Abstract
Thirty six novel heterocyclic derivatives of ethyl 2-(2-pyridylacetate) were efficiently synthesized. The new compounds involve the linkage of a 2-pyridyl ring with thiosemicarbazide (compounds 1–7), 1,2,4-triazole (compounds 1a–7a), 1,3,4-thiadiazole (compounds 1b–7b), and 1,3,4-oxadiazole (compounds 1f–7f) moieties. The last group of compounds 1e–7e involves the connection of a 2-pyridyl ring with 1,2,4-triazole and thiourea. 1H-NMR, 13C-NMR and MS methods were used to confirm the structures of the obtained derivatives. The molecular structures of 3, 3b, 7a and 7f were further confirmed by X-ray crystallography. All obtained compounds were tested in vitro against a number of microorganisms, including Gram-positive cocci, Gram-negative rods and Candida albicans. In addition, the obtained compounds were tested for cytotoxicity and antiviral activity against HIV-1.
Keywords: 1,2,4-triazole; 1,3,4-thiadiazole; 1,3,4-oxadiazole; thiourea; X-ray crystal structure analysis; biological activity
1. Introduction
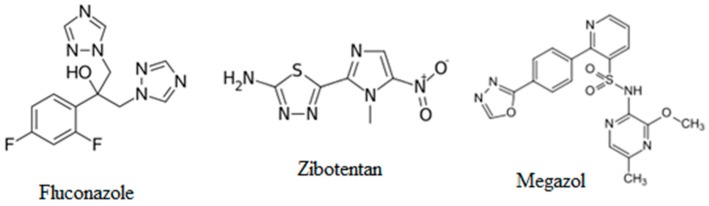
Heterocyclic compounds are well-known for their pharmaceutical importance, and the research for simple and efficient methods of synthesis of compounds incorporating heterocyclic rings has brought a new dimension to drug development [1]. In past decades heterocyclic scaffolds such as the 1,2,4-triazole, 1,3,4-oxadiazole and 1,3,4-thiadiazole rings and their derivatives were intensively investigated by researchers because of their biological activity. Among many heterocyclic compounds, these five-membered structures display unique spatial arrangements, which give possibilities to develop the synthetic pathways for microbiologically active compounds that can have potential therapeutic applications as new medicines. Currently, there are a lot of pharmaceutical products available on the market, which contain the previously mentioned structures such as fluconazole, efinaconazole, terconazole and fosfluconazole. These are used as antifungal agents and their structures are based on the 1,2,4-triazole core. The 1,3,4-thiadiazole scaffold can be found in other active substances like acetazolamide, methazolamide and megazol. Finally, the 1,3,4-oxadiazole arrangement is present in raltegravir and zibotentan. Exemplary structures of some of the previously listed medicines containing mentioned active scaffolds are presented in Figure 1 below.
Figure 1.
Active substances containing 1,2,4-triazole, 1,3,4-thiadiazole and 1,3,4-oxadiazole scaffold.
Currently researchers continue to design and sythesize new 1,2,4-triazole, 1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives because of their high antimicrobial activity against a wide range of Gram-negative and Gram-positive bacteria and yeasts. Compounds that contain one of mentioned heterocyclic motifs are often inhibitory or lethal against different types of microorganisms. Derivatives containing the 1,2,4-triazole core are still intensively studied as they possess potent antifungal properties [2]. Recently, 1,2,4-triazole-based compounds were reported as potential anticonvulsant [3,4,5,6,7] and anti-HIV agents [8,9]. Among the thiadiazole derivatives, those synthesized using the 1,3,4-thiadiazole motif were found to have number of biological activities such as anticonvulsant [10,11], antihypertensive [12,13], local anesthetic [14], anticancer [15,16], anti-HIV [17], anti-inflammatory [18,19], and hypoglycemic activities [20]. 1,3,4-Oxadiazoles are intensively evaluated for their activity against HIV-1 and HIV-2 in MT-4 cells [21,22,23,24].
All three scaffolds are fundamental in designing novel antimicrobial agents. Compounds built on these heterocyclic cores represent the non-steroidal antimicrobial drugs (NSAMDs). A study of the literature confirms that 1,3,4-oxadiazoles, 1,3,4-thiadiazoles, 1,2,4-triazoles and their amino derivatives are recognized as promising candidates for medicines used against bacteria and yeast [25,26,27,28,29]. These compounds often show higher activity than standard antibiotics commonly used as reference materials. Available data presume that the introduction of halogen atoms into the pharmacophore structure can be favourable for preventing the resistant microorganisms from spreading and emerging. These kinds of structural modifications help to increase the lipid-solubility of the studied scaffolds [30,31]. Ureas, thioureas, hydrazones, semicarbazides or thiosemicarbazides are found to posses similar properties and are useful as starting material in the synthesis of the three title heterocyclic arrangements and their structural modification. Prompted by this fact and encouraged by the results of recent studies [32,33,34] we decided to design synthetic paths to produce a battery of 1,3,4-oxadiazole, 1,3,4-thiadiazole, and 1,2,4-triazole derivatives.
2. Results and Discussion
2.1. Chemistry
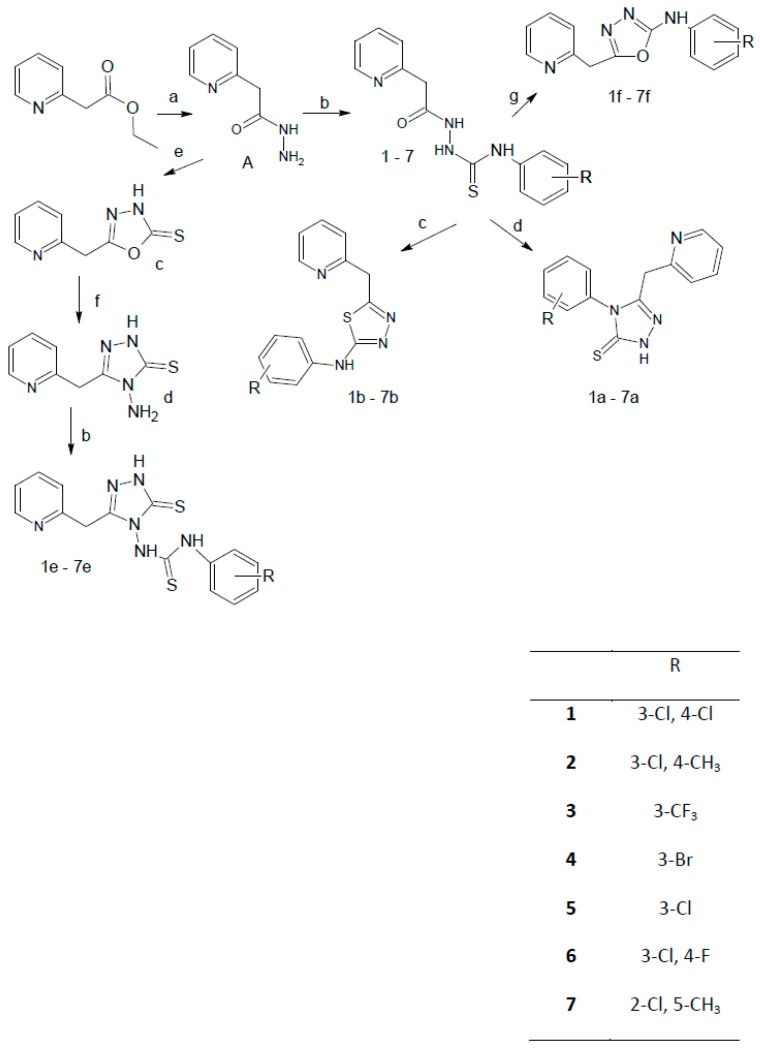
Our aim was to obtain a small library of ethyl 2-(2-pyridylacetate) derivatives. The planned synthetic route is depicted in Scheme 1.
