determined by western blotting (a) and immunofluorescence (b), Scale bar: 20 μm. (c-d) Relative chemosensitivity of ADR-R cells in the presence or absence of YAP knockdown. Cell were treated with 1 μM ADR for 48 h and then subjected to CCK8 analysis (c, n = 5) and caspase-3/7 activity analysis (d, n = 5). Scale bar: 20 μm. (e) Immunofluorescence analysis showing overexpression of YAP in MHCC-97H-YAPS127A and MHCC-97H-YAPS94A cells. (f-g) Relative chemosensitivity of MHCC-97H cells in the presence or absence of YAPS127A or YAPS94A overexpression. Cell were treated with 1 μM ADR for 48 h and then subjected to CCK8 analysis (f, n = 5) and caspase-3/7 activity analysis (g, n = 5). Statistical analysis was performed by Student's t-test. Data were shown as the means ± SD. *, P < .05; **, P < .01; ***, P < .001.
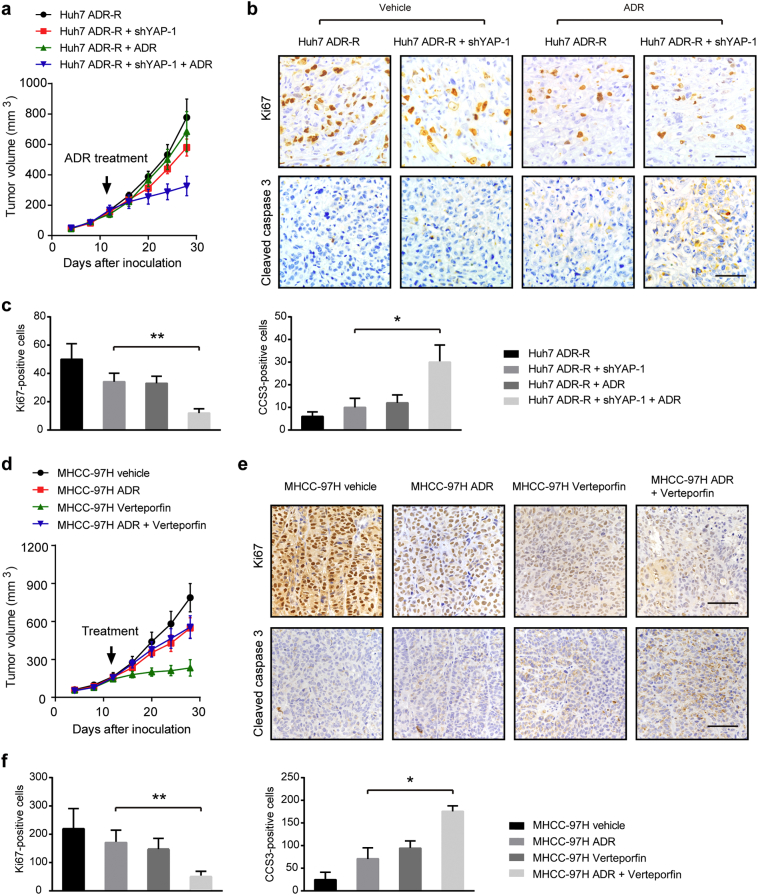
3.3. Targeting YAP1 is able to reverse the chemoresistant phenotype of HCC cells in vivo
To further characterize the involvement of YAP1 in the process of ADR resistant of HCC cells, we examined the influence of consequences of YAP1 silencing and inhibition in the anti-cancer effects of ADR by utilizing an in vivo xenograft model. We implanted Huh7/ADR-R + sh-ctrl and Huh7/ADR-R + sh-YAP1–1 cells into the nude mice (n = 5 per group). ADR was administered at a dose of 10 mg/kg after the tumor volume reached about 150 mm3. Consistent with the observation from in vitro studies, YAP1 knockdown also faintly inhibited tumor growth in vivo (Fig. 3a and Fig. S2a). Meanwhile, YAP-1 knockdown remarkably sensitized Huh7/ADR-R xenograft to ADR treatment. By IHC analysis, we demonstrated that YAP silencing can lead to growth arrest and enhanced cell apoptosis as revealed by Ki67 and cleaved caspase 3 (CCS3) staining, respectively (Fig. 3b-c). Next, we determined whether the YAP1 inhibitor Verteporfin can synergize with ADR to inhibit HCC tumor growth. As shown in Fig. 3d and Fig. S2b, ADR or Verteporfin alone decreased the growth of MHCC-97H xenograft while combined treatment drastically retarded tumor growth (Fig. 3e-f). Consistently, IHC staining of Ki67 and CCS3 also showed that Verteporfin also significantly promoted the anti-prolific and pro-apoptotic effects of ADR (Fig. 3e-f). Collectively, these results demonstrate that YAP1 contributes to the chemoresistant phenotype of HCC cells in vivo.
Fig. 3.
Targeting YAP1 is able to reverse the chemoresistant phenotype of HCC cells in vivo. (a) The effects of YAP knockdown on the growth of HCC xenograft derived from Huh7 ADR-R cells (n = 5 for each group). (b) Representative images of IHC staining of KI67 and cleaved caspase-3 in tumors as shown in panel a. Scale bar: 100 μm. (c) Quantitative analysis of Ki67 and CCS3 staining in xenograft models as shown in panel b. (d) The effects of ADR and Verteporfin on the growth of HCC xenograft derived from MHCC-97H cells (n = 5 for each group). (e) Representative images of IHC staining of KI67 and cleaved caspase-3 in tumors as shown in panel d (n = 5). Scale bar: 100 μm. (f) Quantitative analysis of Ki67 and CCS3 staining in xenograft models as shown in panel e. Statistical analysis was performed by ANOVA test. Data were shown as the means ± SD. *, P < .05; **, P < .01.
