Abstract
Background
Inflammatory bowel diseases (IBD) and asthma share genetic and environmental risk factors. Consequently, several observational studies have explored an association between IBD and asthma. We systematically reviewed and summarized the literature on the co-occurrence of asthma and IBD.
Methods
MEDLINE and EMBASE (to April 2017) were searched to identify observational studies on the association between asthma and IBD. Relative risks (RR) were pooled using random effects models. Heterogeneity was assessed using the I2 and Cochran Q statistics. Meta-regression based on study design, source of patients (population-based vs. tertiary-care center) and study location was conducted to explain between-study heterogeneity.
Results
Eighteen studies were identified (15 Crohn’s disease, 15 ulcerative colitis (UC)). Asthma was associated with both Crohn’s disease (pooled RR 1.30, 95% confidence interval (CI) 1.16–1.47, I2 = 88%) and UC (RR 1.34, 95% CI 1.24–1.44, I2 = 93%). The study design and source of patients and study location explained between-study heterogeneity in Crohn’s disease, but not UC.
Conclusion
Asthma is associated with both Crohn’s disease and UC. Additional research is needed to determine if one disease influences the risk of developing the other or if the frequent co-occurrence of these diseases result from shared genetic, environmental, and microbial risk factors.
Background
Inflammatory bowel disease (IBD) and asthma are both immune-mediated diseases that may be rooted in common pathology, as well as shared genetic and environmental risk factors. Aberrant epithelial barrier function in the lung and gastrointestinal tract, as well as abnormal immune responses to environmental factors and pathogens characterize asthma and IBD1–7. The ‘hygiene hypothesis’ has been proposed for both IBD and asthma, and postulates that children growing up in relatively sterile environments are more likely to develop chronic immune-mediated diseases later in life. Moreover, disruption of early life intestinal microbiota may exacerbate risk of disease development. For example, asthma and IBD are more common among individuals exposed to antibiotics early in life, while breastfeeding decreases the risk of both diseases8–10. Lack of exposure to enteric pathogens early in life may increase the risk of developing immune-mediated diseases, including both asthma and IBD11,12. IBD and asthma also share susceptibility genes, including SMAD3 and IL-23R13–15. Both IBD and asthma have become increasingly common in the Western world during the 20th century and these trends are now being echoed in developing countries16–20.
A previous systematic review and meta-analysis found that individuals with asthma were more likely to have a co-occurring gastrointestinal or urinary condition, but did not specifically evaluate the association between asthma and IBD21. There is growing epidemiologic evidence that asthma and IBD frequently co-occur22–29 and a family history of one disease may influence the risk of developing the other30,31. However, studies evaluating the association between these two diseases have used heterogeneous study designs. Specifically, studies have differed in terms of (1) the relative timing of the two diagnoses and (2) the age of participants included in the study. These two factors have resulted in differing conclusions about the co-occurrence of asthma and IBD.
We conducted a systematic review and meta-analysis to summarize and quantify the association between IBD and asthma, as well as evaluating the impact of study and patient characteristics on the association between these two diseases.
Methods
This systematic review is based on a previously registered protocol32 and is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines33.
Study identification and selection
We searched MEDLINE, including articles available as online only ahead of print, (1946–1 April 2017) and EMBASE + EMBASE Classic (1947–1 April 2017) for observational epidemiologic studies evaluating the association between asthma and IBD (Crohn’s disease, ulcerative colitis (UC), and IBD-type unclassified (IBD-U)). Search terms for IBD and asthma were grouped using the Boolean operator “AND.” The complete search strategy is outlined in Supplementary Table 1. In addition, we hand-searched: (1) references of included studies and relevant review articles; (2) conference abstracts for major gastroenterology meetings (Digestive Diseases Week, American College of Gastroenterology, and United European Gastroenterology Week) and major thoracic meetings (American Thoracic Society Meeting, European Respiratory Society, and American Academy of Chest Physicians Conference) from 2013 to 2017. The review of abstracts and articles identified for full-text review was conducted via crowd sourcing using CrowdScreen SR34. Crowdsourcing has previously been shown to increase the efficiency of the systematic review process, while maintaining high accuracy during the review process34,35. Prior to screening, each member of the CrowdScreen SR Review team was asked to review a test set of 15 abstracts identified by the principal investigator (MEK). The test set included a mix of abstracts that should be included in full-text review and abstracts that should be excluded from further review. Feedback was provided on the accuracy of their initial reviews to enhance performance. Members of the review team were medical students or undergraduate/graduate students in health sciences and all completed the initial test set with a high degree of accuracy. Each abstract and full-text was reviewed independently by at least two members of the review team. Discrepancies for both the abstract and full-text review stages were resolved by MEK.
Studies eligible for inclusion were observational epidemiologic studies (case-control, cohort, or cross-sectional studies) that either compared the rate of asthma in patients with and without IBD or the rate of IBD in patients with and without asthma. No restrictions were placed on date of publication, language, or region of study. Studies reporting on any subtype of IBD (Crohn’s disease, UC, or IBD-U) were included. Case reports and case series were excluded, as were studies reporting on the association between pulmonary function tests or other respiratory illnesses with IBD. If multiple studies reported on the same cohort of patients, the study with the most complete cohort of patients was selected for inclusion in the study.
Data extraction and risk of bias
The following information was extracted from included studies independently by two investigators (MEK, KB) using a piloted data extraction form in REDCap electronic data capture tools hosted at the Children’s Hospital of Eastern Ontario:36 study characteristics, including the country and years in which the study was conducted; study design; method of identifying and recruiting individuals to participate in the study; the association between asthma and IBD (crude and adjusted, where possible, and the confounders adjusted for in the model); definitions used to identify and/or confirm cases of asthma and IBD; the age of study participants; the timing of exposure relative to the study outcome; and characteristics of individuals included in each study. Discrepancies were resolved by EIB. The risk of bias in individual studies was determined using the Newcastle-Ottawa Scale37.
Study design and outcomes
The primary outcomes of our meta-analysis were the association between asthma and either Crohn’s disease, UC, or IBD-U. All analyses were conducted separately for Crohn’s disease, UC, and IBD-U. For the primary analysis, no restriction was placed on the timing of one diagnosis relative to the other.