Scheme 1.
Synthesis of ethyl 2-(2-pyridylacetate) derivatives. Reagents and conditions: (a) NH2NH2 80%, EtOH, rt. 2 h (b) appropriate isothiocyanate, acetonitrile, reflux, 6 h (c) H2SO4(conc.) (d) 2% NaOH, reflux, 1 h (e) KOH, EtOH, CS2 (f) NH2NH2 80%, reflux (g) Et3N, HgCl2, DMF.
In first step the ester ethyl 2-(2-pyridylacetate) was transformed into 2-(pyridin-2-yl)acetohydrazide (A) [35,36]. This was a starting material to obtain the 2-pyridylacetate thiosemicarbazide and oxazole derivatives. Derivatives of N-(phenylsubstituted)-2-(pyridin-2-yl acetyl) hydrazinecarbothioamide (thiosemicarbazide derivatives) were prepared by reaction of hydrazide (A) with an appropriate aryl isothiocyanate. Briefly, 2-(pyridin-2-yl)-acetohydrazide (A) and different isothiocyanates were allowed to react in boiling acetonitrile for 6 h. N-(Phenylsubstituted)-2-(pyridin-2-yl-acetyl)hydrazinecarbothioamide derivatives 1–7 were the starting materials to obtain the corresponding 1,2,4-triazole, 1,3,4-thiadiazole and 1,3,4-oxadiazole derivatives 1a–7a, 1b–7b and 1f–7f, respectively.
The methods for obtaining the 1,2,4-triazole and 1,3,4-thiadiazole derivatives 1a–7a and 1b–7b was similar. For both groups of compounds this required cyclisation, however for 1,2,4-triazole process was accomplished under basic conditions (2% NaOH) while for 1,3,4-thiadiazoles acidic conditions (conc. H2SO4) were used, respectively [37,38].
2-(Pyridin-2-yl)acetohydrazide (A) was also used to obtain 5-(pyridin-2-ylmethyl)-1,3,4-oxadiazole-2(3H)-thione (c). This compound was known [39], but in the present work a different synthesis method was applied. Hydrazide A was added to the solution of ethanol and KOH and next carbon disulfide was added to the mixture. The reaction mixture was refluxed on a steam bath for 5 h, then the solid formed was filtered off and recrystallized from butanol. 1,3,4-Oxadiazole-2-thione (c) was transformed into 4-amino-5-(pyridin-2-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (d) by reaction with hydrazine hydrate. The free amino group offered a possibility for further reaction with isothiocyanates to obtain the thiourea derivatives 1e–7e. This reaction was performed in acetonitrile similar to previously reported thiourea derivative syntheses [32,34].
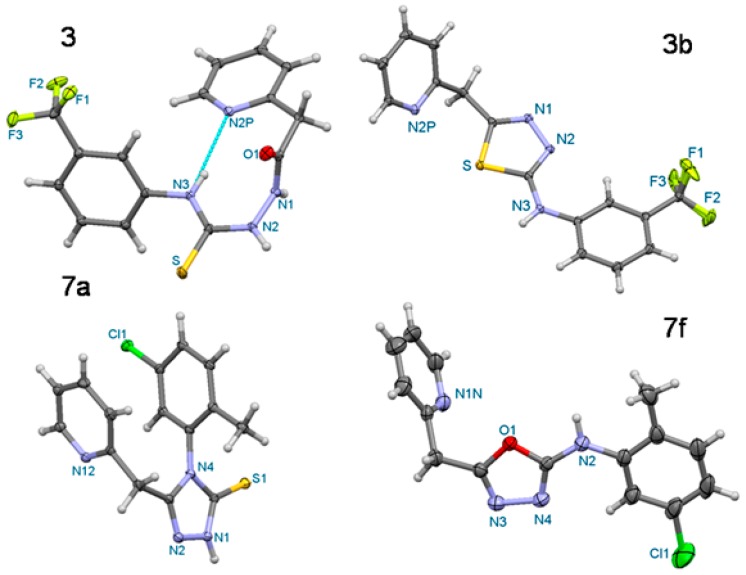
Oxadiazoles 1f–7f were prepared according to a new procedure developed by our team. This synthesis relies on a desulfurization/cyclization reaction of thiosemicarbazides to oxadiazoles using mercuric chloride. Development of this synthetic procedure was inspired by a method already used for preparation of a library of amino acid derived 2-arylamino-[1,3,4]-oxadiazoles [40]. The mechanism of this type of reaction is already established, therefore our goal was to find suitable reaction conditions. It was found that reflux is not needed, furthermore catalytic amounts of triethylamine (1–3 drops) are optional. Applications of this synthetic route will be presented in future publications. The molecular structures of one of the parent derivatives, compound 3, and three representatives of the cyclization products (3b, 7a and 7f) are illustrated in Figure 2. The non-covalent intermolecular contacts in the crystals are shown in the Supplementary Materials. The crystal data and experimental parameters are given in Table 1, below.
Figure 2.
Perspective view of the molecular structures of the derivatives 2-(pyridin-2-yl-acetyl)-N-[3-(trifluoromethyl)phenyl]hydrazinecarbothioamide (3), 5-(pyridin-2-yl-methyl)-N-[3-(trifluoromethyl)phenyl]-1,3,4-thiadiazol-2-amine (3b), 4-(5-chloro-2-methylphenyl)-5-(pyridin-2-yl-methyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (7a) and N-(2-chloro-5-methylphenyl)-5-(pyridin-2-ylmethyl)-1,3,4-oxadiazol-2-amine (7f).
Table 1.
Crystal data and structure refinement parameters for 3, 3b, 7a and 7f.
