Abstract
Increased cerebral monoamine oxidase A (MAO-A) levels have been shown in non-seasonal depression using positron emission tomography (PET). Seasonal affective disorder (SAD) is a sub-form of major depressive disorder and is typically treated with bright light therapy (BLT). The serotonergic system is affected by season and light. Hence, this study aims to assess the relevance of brain MAO-A levels to the pathophysiology and treatment of SAD. Changes to cerebral MAO-A distribution (1) in SAD in comparison to healthy controls (HC), (2) after treatment with BLT and (3) between the seasons, were investigated in 24 patients with SAD and 27 HC using [11C]harmine PET. PET scans were performed in fall/winter before and after 3 weeks of placebo-controlled BLT, as well as in spring/summer. Cerebral MAO-A distribution volume (VT, an index of MAO-A density) did not differ between patients and HC at any of the three time-points. However, MAO-A VT decreased from fall/winter to spring/summer in the HC group (F1, 187.84 = 4.79, p < 0.050), while SAD showed no change. In addition, BLT, but not placebo, resulted in a significant reduction in MAO-A VT (F1, 208.92 = 25.96, p < 0.001). This is the first study to demonstrate an influence of BLT on human cerebral MAO-A levels in vivo. Furthermore, we show that SAD may lack seasonal dynamics in brain MAO-A levels. The lack of a cross-sectional difference between patients and HC, in contrast to studies in non-seasonal depression, may be due to the milder symptoms typically shown by patients with SAD.
Introduction
Seasonal affective disorder (SAD) is characterized by decreased mood and drive in the fall and winter months, followed by spontaneous remission in spring and summer1. Two to three percent of the population suffers from clinically manifest SAD2. Bright light therapy (BLT) is well accepted as the gold standard treatment of this disorder3.
The central contribution of serotonergic changes to depressive pathophysiology, irrespective of seasonality, has been thoroughly demonstrated. Along this line, increased cerebral monoamine oxidase A (MAO-A) levels are considered a central finding in non-seasonal major depressive disorder (MDD)4. MAO-A is a mitochondrial enzyme that is primarily responsible for degradation of serotonin (5-HT) in the brain and is of utmost importance for homeostasis of cerebral 5-HT levels5. Increased MAO-A in MDD is therefore in accordance with the monoamine theory of depressive pathophysiology.
As is the case in MDD6, serotonergic changes are also thought to underlie development of SAD. Numerous molecular components of the serotonergic system are influenced by season and light, including the 5-HT1A-receptor7 and the serotonin transporter (SERT)8–11. Furthermore, seasonal regulation of SERT expression was shown to be altered in SAD12,13. In addition, the efficacy of MAO-A inhibitors in SAD14 emphasizes that changes to MAO-A may play a role in the disorder’s pathophysiology. However, changes to cerebral MAO-A distribution have yet to be investigated in patients with SAD.
This study thus investigates whether brain MAO-A distribution (1) differs between patients with SAD and healthy controls (HC), (2) is sensitive to BLT and (3) changes between fall/winter and spring/summer. In addition, the association between SAD symptom severity and MAO-A was investigated. For this purpose [11C]harmine (7-[11C]methoxy-1-methyl-9H-[3,4-b]indole) positron emission tomography (PET)4,15,16 was used and MAO-A distribution volume (VT), an index auf MAO-A density, was calculated. [11C]harmine is a reversible MAO-A inhibitor, is selective for the MAO-A isoenzyme15 and its metabolites do not pass the blood–brain barrier17. It can therefore be considered the gold standard of currently available MAO-A radioligands for cerebral PET imaging4.
Along the lines of the hyposerotonergic hypothesis of depression4,6 and in reflection of the influence of season and light on other molecules regulating serotonergic neurotransmission, higher MAO-A VT in fall/winter, reduction after BLT and normalization in spring/summer was expected in SAD.
Materials and methods
Subjects
Twenty four patients suffering from unipolar SAD and 27 HC were included in this study. Sample size calculation was performed using G*Power based on effect sizes estimated from Meyer et al.18 using matched pairs t-test with a calculated effect size of 0.7, alpha 0.05 and power 0.95. Diagnosis was determined using the Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; DSM-IV-TR; American Psychiatric Association, 2000) and the Structured Clinical Interview for DSM-IV for Axis I disorders (SCID-I for DSM-IV). The SCID-I was utilized to exclude Axis-I comorbidities in patients and any Axis-I disorders in HC. All patients exhibited a Seasonal Problem Score ≥ 2 together with a Global Seasonality Score ≥ 10 on the Seasonal Pattern Assessment Questionnaire (SPAQ)19. An experienced physician administered the SCID and instructed subjects on how to complete the SPAQ. Patients were free from all psychopharmacologic treatment and BLT within the last 6 months as well as all pharmacologic substances during study participation, including until after PET3. Severe internal or neurologic disorders were excluded using medical history and physical examination, electrocardiography and blood tests. Urine tests were used at screening to exclude current drug abuse, current smoking20 and pregnancy. Breastfeeding women were excluded. During study participation, subjects were instructed to follow their typical routines, so as to limit bias resulting from lifestyle changes. Recruitment of SAD patients and HC was performed via the outpatient clinic at the Department of Psychiatry and Psychotherapy at the Medical University of Vienna and dedicated message boards at the Medical University of Vienna. All participants provided written informed consent and received financial reimbursement for participation. This study was approved by the Ethics Committee of the Medical University of Vienna and performed according to the Declaration of Helsinki.
Experimental design
A first PET scan (PET1) was performed in fall/winter, before start of BLT. Patients and controls were then randomized to active BLT or placebo in a double blind manner (SAD-BLT, SAD-placebo, HC-BLT, HC-placebo). Subjects and study physicians were blinded. Both SAD and HC received placebo-controlled BLT in order to assess if resulting MAO-A changes were an SAD-specific treatment effect, or rather a general neurobiological reaction to BLT. The second scan (PET2) was performed in fall/winter after 3 weeks of daily BLT/placebo. The third PET (PET3) was performed in spring/summer (mean difference PET1–PET2 ± SD = 34.70 ± 12.38 days, mean difference PET2–PET3 ± SD = 155.91 ± 40.18 days, excluding anticyclic subjects). Six of 27 HC were recruited in an anticyclic manner and underwent PET3 in spring/summer first, followed by PET1 and PET2 before and after BLT/placebo in the consecutive fall/winter. A structural MRI scan was performed at any point during study participation for co-registration of PET data. The fall/winter and spring/summer seasons spanned from October 8th to April 22nd and April 17th to September 17th, respectively.