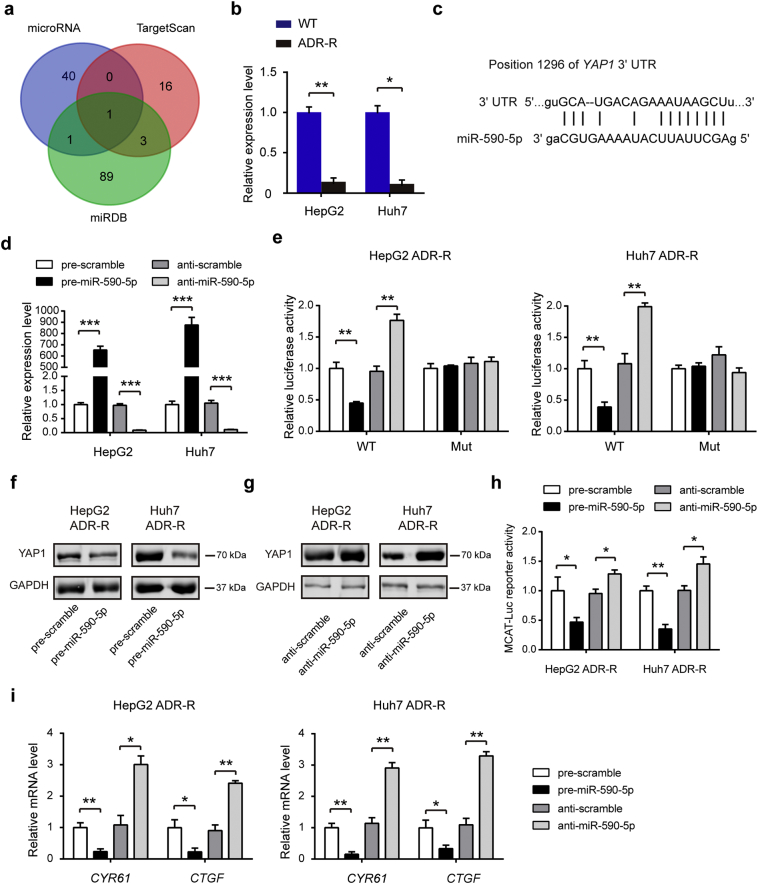
3.4. YAP1 acts as a direct target of miR-590-5p in HCC cells
Given the disparity between YAP1 mRNA and protein level in ADR-R cells, it is quite likely that a post-transcriptional regulatory mechanism of YAP1 expression exists. Because miRNA has been recognized as an important post-transcriptional regulator of gene expression [24, 25], we hypothesized whether miRNAs are involved in the regulation of YAP1 expression in HCC cells. To test this hypothesis, the candidate miRNAs targeting YAP1 were predicted using a combination of three databases: microRNA, TargetScan and miRDB (Table S3). As a result, miR-590-5p was consistently predicted by the three servers (Fig. 4a). Excitingly, miR-590-5p was significantly down-regulated in ADR-R HCC cells in comparison to the WT cells (Fig. 4b). The predicted interactions between miR-590-5p and targeting sites within the 3′-UTR of YAP1 are illustrated in Fig. 4c. To validate the binding of miR-590-5p to the 3′-UTR of YAP1, the full-length 3′-UTR of YAP1 mRNA and its mutant type were amplified and fused downstream of the firefly luciferase gene in a reporter plasmid. Overexpression of miR-590-5p was achieved by transfecting HepG2/ADR-R and Huh7/ADR-R cells with pre-miR-590-5p (synthetic RNA oligonucleotides mimicking miR-590-5p precursors), while knockdown of miR-590-5p was performed by transfecting cells with anti-miR-590-5p (Fig. 4d). The constructed plasmids were transfected into ADR-R cells together with pre-miR-590-5p, pre-scramble, anti-scramble, and anti-miR-590-5p. The luciferase reporter assay showed that miR-590-5p significantly inhibited the luciferase activity of the reporters compared with the cells transfected with control mimics, while anti-miR-590-5p increased approximately 70% the luciferase activity of the reporters compared with the cells transfected with anti-scramble (Fig. 4e). However, no significant difference was observed in the mutant plasmid (Fig. 4e). Moreover, introduction of miR-590-5p in HepG2/ADR-R and Huh7/ADR-R cells led to a marked reduction in protein level of YAP1 in comparison to the pre-scramble (Fig. 4f). In contrast, inhibition of miR-590-5p in HepG2/ADR-R and Huh7/ADR-R cells increased YAP1 protein levels compared with anti-scramble control (Fig. 4g). The YAP1/TEAD luciferase reporter assay (Fig. 4h) and real-time PCR analysis of the downstream targets of YAP1 (Fig. 4i) also confirmed miR-590-5p as a modulator of YAP1 in ADR-R HCC cells. Taken together, these data indicate that miR-590-5p is a direct regulator of YAP in ADR-R HCC cells.
Fig. 4.
YAP acts as a direct target of miR-590-5p in HCC cells. (a) Three bioinformatic softwares (miRDB, microRNA and TargetScan) were used to identify the potential regulatory miRNAs targeting YAP. (b) The expression of miR-590-5p in ADR-R HCC cells and its parental WT cells was analyzed by qRT-PCR. (c) Schematic description of conserved binding site for miR-590-5p with 3’-UTR of human YAP. (d) The expression level of miR-590-5p after transfection with pre-miR-590-5p, pre-scramble, anti-scramble, and anti-miR-590-5p in HepG2 and Huh7 ADR-R cells (n = 3). (e) Relative luciferase activity in ADR-R HCC cells that were transfected with firefly luciferase reporters containing WT or mutant 3′-UTRs of YAP, pre-miR-590-5p, pre-scramble, anti-scramble, and anti-miR-590-5p (n = 3). (f-g) The protein levels of YAP and phosphorylation-YAP (p-YAP) after transfection with pre-miR-590-5p, pre-scramble, anti-scramble, and anti-miR-590-5p in HepG2 and Huh7 ADR-R cells were detected by western blotting. (h) Luciferase reporter assay of YAP1/TEAD binding sites containing single or double TEAD motifs in HepG2 and Huh7 ADR-R cells after transfection with pre-miR-590-5p, pre-scramble, anti-scramble, and anti-miR-590-5p. (i) The mRNA expression level of CYR61 and CTGF after transfection with pre-miR-590-5p, pre-scramble, anti-scramble, and anti-miR-590-5p in HepG2 and Huh7 ADR-R cells (n = 3). Statistical analysis was performed by Student's t-test. Data were shown as the means ± SD. *, P < .05; **, P < .01.