| Compound | 3 | 3b | 7a | 7f |
|---|---|---|---|---|
| Empirical formula | C15H13F3N4OS | C15H11F3N4S | C16H14ClN3S | C15H13ClN4O |
| Formula weight | 354.35 | 336.34 | 315.81 | 300.74 |
| Temperature | 80(2) K | 100(2) K | 120(2) K | 295(2) K |
| Wavelength (Å) | 0.71073 | 0.71073 | 1.54184 | 1.54184 |
| Crystal system | monoclinic | monoclinic | triclinic | monoclinic |
| Space group | P21/n | P21/c | P-1 | I2/a |
| Unit cell dimensions | ||||
| a (Å) | 4.6532(9) | 16.152(5) | 7.417(2) | 15.738(2) |
| b (Å) | 34.942(18) | 7.668(2) | 8.601(2) | 13.945(2) |
| c (Å) | 9.800(3) | 11.594(4) | 12.111(3) | 13.254(2) |
| α (°) | 90 | 90 | 106.72(2) | 90 |
| β (°) | 91.17(5) | 90.99(3) | 92.49(2) | 90.94(1) |
| γ (°) | 90 | 90 | 95.86(2) | 90 |
| Volume (Å−3) | 1593.1(10) | 1435.7(8) | 733.9(3) | 2908.4(7) |
| Z | 4 | 4 | 2 | 8 |
| F(000) | 728 | 688 | 328 | 1248 |
| Density (calcd) (g/cm3) | 1.477 | 1.556 | 1.429 | 1.374 |
| Absorpt. coeff. (mm-1) | 0.246 | 0.263 | 3.591 | 2.364 |
| Max. and min. transmission | 0.985 and 0.8996 | 0.9844 and 0.8886 | 0.990 and 0.9833 | 0.798 and 0.6493 |
| Crystal size (mm) | 0.44 × 0.07 × 0.06 | 0.46 × 0.13 × 0.06 | 0.22 × 0.20 × 0.18 | 0.20 × 0.20 × 0.10 |
| θ range for data coll. (°) | 2.72 to 30.39°. | 2.94 to 28.75°. | 3.82 to 73.65°. | 4.24 to 73.95°. |
| Index ranges | −5 ≤ h ≤ 5, −49 ≤ k ≤ 49, −12 ≤ l ≤ 13 | −21 ≤ h ≤ 21, −9 ≤ k ≤ 10, −11 ≤ l ≤ 15 | −9 ≤ h ≤ 9, −10 ≤ k ≤ 10, −11 ≤ l ≤ 15 | −13 ≤ h ≤ 19, −16 ≤ k ≤ 11, −16 ≤ l ≤ 12 |
| Reflections collected | 8587 | 8611 | 4813 | 3609 |
| Independent reflections | 4085 [R(int) = 0.0466] | 3364 [R(int) = 0.0353] | 2849 [R(int) = 0.0251] | 2419 [R(int) = 0.0189] |
| Data/parameters | 4085/229 | 3364/239 | 2849/195 | 2419/196 |
| Goodness-of-fit on F2 | 1.109 | 1.061 | 1.086 | 1.049 |
| Final R indices [I > 2σ (I)] | R1 = 0.0594 wR2 = 0.1229 |
R1 = 0.0398 wR2 = 0.0956 |
R1 = 0.0432 wR2 = 0.1012 |
R1 = 0.0448 wR2 = 0.1240 |
| R indices (all data) | R1 = 0.0871 wR2 = 0.1354 |
R1 = 0.0600 wR2 = 0.1001 |
R1 = 0.0490 wR2 = 0.1050 |
R1 = 0.0580 wR2 = 0.1382 |
| Extinction coefficient | -- | -- | -- | 0.0017(2) |
| Δρmax/min (e Å−3) | 0.38/−0.38 | 0.32/−0.24 | 0.58/−0.33 | 0.18/−0.29 |
| CCDC No. | 1539080 | 1539081 | 1539083 | 1539082 |
2.2. Biological Studies
All obtained compounds were tested in vitro against a number of bacteria, including Gram-positive cocci, Gram-negative rods and fungi. Microorganisms used in this study have common applications in the antimicrobial tests for many substances like antibiotics, antiseptic drugs and in the search for new antimicrobial agents [32,33,34,41]. The procedure started with a preliminary screening by the disc diffusion method, in order to select derivatives with antimicrobial properties. All obtained compounds are inactive in microbiological screening.
With the aim to evaluate more widely the biological properties of synthesized compounds (1–7, 1a–7a and 1b–7b) on the basis of reported anti-HIV activities of the similar derivatives, compounds were tested in cell-based assay against the human immunodeficiency virus type-1 (HIV-1), using efavirenz as reference inhibitor. The cytotoxicity against the MT-4 cells was evaluated in parallel with the antiviral activity (Table 2). None of the tested compounds showed selective anti-HIV-1 activity. Almost all tested compounds are non-cytotoxic for exponentially growing MT-4 cells (CC50 >100 µM).
Table 2.
Cytotoxicity and anti-HIV-1 activity of compounds (1–7, 1a–7a and 1b–7b).
| Compounds | MT-4 | HIV-1IIIB |
|---|---|---|
| a CC50 | b EC50 | |
| (μM) | ||
| 1 | >100 | >100 |
| 2 | >100 | >100 |
| 3 | >100 | >100 |
| 4 | >100 | >100 |
| 5 | >100 | >100 |
| 6 | >100 | >100 |
| 7 | >100 | >100 |
| 1a | >100 | >100 |
| 2a | >100 | >100 |
| 3a | >100 | >100 |
| 4a | >100 | >100 |
| 5a | >100 | >100 |
| 6a | >100 | >100 |
| 7a | >100 | >100 |
| 1b | >100 | >100 |
| 2b | 47 | >47 |
| 3b | 70.6 | >70.6 |
| 4b | 74.0 | >74.0 |
| 5b | >100 | >100 |
| 6b | >100 | >100 |
| 7b | >100 | >100 |
| EFV | 45.0 | 0.003 |
a Compound concentration (µM) required to reduce the viability of mock-infected MT-4 cells by 50%, as determined by the MTT method; b Compound concentration (μM) required to achieve 50% protection of MT-4 cells from the HIV-1 induced cytopathogenicity, as determined by the MTT method.
3. Materials and Methods
3.1. Apparatus, Materials and Analysis
The NMR spectra were recorded on an AVANCE DMX 400 spectrometer (Bruker, Billerica MA, USA) operating at 300 MHz (1H-NMR) and 75 MHz (13C-NMR). The chemical shift values are expressed in ppm relative to TMS used as an internal standard. Mass spectral ESI measurements were carried out on ZQ Micromass instrument (Waters, Milford, MA, USA) equipped with a quadrupole mass analyzer. The spectra were run in the positive ion mode at a declustering potential of 40–60 V. The samples were previously separated on a UPLC column (C18) using an UPLC ACQUITY™ system by Waters connected with a DPA detector. Flash chromatography was performed on silica gel 60 (200–400 mesh, Merck, Kenilworth, NJ, USA) using chloroform/methanol (19:1 vol) mixture as eluent. Analytical TLC was carried out on silica gel F254 (Merck) plates (0.25 mm thickness). The diffraction data for 3 and 3b were collected on an Xcalibur instrument (Thermo Fisher Scientific, Waltham, MA, USA), while that for 7a and 7f was collected on a SuperNova diffractometer (Agilent Technologies, Santa Clara, CA, USA). The CRYSALIS program system [42] was used for data collection, cell refinement and data reduction. The data were corrected for Lorentz and polarization effects. A multi-scan absorption correction was applied. The structure was solved using direct methods using the SHELXS-97, and refined by the full-matrix least-squares on F2 with the SHELXL-97 program [43]. All non-H atoms were refined with anisotropic displacement parameters. The H-atoms attached to carbon were positioned geometrically and refined using the riding model with Uiso(H) = 1.2Ueq(C). The nitrogen bonded H-atoms were found in the difference-Fourier map and refined with isotropic displacement parameters. The crystal data and experimental parameters used in the X-ray structure analysis are given in Table 1.