Bright light therapy
BLT was performed over the course of 3 weeks using an artificial white light source (PhysioLight LD220 by Davita Medical Products, Kranenburg, Germany) with full-spectrum 10,000 lux light intensity (at a distance of 50 cm from the light source). Treatment was administered daily, for 30 min, before noon. This treatment scheme was based on previous studies3,21. Three weeks were chosen as the treatment time-frame because changes to other serotonergic molecules have been shown after a similar time-period of BLT10. To promote compliance, therapy was addressed at following visits. Placebo control consisted of a non-biologically active light source22. The placebo lamp was the same in size and shape as the therapeutic device, however the light intensity was reduced to <400 lux at 50 cm using a transparent filter within the device.
Imaging methods
PET
[11C]harmine (7-[11C]methoxy-1-methyl-9H-[3,4-b]indole) synthesis and quality control was performed according to procedures described by Philippe et al.23 All PET data was acquired using a GE Advance full-ring scanner at the PET Center at the Medical University of Vienna and in accordance with the center’s standard protocols. For tissue attenuation correction, a transmission scan of 5 min was carried out with 68GE rod sources. An intravenous bolus of [11C]harmine (4.6 MBq/kg body weight) was administered simultaneously with the start of PET. Dynamic PET was performed in 3D mode and reconstructed in 35 transaxial section volumes (128 × 128 matrix) with an iterative filtered backprojection algorithm (FORE-ITER) with a spatial resolution of 4.36 mm full-width at half maximum next to the center of the field of view. Fifty one successive time frames (12 frames of 5 s, 6 frames of 10 s, 3 frames of 20 s, 6 frames of 30 s, 9 frames of 1 min and 15 frames of 5 min) were collected, resulting in a total acquisition time of 90 min, optimized for modeling of [11C]harmine. Arterial blood samples for [11C]harmine quantification were drawn automatically for the first 10 min and manually at standardized intervals after [11C]harmine injection15.
MRI
All subjects underwent one T1-weighted structural magnetic resonance imaging (MRI) scan for co-registration of PET data. Structural co-registration was performed using SPM12 (Wellcome Trust Centre for Neuroimaging, London, United Kingdom; http://www.fil.ion.ucl.ac.uk/spm/). MRI was performed using a 3 T PRISMA MR Scanner (Siemens Medical, Erlangen, Germany, 1 × 1 mm voxel size, 1.1 mm slice thickness, 200 slices) or a 3 T Achieva MR Scanner (Philipps, Best, Netherlands, 0.47 × 0.47 mm voxel size, 0.88 mm slice thickness, 180 slices).
Quantification of monoamine oxidase A
Manual arterial blood samples were processed according to Ginovart et al15. A gamma counter, cross-calibrated with the PET scanner and the arterial blood sampling system, was used to calculate radioactivity concentrations. Quantification was carried out using PMOD 3.509 (PMOD Technologies Ltd., Zurich, Switzerland; www.pmod.com). Whole blood activity was multiplied with plasma-to-whole blood ratio and the fraction of intact radioligand in the plasma to obtain the final arterial input function (AIF). VT of specifically bound radioligand was quantified using a constrained two-tissue compartment model (2TCM) by coupling K1/k2 over several regions of interest (ROIs)15. Constrained 2TCM was used because this model resulted in more stable standard error of the fit across regions in this data set than the unconstrained method. For quantification, regions were taken from an automated anatomical labeling-based atlas (amygdala and hippocampal complex, cingulate cortex, frontal, temporal, parietal and occipital area, insula, midbrain, striatum, thalamus and cerebellar gray matter)24. Next, voxel-wise quantification was carried out using Logan plot, whereby the AIF and the time activity curve of the thalamus, a stable high uptake region, served as input. From the resulting distribution images, all regional VT except midbrain were extracted using the Harvard-Oxford probabilistic atlas and averaged for both hemispheres. ROIs for statistical analyses were selected based on the investigation by Meyer et al.4 and included the frontal and temporal pole, anterior and posterior cingulate gyrus, caudate, putamen, thalamus, hippocampus and midbrain. A global ROI consisting of the above-mentioned ROIs and weighted for ROI size was calculated for regression analyses.
To demonstrate the consistency of the quantification methods used, and because VT may be underestimated by Logan plot, VT values calculated for coupling of K1/k2 and VT values used for statistical analyses were compared. Furthermore, repeated measures analyses of variance (ANOVA) were performed with K1/k2 values and free fraction of tracer in blood to demonstrate that changes shown in VT can be attributed to changes in k3/k4 (Supplement).
Psychometric assessment
For assessment of depressive symptom severity at PET1, PET2 and PET3, the Beck Depression Inventory (BDI)25 was used for self-rating and the 24-item Hamilton Depression Rating Scale (HAMD) was used for external rating26. An experienced physician administered the HAMD and instructed subjects on how to complete the BDI. All psychological scores including those performed at the screening visit (SCID, SPAQ) were performed in the clinical setting using paper-based report forms.
Statistical analyses
In order to assess changes to symptom severity (BDI, HAMD), repeated measures ANOVA with measurement (PET1, PET2, PET3) as within subject factor, therapy arm (BLT, placebo) and group (SAD, HC) as between subject factors and symptom severity scores (BDI, HAMD) as dependent variables were performed.
Regression analyses were performed in order to assess if certain clinical variables had a significant influence on MAO-A VT. Variables showing significant effects on MAO-A VT were then included as covariates in subsequent mixed model analyses of MAO-A VT data. Regression analyses with age, sex, perimenopausal status and symptom severity (BDI, HAMD) as independent variables and global MAO-A VT as the dependent variable were performed at PET1, PET2 and PET3. Perimenopausal status was defined as female sex and age ≥ 41 years.
In order to assess changes to MAO-A VT, linear mixed model analyses using measurement (PET1, PET2, PET3), therapy arm (BLT, placebo), group (SAD, HC) and region (9 ROIs) as fixed factors, subject as random factor and MAO-A VT as dependent variable were performed. Clinical variables showing significant effects on MAO-A VT in the regression analyses described above were included as covariates.
Furthermore, initial mixed model analyses were repeated without the three subjects (1 SAD, 2 HC) who were scanned between 17th and 22nd of April to exclude the relevance of overlapping scan dates between the seasons.