Sensitivity analyses evaluating the temporal associations between asthma and IBD were conducted. Specifically, we conducted two sensitivity analyses in which included studies were limited to (1) those in which the diagnosis of asthma preceded the diagnosis with IBD; and (2) those in which the diagnosis of IBD preceded the diagnosis with asthma. Subgroup analyses were conducted based on the age of diagnosis of IBD, defined according to the Montreal classification (pediatric-onset: ≤16, young adult-onset: 17–40, and older adult-onset: >40)38 and/or asthma.
Statistical analysis
Analyses were conducted separately for the association between asthma and (1) Crohn’s disease; (2) UC; and (3) IBD-U. Relative risks (RR) and their 95% confidence intervals (CI) were pooled to estimate the association between asthma and IBD. Random effects models were used to account for expected heterogeneity across study designs. The most adjusted estimate was used. Odds ratios were assumed to approximate the RR due to the rare prevalence of IBD and asthma. We performed a sensitivity analysis separating case-control from cohort studies.
Between-study heterogeneity was assessed using the I2 statistic and the Cochran Q statistic with p < 0.1 being considered statistically significant. Meta-regression was conducted to explore sources of study heterogeneity based on the source of patients involved in the study (population-based vs. recruited from tertiary-care center) and the country in which the study was conducted.
All statistical analyses were conducted using the meta and metafor packages in R Software version 3.4.239–41.
Results
Description of included studies
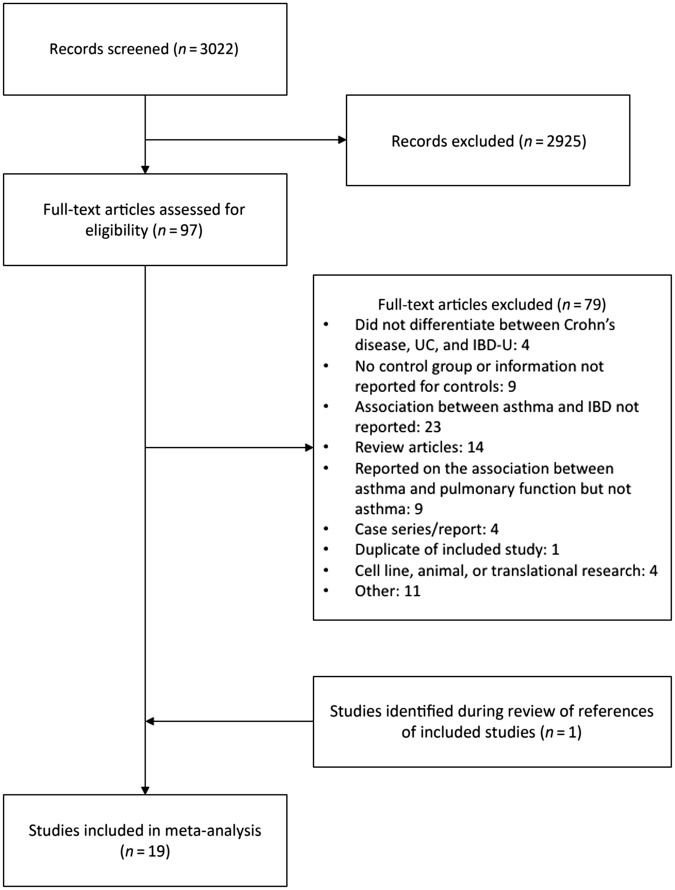
There were 3975 citations identified from the search of MEDLINE and EMBASE. After removing duplicates, 3022 references remained. Of the 97 studies identified for full-text review, 18 of these were included in the meta-analysis (Fig. 1). One additional study was identified after reviewing references of included studies. Fifteen studies reported the association between asthma and Crohn’s disease22–24,26–29,42–49. Sixteen studies reported the association between asthma and UC22–24,26–30,42–44,48–52. One study reported on the association between asthma and IBD-U27. Characteristics of included studies are described in Table 1.
Fig. 1. PRISMA flow diagram.
.
Table 1.
Characteristics of included studies
| Study | Country | Study design | Data source (years of study) | Definition of IBD | Definition of asthma | Relative timing of diagnoses | Type of IBD | Matched variables and covariates | Age of participants | Sample size |
|---|---|---|---|---|---|---|---|---|---|---|
| Bernstein23 | Canada | Matched case-control | Provincial health administrative data (1984–2003) | Validated algorithm | ≥5 health care contacts for asthma | Asthma diagnosed before or after IBD | CD, UC | Matched on age, sex, and rural/urban residence | Pediatric and adult | IBD: 8072 Controls: 80,489 |
| Boneberger52 | Chile | Case-control | Tertiary-care center (2009–2010) | Routine clinical practice | Not specified | Not specified | UC | Adjusted for age and sex | Range: 6 to 45 years at study entry | IBD: 52 Controls: 174 |
| Brassard42 | Canada | Retrospective cohort | Provincial health administrative data (2001–2006) | Externally validated algorithm | ≥3 prescriptions for respiratory medication within 1 year, on at least two separate occasions; the third prescription must have occurred at ≤40 years of age | Asthma diagnosed before IBD | CD, UC | Incidence rates were standardized based on age and sex | ≤40 years at asthma diagnosis | Asthma: 136,178a |
| D’Arienzo50 | Italy | Case-control | Tertiary-care center (1998) | Standard diagnostic criteria | Standard diagnostic criteria | Not specified | UC | None | Range: 16 to 69 years | IBD: 50 Controls: 50 |
| D’Arienzo51 | Italy | Matched case-control | Tertiary-care center (2000) | Standard diagnostic criteria | Standard diagnostic criteria | Not specified | UC | Matched controls were partners of cases | Adult | IBD: 45 Controls: 37 |
| Gearry24 | New Zealand | Frequency matched case-control | Canterbury IBD Study (2003–2005) | Standard diagnostic criteria | Not specified | Not specified | CD, UC | Frequency matched based on age (at recruitment) and sex. Adjusts for family history of IBD, smoking status, age, social class at birth, sex, and ethnicity | Adult | IBD: 1291 Controls: 600 |
| Hammer43 | United Kingdom | Matched case-control | Tertiary-care center (1952–1965) | Standard diagnostic criteria | Questionnaire or interview | Not specified | Colonic CD, UC | None | Not specified | IBD: 243 Controls: 319 |
| Hemminki28 | Sweden | Prospective cohort | National health administrative data (1964–2007) | Hospitalization with an ICD code for IBD | Hospitalization with an ICD code for asthma | IBD diagnosed after asthma | CD, UC | Standardized incidence rates used expected number of cases based on age, sex, year, region, and socioeconomic status | Children and adults | Asthma: 148,295c |
| Kappelman44 | United States | Matched case-control | PharMetrics Patient-Centric Database (2003–2004) | Validated algorithm | ICD code for asthma | Not specified | CD, UC | Matched on age, sex, health plan type, and geographic region | Children only | IBD: 1242 Controls: 3353 |