3.2. General Procedure of the Synthesis of N-(Phenylsubstituted)-2-(pyridin-2-ylacetyl)hydrazinecarbo-thioamide Derivatives
2-(Pyridin-2-yl)acetohydrazide (A 0.01 mol, 1.51 g) and 0.01 mol of an appropriate isothiocyanate were refluxed in acetonitrile for 6 h. The product was filtered and washed carefully with diethyl ether in order to remove unreacted isothiocyanate, and then with water in order to remove hydrazide residues. The product was dried and then crystallized from an ethanol solution.
N-(3,4-Dichlorophenyl)-2-(pyridin-2-ylacetyl)hydrazinecarbothioamide (1). Yield 85%. m.p. 173–174 °C. 1H-NMR (DMSO-d6) δ (ppm): 3.72 (s, 2H, CH2); 7.24 (t, 1H, CHarom., J = 6.20 Hz); 7.37 (d, 1H, CHarom., J = 7.60 Hz); 7.44–7.46 (dd, 1H, CHarom., J1 = 3.46 Hz, J2 = 1.50 Hz); 7.59 (d, 1H, CHarom., J = 8.80 Hz); 7.74 (t, 1H, CHarom., J = 7,40 Hz), 7.80 (s, 1H, CHarom.); 8.33 (s, 1H, CHarom.); 9.95 (s, 1H, NH); 9.98 (s, 1H, NH); 10.32 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 42.73, 122.17, 124.18, 125.46, 127.0, 130.08 (2C), 130.26, 136.9, 139.31, 148.8, 155.65, 168.82, 180.72. ESI MS: m/z = 377.00 [M + Na] (100%).
N-(3-Chloro-4-methylphenyl)-2-(pyridin-2-ylacetyl)hydrazinecarbothioamide (2). Yield 83%. m.p. 183–184 °C. 1H-NMR (DMSO-d6) δ (ppm): 2.36 (s, 3H, CH3), 3.90 (s, 2H, CH2); 7.21 (t, 1H, CHarom., J = 6.20 Hz); 7.30 (d, 1H, CHarom., J = 7.60 Hz); 7.20 (m, 2H, CHarom.); 7.52 (s, 1H, CHarom.); 7.72 (t, 1H, CHarom., J = 7,40 Hz); 8.40 (s, 1H, CHarom.); 9.19 (s, 1H, NH); 9.21 (s, 1H, NH); 10.30 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 19.07, 42.69, 122.16, 124.19, 125.71, 130.66, 132.27, 136.94, 138.22 (2C), 148.74 (2C), 155.76, 168.73, 180.72. ESI MS: m/z = 357.00 [M + Na] (100%).
2-(Pyridin-2-ylacetyl)-N-[3-(trifluoromethyl)phenyl]hydrazinecarbothioamide (3). Yield 72%. m.p. 177–178 °C. 1H-NMR (DMSO-d6) δ (ppm): 3.94 (s, 2H, CH2); 7.32 (d, 1H, CHarom., J = 8.00 Hz); 7.50 (m, 2H, CHarom.); 7.70 (s, 2H, CHarom.); 8.39 (d, 1H, CHarom., J = 4.00 Hz); 9.49 (s, 1H, NH); 9.52 (s, 1H, NH); 10.30 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 42.75, 118.63 (q) 121.67 (q, J = 3.70 Hz), 122.14, 124.2, 125.85 (q, J = 272.80 Hz), 128.63 (q, J = 33.40 Hz), 129.42, 168.86, 180.84. ESI MS: m/z = 377.00 [M + Na] (100%).
N-(3-Bromophenyl)-2-(pyridin-2-ylacetyl)hydrazinecarbothioamide (4). Yield 80%. m.p. 176–177 °C. 1H-NMR (DMSO-d6) δ (ppm): 3.76 (s, 2H, CH2); 7.35–7.49 (m, 5H, CHarom.); 7.75–7.80 (m, 2H, CHarom.); 8.35 (d, 1H, CHarom., J = 4.00 Hz); 9.49 (s, 1H, NH); 9.96 (s, 1H, NH); 10.33 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 42.72, 120.59, 121.82, 122.16, 124.19, 127.87, 130.16 (2C), 136.92, 140.76, 148.75, 155.72, 148.76, 168.79, 180.68. ESI MS: m/z = 366.30 [M + H] (100%).
N-(3-Chlorophenyl)-2-(pyridin-2-ylacetyl)hydrazinecarbothioamide (5). Yield 78%. m.p. 171–172 °C. 1H-NMR (DMSO-d6) δ (ppm): 3.76 (s, 2H, CH2); 7.25–7.44 (m, 5H, CHarom.); 7.64 (br. s, 1H, CHarom.); 7.78 (tt, 1H, CHarom., J = 4.56 Hz); 8.35 (d, 1H, CHarom., J = 4.00 Hz); 9.90 (s, 1H, NH); 9.97 (s, 1H, NH); 10.34 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 42.71, 122.15, 124.18, 124.98, 129.87, 132.25, 136.9, 140.63 (2C), 148.76 (2C), 155.72, 168.77, 180.73. ESI MS: m/z = 319.30 [M − H] (100%).
N-(3-Chloro-4-fluorophenyl)-2-(pyridin-2-ylacetyl)hydrazinecarbothioamide (6). Yield 72%. m.p. 188–189 °C. 1H-NMR (DMSO-d6) δ (ppm): 3.76 (s, 2H, CH2); 7.25–7.29 (dd, 1H, CHarom., J1 = 5.10 Hz, J2 = 1.50 Hz); 7.41 (m, 3H, CHarom.); 7.71 (d, 1H, CHarom., J = 6.80 Hz); 7.78 (tt, 1H, CHarom., J = 4.56 Hz); 8.34 (d, 1H, CHarom., J = 4.00 Hz); 9.91 (s, 1H, NH); 9.93 (s, 1H, NH); 10.33 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 42.7, 116.51, 118.66, 122.15, 124.18, 126.17, 127.58, 136.36, 148.77 (2C), 153.05, 155.69, 168.78, 180.99. ESI MS: m/z = 337.30 [M − H] (100%).
N-(5-Chloro-2-methylphenyl)-2-(pyridin-2-ylacetyl)hydrazinecarbothioamide (7). Yield 78%. m.p. 192–193 °C. 1H-NMR (DMSO-d6) δ (ppm): 2.24 (s, 3H, CH3), 3.90 (s, 2H, CH2); 7.18–7.22 (dd, 1H, CHarom., J1 = 4.53 Hz, J2 = 1.80 Hz); 7.30 (d, 1H, CHarom., J = 10.80 Hz); 7.2 (t, 1H, CHarom., J = 8.40 Hz); 7.62 (d, 1H, CHarom., J = 10.00 Hz); 7.52 (tt, 1H, CHarom., J = 4.48 Hz); 8.2 (d, 1H, CHarom., J = 2.80 Hz); 8.40 (d, 1H, CHarom., J = 4.00 Hz); 9.18 (s, 1H, NH); 9.21 (s, 1H, NH); 10.30 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 17.05, 43.07, 122.2, 124.19, 126.67, 128.57, 129.5, 131.68, 134.82, 136.98, 139.3, 148.83, 156.16, 168.7, 181.47. ESI MS: m/z = 357.00 [M + Na] (100%).