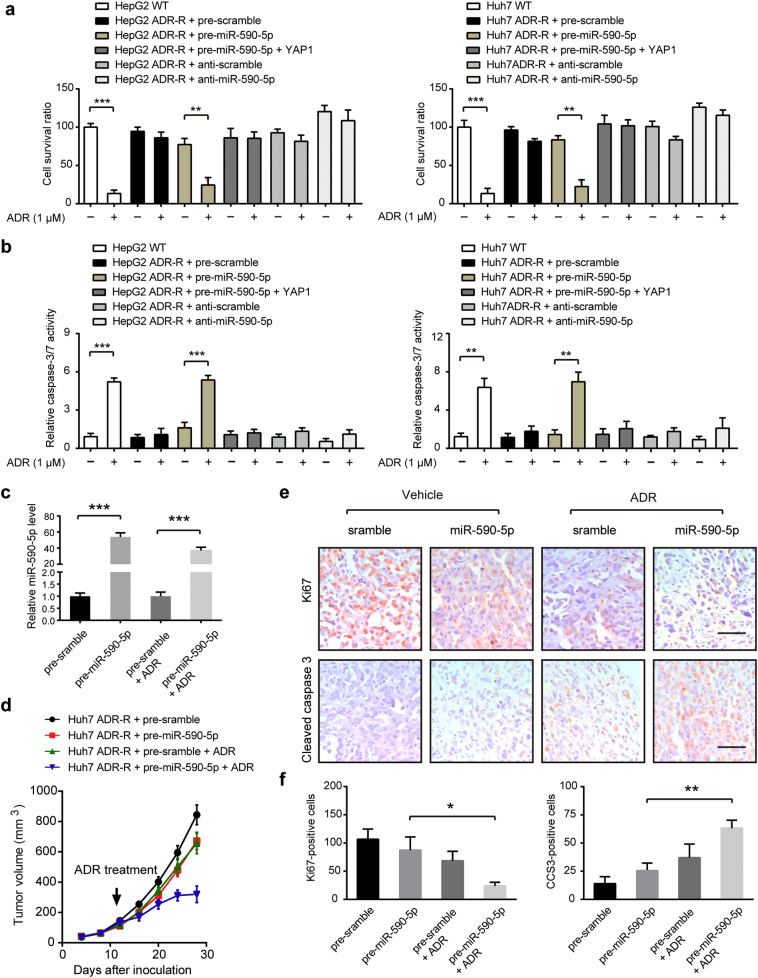
3.5. MiR-590-5p can sensitize ADR-R HCC cells to ADR in vitro and in vivo
By CCK-8 cell viability assay and cell apoptosis assay, we found that overexpression of miR-590-5p in HepG2/ADR-R and Huh7/ADR-R cells mimic the effects induced by YAP1 knockdown. Overexpression of miR-590-5p significantly improved the chemosensitivity of ADR-R HCC cells to ADR treatment, while inhibition of miR-590-5p reduced the chemosensitivity of ADR-R HCC cells to ADR treatment (Fig. 5a-b). Meanwhile, restoration of YAP1 largely compromised the inhibitory effects of miR-590-5p on HCC chemoresistance, suggesting the regulatory roles of miR-590-5p are, at least, partially mediated by YAP1 (Fig. 5a-b). To further validate the effect of miR-590-5p on ADR resistant, the mirVana™ miRNA mimic miR-590-5p or its negative control complexed with Invivofectamine@2.0 Reagent were injected into the tail veins of nude mice. The results showed that introduction of miR-590-5p significantly elevated miR-590-5p level in tumor tissues (Fig. 5c) and partially inhibited tumor growth (Fig. 5d and Fig. S2c). Moreover, Huh7/ADR-R cells treated with miRNA mimic miR-590-5p were more sensitive to the ADR, as demonstrated by tumor growth curves (Fig. 5d), decrease of cell proliferation index Ki67 and increase of cell apoptosis marker CCS3 (Fig. 5e-f). Taken together, we establish that miR-590-5p is involved in the chemoresistant phenotype of HCC cells.
Fig. 5.
MiR-590-5p can sensitize ADR-R HCC cells to ADR in vitro and in vivo. (a-b) Relative chemosensitivity of ADR-R HCC cells in the presence or absence of pre-miR-590-5p and anti-miR-590-5p. Cell were treated with 1 μM ADR for 48 h and then subjected to CCK8 analysis (a, n = 5) and caspase-3/7 activity analysis (b, n = 5). Statistical analysis was performed by Student's t-test. (c) The delivery efficiency of miR-590-5p in HCC tumor tissues. (d) The effects of introduction of miR-590-5p on the growth of HCC xenograft derived from Huh7/ADR-R cells (n = 5 for each group). Statistical analysis was performed by Student's t-test. (e) Representative images of IHC staining of Ki67 and cleaved caspase-3 in tumors as shown in panel c. Scale bar: 100 μm. (f) Quantitative analysis of Ki67 and CCS3 staining in xenograft models as shown in panel d (n = 5, ANOVA test). Data were shown as the means ± SD. *, P < .05; **, P < .01; ***, P < .001.