| Kuenzig22 | Canada | Case-control | Population-based health administrative data (1994–2010) | Validated algorithm | Externally validated algorithm | 1. Asthma before IBD 2. Asthma diagnosed before or after IBD |
CD, UC | Age, sex, rural/urban residence, socioeconomic status | Children and adults | IBD: 5464 Controls: 402,800 |
| Livnat45 | Israel | Case-control | Tertiary-care center (2008–2009b) | Standard diagnostic criteria | Self-report | Not specified | CD | None | Children and young adults | IBD: 23 Controls: 24 |
| Myrelid46 | Sweden | Matched case-control | Cases: Unclear controls: Southeastern Region Population Registry (2000) | Standard diagnostic criteria | Self-report | Not specified | CD | Age, gender, place of residence, and other atopic manifestations (allergic rhinitis, eczema) | Range: 18 to 50 years | IBD: 275 Controls: 777 |
| Nakamura30 | Japan | Matched case-control | Cases: Patients receiving financial aid from the Japanese government for the treatment of UC (1988–1990) Controls: Patients on the roster of a health check-up program | Diagnosis of UC by treating physician, and confirmation of diagnosis by independent group for approval of financial aid | Self-report questionnaire | Asthma before IBD | UC | Matched on age and sex | Children and adults | IBD: 384 Controls: 384 |
| Neilly47 | Scotland | Matched case-control | Not specified | Not specified | Previous physician-diagnosed asthma and/or history of persistent or episodic wheeze with breathlessness, responsive to bronchodilator therapy | Not specified | CD | Matched on age, sex, and smoking history | Adults | IBD: 29 Controls: 29 |
| Peng29 | Taiwan | Frequency matched retrospective cohort | National health administrative data (2000–2011) | ICD codes for IBD | ICD code for asthma and treated with inhaled corticosteroids, systemic corticosteroids, or inhaled short-acting ß2 agonists | Asthma diagnosed after IBD | CD, UC | Frequency matched on age, sex, and index year. Adjusted for age, sex, and other comorbidities (rhinitis, chronic sinusitis, atopic dermatitis, and chronic obstructive pulmonary disease) | Adults | IBD: 319 Controls: 807 |
| Pugh48 | UK | Matched case-control | Tertiary care center; Ileostomy Association | Standard diagnostic criteria | Self-report | Not specified | CD, UC | Some controls were partners of cases. Others were matched to cases based on age and sex | Not specified | IBD: 500 Controls: 500 |
| Raj49 | UK | Cohort | Tertiary-care center (1995–2005) | Standard diagnostic criteria | Consistent clinical picture with objective evidence of variable outflow obstruction and/or airway hyper-responsiveness | IBD preceded the onset of respiratory disease in all cases but 1 | CD, UC | None | Mean age: • UC: 61 • CD: 60 |
Asthma: 893a |
| Virta26 | Finland | Matched case-control | National health administrative data (1994–2010) | Received special reimbursement for IBD | Received special reimbursement for asthma | Asthma diagnosed before IBD | CD, UC | Matched on date of birth, sex, and place of residence | Pediatric | IBD: 595 Controls: 2380 |
| Weng27 | United States | Matched case-control | Kaiser Permanente Medical Care Program (1996–2005) | ≥2 inpatient or outpatient ICD codes for IBD | ≥2 inpatient or outpatient ICD codes for asthma | Asthma could be diagnosed either before or after the diagnosis with IBD | CD, UC, IBD-U | Matched on age, sex, and length of enrolment in health maintenance organization. Adjusted for smoking. | Children and adults | IBD: 12,601 Controls: 50,404 |
CD Crohn’s disease, IBD inflammatory bowel disease, IBD-U inflammatory bowel disease type unclassified, ICD International Classification of Diseases, UC ulcerative colitis
aThe risk of IBD in patients with asthma was compared to the previously reported risk of IBD in the general population
bYears of study obtained from study protocol on clinicaltrials.gov
cThe risk of IBD in patients with asthma was compared to the number of cases that would be expected if there was no association between asthma and IBD
Risk of bias of included studies
The risk of bias among included case-control and cohort studies is summarized in Supplementary Tables 3 and 4, respectively. The majority of studies were conducted using population-based health administrative data or tertiary-care studies enrolling consecutive patients. Bias may have been introduced to case-control studies based on the controls included in the studies: one study included hospital staff50, one included partners of cases51, four included hospitalized patients or patients visiting a clinic for reasons not related to IBD or asthma30,43,45,52, and one included a mix of partners of cases and non-IBD patients48. Nine case-control studies did not explicitly report that controls did not have IBD24,30,43,45–48,52,53. Two of the four cohort studies included compared the frequency with which asthma and IBD co-occurred to previously published estimates of the rate of either asthma or IBD in the general population42,49.
Association between asthma and IBD
Asthma and Crohn’s disease were associated (pooled RR 1.31, 95% CI 1.16 –1.47, 15 studies, 824,173 participants; heterogeneity: I2 = 88%, p < 0.0001; Fig. 2). There was a significant association between UC and asthma (pooled RR 1.30, 95% CI 1.21–1.40, 16 studies, 819,714 participants; heterogeneity: I2 = 93%, p < 0.0001; Fig. 3). Asthma and IBD-U were associated in a single study (OR 1.9, 95% CI 1.6–2.4, 51,459 participants)27.
Fig. 2. Association between asthma and Crohn’s disease.