3.3. General Procedure for the Synthesis of 5-(Pyridin-2-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione Derivatives
N-(Substituted-phenyl)-2-(pyridin-2-ylacetyl)hydrazinecarbothioamide (0.01 mol) was refluxed with 2% NaOH solution (40–50 mL) for 4 h. After cooling the solution was neutralized with dilute hydrochloric acid. The precipitated compound was filtered and then crystallized from ethanol.
4-(3,4-Dichlorophenyl)-5-(pyridin-2-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (1a). Yield 79%. m.p. 192–193 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.13 (s, 2H, CH2); 7.08 (d, 1H, CHarom., J = 10.40 Hz); 7.19 (dd, 1H, CHarom., J1 = 5.20 Hz, J2 = 2.40 Hz); 7.28 (d, 1H, CHarom., J = 4.93 Hz); 7.57 (d, 1H, CHarom., J = 3.20 Hz); 7.61 (tt, 1H, CHarom., J = 4.08 Hz), 7.68 (d, 1H, CHarom., J = 11.20 Hz); 8.37 - 8.39 (dd, 1H, CHarom., J1 = 4.80 Hz, J2 = 0.60 Hz); 13.88 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 33.99, 122.15, 123.41, 128.88, 130.51, 130.93, 131.34, 132.28, 133.46, 136.64, 148.99, 150.25, 154.59, 167.82. ESI MS: m/z = 361.00 [M + Na] (100%).
4-(3-Chloro-4-methylphenyl)-5-(pyridin-2-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (2a). Yield 75%. m.p. 188–189 °C. 1H-NMR (DMSO-d6) δ (ppm): 2.32 (s, 3H, CH3); 4.07 (s, 2H, CH2); 7.07 (d, 1H, CHarom., J = 10.40 Hz); 7.11–7.12 (dd, 1H, CHarom., J1 = 8.10 Hz, J2 = 1.80 Hz); 7.17–7.21 (dd, 1H, CHarom., J1 = 7.20 Hz, J2 = 2.10 Hz); 7.28 (d, 1H, CHarom., J = 2.40 Hz); 7.38 (d, 1H, CHarom., J = 10.80 Hz); 7.61 (tt, 1H, CHarom., J = 4.56 Hz); 8.39 (d, 1H, CHarom., J = 4.80 Hz); 13.81 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 19.31, 34.01, 122.12, 123.33, 127.03, 128.58, 131.41, 132.43, 133.15, 136.6, 136.96, 148.97, 150.36, 154.72, 167.90. ESI MS: m/z = 339.00 [M + Na] (100%).
5-(Pyridin-2-ylmethyl)-4-[3-(trifluoromethyl)phenyl]-2,4-dihydro-3H-1,2,4-triazole-3-thione (3a). Yield 70%. m.p. 178–179 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.13 (s, 2H, CH2); 6.99 (d, 1H, CHarom., J = 10.40 Hz); 7.13–7.17 (dd, 1H, CHarom., J1 = 6.60 Hz, J2 = 1.50 Hz); 7.52–7.64 (m, 4H, CHarom.); 7.77 (d, 1H, CHarom., J = 9.60 Hz); 8.35 (d, 1H, CHarom., J = 4.80 Hz); 13.90 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.08, 118.01 (q), 121.62 (q, J = 4.20 Hz), 122.07, 123.29, 125.6 (q, J = 272.60 Hz), 126. 03 (q, 30.70 Hz) 129.51 (q, 30.80 Hz), 134.41, 136.6, 154.56, 167.91. ESI MS: m/z = 359.00 [M + Na] (100%).
4-(3-Bromophenyl)-5-(pyridin-2-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (4a). Yield 79%. m.p. 222–223 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.09 (s, 2H, CH2); 7.02 (d, 1H, CHarom., J = 10.40 Hz); 7.17–7.21 (dd, 1H, CHarom., J1 = 6.60 Hz, J2 = 1.80 Hz); 7.27 (m, 1H, CHarom.); 7.36 (t, 1H, CHarom., J = 10.80 Hz); 7.42 (t, 1H, CHarom., J = 2.40 Hz); 7.57–7.62 (m, 2H, CHarom.); 8.38 (d, 1H, CHarom., J = 4.80 Hz); 13.83 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.04, 121.24, 122.11, 123.34, 127.55, 130.87, 131.15, 132.24, 134.95, 136.62, 148.96, 150.27, 154.59, 167.82. ESI MS: m/z = 347.20 [M] (100%).
4-(3-Chlorophenyl)-5-(pyridin-2-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (5a). Yield 73%. m.p. 223–224 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.10 (s, 2H, CH2); 7.03 (d, 1H, CHarom., J = 10.40 Hz); 7.16–7.23 (m, 2H, CHarom.); 7.31 (t, 1H, CHarom., J = 2.40 Hz); 7.39–7.50 (m, 2H, CHarom.); 7.60 (tt, 1H, CHarom., J = 4.56 Hz); 8.38 (d, 1H, CHarom., J = 5.20 Hz); 13.84 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.04, 119.93, 122.14, 123.41, 127.19, 130.59, 133.1, 134.89, 136.61, 149.01, 150.3, 154.6, 167.98. ESI MS: m/z = 334.90 [M + Na] (100%).
4-(3-Chloro-4-fluorophenyl)-5-(pyridin-2-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (6a). Yield 74%. m.p. 224–225 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.11 (s, 2H, CH2); 7.05 (d, 1H, CHarom., J = 10.40 Hz); 7.17–7.21 (dd, 1H, CHarom., J1 = 6.60 Hz, J2 = 1.50 Hz); 7.26–7.31 (m, 1H, CHarom.); 7.46 (t, 1H, CHarom., J = 11.00 Hz); 7.51 (t, 1H, CHarom., J = 4.40 Hz); 7.61 (tt, 1H, CHarom., J = 4.56 Hz); 8.39 (d, 1H, CHarom., J = 5.10 Hz); 13.85 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.03, 117.40, 119.90, 122.13, 123.40, 129.63, 130.63, 136.64, 149.01, 150.40, 154.59, 155.71, 159.01, 167.97. ESI MS: m/z = 319.30 [M] (100%).
4-(5-Chloro-2-methylphenyl)-5-(pyridin-2-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (7a). Yield 73%. m.p. 225 °C. 1H-NMR (DMSO-d6) δ (ppm): 1.81 (s, 3H, CH3); 4.00 (s, 2H, CH2); 6.95 (d, 1H, CHarom., J = 10.40 Hz); 7.18–7.22 (dd, 1H, CHarom., J1 = 6.60 Hz, J2 = 1.80 Hz); 7.25–7.28 (m, 2H, CHarom.); 7.40–7.43 (dd, 1H, CHarom., J1 = 8.10 Hz, J2 = 2.10 Hz); 7.59 (tt, 1H, CHarom., J = 4.56 Hz); 8.39 (d, 1H, CHarom., J = 5.10 Hz); 13.86 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 16.60, 34.11, 122.24, 123.32, 128.43, 129.79, 130.47, 132.21, 133.52, 135.76, 136.63, 149.03, 150.20, 154.37, 167.24. ESI MS: m/z = 339.00 [M + Na] (100%).