In an exploratory analysis, mixed models were repeated without covariates. In addition, an exploratory mixed model analysis with therapy arm (BLT, placebo), group (SAD, HC) and region (9 ROIs) as fixed factors, subject as random factor and the difference between MAO-A VT at PET1 and PET3 as dependent variable, was performed to assess if seasonal change in MAO A VT differed between SAD and HC or therapy arms.
Prerequisites for statistical tests were evaluated (i.e., normal distribution, homogeneity of variance) and violations were accounted for (i.e., choice of covariance structure). For repeated measures ANOVA of symptom severity scores (BDI, HAMD) and mixed model analyses of MAO-A VT, post hoc models were corrected using Fisher’s least significant difference procedure in accordance with the closed test principle if factors showed a significant interaction in the primary model. Post-hoc models were declared nonsignificant if the p value of the primary interaction effect was nonsignificant, but carried out without further correction in case of a significant primary interaction effect. For all regression analyses, multiple testing was corrected for using the Holm–Bonferroni method, unless otherwise stated.
Results
PET data was acquired in 26 SAD and 36 HC and could be analyzed in 24 SAD (mean age ± SD = 34.21 ± 10.57 years, 8 male) and 27 HC (33.52 ± 10.57 years, 11 male). Of the 24 SAD who underwent PET1, 21 and 18 underwent PET2 and PET3, respectively. Of the 27 HC included in this study, PET data was available at PET1 and PET2 for 26 and at PET3 for 24 (Table 1).
Table 1.
Clinical variables
| SAD | HC | ||
|---|---|---|---|
| BDI (PET1) | |||
| BLT | 17.08 ± 5.63* |
 **
**
|
0.83 ± 1.40 |
| Placebo | 11.91 ± 5.86 | 1.38 ± 2.10 | |
| BDI (PET2) | |||
| BLT | 11.17 ± 7.27*** |
 **
**
|
0.92 ± 1.78 |
| Placebo | 7.75 ± 5.65 | 0.21 ± 0.58*** | |
| BDI (PET3) | |||
| BLT | 4.27 ± 3.47*** |
 **
**
|
0.70 ± 1.16 |
| Placebo | 2.29 ± 2.69*** | 0.77 ± 1.53 | |
| HAMD (PET1) | |||
| BLT | 11.15 ± 3.80 |
 **
**
|
0.25 ± 0.45 |
| Placebo | 8.91 ± 4.55 | 0.23 ± 0.60 | |
| HAMD (PET2) | |||
| BLT | 9.23 ± 6.87 |
 **
**
|
0.5 ± 1.17 |
| Placebo | 5.75 ± 4.17 | 0.14 ± 0.36 | |
| HAMD (PET3) | |||
| BLT | 3.00 ± 3.61 |
 **,***
**,***
|
1.10 ± 1.45 |
| Placebo | 2.71 ± 2.93 | 0.23 ± 0.44 | |
| Group size | 24 | 27 | |
| Age at baseline (mean years±SD) | 34.21 ± 10.57 | 33.52 ± 10.57 | |
| Sex (male/female) | 8/16 | 11/16 | |
| Therapy arm (BLT/placebo) | 13/11 | 12/15 | |
SAD: seasonal affective disorder, HC: healthy controls, PET: positron emission tomography, BLT: bright light therapy, BDI: Beck Depression Inventory, HAMD: Hamilton Depression Rating Scale
*Significant difference vs. placebo (p < 0.050)
**Significant difference vs. HC (p < 0.010)
***Significant difference vs. PET1 (p < 0.050)
Repeated measures ANOVA showed that BDI scores decreased from PET1 to PET2 in the SAD-BLT group (F1, 11.46 = 7.06, p = 0.022) and in the HC-placebo group (F1, 13.12 = 5.74, p = 0.032). Between PET1 and PET3, BDI scores improved in SAD-BLT (F1, 9.53 = 59.23, p < 0.001) and SAD-placebo (F1, 9.60 = 25.91, p = 0.001) groups while HAMD improved in SAD (F1, 21.20 = 61.97, p < 0.001) in general. At PET1, BDI scores differed between SAD-BLT and SAD-placebo, with lower values in the latter (F1, 22.00 = 4.84, p = 0.039). This was no longer the case at PET2, after BLT. SAD showed higher BDI and HAMD scores than HC at all three PET time points (all p < 0.010) (Table 1). As BDI and HAMD were, as to be expected, strewn around 0 in the HC group, significant clinical results were confirmed using non-parametric testing (all p < 0.050).
Regression analyses showed that global MAO-A VT was not significantly influenced by age, sex, perimenopausal status, nor HAMD scores at PET1, PET2 or PET3. However, BDI had a significant influence on MAO-A VT at PET2 (R2 = 0.088, adjusted R2 = 0.067, p = 0.045, uncorrected). This association was negative, with lower MAO-A VT related to higher BDI, and was significant when SAD and HC were investigated together. Based on the results of these regression analyses, BDI was included as a covariate in all subsequent mixed model analyses of MAO-A VT.
Mixed model analysis of MAO-A VT data from all three time points revealed a three-way interaction between measurement, therapy and group (F2, 555.84 = 4.28, p = 0.014). Between PET1 and PET2, a two-way interaction between measurement and therapy was shown (F1, 385.58 = 19.16, p < 0.001). MAO-A VT fell significantly after BLT (F1, 208.92 = 25.96, p < 0.001) and no change was shown after placebo (Figs. 1 and 2). Between PET1 and PET3 a three-way interaction between measurement, therapy, and group (F1, 341.23 = 6.46, p = 0.011) was shown. MAO-A VT decreased significantly in HC (F1, 187.84 = 4.79, p = 0.030) from fall/winter before treatment (PET1) to spring/summer (PET3), while SAD did not differ in MAO-A VT between these time points (Fig. 3). MAO-A VT did not differ between group (SAD, HC) or therapy (BLT, placebo) at any of the three PET time points. For average regional MAO-A VT see Table 2.
Fig. 1. MAO-A VT reduction after BLT.