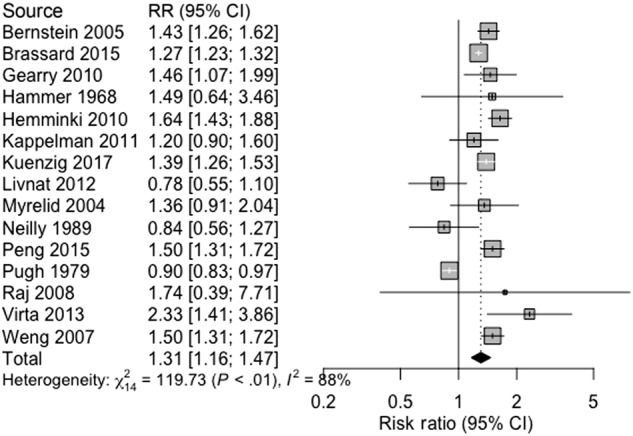
.
Fig. 3. Association between asthma and ulcerative colitis.
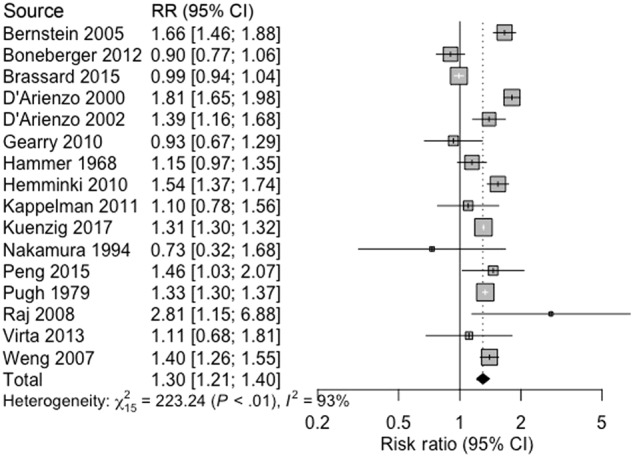
.
Explaining heterogeneity: study design
The association between asthma and IBD was consistently elevated in both case-control and cohort studies (Table 2). Although 22% of heterogeneity was accounted for by study design in studies evaluating the association between asthma and UC, significant heterogeneity persisted (residual heterogeneity: I2 = 91%, p < 0.0001). Separately analyzing case-control and cohort studies in Crohn’s disease did not account for any heterogeneity (residual heterogeneity: I2 = 88%, p < 0.0001).
Table 2.
Results of subgroup analyses and meta-regression based on study design
| Type of IBD | Study design | RR (95% CI) | Heterogeneity | Number of studies | Number of participants | Subgroup differences | Residual heterogeneity |
|---|---|---|---|---|---|---|---|
| Crohn’s disease | Case-control | 1.25 (1.05–1.50) | I2 = 89% | 11 | 513,008 | p = 0.24 REF |
I2 = 69% R2 = 0% p < 0.0001 |
| Cohort | 1.45 (1.23–1.71) | I2 = 82% | 4 | 311,165 | β 1.6 (95% CI−0.14 to 0.46) | ||
| Ulcerative colitis | Case-control | 1.33 (1.24–1.42) | I2 = 89% | 12 | 512,807 | p = 0.78 REF |
I2 = 91% R2 = 22% p < 0.0001 |
| Cohort | 1.40 (0.99–1.98) | I2 = 94% | 4 | 306,907 | β −0.046 (95% CI −0.21 to 0.12) |
Explaining heterogeneity: data source
Among studies evaluating the association between asthma and Crohn’s disease, there was a significantly elevated association in population-based studies (pooled RR 1.43, 95% CI 1.32–1.56, 10 studies, 822,775 participants; heterogeneity: I2 = 69%) but a protective association in studies recruiting patients from tertiary-care centers (pooled RR 0.89, 95% CI 0.83–0.97, 5 studies, 1398 participants; heterogeneity: I2 = 0%). Meta-regression based on source of patients (population-based or tertiary-care) accounted for 77% of heterogeneity among studies evaluating the association between asthma and Crohn’s disease. However, significant heterogeneity between studies remained (residual heterogeneity: I2 = 59%, p < 0.0001). No heterogeneity could be accounted for in studies evaluating the association between asthma and ulcerative colitis (residual heterogeneity: I2 = 94%, p < 0.0001). Results of meta-regression and subgroup analyses based on source of patients are summarized in Table 3.
Table 3.
Results of subgroup analyses and meta-regression based on source of study participants
| Type of IBD | Data source | RR (95% CI) | Heterogeneity | Number of studies | Number of participants | Subgroup differences | Residual heterogeneity |
|---|---|---|---|---|---|---|---|
| Crohn’s disease | Population-based or health maintenance organization | 1.43 (1.32–1.56) | I2 = 69% | 10 | 822,775 | p < 0.0001 REF |
I2 = 59% R2 = 77% p = 0.0024 |
| Tertiary-care center, not stated, or other | 0.89 (0.83–0.97) | I2 = 0% | 5 | 1398 | β −0.48 (95% CI −0.65 to −0.30) | ||
| Ulcerative colitis | Population-based or health maintenance organization | 1.29 (1.13–1.46) | I2 = 94% | 9 | 816,528 | p = 0.94 REF |
I2 = 94% R2 = 0% p < 0.0001 |
| Tertiary-care center, not stated, or other | 1.30 (1.08–1.56) | I2 = 92% | 7 | 3186 | β 0.012 (95% CI −0.20 to 0.22) |
Explaining heterogeneity: location of study
Differences across countries were observed in the association between asthma and Crohn’s disease (p < 0.0001). Between country differences accounted for 92% of the heterogeneity across case-control studies (residual heterogeneity: I2 = 33%, p = 0.16) evaluating the association between asthma and Crohn’s disease (Table 4).
Table 4.