3.4. General Procedure for the Synthesis of 5-(Pyridin-2-ylmethyl)-1,3,4-thiadiazol-2-amine Derivatives
N-(Substituted-phenyl)-2-(pyridin-2-ylacetyl)hydrazinecarbothioamide (0.01 mol) was mixed for 4 h with concentrated sulfuric acid (0.5 mL). Then to the solution crushed ice was added. After cooling the solution was neutralized with dilute NaOH. The precipitated compound was filtered and then crystallized from ethanol.
N-(3,4-Dichlorophenyl)-5-(pyridin-2-ylmethyl)-1,3,4-thiadiazol-2-amine (1b). Yield 71%. m.p. 201–202 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.45 (s, 2H, CH2); 7.28–7.32 (m, 1H, CHarom.); 7.42–7.46 (m, 2H, CHarom.); 7.55 (d, 2H, Carom., J = 8.70 Hz); 7.78 (tt, 1H, CHarom., J = 4.56 Hz); 8.05 (d, 1H, CHarom., J = 2.40 Hz); 8.54 (d, 1H, CHarom., J = 6.40 Hz); 10.55 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 38.01, 117.38, 118.30, 122.31, 122.70, 123.33, 130.78, 131.22, 137.18, 140.55, 149.26, 156.58, 158.08, 164.19. ESI MS: m/z = 361.00 [M + Na] (100%).
N-(3-Chloro-4-methylphenyl)-5-(pyridin-2-ylmethyl)-1,3,4-thiadiazol-2-amine (2b). Yield 78%. m.p. 181–182 °C. 1H-NMR (DMSO-d6) δ (ppm): 2.26 (s, 3H, CH3); 4.43 (s, 2H, CH2); 7.25–7.33 (m, 3H, CHarom.); 7.43 (d, 1H, CHarom., J = 8.00 Hz); 7.79 (tt, 1H, CHarom., J = 4.56 Hz); 7.59 (d, 1H, CHarom., J = 2.80 Hz); 8.55 (d, 1H, CHarom., J = 6.40 Hz); 10.31 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 18.77, 38.08, 115.98, 117.18, 122.28, 123.32, 128.0, 131.37, 133.31, 137.16, 139.78, 149.27, 156.7, 157.4, 164.52. ESI MS: m/z = 339.00 [M + Na] (100%).
5-(Pyridin-2-ylmethyl)-N-[3-(trifluoromethyl)phenyl]-1,3,4-thiadiazol-2-amine (3b). Yield 69%. m.p. 179–180 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.46 (s, 2H, CH2); 7.28–7.33 (m, 2H, CHarom.); 7.44 (d, 1H, CHarom., J = 10.40 Hz); 7.55 (t, 1H, CHarom., J = 10.60 Hz); 7.71–7.75 (dd, 1H, CHarom., J1 = 8.40 Hz, J2 = 1.50 Hz); 7.81 (tt, 1H, CHarom., J = 4.56 Hz); 8.15 (s, 1H, CHarom.,); 8.55 (d, 1H, CHarom., J = 6.60 Hz); 10.59 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 38.05, 113.18 (q, 4.20 Hz), 117.75 (q, 4.20 Hz), 120.77, 122.37, 123.35, 125.97 (q, 271.20 Hz), 129.79 (q, 33.20 Hz), 130.14. 137.19, 141.29, 149.27, 156.63, 157.96, 164.43. ESI MS: m/z = 359.0 [M + Na] (100%).
N-(3-Bromophenyl)-5-(pyridin-2-ylmethyl)-1,3,4-thiadiazol-2-amine (4b). Yield 76%. m.p. 156–157 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.45 (s, 2H, CH2); 6.99–7.03 (m, 1H, CHarom.); 7.28–7.45 (m, 4H, CHarom.); 7.78 (tt, 1H, CHarom., J = 4.56 Hz); 7.86 (t, 1H, CHarom., J = 2.60 Hz); 8.54 (d, 1H, CHarom., J = 6.40 Hz); 10.44 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 37.31, 116.10, 119.46, 121.92, 122.86, 124.06, 130.88, 138.63, 142.09, 148.15, 148.75, 155.85, 157.38, 164.47. ESI MS: m/z = 347.20 [M] (100%).
N-(3-Chlorophenyl)-5-(pyridin-2-ylmethyl)-1,3,4-thiadiazol-2-amine (5b). Yield 78%. m.p. 149–150 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.44 (s, 2H, CH2); 6.96–7.08 (m, 1H, CHarom.); 7.27–7.40 (m, 4H, CHarom.); 7.79 (tt, 1H, CHarom., J = 4.56 Hz); 7.85 (t, 1H, CHarom., J = 2.60 Hz); 8.54 (d, 1H, CHarom., J = 6.40 Hz); 10.48 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 37.94, 115.69, 116.62, 121.12, 122.41, 123.47, 130.58, 133.43, 137.43, 142.00, 149.08, 156.53, 157.74, 164.43. ESI MS: m/z = 311.90 [M] (100%).
N-(3-Chloro-4-fluorophenyl)-5-(pyridin-2-ylmethyl)-1,3,4-thiadiazol-2-amine (6b). Yield 75%. m.p. 196–197 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.44 (s, 2H, CH2); 6.28–7.45 (m, 4H, CHarom.); 7.79 (tt, 1H, CHarom., J = 4.64 Hz); 7.97–7.99 (dd, 1H, CHarom., J1 = 6.00 Hz, J2 = 2.70 Hz); 8.54 (d, 1H, CHarom., J = 2.76 Hz); 10.43 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 38.06, 116.97 (d, 22.20 Hz), 117.45 (d, 7.80 Hz) 118.38, 119.48 (d, 18.60 Hz), 122.29, 123.32, 137.16, 137.91 (d, 3.30 Hz), 149.26, 153.62 (242.60 Hz), 156.63, 157.68, 164.49. ESI MS: m/z = 319.30 [M + H] (100%).
N-(5-Chloro-2-methylphenyl)-5-(pyridin-2-ylmethyl)-1,3,4-thiadiazol-2-amine (7b). Yield 71%. m.p. 145–146 °C. 1H-NMR (DMSO-d6) δ (ppm): 2.24 (s, 3H, CH3); 4.53 (s, 2H, CH2); 6.99 (dd, 1H, CHarom., J1 = 8.10 Hz, J2 = 2.10 Hz); 7.22 (d, 1H, CHarom., J = 10.80 Hz); 7.51 (t, 1H, CHarom., J = 8.40 Hz); 7.63 (d, 1H, CHarom., J = 10.00 Hz); 8.02 (tt, 1H, CHarom., J = 4.48 Hz); 8.20 (d, 1H, CHarom., J = 2.80 Hz); 8.65 (d, 1H, CHarom., J = 6.40 Hz); 10.31 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 17.62, 36.64, 119.01, 122.21, 123.32, 124.70, 125.99, 130.69, 131.83, 139.48, 140.01, 147.19, 155.23, 157.24, 165.65. ESI MS: m/z = 334.80 [M] (100%).