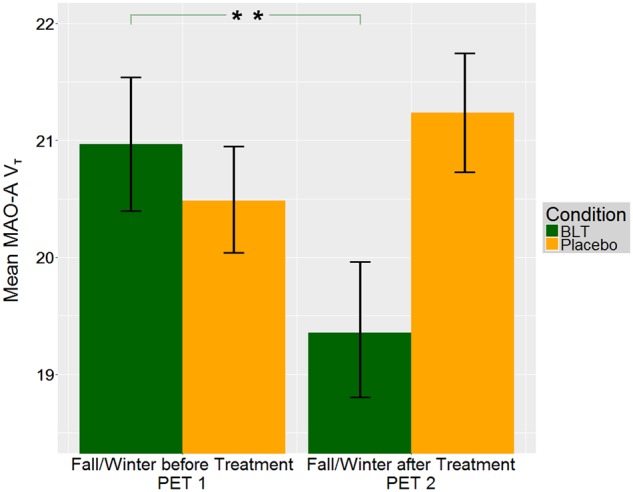
Three weeks of BLT resulted in a reduction in MAO-A VT (F1, 208.92 = 25.96, p < 0.001), while placebo did not. Bars denote mean MAO-A VT for BLT (green, SAD and HC pooled) and placebo (yellow, SAD and HC pooled), brackets denote standard error. Illustrated MAO-A VT is averaged for all ROIs. Effects of BLT and placebo are pooled for HC and SAD because mixed model analyses of PET1 and PET2 data revealed a two-way interaction between measurement and therapy (F1, 385.58 = 19.16, p < 0.001). PET1 fall/winter before BLT/placebo, PET2 fall/winter after BLT/placebo. **p ≤ 0.001. MAO-A VT: monoamine oxidase A distribution volume, BLT: bright light therapy, SAD: seasonal affective disorder, HC: healthy controls, 5-HT: Serotonin
Fig. 2. Brain maps of MAO-A VT before and after BLT in SAD and HC.
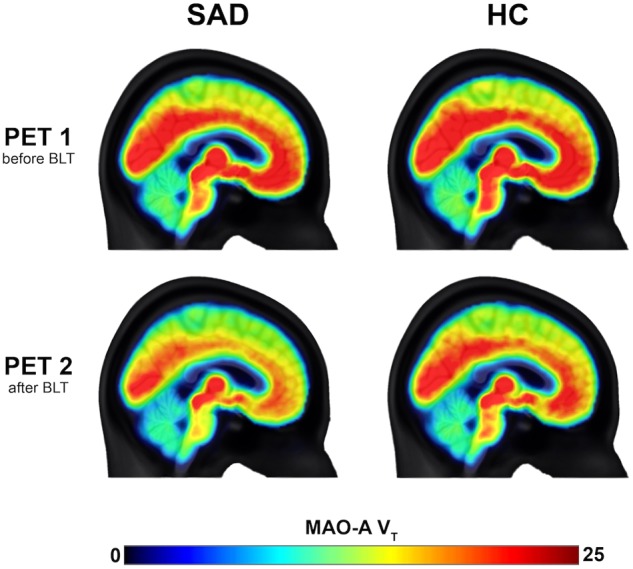
MAO-A VT was reduced significantly after BLT (F1, 208.92 = 25.96, p < 0.001), though not after placebo. MAO-A VT brain maps are shown for SAD (left) and HC (right) before and after BLT. Color bar denotes MAO-A VT. MAO-A VT: monoamine oxidase A distribution volume, BLT: bright light therapy, SAD: seasonal affective disorder, HC: healthy controls
Fig. 3. MAO-A VT decrease from fall/winter to spring/summer in HC.
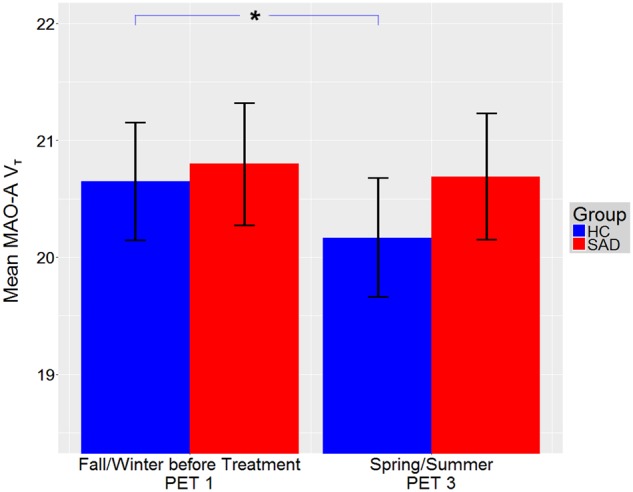
MAO-A VT decreased in HC from fall/winter (PET1, before BLT/placebo) to spring/summer (F1, 187.84 = 4.79, p = 0.030), while no change was shown in SAD. Though higher MAO-A VT in HC in fall/winter compared to spring/summer appears contrary to the hyposerotonergic hypothesis of depression, it may be reflective of results from previous studies showing that MAO-A levels are dependent on substrate availability31. Higher MAO-A VT in fall/winter may therefore be secondary to other mechanisms that increase serotonin, hereby preventing development of SAD. Bars denote mean MAO-A VT for HC (blue, BLT and placebo pooled) and SAD (red, BLT and placebo pooled), brackets denote standard error. Illustrated MAO-A VT is averaged for all ROIs. *p ≤ 0.050. MAO-A VT: monoamine oxidase A distribution volume, BLT: bright light therapy, SAD: seasonal affective disorder, HC: healthy controls
Table 2.