Results of subgroup analyses and meta-regression based on study location
| Type of IBD | Country | RR (95% CI) | Heterogeneity | Number of studies | Number of participants | Subgroup differences | Residual heterogeneity |
|---|---|---|---|---|---|---|---|
| Crohn’s Disease | Canada | 1.34 (1.24–1.45) | I2 = 64% | 3 | 588,067 | p < 0.0001 REF |
I2 = 33% R2 = 92% p = 0.16 |
| Finland | 2.33 (1.41–3.86) | – | 1 | 1165 | β 0.55 (0.036 to 1.07) | ||
| Israel | 0.78 (0.55–1.10) | – | 1 | 47 | β −0.54 (−0.91 to −0.17) | ||
| New Zealand | 1.46 (1.07–1.99) | – | 1 | 1238 | β 0.087 (−0.25 to 0.42) | ||
| Sweden | 1.61 (1.41–1.83) | I2 = 0% | 2 | 148,347 | β 0.18 (0.0014 to 0.35) | ||
| Taiwan | 1.50 (1.31–1.72) | – | 1 | 25,799 | β 0.11 (−0.071 to 0.30) | ||
| United Kingdom | 0.90 (0.83–0.97) | I2 = 0% | 4 | 1351 | β −0.39 (−0.54 to −0.25) | ||
| United States | 1.39 (1.13–1.71) | I2 = 47% | 2 | 57,159 | β 0.062 (−0.10 to 0.23) | ||
| Ulcerative colitis | Canada | 1.28 (1.03–1.60) | I2 = 98% | 3 | 583,902 | p < 0.0001 REF |
I2 = 96% R2 = 0% p < 0.0001 |
| Chile | 0.90 (0.77–1.06) | – | 1 | 226 | β −0.35 (−0.80 to 0.099) | ||
| Finland | 1.11 (0.68–1.81) | – | 1 | 1810 | β −0.14 (−0.79 to 0.50) | ||
| Italy | 1.61 (1.25–2.07) | I2 = 84% | 2 | 182 | β 0.22 (−0.13 to 0.57) | ||
| Japan | 0.73 (0.32–1.68) | – | 1 | 768 | β −0.56 (−1.50 to 0.37) | ||
| New Zealand | 0.93 (0.67–1.29) | – | 1 | 1253 | β −0.32 (−0.85 to 0.21) | ||
| Sweden | 1.54 (1.37–1.74) | – | 1 | 148,295 | β 0.18 (−0.26 to 0.62) | ||
| Taiwan | 1.46 (1.03–2.07) | – | 1 | 21,541 | β 0.13 (−0.42 to 0.68) | ||
| United Kingdom | 1.29 (1.09–1.54) | I2 = 66% | 3 | 2010 | β 0.031 (−0.31 to 0.37) | ||
| United States | 1.32 (1.07–1.62) | I2 = 41% | 2 | 59,727 | β 0.0018 (−0.37 to 0.37) |
Similarly, differences between countries were noted for the association between asthma and UC (p < 0.0001; Table 4). However, no heterogeneity could be accounted for by country (residual heterogeneity: I2 = 96%; p < 0.0001).
Sensitivity analysis: relative timing of diagnoses
When restricting the analysis to studies in which the diagnosis of asthma preceded the diagnosis of Crohn’s disease, there was a significant association (pooled RR 1.49, 95% CI 1.27–1.74, 4 studies, 691,525 participants; heterogeneity: I2 = 86%, p < 0.0001; Supplementary Figure 1). In studies in which the diagnosis of asthma preceded the diagnosis of UC, the two diseases were not significantly associated (pooled RR 1.21, 95% CI 0.98–1.51, 5 studies, 692,228 participants; I2 = 97%, p < 0.0001; Supplementary Figure 2).
Patients previously diagnosed with both Crohn’s disease and UC were at an increased risk of new-onset asthma in a single study (Crohn’s disease: hazard ratio (HR) 1.50, 95% CI 1.31–1.72; UC: HR 1.46, 95% CI 1.03–2.07)29.
Subgroup analysis: age at IBD diagnosis
The impact of age at IBD diagnosis on the association between asthma and IBD was evaluated in three studies: one case-control study limited to the association between asthma and UC (408,264 participants)22, and one cohort study evaluating the association between asthma and both Crohn’s disease and UC (136,178 participants)42, and one cohort study evaluating the association between asthma and IBD (26,300 participants)29. An additional three studies were limited to pediatric patients and were included in subgroup analyses of pediatric-onset IBD (7554 participants)26,44,45. There was no association between asthma and pediatric-onset Crohn’s disease (pooled RR 1.35, 95% CI 0.94–1.93; heterogeneity: I2 = 92%, Supplementary Figure 3). Adult-onset Crohn’s disease was associated with asthma (20–29 years at diagnosis: incident rate ratio (IRR) 1.18, 95% CI 1.10–1.26; 30–39 years: IRR 1.42, 95% CI 1.32–1.53; 40–49 years: IRR 1.31, 95% CI 1.23–1.41). Pooling across age groups in early adulthood (20–29 and 30–39 years), Crohn’s disease and asthma were associated in adults diagnosed in young adulthood (pooled RR 1.29, 95% CI 1.08–1.55; heterogeneity: I2 = 92%). Meta-regression based on age group did not account for any heterogeneity (residual heterogeneity: I2 = 92%, p < 0.0001).
Asthma and UC were associated in patients diagnosed with UC as young adults (≤40 years: pooled RR 1.11, 95% CI 1.04–1.19; I2 = 0%; Supplementary Figure 4). There was no association between asthma and UC diagnosed during childhood (pooled RR 1.11, 95% CI 0.97–1.28; I2 = 0%) or after 40 years of age (pooled RR 1.07, 95% CI 0.57–2.00; I2 = 98%). Among studies analyzing the association between asthma and UC in those diagnosed as older adults, one study suggested there was an increased association while the other identified a protective association. Significant heterogeneity persisted following meta-regression based on age groups (residual heterogeneity: I2 = 87%, p < 0.0001).
Discussion
This systematic review and meta-analysis suggests the frequent co-occurrence of asthma with both Crohn’s disease and UC. IBD has also been associated with other respiratory disorders (e.g., chronic obstructive pulmonary disease)42,54. Further, both asthma and IBD have been associated with other immune-mediated and atopic conditions, including multiple sclerosis, rheumatoid arthritis, diabetes, eczema, and rhinitis28,44,46,48,55–59. However, there was a high degree of heterogeneity between studies, suggesting that the association between asthma and IBD may vary across populations and be impacted by differences in study methodology.
The association between Crohn’s disease and asthma was consistent regardless of the relative timing of the diagnoses of the two diseases (i.e., asthma preceding Crohn’s disease or Crohn’s disease preceding asthma). However, the risk of asthma was elevated among patients with existing UC but patients with existing asthma did not appear to be at an increased risk of UC. Based on these findings, it is not clear if one disease results in a predisposition to the other or the co-occurrence of these diseases simply occurs due to the commonalities in the physiology of the gut and the lung, as well as shared genetic and environmental risk factors.