3.5. 5-(Pyridin-2-ylmethyl)-1,3,4-oxadiazole-2(3H)-thione (c)
3.6. 4-Amino-5-(pyridin-2-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione
5-(Pyridin-2-ylmethyl)-1,3,4-oxadiazole-2(3H)-thione (c, 0.01 mole, 1.93 g) was refluxed with 80% hydrazine hydrate (0.5 mL) and ethanol (20 mL) for 4 h. The precipitated compound was filtered and then crystallized from ethanol. Yield 80%. m.p. 210–211 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.20 (s, 2H, CH2); 5.5 (br. s, 2H, NH2); 7.27 (d, 1H, CHarom. J = 4.10 Hz); 7.35 (d, 1H, CHarom. J = 7.80 Hz); 7.75 (tt, 1H, CHarom. J = 3.43 Hz); 8.45 (d, 1H, CHarom. J = 3.90 Hz); 13.53 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 33.47, 122.32, 123.65, 136.88, 139.40, 149.10, 167, 31, 181.70. ESI MS: m/z = 206.50 [M − H] (100%).
3.7. General Procedure for the Synthesis of 1-(Subtituted phenyl)-3-[3-(pyridin-2-ylmethyl)-5-thioxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]thiourea Derivatives
4-Amino-5-(pyridin-2-ylmethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (0.01 mol, 2.0 g) and an appropriate isothiocyanate (0.01 mol) were refluxed in acetonitrile for 6 h. The product was filtered and washed carefully with diethyl ether in order to remove unreacted isothiocyanate. The product was then dried and then the residue was purified by column chromatography (eluent: chloroform–methanol 9.5:0.5).
1-(3,4-Dichlorophenyl)-3-[3-(pyridin-2-ylmethyl)-5-thioxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]-thiourea (1e). Yield 68%. m.p. 156–157 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.14 (s, 2H, CH2); 7.28 (d, 1H, CHarom., J = 7.80 Hz); 7.37 (d, 1H, CHarom., J = 8.50 Hz); 7.48 (m, 1H, CHarom.); 7.59 (d, 1H, CHarom., J = 9.00 Hz); 7.56 (tt, 1H, CHarom., J = 4.08 Hz), 7.68 (m, 1H, CHarom.); 8.49 (d, 1H, CHarom., J = 2.80 Hz); 10.78 (br. s, 2H, NH); 13.72 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 33.47, 122.35, 122.78, 123.63, 127.25, 130.30, 131.20, 136.71, 136.90, 138.62, 149.11, 151.15, 154.48, 167.25, 181.72. ESI MS: m/z = 409.90 [M − H] (100%).
1-(3-chloro-4-methylphenyl)-3-[3-(pyridin-2-ylmethyl)-5-thioxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]thiourea (2e). Yield 65%. m.p. 177–178 °C. 1H-NMR (DMSO-d6) δ (ppm): 2.30 (s, 3H, CH3); 4.14 (s, 2H, CH2); 7.29 (d, 1H, CHarom., J = 10.40 Hz); 7.31 (s, 1H, CHarom.); 7.34 (d, 1H, CHarom., J = 5.40 Hz); 7.37 (d, 1H, CHarom., J = 2.40 Hz); 7.40 (d, 1H, CHarom., J = 10.80 Hz); 7.76 (tt, 1H, CHarom., J = 4.56 Hz); 8.49 (d, 1H, CHarom., J = 2.40 Hz); 10.00 (br. s, 1H, NH); 10.59 (br. s, 1H, NH); 13.70 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 19.07, 33.42, 116.73, 122.34, 123.62, 127.42, 131.33, 131.41, 133.31, 136.92, 137.52, 149.06, 154.50, 167.26, 181.20. ESI MS: m/z = 389.00 [M − H] (100%).
1-[3-(pyridin-2-ylmethyl)-5-thioxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]-3-[3-(trifluoromethyl)-phenyl]thiourea (3e). Yield 80%. m.p. 172–173 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.15 (s, 2H, CH2); 2.26 (d, 1H, CHarom., J = 8.50 Hz); 7.38 (d, 1H, CHarom., J = 7.80 Hz); 7.49–7.64 (m, 4H, CHarom.); 7.75 (tt, 1H, CHarom., J = 9.60 Hz); 8.46 (d, 1H, CHarom., J = 2.40 Hz); 10.70 (br. s, 2H, NH); 13.83 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 33.44, 121.81 (q), 122.32, 123.65 (q), 125.77 (q, 270.20 Hz), 129.66 (q, J = 33.2 Hz), 136.88, 139.40, 149.09, 151.07, 154.47, 167.31, 180.31. ESI MS: m/z = 409.0 [M − H] (100%).
1-(3-Bromophenyl)-3-[3-(pyridin-2-ylmethyl)-5-thioxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]-thiourea (4e). Yield 75%. m.p. 178–179 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.14 (d, 2H, CH2, J = 8.40 Hz); 7.29 (m, 6H, CHarom.); 7.76 (tt, 1H, CHarom., J = 2.40 Hz); 8.49 (d, 1H, CHarom. J = 8.40 Hz.); 10.67 (br. s, 2H, NH); 13.71(s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.47, 121.24, 122.37, 123.64, 128.05, 130.43, 136.29, 140.11, 149.12, 151.22, 154.52, 155.2, 167.28, 181.41. ESI MS: m/z = 429.90 [M − H] (100%)
1-(3-Chlorophenyl)-3-[3-(pyridin-2-ylmethyl)-5-thioxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]-thiourea (5e). Yield 66%. m.p. 170 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.15 (d, 2H, CH2, J = 9.00 Hz); 7.14–7.39 (m, 6H, CHarom.); (tt, 1H, CHarom., J = 4.56 Hz); 8.47 (d, 1H, CHarom., J = 5.20 Hz); 10.67 (br. s, 2H, NH); 13.71(s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.45, 122.37, 123.81, 125.15, 127.19, 130.15, 132.60, 133.39, 136.87, 137.88, 139.96, 148.84, 151.13, 154.52, 167.98, 181.36. ESI MS: m/z = 375.00 [M − H] (100%).
1-(3-Chloro-4-fluorophenyl)-3-[3-(pyridin-2-ylmethyl)-5-thioxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]thiourea (6e). Yield 65%. m.p. 190–192 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.15 (d, 2H, CH2, J = 9.00 Hz); 7.29 (t, 1H, CHarom., J = 6.00 Hz); 7.39–7.42 (m, 4H, CHarom.); 7.75 (tt, 1H, CHarom., J = 9.00 Hz); 8.49 (d, 1H, CHarom., J = 6.00 Hz); 10.71 (br. s, 2H, NH); 13.72 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 33.43, 116.44 (d, 22.40 Hz), 118.73 (d, 19.40 Hz), 121.22 (d), 121.42, 123.63, 125.08 (d), 135.59 (d), 136.9, 149.09, 152.16, 154.47 (d, 24.30 Hz), 156.37, 167.97, 181.79. ESI MS: m/z = 393.00 [M − H] (100%).
1-(2-Chloro-5-methylphenyl)-3-[3-(pyridin-2-ylmethyl)-5-thioxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]thiourea (7e). Yield 63%. m.p. 180–181 °C. 1H-NMR (DMSO-d6) δ (ppm): 2.07 (s, 3H, CH3); 4.37 (s, 2H, CH2); 7.27 (s, 1H, CHarom.); 7.65–7.72 (m, 3H, CHarom.); 8.17 (t, 1H, CHarom., J = 4.56 Hz); 8.70 (d, 1H, CHarom., J = 2.40 Hz); 10.37 (br. s, 1H, NH); 10.69 (br. s, 1H, NH); 13.82 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 17.41, 29.65, 118.09, 125.25, 126.95, 127.23, 130.47, 132.85, 142.94, 144.76, 148.62, 150.38, 150.87, 167.44, 181.67. ESI MS: m/z = 389.00 [M − H] (100%).