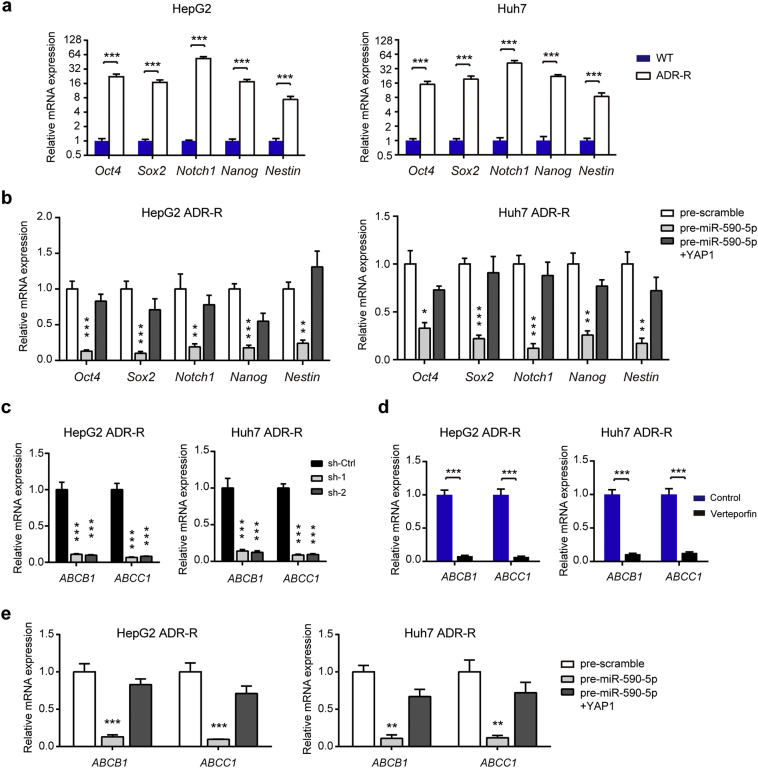
3.6. YAP1 regulates expression of stemness markers and ABC transporters in ADR-R HCC cells
It is well-established that chemoresistance can be developed by two main mechanisms. One is a minority of resistance cells with stem-cell like properties that have inherent resistance to anti-tumor drugs and the other one is acquire resistance as a response to chemotherapy treatment, especially the increased expression of drug efflux pumps, such as the ATP-binding cassette (ABC) transporters family [32, 33]. Therefore, we deduced that increased stem-cell like properties and drug efflux may contribute to YAP1-mediated chemoresistance. By real-time PCR analysis, we showed that the stem cell markers OCT4, SOX-2, Notch-1, Nanog and Nestin were all highly expressed in ADR-R HCC cells compared with the their parental cells (Fig. 6a). Interestingly, miR-590-5p significantly inhibited the expression of these stem cell markers in ADR-R cells, while ectopic expression of YAP1 largely compromised the inhibitory role of miR-590-5p (Fig. 6b). Then, we screened the expression levels of 18 ABC transporter family members in both HepG2/ADR-R and Huh7/ADR-R cells (Fig. S3). Of these ABC transporter members, ABCB1 and ABCC1 were remarkably up-regulated in ADR-R cells compared with WT cells (Fig. S2). Genetic silencing (Fig. 6c) or pharmacological inhibition (Fig. 6d) of YAP1 largely attenuated the mRNA level of ABCB1 and ABCC1 in both two cell lines. In addition, ABCB1 and ABCC1 mRNA level were also down-regulated by miR-590-5p and restored by introduction of YAP1 (Fig. 6e). Together, these data suggest the regulatory role of miR-590-5p/YAP1axis on stemness markers and ABC transporters in HCC chemoresistance.
Fig. 6.
YAP1 regulates expression of stemness markers and ABC transporters in ADR-R HCC cells. (a) The mRNA expression of several stemness markers in ADR-R HCC cells and its parental WT cells were analyzed by qRT-PCR (n = 3, Student's t-test). (b) The effects of miR-590-5P/YAP1 axis on the expression of stemness markers were determined by qRT-PCR (n = 3, ANOVA test). (c) The effects of YAP1 knockdown on the expression of ABC transporters (n = 3, ANOVA test). (d) The effects of YAP1 inhibition by Verteporfin on the expression of ABC transporters (n = 3, Student's t-test). (e) The effects of miR-590-5P/YAP1 axis on the expression of ABC transporters were determined by qRT-PCR (n = 3, ANOVA test). Data were shown as the means ± SD. *, P < .05; **, P < .01; ***, P < .001.
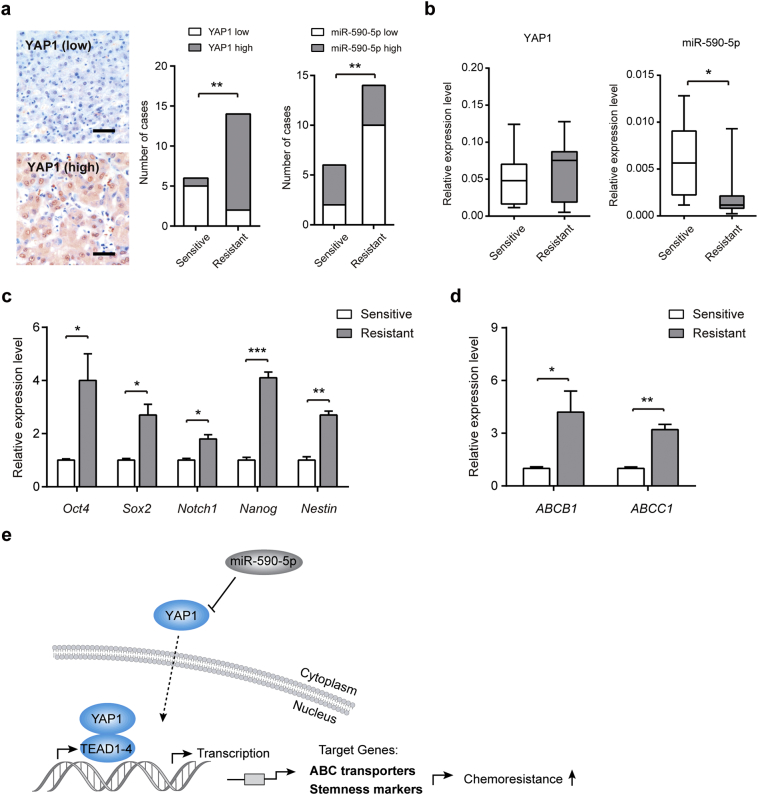
3.7. MiR-590-5p/YAP1 axis correlates with chemotherapy response of HCC
Finally, we determined whether the miR-590-5p/YAP1 axis is correlated with chemotherapy response in HCC patients. For this experiment, needle tumor tissues obtained from 20 HCC patients with transarterial chemoembolization (TACE) were analyzed [28]. As shown in Fig. 7a, 83.3% HCC patients with lower YAP1 expression were response to chemotherapy, while 85.7% HCC patients with higher YAP1 expression are resistant to chemotherapy. A favorable chemotherapy response was found in patients with higher miR-590-5p expression (Fig. 7a). Specifically, intensely expressed nucleus YAP1 was revealed in the chemoresistant group (Fig. 7a). Compared with the chemoresistant group (n = 13), miR-590-5p was significantly down-regulated in the chemosensitive group (n = 7). In contrast, the mRNA levels of YAP1 did not differ significantly (Fig. 7b). Furthermore, the expression of stemness markers (Fig. 7c) and ABC transporters (Fig. 7d) were also highly expressed in tissues from the chemoresistant group compared with that in the chemosensitive group.