Analyses stratified by age at diagnosis of IBD were inconsistent, with some studies suggesting a consistently elevated association between the two diseases across all ages, while others found contradictory age-specific associations. For example, one study found that the association between asthma and UC was decreased among people who were diagnosed with UC between 40 and 49 years of age42, while another study found an increased risk among patients diagnosed with UC > 40 years of age22. As both studies used health administrative data, and neither used an internally validated algorithm to identify cases of asthma, misclassification of asthma may have resulted in bias.
Additionally, failure to account for smoking status may have influenced the findings of these studies. Smoking is associated with an increased risk of Crohn’s disease but a decreased risk of UC60. Of the three included studies that adjusted for smoking status, two reported elevated associations between asthma and Crohn’s disease while one suggested that there may be a protective effect of the two diseases but was underpowered to detect a difference24,27,47. Asthma and UC were associated in one study that adjusted for smoking but not the other24,27. Differential rates of smoking across ages at IBD diagnosis may also contribute to differences observed in the association between asthma and IBD. For example, smoking is significantly more common among patients who are older at the time of Crohn’s disease diagnosis61. As a result, smokers with Crohn’s disease may be at an elevated risk of respiratory disease due to smoking but not their IBD. The decreased rate of smoking among patients with UC may explain the protective association seen in one study42. Similarly, other environmental risk factors (e.g., air pollution) demonstrate age-specific associations with both asthma and IBD and may contribute to the differences in the age-specific associations between asthma and IBD62,63.
A limitation of studies evaluating the associations between two conditions is that patients diagnosed with one condition have higher health services utilization than healthy individuals and may subsequently be more likely to be diagnosed with another condition64. No study included in our systematic review accounted for increased health care use. As a result, our findings of an increased association between asthma and IBD may have resulted from detection bias.
The association between asthma and Crohn’s disease varied across geographic regions. In Canada, New Zealand, Finland, Sweden, Taiwan, and the United States, the association between asthma and Crohn’s disease was elevated. However, there was a negative association in the United Kingdom and Israel. Regional differences in the association between asthma and UC were less pronounced. Although the reasons for these regional differences are not known, it is possible that differing penetrance of genetic or environmental risk factors may contribute. For example, the high prevalence of early-life exposure to peanuts has been associated with a decreased risk of peanut allergy in Israel65. This decreased risk of atopy may also result in a decreased risk of asthma among these children but not impact their elevated risk of developing IBD, due to genetic predisposition of IBD amongst Ashkenazi Jews.
As this is a systematic review, our ability to make conclusions about the co-occurrence of asthma and IBD is limited by the availability and quality of previous studies evaluating the association between these two diseases. Although we included 19 studies in our review, there was high degree of heterogeneity across studies. Heterogeneity was reduced when accounting for study methodology (i.e., study design and source of study participants) in studies analyzing the association between asthma and Crohn’s disease but not in the association between asthma and UC. In fact, there was an elevated association between asthma and Crohn’s disease in population-based studies, but a protective association between the two diseases in studies conducted using patients recruited from tertiary-care centers. All samples recruiting patients from tertiary-care centers were conducted either in Israel or the United Kingdom. This suggests that there are other underlying differences between studies and populations when it comes to understanding the association between asthma and IBD and we are unable to ascertain if the differences we observed result from differing study design or differences across populations. Specifically, the majority of studies recruiting patients from tertiary-care centers may have introduced selection bias by their choice of controls (e.g., partners of cases). However, similar control groups were selected in studies evaluating the association between UC and asthma included control groups that could similarly have introduced selection bias, yet the association remained similar regardless of the source of patients. In addition, there may be other differences in study design that were not accounted for (e.g., differences in the case definitions for IBD and asthma and variables adjusted for), underlying differences in the study population, or the phenotypes of asthma and IBD identified in the study (i.e., disease severity, behavior, management approach).
Conclusions
Asthma is associated with both Crohn’s disease and ulcerative colitis. Geographic differences, as well as differences in study design, contribute to this heterogeneity. We were unable to determine whether one disease increases the risk of the other or if both arise due to commonalities in pathology and shared risk factors. Future well-designed observational research should attempt to address these issues in their study design.
Study Highlights
What is current knowledge
Asthma and the inflammatory bowel diseases share environmental, genetic, and microbial risk factors
What is new here
Asthma is associated with both Crohn’s disease and ulcerative colitis
The relationship between these two diseases appears to vary by region
Electronic supplementary material
Acknowledgements
The following individuals were members of the CrowdScreen SR Review Team and participated in the review of abstracts and full-text: Jessica Bui; Adrijana D’Silva; Kainat Bashir, Jeanette Cheng, Yi Tong, Zhuo Qian (George) Cao, Matthew Peacock, Melissa Phuong; Melissa Wan; Meng Yang (Sunny) Xia; and Hsin Yun Yang. The authors would like to thank Pär Myrelid for providing additional data to enable the inclusion of Myrelid et al. in the meta-analysis46 and Nima Hamidi for translating Sharifpour et al.66 (excluded).
Specific author contributions
Study concept and design: M.E.K., G.G.K., E.I.B., Data acquisition: M.E.K., K.B., Crowdscreen SR Review Team, Statistical analysis: M.E.K., Interpretation of the data: M.E.K., G.G.K., E.I.B., Drafting of the manuscript: M.E.K., Critical revision of the manuscript for intellectual content: M.E.K., K.B., R.L., G.G.K., E.I.B., Final approval of the manuscript: M.E.K., K.B., R.L., G.G.K., E.I.B.
Financial support
M.E.K. was supported by a Post-Doctoral Fellowship Award from the Canadian Institutes of Health Research (CIHR), Canadian Association of Gastroenterology (CAG), and Crohn’s and Colitis Canada. G.G.K. was supported by a CIHR Embedded Clinician Research Award and a Population Health Investigator Award from Alberta Innovates - Health Solutions. E.I.B. was supported by a New Investigator Award from the CIHR, CAG and Crohn’s and Colitis Canada. E.I.B. was also supported by the Career Enhancement Program of the Canadian Child Health Clinician Scientist Program.
Guarantor of the article
M. Ellen Kuenzig.
Potencial competing interests
None
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
The online version of this article (10.1038/s41424-018-0054-z) contains supplementary material, which is available to authorized users.