Monoamine oxidase A distribution volume
| ROI | SAD | HC |
|---|---|---|
| PET1 (fall/winter before BLT/placebo) | ||
| Frontal pole | 18.65 ± 4.49 | 18.61 ± 4.05 |
| Temporal pole | 18.58 ± 4.04 | 17.81 ± 3.82 |
| Anterior cingulate gyrus | 21.48 ± 4.76 | 21.08 ± 4.94 |
| Posterior cingulate gyrus | 21.30 ± 5.14 | 21.30 ± 4.52 |
| Thalamus | 24.58 ± 5.88 | 25.21 ± 6.35 |
| Caudate | 15.67 ± 3.95 | 15.44 ± 3.15 |
| Putamen | 21.36 ± 5.15 | 21.06 ± 4.55 |
| Hippocampus | 20.96 ± 5.02 | 20.70 ± 4.60 |
| Midbrain | 24.71 ± 5.79 | 24.39 ± 6.05 |
| PET2 (fall/winter after BLT) | ||
| Frontal pole | 16.81 ± 5.14 | 18.00 ± 5.13 |
| Temporal pole | 16.99 ± 5.44 | 17.25 ± 5.33 |
| Anterior cingulate gyrus | 19.65 ± 5.97 | 20.04 ± 6.56 |
| Posterior cingulate gyrus | 19.47 ± 6.22 | 20.07 ± 6.36 |
| Thalamus | 22.46 ± 6.88 | 23.84 ± 6.02 |
| Caudate | 14.39 ± 4.84 | 14.42 ± 3.94 |
| Putamen | 19.69 ± 5.97 | 20.44 ± 5.76 |
| Hippocampus | 18.95 ± 6.00 | 19.80 ± 5.69 |
| Midbrain | 22.49 ± 6.16 | 23.66 ± 7.17 |
| PET2 (fall/winter after placebo) | ||
| Frontal pole | 18.89 ± 4.17 | 19.13 ± 3.22 |
| Temporal pole | 18.33 ± 3.63 | 18.45 ± 3.77 |
| Anterior cingulate gyrus | 21.62 ± 4.60 | 22.58 ± 4.63 |
| Posterior cingulate gyrus | 21.50 ± 4.57 | 22.75 ± 4.08 |
| Thalamus | 24.79 ± 5.37 | 26.19 ± 5.01 |
| Caudate | 16.07 ± 4.07 | 16.36 ± 2.86 |
| Putamen | 21.10 ± 4.10 | 21.82 ± 4.08 |
| Hippocampus | 21.15 ± 4.42 | 21.73 ± 3.92 |
| Midbrain | 24.67 ± 5.18 | 25.12 ± 5.26 |
| PET3 (spring/summer) | ||
| Frontal pole | 18.67 ± 3.50 | 18.23 ± 3.99 |
| Temporal pole | 18.85 ± 3.73 | 17.63 ± 4.16 |
| Anterior cingulate gyrus | 22.33 ± 4.95 | 21.14 ± 5.41 |
| Posterior cingulate gyrus | 21.81 ± 4.31 | 20.88 ± 4.91 |
| Thalamus | 23.73 ± 4.71 | 24.01 ± 4.71 |
| Caudate | 14.68 ± 2.55 | 14.47 ± 3.14 |
| Putamen | 21.01 ± 3.77 | 20.44 ± 4.33 |
| Hippocampus | 20.41 ± 3.39 | 20.18 ± 3.94 |
| Midbrain | 25.36 ± 5.35 | 24.70 ±6.05 |
Monoamine oxidase A distribution volume (MAO-A VT) reported as mean ± SD. ROI: region of interest, SAD: seasonal affective disorder, HC: healthy controls, BLT: bright light therapy, PET: positron emission tomography
In mixed model analyses excluding subjects with overlapping scan dates (1 SAD, 2 HC) MAO-A VT also increased in SAD-BLT (F1, 84.63 = 22.60, p < 0.001) from PET1 to PET3. All other effects were unchanged from the main analyses.
When performed without BDI as a covariate, mixed model analysis of MAO-A VT data from all three time points revealed a two-way interaction between measurement and therapy (F2, 556.22 = 14.57, p < 0.001) as well as measurement and group (F2, 556.22 = 10.84, p < 0.001), rather than a 3-way measurement by therapy by group interaction. All other effects were unchanged from the main analyses. Mixed model analyses utilizing the difference in MAO-A VT between PET1 and PET3 as dependent variable revealed no significant differences between group or therapy arm in change to MAO-A VT between PET1 and PET3.
Discussion
In our study, cerebral MAO-A VT did not differ between SAD and HC in fall/winter before treatment, fall/winter after treatment, or in spring/summer. However, cerebral MAO-A VT decreased in HC from fall/winter (before treatment) to spring/summer, while it remained unchanged in SAD. In addition, this is the first study to demonstrate reduction of cerebral MAO-A VT after BLT, in vivo.
Our study was motivated by experiments showing increased MAO-A levels in non-seasonally depressed patients. These findings are a fundament of the hyposerotonergic hypothesis of depression4,6. Furthermore, extensive evidence demonstrates that the human serotonergic system is affected by season8,11 and light7,9,10. Seasonality of symptoms in SAD has been linked to the influence of light27 which is underscored by the efficacy of BLT3. We therefore hypothesized that the MAO-A may serve as an endophenotypic link between light, SAD symptoms and their treatment.
However, we did not find a difference in MAO-A VT between SAD and HC in fall/winter before (PET1) or after BLT treatment (PET2), or in spring/summer (PET3). On the one hand, our findings suggest that MAO-A VT is not altered in SAD. This explanation would be in accordance with previous studies that show unaltered MAO-A VT in non-seasonal MDD. However, comparability with these earlier studies is limited because they were performed in other brain regions and after suicide28. Furthermore, our patient group showed mild typical depressive symptoms, as is often the case in SAD. Therefore, the lack of a statistically significant difference in MAO-A VT between SAD and HC, in contrast to studies in MDD4, may reflect the observation that typical depressive symptoms are less severe in SAD than in non-seasonal depression29. Studies linking more pronounced symptom severity to higher MAO-A in MDD support this concept30.
Our results suggest that changes to MAO-A VT in SAD may instead be based on dysregulation of seasonal changes to cerebral expression levels. Although SAD and HC did not show cross-sectional differences in MAO-A VT, HC showed a decrease in MAO-A VT from fall/winter to spring/summer, while SAD did not. Our finding that HC, but not SAD, showed a seasonal dynamic is in line with a previous study showing SERT downregulation in fall/winter in HC, but not in SAD12. However, these results are in contrast with another study, which instead showed increased seasonal SERT variation in SAD in comparison to healthy individuals13. In our study, while HC showed seasonal change and SAD did not, the seasonal (fall/winter to spring/summer) difference in MAO-A VT did not differ between SAD and HC. In summary, these results suggest that the serotonergic pathology in SAD might rather be understood as altered dynamic regulation of expression, rather than a static change, which is in line with previous studies12,13.
In the sub-analysis without the subjects responsible for the seasonal overlap between fall/winter after treatment (PET2) and spring/summer (PET3), the SAD-BLT group also showed seasonal changes. In this group, MAO-A VT increased from fall/winter before treatment (PET1) to spring/summer (PET3). Therefore, the change in MAO-A VT was in the opposite direction as in the HC group. On the one hand, this effect might be understood as an overcompensation, after levels of the enzyme were decreased by BLT. Alternatively, this finding may reflect that BLT treatment activates dynamic regulation of MAO-A VT expression.