Fig. 7.
miR-590-5p/YAP1 axis correlates with chemotherapy response of HCC. (a) Representative immunohistochemical staining of YAP1 in tumor tissues of HCC patients with transarterial chemoembolization (TACE) (left panel), Scale bar: 100 μm. The relationship between miR-590-5p/YAP1 axis and chemotherapy response in HCC patients (right panel). Statistical analysis was performed by the two-tailed Fisher's exact test. (b) The relative expression levels of YAP1 mRNA and miR-590-5p in HCC patients responsive to TACE (sensitive, n = 7) and unresponsive to TACE (resistant, n = 13) were determined by qRT-PCR. (c) The relative mRNA expression levels of stem cell markers in the TACE-sensitive (n = 7) and resistant group (n = 13). (d) The relative mRNA expression levels of ABC transporters in the TACE-sensitive (n = 7) and resistant group (n = 13). Statistical analysis was performed by Student's t-test. Data were shown as the means ± SEM. *, P < .05; **, P < .01; ***, P < .001.
4. Discussion
Emerging studies have shown that resistant to systemic chemotherapy is one of the major reasons for the poor prognosis of patients with advanced HCC. Previously, several oncogenic signaling pathways, including phosphoinositide 3 kinase (PI3K)/Akt [34], extracellular signal-regulated kinase 1/2 (ERK1/2) [35], Notch [36], and Wnt/β-catenin [37], have been demonstrated to be involved in HCC chemoresistance. In this study, we demonstrated that dysregulated YAP1 expression plays a role in HCC chemoresistance.
Firstly, by establishing two cell models with acquired ADR resistance, we screened the key components in Hippo signaling pathway and identified YAP1 as the major dysregulated molecule in ADR-R cells. The Hippo pathway, initially identified in Drosophila, has been shown to regulate organ size and tissue homeostasis in different model organisms [12, 14]. Moreover, accumulated studies have shown that dysregulated Hippo pathway is widely implicated in tumorigenesis, such as breast cancer [38], colorectal cancer [14], lung cancer [39], and liver cancer [13]. Notably, liver specific genetic manipulation of multiple components of Hippo signaling pathway uniformly leads to liver enlargement and tumor development [40, 41]. As the well-characterized downstream transcriptional coactivator of the Hippo pathway, YAP1 has been demonstrated to be highly activated in many human cancers. Upregulated YAP1 was frequently associated with reduced overall survival and disease-free survival in patients with cancer [40]. Meanwhile, YAP1 can promote cancer cell proliferation, migration, invasion, stemness and chemoresistance through multiple mechanisms [40]. Recently, several reports have uncovered the role of Hippo-YAP1-TEAD signaling pathway the chemoresistant phenotype of cancer cells. For example, phosphorylation-defective YAP1 makes ovarian cancer cells much more resistant to cisplatin and inhibition of YAP1 by siRNA increases sensitivity of erlotinib-resistant non-small cell lung cancer cell line H1975 to erlotinib [42, 43]. In this study, we found that suppression of YAP1 resentisized ADR-R cells to ADR and introduction of consecutive active YAP1 enhanced HCC chemoresistance. The in vitro and in vivo growth rates of the ADR-R cells without ADR treatment were similar to their parental WT cells, indicating that the selection process did not alter the proliferation and tumorigenesis of these two sublines. Consistent with previous reports [44], YAP1 indeed contributes to cell proliferation in vitro and tumor growth in vivo. However, the growth-promoting and anti-apoptotic effects of YAP1 were remarkably amplified in the presence of ADR treatment, suggesting its role in regulating HCC chemoresistance. Given other Hippo components were not altered in ADR-R cells, we postulated that YAP1 is activated by Hippo independent mechanisms.