References
- 1.de Souza HSP, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2015;13:13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 2.Xiao C, et al. Defective epithelial barrier function in asthma. J. Allergy Clin. Immunol. 2011;128:549–566. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 3.Holgate ST. Epithelium dysfunction in asthma. J. Allergy Clin. Immunol. 2007;120:1233–1244. doi: 10.1016/j.jaci.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Henderson P, et al. Function of the intestinal epithelium and its dysregulation in inflammatory bowel disease. Inflamm. Bowel Dis. 2011;17:382–395. doi: 10.1002/ibd.21379. [DOI] [PubMed] [Google Scholar]
- 5.Hackett TL, et al. Intrinsic phenotypic differences of asthmatic epithelium and its inflammatory responses to respiratory syncytial virus and air pollution. Am. J. Respir. Cell Mol. Biol. 2011;45:1090–1100. doi: 10.1165/rcmb.2011-0031OC. [DOI] [PubMed] [Google Scholar]
- 6.Wark PAB, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 2005;201:937–947. doi: 10.1084/jem.20041901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geremia A, et al. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014;13:3–10. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Frolkis AD, et al. Environment and the inflammatory bowel diseases. Can. J. Gastroenterol. 2013;27:e18–e24. doi: 10.1155/2013/102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aujnarain A, Mack DR, Benchimol EI. The role of the environment in the development of pediatric inflammatory bowel disease topical collection on pediatric gastroenterology. Curr. Gastroenterol. Rep. 2013;15:326. doi: 10.1007/s11894-013-0326-4. [DOI] [PubMed] [Google Scholar]
- 10.Dogaru CM, et al. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am. J. Epidemiol. 2014;179:1153–1167. doi: 10.1093/aje/kwu072. [DOI] [PubMed] [Google Scholar]
- 11.Daley D. The evolution of the hygiene hypothesis. Curr. Opin. Allergy Clin. Immunol. 2014;14:390–396. doi: 10.1097/ACI.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 12.Stiemsma L, et al. The hygiene hypothesis: current perspectives and future therapies. Immunotargets Ther. 2015;4:143–157. doi: 10.2147/ITT.S61528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lees CW, et al. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739–1753. doi: 10.1136/gut.2009.199679. [DOI] [PubMed] [Google Scholar]
- 14.Zhong Y, Kinio A, Saleh M. Functions of NOD-like receptors in human diseases. Front. Immunol. 2013;4:333. doi: 10.3389/fimmu.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdollahi E, et al. Protective role of R381Q (rs11209026) polymorphism in IL-23Rgene in immune-mediated diseases: a comprehensive review. J. Immunotoxicol. 2015;13:286–300. doi: 10.3109/1547691X.2015.1115448. [DOI] [PubMed] [Google Scholar]
- 16.Molodecky NA, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 18.Benchimol EI, et al. Epidemiology of pediatric inflammatory bowel disease: A systematic review of international trends. Inflamm. Bowel Dis. 2011;17:423–439. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 19.Masoli M, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 20.Ng SC, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 21.Su X, et al. Prevalence of comorbidities in asthma and nonasthma patients. Medicine. 2016;95:e3459–7. doi: 10.1097/MD.0000000000003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuenzig M. Ellen, Barnabe Cheryl, Seow Cynthia H., Eksteen Bertus, Negron Maria E., Rezaie Ali, Panaccione Remo, Benchimol Eric I., Sadatsafavi Mohsen, Aviña-Zubieta J. Antonio, Kaplan Gilaad G. Asthma Is Associated With Subsequent Development of Inflammatory Bowel Disease: A Population-based Case–Control Study. Clinical Gastroenterology and Hepatology. 2017;15(9):1405-1412.e3. doi: 10.1016/j.cgh.2017.02.042. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827–836. doi: 10.1053/j.gastro.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Gearry RB, et al. Population-based cases control study of inflammatory bowel disease risk factors. J. Gastroenterol. Hepatol. 2010;25:325–333. doi: 10.1111/j.1440-1746.2009.06140.x. [DOI] [PubMed] [Google Scholar]
- 25.Kauppi P, et al. Chronic comorbidities contribute to the burden and costs of persistent asthma. Mediat. Inflamm. 2015;2015:819194. doi: 10.1155/2015/819194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virta LJ, Ashorn M, Kolho KL. Cowʼs milk allergy, asthma, and pediatric IBD. J. Pediatr. Gastroenterol. Nutr. 2013;56:649–651. doi: 10.1097/MPG.0b013e318285e9d8. [DOI] [PubMed] [Google Scholar]
- 27.Weng X, et al. Clustering of inflammatory bowel disease with immune mediated diseases among members of a Northern California-managed care organization. Am. J. Gastroenterol. 2007;102:1429–1435. doi: 10.1111/j.1572-0241.2007.01215.x. [DOI] [PubMed] [Google Scholar]
- 28.Hemminki K, et al. Subsequent autoimmune or related disease in asthma patients: clustering of diseases or medical care? Ann. Epidemiol. 2010;20:217–222. doi: 10.1016/j.annepidem.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Peng YH, et al. Association of inflammatory bowel disease with asthma risk: A nationwide cohort study. Allergy Asthma Proc. 2015;36:92–98. doi: 10.2500/aap.2015.36.3869. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Y, et al. A case-control study of ulcerative colitis in Japan. J. Clin. Gastroenterol. 1994;18:72–79. doi: 10.1097/00004836-199401000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Hemminki K, et al. Familial association of inflammatory bowel diseases with other autoimmune and related diseases. Am. J. Gastroenterol. 2010;105:139–147. doi: 10.1038/ajg.2009.496. [DOI] [PubMed] [Google Scholar]
- 32.Kuenzig, E. The association between asthma and the inflammatory bowel diseases. PROSPERO 2017;CRD42017052738 https://www.crd.york.ac.uk/prospero/display_record.asp?ID=CRD42017052738
- 33.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS. Med. 2009;6:e1000097–6. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nama N, et al. A pilot validation study of crowdsourcing systematic reviews: update of a searchable database of pediatric clinical trials of high-dose vitamin D. Transl. Pediatr. 2017;5:18–26. doi: 10.21037/tp.2016.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mortensen ML, et al. An exploration of crowdsourcing citation screening for systematic reviews. Res. Syn. Meth. 2017;8:366–386. doi: 10.1002/jrsm.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris, P. A. et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. 2009;42:377–381. http://linkinghub.elsevier.com/retrieve/pii/S1532046408001226 [DOI] [PMC free article] [PubMed]
- 37.Wells, G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. ohri.ca http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 30 Jan 2017.