HC showed higher MAO-A VT in fall/winter than in spring/summer. Increased serotonergic degradation in healthy individuals appears contrary to previous studies showing increased MAO-A VT in depression. However, this pattern may be reflective of findings showing that MAO-A levels are dependent on availability of 5-HT and other substrates such as dopamine31. MAO-A levels were decreased after reduction of 5-HT via tryptophan depletion and increased after application of carbidopa–levodopa31. Based on the monoaminergic hypothesis of depression, 5-HT and other monoamine levels may be low in SAD, but not in HC6. Therefore, other mechanisms in HC may in fact elevate, or prevent reduction of, 5-HT12, though these regulatory processes are likely to be complex32. In theory, this would result in an increase in MAO-A VT31. In summary, the increased MAO-A VT seen in HC in fall/winter in comparison to spring/summer might reflect that seasonal MAO-A VT changes are secondary to other changes within the serotonergic system.
MAO-A VT decreased after BLT. Lower MAO-A VT would in theory result in a reduction in 5-HT degradation5, resulting in higher 5-HT levels and ultimately antidepressant effects. This is in analogy to the effects of monoamine oxidase inhibitors33 and other serotonergic antidepressants such as selective serotonin reuptake inhibitors6. MAO-A VT reduction was not shown after placebo, thus demonstrating that the effect is specific to BLT and not merely an effect of time. However, downregulation of MAO-A VT after BLT was not specific to the SAD group, as demonstrated by the measurement by therapy, rather than measurement by therapy by group interaction, between PET1 and PET2. Therefore, we suggest that reduction of MAO-A VT may be understood as an unspecific neurobiological correlate of intense light exposure, in this case via BLT, because the effect was not specific to underlying pathology.
Several limitations must be discussed. The BDI and the 24-item HAMD are beneficial as they encompass self- and external ratings. However, although they do assess some atypical depressive symptoms such as fatigue, not all are addressed. This factor may explain why we did not find a more pronounced association between symptom severity and MAO-A VT in SAD, particularly as MAO-A levels have previously been linked to atypical depressive symptoms30. Furthermore, BDI differed between the SAD-placebo and SAD-BLT arms before treatment. However, this point was assessed via inclusion of BDI as a covariate in the subsequent mixed model analyses of MAO-A VT. HC showed particularly low BDI and HAMD scores. As a result, the effect of BLT and season on the HC’s BDI scores may not be adequately assessed while changes to BDI and HAMD in SAD may be overestimated. For placebo treatment, a light intensity of 400 lux was chosen because this intensity was previously described as not biologically active22, albeit similar enough to verum BLT (10,000 lux) so as not to unblind treatment. However, we cannot exclude the possibility that this low intensity light has some therapeutic effect. Therefore, the difference in MAO-A VT between BLT and placebo may in fact be more pronounced. Another potential limitation is the minor overlap between the fall/winter and spring/summer seasons. This point was addressed through a sub-analysis which excluded affected subjects and replicated original results. In addition, though it is a widely utilized method, implemented in PMOD (PMOD Technologies Ltd., Zurich, Switzerland; www.pmod.com), the Logan plot may underestimate MAO-A VT at the voxel level34, an effect which is dependent on signal noise level and the extent of the VT35. However, potential bias would be circumvented by the fact that MAO-A VT was quantified utilizing the same methodology in all subjects.
In addition, if we assume that differences in MAO-A VT between SAD and HC are discrete, in accordance with the concept of SAD as a less severe sub-set of MDD, the subject size may be considered a limitation, though it is similar to previous [11C]harmine PET studies4. Along this line, the effects shown in our study were statistically significant when all regions were investigated together, but not on the individual region level. This finding may be a result of discrete differences in MAO-A VT within individual regions that are elucidated when statistical power is increased by joint evaluation of all regions.
In order to address potential variability and reduce bias in our sample, numerous clinical variables including age, sex, symptom severity, perimenopausal status and smoking20 were corrected for, either through regression analyses (age, sex, symptom severity, perimenopausal status) and subsequent inclusion as a covariate when significant (this was only the case for BDI), or through definition as an exclusion criterion (smoking). The influence of BLT on MAO-A VT, yet not placebo, as well as the seasonal effects in HC, yet not SAD, were also significant in an exploratory analysis in which BDI was not included as a covariate. This suggests that the influence of BDI on MAO-A VT that was elucidated in regression analysis is likely discrete. In addition, it emphasizes that the influence of BLT and the seasonal effects in HC are likely specific effects of light and season, and not directly related to symptom severity. Previous studies illustrate a lack of an influence of sex on MAO-A VT4, as was the case in our study. However, the MAO-A gene is encoded on the X-chromosome36 and an influence of estrogen37 and testosterone38 on MAO-A expression has been demonstrated. Increased MAO-A has been shown in perimenopause, a phase which is characterized by a drop in estrogen levels, which has been linked to depressive symptoms37. Though we corrected for various clinical factors, it is possible that the factors discussed above, such as sex, exert influence on MAO-A VT through interaction with other clinical variables that were not assessed. We base this assumption on studies which demonstrate a gender specific influence of MAO-A genotype on various clinical outcomes39,40. In addition, genetic variables that are not accounted for in this study may play a role. For example, an influence of genetics on how the serotonergic system is influenced by light has been demonstrated with the 5-HTTLPR41,42. An influence of MAO-A genotype on propensity for seasonal variation in protein expression has to our knowledge yet to be shown, but cannot be excluded. Furthermore, various components of the serotonergic system demonstrate diurnal patterns of variation11. In fact, circadian changes to MAO-A activity have been demonstrated in animal studies43. Therefore, it must be considered a limitation that not all PET measurements took place during the same time of day. However, time of day of PET measurement varied within groups (SAD, HC), treatment-arms (BLT, placebo) and measurement (PET1, PET2, PET3), which likely mitigates a potential effect of this factor on our results.
In summary, we did not find differences in cerebral MAO-A VT between SAD patients and controls when our data was assessed cross-sectionally, i.e., SAD and HC did not differ in fall/winter before treatment, after treatment or in spring/summer. The lack of a difference in MAO-A VT between these two groups, which is in contrast with previous studies in non-seasonal depression, may result from lower symptom severity in SAD in comparison to MDD29. However, HC showed a seasonal dynamic to MAO-A expression, which was not shown by SAD patients. This suggests that SAD may lack seasonal dynamics in brain MAO-A levels. This pattern is consistent with that shown in previous studies on the SERT in SAD12. We also demonstrate that BLT results in reduction in MAO-A VT, which is likely to result in an increase of 5-HT levels5, and is thus in accordance with the serotonergic hypothesis of depression and antidepressant treatment. However, this effect was not specific to the patient group, suggesting that these results reflect a general, neurobiological effect of BLT on the serotonergic system rather than a specific treatment effect.