Secondly, we identified miR-590-5p as a modulator of YAP1 in ADR-R cells. Dysregulation of miR-590-5p have been documented in carcinogenesis and in the progression of several types of malignances [[45], [46], [47]]. In gastric cancer, miR-590-5p regulates gastric cancer chemosensitivity through RECK and the AKT/ERK pathway [47]. In cervical cancer, miR-590-5p acts as an oncogene by targeting the CHL1 gene and promotes cervical cancer proliferation [46]. In malignant melanoma, lncRNA-ATB functions as a competing endogenous RNA to promote YAP1 expression by sponging miR-590-5p [48]. In colorectal cancer, miR-590-5p suppresses tumorigenesis by targeting YAP1 [45]. In this study, Ou et al. showed that increased cell density led to elevated expression of RNase III endonuclease, DICER1, which increases miR-590 biogenesis and miR-590-5p expression; YAP1 is elevated in colorectal cancer tissues and associated with tumorigenesis; and miR-590-5p acts as a direct functional modulator of YAP1 in colorectal cancer [45]. Consistent with this report, we showed here that miR-590-5p directly targets YAP1 in HCC, and we revealed the regulatory of miR-590-5p/YAP1 axis in HCC chemoresistance from both in vitro and in vivo studies. Overexpression of miR-590-5p was sufficient to resentisize ADR-R cells to ADR treatment. Importantly, miR-590-5p may act as novel biomarker or predictor of radio- or chemo-responsiveness in several cancers [[49], [50], [51]]. Therefore, from both the therapeutic and diagnostic point of view, miR-590-5p is critically involved in the chemoresistant phenotype of cancers. MiR-590-5p acts as a density-sensitive microRNA in colorectal cancer [45], however, we failed to found similar observation in HCC (data not shown), suggesting a tissue-specific manner of miR-590-5p regulation in cancers. In addition, we also determined the DNA copy number alteration, DNA methylation level, and expression of Dicer and Drosha in ADR-R cells and control cells. However, we did not get a positive result. Thus, the detailed reason for dysregulated miR-590-5p in HCC warrants further investigation.
Thirdly, we correlated YAP1-mediated chemoresistance in HCC cells with increased expression of stemness markers and ABC transporters. A minority of cancer cells with stem-cell like properties can repopulate the tumor after anti-tumor drug treatment. ABC transporters can reduce the intracellular accumulation of chemotherapy drugs and are frequently increased following chemotherapy [32]. In ovarian cancer, YAP1/TEAD co-activator can regulate ovarian cancer initiated cell pluripotency and chemo-resistance by up-regulating stemness markers and ABC transporters [21]. Meanwhile, YAP activation can promote cancer stem cells self-renewal and chemoresistance in cancer cells [52]. Consistent with these observations, we certified that YAP1 was able to promote the expression of various stem-cell markers and ABC transporters in ADR-R cells. Given that ABC transporters remain attractive potential adjuvant to chemotherapy, our study provides an evidence for the development of potential targeted therapies focused on ABC transporters.
Finally, we correlated the expression of miR-590-5p/YAP1 axis with the chemotherapy response in clinical HCC patient with TACE treatment. Currently, TACE has been widely performed to improve the outcome of patients with unresectable HCC [53]. However, the prognosis of advanced HCC patients from TACE treatment is still unsatisfactory because of acquired chemoresistance during TACE treatment [53, 54]. In this study, we found that the protein expression level of YAP1 in HCC patients who were poorly responsive to TACE treatment was significantly higher than that in the responsive HCC patients, indicating that increased YAP expression may be contribute to the acquired chemoresistance of HCC patients after chemotherapy. Meanwhile, miR-590-5p was significantly down-regulated in the chemosensitive group compared with the chemoresistant group. Combined with previous observations from cell lines and mouse models, we concluded that miR-590-5p/YAP1 axis promotes the development of chemoresistance in HCC. However, the sample size of patients received TACE treatment in this study was insufficient to draw a solid conclusion. Further large-scale and multi-center studies are warranted to make a precise conclusion.
In conclusion, our findings provide new insight into the role of miR-590-5p/YAP1 dysregulation in the chemoresistance of HCC cells and imply a strategy that combines the use of targeting miR-590-5p/YAP1 with chemotherapy.
Funding sources
The work was supported by the National Natural Science Foundation of China (No. 81573657), Zhejiang Province Medical and Health Care Key Project (No. 2016146810), Experimental Animal Science and Technology Projects of Zhejiang Province (No: 2017C37178, 2016C37101), Public Welfare Technology Application Research Project of Lishui (No: 2016GYX36, 2016GYX37), and Chinese Medicine Science and Technology Projects of Zhejiang Province (Nos. 2015ZA228 and 2016ZA209).
Conflicts of interest
The authors declare no conflicts of interest.
Authors' contributions
Conception and design: MJC, MX, JSJ.
Acquisition of data: MJC, LMW, JFT, ZWZ, XXF, JTM, QYW, XLW.
Analysis and interpretation of data: MJC, LMW, JFT.
Writing, review, and/or revision of the manuscript: MJC, MX, LH, JSJ.
Study supervision: MX, LH, JSJ.
Final approval of manuscript: All authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.08.010.
Contributor Information
Li Huang, Email: 13817168836@163.com.
Min Xu, Email: lschrxm@163.com.