- 38.Silverberg MS, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. 2005;19(Suppl A):5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 39.R. Core Team. R: A language and environment for statistical computing. http://www.R-project.org/
- 40.Schwarzer G. meta: An R package for meta-analysis. R. News. 2007;7:40–45. [Google Scholar]
- 41.Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36:1–48. (2010).
- 42.Brassard P, et al. Increased incidence of inflammatory bowel disease in Québec residents with airway diseases. Eur. Respir. J. 2015;45:962–968. doi: 10.1183/09031936.00079414. [DOI] [PubMed] [Google Scholar]
- 43.Hammer B, Ashurst P, Naish J. Diseases associated with ulcerative colitis and Crohn’s disease. Gut. 1968;9:17–21. doi: 10.1136/gut.9.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kappelman MD, et al. Association of paediatric inflammatory bowel disease with other immune-mediated diseases. Arch. Dis. Child. 2011;96:1042–1046. doi: 10.1136/archdischild-2011-300633. [DOI] [PubMed] [Google Scholar]
- 45.Livnat G, et al. Bronchial reactivity and fractional exhaled NO in Crohn’s disease in the era of immunomodulating treatment. Acta Paediatr. 2012;101:e399–e404. doi: 10.1111/j.1651-2227.2012.02751.x. [DOI] [PubMed] [Google Scholar]
- 46.Myrelid P, et al. Atopic manifestations are more common in patients with Crohn disease than in the general population. Scand. J. Gastroenterol. 2009;39:731–736. doi: 10.1080/00365520410005955. [DOI] [PubMed] [Google Scholar]
- 47.Neilly JB, et al. Pulmonary abnormalities in Crohn’s disease. Respir. Med. 1989;83:487–491. doi: 10.1016/S0954-6111(89)80132-5. [DOI] [PubMed] [Google Scholar]
- 48.Pugh SM, et al. Atopic disease in ulcerative colitis and Crohn’s disease. Clin. Allergy. 1979;9:221–223. doi: 10.1111/j.1365-2222.1979.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 49.Raj AA, et al. Prevalence of inflammatory bowel disease in patients with airways disease. Respir. Med. 2008;102:780–785. doi: 10.1016/j.rmed.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 50.D’Arienzo A. Allergy and mucosal eosinophil infiltrate in ulcerative colitis. Scand. J. Gastroenterol. 2000;35:624–631. doi: 10.1080/003655200750023598. [DOI] [PubMed] [Google Scholar]
- 51.D’Arienzo A, et al. Ulcerative. Scand. J. Gastroenterol. 2002;37:1156–1163. doi: 10.1080/003655202760373362. [DOI] [PubMed] [Google Scholar]
- 52.Boneberger A, et al. Atopic manifestations in patients with ulcerative colitis: a report from Chile. J. Investig. Allergol. Clin. Immunol. 2012;22:73–75. [PubMed] [Google Scholar]
- 53.Virta LJ, Kolho KL. Antidepressant use among paediatric patients with recent-onset inflammatory bowel disease: a nationwide case control study in Finland. J. Paediatr. Child Health. 2014;50:562–565. doi: 10.1111/jpc.12516. [DOI] [PubMed] [Google Scholar]
- 54.Haapamäki J, et al. Increased risk for coronary heart disease, asthma, and connective tissue diseases in inflammatory bowel disease. J. Crohns Colitis. 2011;5:41–47. doi: 10.1016/j.crohns.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Shen TC, et al. The risk of asthma in rheumatoid arthritis: a population-based cohort study. QJM. 2014;107:435–442. doi: 10.1093/qjmed/hcu008. [DOI] [PubMed] [Google Scholar]
- 56.Hsiao YT, et al. Type 1 diabetes and increased risk of subsequent asthma. Medicine. 2015;94:e1466–e1466. doi: 10.1097/MD.0000000000001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edwards LJ, Constantinescu CS. A prospective study of conditions associated with multiple sclerosis in a cohort of 658 consecutive outpatients attending a multiple sclerosis clinic. Mult. Scler. 2004;10:575–581. doi: 10.1191/1352458504ms1087oa. [DOI] [PubMed] [Google Scholar]
- 58.Yun HD, et al. Asthma and proinflammatory conditions: a population-based retrospective matched cohort study. Mayo Clin. Proc. 2012;87:953–960. doi: 10.1016/j.mayocp.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinart M, et al. Comorbidity of eczema, rhinitis, and asthma in IgE-sensitised and non-IgE-sensitised children in MeDALL: a population-based cohort study. Lancet Respir. Med. 2014;2:131–140. doi: 10.1016/S2213-2600(13)70277-7. [DOI] [PubMed] [Google Scholar]
- 60.Mahid SS, et al. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin. Proc. 2006;81:1462–1471. doi: 10.4065/81.11.1462. [DOI] [PubMed] [Google Scholar]
- 61.Kuenzig ME, et al. The NOD2-Smoking interaction in Crohn’s Disease is likely specific to the 1007fs mutation and may be explained by age at diagnosis: a meta-analysis and case-only study. EBioMedicine. 2017;21:188–196. doi: 10.1016/j.ebiom.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaplan GG, et al. The inflammatory bowel diseases and ambient air pollution: a novel association. Am. J. Gastroenterol. 2010;105:2412–2419. doi: 10.1038/ajg.2010.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Altzibar JM, et al. Epidemiology of asthma exacerbations and their relation with environmental factors in the Basque Country. Clin. Exp. Allergy. 2015;45:1099–1108. doi: 10.1111/cea.12419. [DOI] [PubMed] [Google Scholar]
- 64.Gross A, et al. Use of medical care biases associations between Parkinson disease and other medical conditions. Neurology. 2018;90:e2155–e2165. doi: 10.1212/WNL.0000000000005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toit DuG, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J. Allergy Clin. Immunol. 2008;122:984–991. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 66.Sharifpour A, et al. Evaluation of correlation between ulcerative colitis with asthma and air way hyper responsiveness. J. Mazand Univ. Med Sci. 2010;20:21–28. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.