Electronic supplementary material
Acknowledgements
We would like to thank J. Unterholzner, M.G. Godbersen and C. Kraus for clinical assistance, A. Hahn, S. Ganger and M. Kloebl for technical support, S. Avramidis and J. Jungwirth for administrative support, as well as J. Peters and K. Eineckel for data acquisition. We would also like to thank N. Berroterán-Infante for assistance with radioligand synthesis, K. Rebhan for radioligand metabolite processing, T. Zenz and A. Krcal für PET technical assistance, and I. Leitinger, H. Ibeschitz, V. Weiss, and F. Pichler for assistance with PET measurements. This work is funded by Austrian Science Fund (FWF) grants to D. Winkler (P24359) and to R. Lanzenberger (P27141, APKLI504FW).
Conflict of interest
M.S. has received travel grants from Janssen, Eli Lilly, and AOP Orphan Pharmaceuticals, speaker honoraria from Janssen and Austroplant, workshop participation from Eli Lilly, and is recipient of a 2015 NARSAD Young Investigator Grant (23741). M.H. has received financial support from the Austrian Science Fund and the Jubilee Fund of the Austrian National Bank. G.G. is recipient of a DOC Fellowship of the Austrian Academy of Sciences at the Medical University of Vienna. G.S.K. received travel grants from Roche and Pfizer. P.B.-M. received a travel grant from AOP Orphan Pharmaceuticals AG and speaker honoraria from Janssen. T.V. received travel grants and compensation for workshop participation from Pfizer and Eli Lilly and speaker honoraria from Eli Lilly. W.W. is an employee at CBmed Ltd., Graz, Austria, has received speaker honoraria from GE Healthcare, Vienna, Austria and research support from DSD, Vienna, Austria, BSM Diagnostica, Vienna, Austria, ABX, Radeberg, Germany, and Rotem GmbH, Leipzig, Germany. S.K. has received grants/research support, consulting fees and/or honoraria within the last three years from Angelini, AOP Orphan Pharmaceuticals AG, AstraZeneca, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, and Servier. R.L. has received travel grants and conference speaker honoraria from AstraZeneca, Lundbeck A/S, Roche Austria GmbH, Dr. Willmar Schwabe GmbH & Co. KG, AOP Orphan Pharmaceuticals, and Janssen-Cilag Pharma Gmbh. D.W. has received lecture fees from Angelini, Lundbeck and Pfizer. The remaining authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41398-018-0227-2).
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.) (APA, Washington, DC, 2000).
- 2.Pjrek E, et al. Epidemiology and socioeconomic impact of seasonal affective disorder in Austria. Eur. Psychiatry. 2016;32:28–33. doi: 10.1016/j.eurpsy.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Golden RN, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am. J. Psychiatry. 2005;162:656–662. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- 4.Meyer JH, et al. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch. Gen. Psychiatry. 2006;63:1209–1216. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- 5.Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu. Rev. Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spies M, Knudsen GM, Lanzenberger R, Kasper S. The serotonin transporter in psychiatric disorders: insights from PET imaging. Lancet Psychiatry. 2015;2:743–755. doi: 10.1016/S2215-0366(15)00232-1. [DOI] [PubMed] [Google Scholar]
- 7.Spindelegger C, et al. Light-dependent alteration of serotonin-1A receptor binding in cortical and subcortical limbic regions in the human brain. World J. Biol. Psychiatry. 2012;13:413–422. doi: 10.3109/15622975.2011.630405. [DOI] [PubMed] [Google Scholar]
- 8.Praschak-Rieder N, Willeit M, Wilson AA, Houle S, Meyer JH. Seasonal variation in human brain serotonin transporter binding. Arch. Gen. Psychiatry. 2008;65:1072–1078. doi: 10.1001/archpsyc.65.9.1072. [DOI] [PubMed] [Google Scholar]
- 9.Harrison SJ, et al. Light therapy and serotonin transporter binding in the anterior cingulate and prefrontal cortex. Acta Psychiatr. Scand. 2015;132:379–388. doi: 10.1111/acps.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyrer AE, et al. Serotonin transporter binding is reduced in seasonal affective disorder following light therapy. Acta Psychiatr. Scand. 2016;134:410–419. doi: 10.1111/acps.12632. [DOI] [PubMed] [Google Scholar]
- 11.Matheson GJ, et al. Diurnal and seasonal variation of the brain serotonin system in healthy male subjects. NeuroImage. 2015;112:225–231. doi: 10.1016/j.neuroimage.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Mc Mahon B, et al. Seasonal difference in brain serotonin transporter binding predicts symptom severity in patients with seasonal affective disorder. Brain. 2016;139:1605–1614. doi: 10.1093/brain/aww043. [DOI] [PubMed] [Google Scholar]
- 13.Tyrer AE, et al. Increased seasonal variation in serotonin transporter binding in seasonal affective disorder. Neuropsychopharmacology. 2016;41:2447–2454. doi: 10.1038/npp.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lingjaerde O, et al. Treatment of winter depression in Norway. II. A comparison of the selective monoamine oxidase A inhibitor moclobemide and placebo. Acta Psychiatr. Scand. 1993;88:372–380. doi: 10.1111/j.1600-0447.1993.tb03476.x. [DOI] [PubMed] [Google Scholar]
- 15.Ginovart N, et al. Positron emission tomography quantification of [11C]-harmine binding to monoamine oxidase-A in the human brain. J. Cereb. Blood Flow Metab. 2006;26:330–344. doi: 10.1038/sj.jcbfm.9600197. [DOI] [PubMed] [Google Scholar]
- 16.Bergstrom M, Westerberg G, Langstrom B. 11C-harmine as a tracer for monoamine oxidase A (MAO-A): in vitro and in vivo studies. Nucl. Med. Biol. 1997;24:287–293. doi: 10.1016/s0969-8051(97)00013-9. [DOI] [PubMed] [Google Scholar]