Jiansong Ji, Email: lschrjjs@163.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.White D.L., Thrift A.P., Kanwal F., Davila J., El-Serag H.B. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology. 2017;152(4):812–820. doi: 10.1053/j.gastro.2016.11.020. [e5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez-Alejandro R., Levstik M.A., Linehan D.C. Hepatectomy for early hepatocellular carcinoma: not if, but when and how? JAMA Surg. 2017;152(3) doi: 10.1001/jamasurg.2016.5042. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda M., Mitsunaga S., Ohno I., Hashimoto Y., Takahashi H., Watanabe K. Systemic chemotherapy for advanced hepatocellular carcinoma: past, present, and future. Diseases. 2015;3(4):360–381. doi: 10.3390/diseases3040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Dika I., Abou-Alfa G.K. The role (if any) of chemotherapy in hepatocellular carcinoma. The Lancet Gastroenterology & Hepatology. 2017;2(6):387–389. doi: 10.1016/S2468-1253(17)30104-8. [DOI] [PubMed] [Google Scholar]
- 6.Pan L.H., Zhao C., Ma Y.L. Is Y90 Radioembolization superior or comparable to Transarterial chemoembolization for treating hepatocellular carcinoma? Gastroenterology. 2017;152(6):1627–1628. doi: 10.1053/j.gastro.2016.10.048. [DOI] [PubMed] [Google Scholar]
- 7.Peck-Radosavljevic M. Drug therapy for advanced-stage liver cancer. Liver Cancer. 2014;3(2):125–131. doi: 10.1159/000343868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Govaere O., Wouters J., Petz M., Vandewynckel Y.P., Van den Eynde K., Van den Broeck A. Laminin-332 sustains chemoresistance and quiescence as part of the human hepatic cancer stem cell niche. J Hepatol. 2016;64(3):609–617. doi: 10.1016/j.jhep.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Li Y., Ye Y., Feng B., Qi Y. Long noncoding RNA lncARSR promotes doxorubicin resistance in hepatocellular carcinoma via modulating PTEN-PI3K/Akt pathway. J Cell Biochem. 2017;118(12):4498–4507. doi: 10.1002/jcb.26107. [DOI] [PubMed] [Google Scholar]
- 10.Zanconato F., Cordenonsi M., Piccolo S. YAP/TAZ at the roots of Cancer. Cancer Cell. 2016;29(6):783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badouel C., McNeill H. SnapShot: the hippo signaling pathway. Cell. 2011;145(3):484. doi: 10.1016/j.cell.2011.04.009. [e1] [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Yu A., Yu F.X. The hippo pathway in tissue homeostasis and regeneration. Protein Cell. 2017;8(5):349–359. doi: 10.1007/s13238-017-0371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel S.H., Camargo F.D., Yimlamai D. Hippo signaling in the liver regulates organ size, cell fate, and carcinogenesis. Gastroenterology. 2017;152(3):533–545. doi: 10.1053/j.gastro.2016.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong A.W., Meng Z., Guan K.L. The hippo pathway in intestinal regeneration and disease. Nat Rev Gastroenterol Hepatol. 2016;13(6):324–337. doi: 10.1038/nrgastro.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishio M., Sugimachi K., Goto H., Wang J., Morikawa T., Miyachi Y. Dysregulated YAP1/TAZ and TGF-beta signaling mediate hepatocarcinogenesis in Mob1a/1b-deficient mice. Proc Natl Acad Sci U S A. 2016;113(1):E71–E80. doi: 10.1073/pnas.1517188113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae J.S., Kim S.M., Lee H. The hippo signaling pathway provides novel anti-cancer drug targets. Oncotarget. 2017;8(9):16084–16098. doi: 10.18632/oncotarget.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D.Y., Wu Y.N., Huang J.Q., Wang W., Xu M., Jia J.P. Hippo/YAP signaling pathway is involved in osteosarcoma chemoresistance. Chin J Cancer. 2016;35:47. doi: 10.1186/s40880-016-0109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartucci M., Dattilo R., Moriconi C., Pagliuca A., Mottolese M., Federici G. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34(6):681–690. doi: 10.1038/onc.2014.5. [DOI] [PubMed] [Google Scholar]
- 19.Chen M., Wang M., Xu S., Guo X., Jiang J. Upregulation of miR-181c contributes to chemoresistance in pancreatic cancer by inactivating the hippo signaling pathway. Oncotarget. 2015;6(42):44466–44479. doi: 10.18632/oncotarget.6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song S., Honjo S., Jin J., Chang S.S., Scott A.W., Chen Q. The hippo coactivator YAP1 mediates EGFR overexpression and confers Chemoresistance in esophageal Cancer. Clin Cancer Res. 2015;21(11):2580–2590. doi: 10.1158/1078-0432.CCR-14-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia Y., Zhang Y.L., Yu C., Chang T., Fan H.Y. YAP/TEAD co-activator regulated pluripotency and chemoresistance in ovarian cancer initiated cells. PloS One. 2014;9(11) doi: 10.1371/journal.pone.0109575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marti P., Stein C., Blumer T., Abraham Y., Dill M.T., Pikiolek M. YAP promotes proliferation, chemoresistance, and angiogenesis in human cholangiocarcinoma through TEAD transcription factors. Hepatology. 2015;62(5):1497–1510. doi: 10.1002/hep.27992. [DOI] [PubMed] [Google Scholar]
- 23.Mao B., Hu F., Cheng J., Wang P., Xu M., Yuan F. SIRT1 regulates YAP2-mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene. 2014;33(11):1468–1474. doi: 10.1038/onc.2013.88. [DOI] [PubMed] [Google Scholar]
- 24.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 26.Kota J., Chivukula R.R., O'Donnell K.A., Wentzel E.A., Montgomery C.L., Hwang H.W. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137(6):1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song J.H., Meltzer S.J. MicroRNAs in pathogenesis, diagnosis, and treatment of gastroesophageal cancers. Gastroenterology. 2012;143(1):35–47. doi: 10.1053/j.gastro.2012.05.003. [e2] [DOI] [PubMed] [Google Scholar]