- 17.Tweedie DJ, Burke MD. Metabolism of the beta-carbolines, harmine and harmol, by liver microsomes from phenobarbitone- or 3-methylcholanthrene-treated mice. Identification and quantitation of two novel harmine metabolites. Drug Metab. Dispos. 1987;15:74–81. [PubMed] [Google Scholar]
- 18.Meyer JH, et al. Brain monoamine oxidase A binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch. Gen. Psychiatry. 2009;66:1304–1312. doi: 10.1001/archgenpsychiatry.2009.156. [DOI] [PubMed] [Google Scholar]
- 19.Raheja SK, King EA, Thompson C. The seasonal pattern assessment questionnaire for identifying seasonal affective disorders. J. Affect. Disord. 1996;41:193–199. doi: 10.1016/s0165-0327(96)00087-0. [DOI] [PubMed] [Google Scholar]
- 20.Fowler JS, et al. Brain monoamine oxidase A inhibition in cigarette smokers. Proc. Natl Acad. Sci. USA. 1996;93:14065–14069. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Praschak-Rieder N, Willeit M. Treatment of seasonal affective disorders. Dialog. Clin. Neurosci. 2003;5:389–398. doi: 10.31887/DCNS.2003.5.4/npraschakrieder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brainard GC, et al. Sensitivity of the human circadian system to short-wavelength (420-nm) light. J. Biol. Rhythms. 2008;23:379–386. doi: 10.1177/0748730408323089. [DOI] [PubMed] [Google Scholar]
- 23.Philippe C, et al. Parameter evaluation and fully-automated radiosynthesis of [(11)C]harmine for imaging of MAO-A for clinical trials. Appl. Radiat. Isot. 2015;97:182–187. doi: 10.1016/j.apradiso.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savli M, et al. Normative database of the serotonergic system in healthy subjects using multi-tracer PET. Neuroimage. 2012;63:447–459. doi: 10.1016/j.neuroimage.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molin J, Mellerup E, Bolwig T, Scheike T, Dam H. The influence of climate on development of winter depression. J. Affect. Disord. 1996;37:151–155. doi: 10.1016/0165-0327(95)00090-9. [DOI] [PubMed] [Google Scholar]
- 28.Ordway GA, et al. Quantitative distribution of monoamine oxidase A in brainstem monoamine nuclei is normal in major depression. Brain Res. 1999;847:71–79. doi: 10.1016/s0006-8993(99)02043-0. [DOI] [PubMed] [Google Scholar]
- 29.Thalen BE, Kjellman BF, Morkrid L, Wetterberg L. Seasonal and non-seasonal depression. A comparison of clinical characteristics in Swedish patients. Eur. Arch. Psychiatry Clin. Neurosci. 1995;245:101–108. doi: 10.1007/BF02190736. [DOI] [PubMed] [Google Scholar]
- 30.Chiuccariello L, et al. Elevated monoamine oxidase a binding during major depressive episodes is associated with greater severity and reversed neurovegetative symptoms. Neuropsychopharmacology. 2014;39:973–980. doi: 10.1038/npp.2013.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacher J, et al. Dynamic, adaptive changes in MAO-A binding after alterations in substrate availability: an in vivo [(11)C]-harmine positron emission tomography study. J. Cereb. Blood Flow Metab. 2012;32:443–446. doi: 10.1038/jcbfm.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert GW, Reid C, Kaye DM, Jennings GL, Esler MD. Effect of sunlight and season on serotonin turnover in the brain. Lancet. 2002;360:1840–1842. doi: 10.1016/s0140-6736(02)11737-5. [DOI] [PubMed] [Google Scholar]
- 33.Miura H, Naoi M, Nakahara D, Ohta T, Nagatsu T. Effects of moclobemide on forced-swimming stress and brain monoamine levels in mice. Pharmacol. Biochem. Behav. 1996;53:469–475. doi: 10.1016/0091-3057(95)02019-5. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, et al. A consistent and efficient graphical analysis method to improve the quantification of reversible tracer binding in radioligand receptor dynamic PET studies. Neuroimage. 2009;44:661–670. doi: 10.1016/j.neuroimage.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slifstein M, Laruelle M. Effects of statistical noise on graphic analysis of PET neuroreceptor studies. J. Nucl. Med. 2000;41:2083–2088. [PubMed] [Google Scholar]
- 36.Pintar JE, et al. Gene for monoamine oxidase type A assigned to the human X chromosome. J. Neurosci. 1981;1:166–175. doi: 10.1523/JNEUROSCI.01-02-00166.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rekkas PV, et al. Greater monoamine oxidase a binding in perimenopausal age as measured with carbon 11-labeled harmine positron emission tomography. JAMA Psychiatry. 2014;71:873–879. doi: 10.1001/jamapsychiatry.2014.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purves-Tyson TD, et al. Testosterone regulation of sex steroid-related mRNAs and dopamine-related mRNAs in adolescent male rat substantia nigra. BMC Neurosci. 2012;13:95. doi: 10.1186/1471-2202-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verhoeven FE, et al. The effects of MAOA genotype, childhood trauma, and sex on trait and state-dependent aggression. Brain Behav. 2012;2:806–813. doi: 10.1002/brb3.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Priess-Groben HA, Hyde JS. 5-HTTLPR X stress in adolescent depression: moderation by MAOA and gender. J. Abnorm. Child Psychol. 2013;41:281–294. doi: 10.1007/s10802-012-9672-1. [DOI] [PubMed] [Google Scholar]
- 41.Rosenthal NE, et al. Role of serotonin transporter promoter repeat length polymorphism (5-HTTLPR) in seasonality and seasonal affective disorder. Mol. Psychiatry. 1998;3:175–177. doi: 10.1038/sj.mp.4000360. [DOI] [PubMed] [Google Scholar]
- 42.Kalbitzer J, Kalbitzer U, Knudsen GM, Cumming P, Heinz A. How the cerebral serotonin homeostasis predicts environmental changes: a model to explain seasonal changes of brain 5-HTT as intermediate phenotype of the 5-HTTLPR. Psychopharmacology. 2013;230:333–343. doi: 10.1007/s00213-013-3308-1. [DOI] [PubMed] [Google Scholar]
- 43.Chevillard C, Barden N, Saavedra JM. Twenty-four hour rhythm in monoamine oxidase activity in specific areas of the rat brain stem. Brain Res. 1981;223:205–209. doi: 10.1016/0006-8993(81)90825-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