- 28.Xu M., Zhao Z., Song J., Lan X., Lu S., Chen M. Interactions between interleukin-6 and myeloid-derived suppressor cells drive the chemoresistant phenotype of hepatocellular cancer. Exp Cell Res. 2017;351(2):142–149. doi: 10.1016/j.yexcr.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Stein C., Bardet A.F., Roma G., Bergling S., Clay I., Ruchti A. YAP1 exerts its transcriptional control via TEAD-mediated activation of enhancers. PLoS Genet. 2015;11(8) doi: 10.1371/journal.pgen.1005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi X., Zhu H.R., Liu T.T., Shen X.Z., Zhu J.M. The hippo pathway in hepatocellular carcinoma: non-coding RNAs in action. Cancer Lett. 2017;400:175–182. doi: 10.1016/j.canlet.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 31.Sohn B.H., Shim J.J., Kim S.B., Jang K.Y., Kim S.M., Kim J.H. Inactivation of hippo pathway is significantly associated with poor prognosis in hepatocellular carcinoma. Clin Cancer Res. 2016;22(5):1256–1264. doi: 10.1158/1078-0432.CCR-15-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ween M.P., Armstrong M.A., Oehler M.K., Ricciardelli C. The role of ABC transporters in ovarian cancer progression and chemoresistance. Crit Rev Oncol Hematol. 2015;96(2):220–256. doi: 10.1016/j.critrevonc.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Vasiliou V., Vasiliou K., Nebert D.W. Human ATP-binding cassette (ABC) transporter family. Hum Genomics. 2009;3(3):281–290. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tripathi S.C., Fahrmann J.F., Celiktas M., Aguilar M., Marini K.D., Jolly M.K. MCAM mediates Chemoresistance in small-cell lung Cancer via the PI3K/AKT/SOX2 signaling pathway. Cancer Res. 2017;77(16):4414–4425. doi: 10.1158/0008-5472.CAN-16-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan C.W., Jin X., Zhao Y., Pan Y., Yang J., Karnes R.J. AKT-phosphorylated FOXO1 suppresses ERK activation and chemoresistance by disrupting IQGAP1-MAPK interaction. EMBO J. 2017;36(8):995–1010. doi: 10.15252/embj.201695534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambrogio C., Gomez-Lopez G., Falcone M., Vidal A., Nadal E., Crosetto N. Combined inhibition of DDR1 and notch signaling is a therapeutic strategy for KRAS-driven lung adenocarcinoma. Nat Med. 2016;22(3):270–277. doi: 10.1038/nm.4041. [DOI] [PubMed] [Google Scholar]
- 37.Cai J., Fang L., Huang Y., Li R., Xu X., Hu Z. Simultaneous overactivation of Wnt/beta-catenin and TGFbeta signalling by miR-128-3p confers chemoresistance-associated metastasis in NSCLC. Nat Commun. 2017;8:15870. doi: 10.1038/ncomms15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cordenonsi M., Zanconato F., Azzolin L., Forcato M., Rosato A., Frasson C. The hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147(4):759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 39.Lau A.N., Curtis S.J., Fillmore C.M., Rowbotham S.P., Mohseni M., Wagner D.E. Tumor-propagating cells and yap/Taz activity contribute to lung tumor progression and metastasis. EMBO J. 2014;33(5):468–481. doi: 10.1002/embj.201386082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harvey K.F., Zhang X., Thomas D.M. The hippo pathway and human cancer. Nat Rev Cancer. 2013;13(4):246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 41.Lee K.P., Lee J.H., Kim T.S., Kim T.H., Park H.D., Byun J.S. The hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107(18):8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu P.C., You B., Yang Y.L., Zhang W.Q., Wang Y.C., Xu Z. YAP promotes erlotinib resistance in human non-small cell lung cancer cells. Oncotarget. 2016;7(32):51922–51933. doi: 10.18632/oncotarget.10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall C.A., Wang R., Miao J., Oliva E., Shen X., Wheeler T. Hippo pathway effector yap is an ovarian cancer oncogene. Cancer Res. 2010;70(21):8517–8525. doi: 10.1158/0008-5472.CAN-10-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huo X., Zhang Q., Liu A.M., Tang C., Gong Y., Bian J. Overexpression of yes-associated protein confers doxorubicin resistance in hepatocellullar carcinoma. Oncol Rep. 2013;29(2):840–846. doi: 10.3892/or.2012.2176. [DOI] [PubMed] [Google Scholar]
- 45.Ou C., Sun Z., Li X., Li X., Ren W., Qin Z. MiR-590-5p, a density-sensitive microRNA, inhibits tumorigenesis by targeting YAP1 in colorectal cancer. Cancer Lett. 2017;399:53–63. doi: 10.1016/j.canlet.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Chu Y., Ouyang Y., Wang F., Zheng A., Bai L., Han L. MicroRNA-590 promotes cervical cancer cell growth and invasion by targeting CHL1. J Cell Biochem. 2014;115(5):847–853. doi: 10.1002/jcb.24726. [DOI] [PubMed] [Google Scholar]
- 47.Shen B., Yu S., Zhang Y., Yuan Y., Li X., Zhong J. miR-590-5p regulates gastric cancer cell growth and chemosensitivity through RECK and the AKT/ERK pathway. OncoTargets and therapy. 2016;9:6009–6019. doi: 10.2147/OTT.S110923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mou K., Liu B., Ding M., Mu X., Han D., Zhou Y. lncRNA-ATB functions as a competing endogenous RNA to promote YAP1 by sponging miR-590-5p in malignant melanoma. International j. of oncology. 2018;53(3):1094–1104. doi: 10.3892/ijo.2018.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Summerer I., Niyazi M., Unger K., Pitea A., Zangen V., Hess J. Changes in circulating microRNAs after radiochemotherapy in head and neck cancer patients. Radiat Oncol. 2013;8:296. doi: 10.1186/1748-717X-8-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watson J.A., Bryan K., Williams R., Popov S., Vujanic G., Coulomb A. miRNA profiles as a predictor of chemoresponsiveness in Wilms' tumor blastema. PloS One. 2013;8(1):e53417. doi: 10.1371/journal.pone.0053417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kheirelseid E.A., Miller N., Chang K.H., Curran C., Hennessey E., Sheehan M. miRNA expressions in rectal cancer as predictors of response to neoadjuvant chemoradiation therapy. Int J Colorectal Dis. 2013;28(2):247–260. doi: 10.1007/s00384-012-1549-9. [DOI] [PubMed] [Google Scholar]
- 52.Sorrentino G., Ruggeri N., Zannini A., Ingallina E., Bertolio R., Marotta C. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat Commun. 2017;8:14073. doi: 10.1038/ncomms14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lencioni R., de Baere T., Soulen M.C., Rilling W.S., Geschwind J.F. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi: 10.1002/hep.28453. [DOI] [PubMed] [Google Scholar]
- 54.Sieghart W., Hucke F., Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62(5):1187–1195. doi: 10.1016/j.jhep.2015.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material