3.8. General Procedure for the Synthesis of N-(Substituted-phenyl)-5-(pyridin-2-ylmethyl)-1,3,4-oxadiazol-2-amine Derivatives
Triethylamine (2–3 drops) was added to a suspension of thiosemicarbazide (1 mmol) and mercuric chloride (1.25 mmol) in dry DMF (5 mL). The resulting mixture was stirred for a maximum of 8 h at room temperature or until TLC showed the end of the reaction. The suspension was filtered through paper filter, then washed with CHCl3. The filtrate was diluted with water, extracted three times with CHCl3 (15 mL), the combined organic fractions were dried over MgSO4, filtered and concentrated under reduced pressure. The resulting residue was purified by silica gel chromatography (chloroform–methanol; 9.5:0.5).
N-(3,4-Dichlorophenyl)-5-(pyridin-2-ylmethyl)-1,3,4-oxadiazol-2-amine (1f). Yield 72%. m.p. 198–199 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.36 (s, 2H, CH2); 7.28–7.33 (m, 1H, CHarom.); 7.41–7.45 (dd, 2H, CHarom., J1 = 11.40 Hz, J2 = 2.70 Hz); 7.55–7.58 (d, 1H, CHarom., J = 8.70 Hz); 7.71–7.72 (td, 1H, CHarom., J = 5.70 Hz); 7.85–7.86 (d, 1H, CHarom., J = 2.40 Hz); 8.49–8.52 (m, 1H, CHarom.); 10.81 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 33.48, 117.11, 117.93, 122.48, 123.05, 123.39, 130.90, 131.31, 137.12, 138.82, 149.37, 154.75, 158.30, 159.44. ESI MS: m/z = 319.00 [M − H] (100%).
N-(3-Chloro-4-methylphenyl)-5-(pyridin-2-ylmethyl)-1,3,4-oxadiazol-2-amine (2f). Yield 75%. m.p. 176–177 °C. 1H-NMR (DMSO-d6) δ (ppm): 1.22 (s, 3H, CH3); 4.34 (s, 2H, CH2); 7.26–7.35 (m, 2H, CHarom.); 7.38–7.44 (m, 1H, CHarom.); 7.67–7.68 (d, 1H, CHarom., J = 0.90 Hz); 7.71-7.72 (td, 1H, CHarom., J = 2.90 Hz); 7.95 (s, 1H, CHarom.); 8.50–8.51 (m, 1H, CHarom.); 10.52 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 18.74, 33.50, 115.65, 116.67, 122.47, 123.37, 128.09, 131.49, 133.35, 137.12, 137.88, 149.36, 154.83, 157.98, 159.92 ESI MS: m/z = 301.20 [M] (100%).
5-(Pyridin-2-ylmethyl)-N-[3-(trifluoromethyl)phenyl]-1,3,4-oxadiazol-2-amine (3f). Yield 67%. m.p. 145–146 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.37 (s, 2H, CH2); 7.29–7.33 (m, 2H, CHarom.); 7.42–7.45 (m, 1H, CHarom.); 7.53–7.58 (t, 1H, CHarom., J = 4.20 Hz); 7.71–7.75 (m, 1H, CHarom.); 7.77–7.83 (td, 1H, CHarom., J = 3.90 Hz); 7.97–7.98 (m, 1H, CHarom.); 8.49–8.52 (dq, 1H, CHarom., J = 2.40 Hz); 10.83 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 33.51, 112.69, 117.90 (q, 4.00 Hz), 120.54 (q, 4.10 Hz), 123.40 (q, 27.40 Hz), 125.91, 130.03 (q, 33.20 Hz), 130.26 (q, 33.20 Hz), 137.13, 139.52, 149.37, 154.80, 158.25, 159.66. ESI MS: m/z = 319.10 [M − H] (100%).
N-(3-Bromophenyl)-5-(pyridin-2-ylmethyl)-1,3,4-oxadiazol-2-amine (4f). Yield 70%. m.p. 164–165 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.36 (s, 2H, CH2); 7.13–7.17 (m, 1H, CHarom.); 7.25–7.33 (m, 2H, CHarom.); 7.42–7.47 (m, 2H, CHarom.); 7.77–7.84 (m, 2H, CHarom., J = 3.90 Hz); 8.49–8.52 (m, 1H, CHarom.); 10.65 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 33.51, 115.78, 119.01, 121.94, 122.47, 123.37, 124.19, 130.97, 137.11, 140.30, 149.36, 154.80, 158.14, 159.59. ESI MS: m/z = 343.10[M − H] (100%).
N-(3-Chlorophenyl)-5-(pyridin-2-ylmethyl)-1,3,4-oxadiazol-2-amine (5f). Yield 72%. m.p. 147–148 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.36 (s, 2H, CH2); 7.00–7.03 (m, 1H, CHarom.); 7.28–7.45 (m, 4H, CHarom.); 7.69–7.70 (t, 1H, CHarom., J = 2.10 Hz); 7.76–7.82 (m, 1H, CHarom.); 8.50–8.51 (d, 1H, CHarom. J = 4.80 Hz); 10.67 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 33.51, 115.42, 116.20, 121.29, 122.47, 123.38, 130.68, 133.48, 137.11, 140.18, 149.37, 154.81, 158.14, 159.64. ESI MS: m/z = 329.10 [M − H] (100%).
N-(3-Chloro-4-fluorophenyl)-5-(pyridin-2-ylmethyl)-1,3,4-oxadiazol-2-amine (6f). Yield 72%. m.p. 178–179 °C. 1H-NMR (DMSO-d6) δ (ppm): 4.35 (s, 2H, CH2); 7.28–7.45 (m, 4H, CHarom.); 7.76-7.22 (m, 2H, CHarom.); 8.49–8.51 (d, 1H, CHarom., J = 2.40 Hz); 10.66 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 33.52, 117.10, 117.90, 122.47, 123.38, 136.03, 136.06, 137.11, 149.37, 150.60, 153.79, 154.80, 158.12, 159.66. ESI MS: m/z = 317.10 [M − H] (100%).
N-(2-Chloro-5-methylphenyl)-5-(pyridin-2-ylmethyl)-1,3,4-oxadiazol-2-amine (7f). Yield 69%. m.p. 215–216 °C. 1H-NMR (DMSO-d6) δ (ppm): 3.36 (bs, 3H, CH3); 4.36 (s, 2H, CH2); 7.28–7.33 (m, 1H, CHarom.); 7.41–7.45 (m, 2H, CHarom.); 7.55–7.57 (d, 1H, CHarom., J = 4.30 Hz); 7.77–7.82 (td, 1H, CHarom., J = 3.90 Hz); 7.85–7.86 (d, 1H, CHarom., J = 1.20 Hz); 8.49–8.52 (m, 1H, CHarom.). 13C-NMR (DMSO-d6) δ (ppm): 33.49, 33.51, 117.14, 117.95, 122.49, 123.03, 123.40, 130.91, 131.31, 137.14, 138.87, 149.37, 154.37, 158.30, 159.48. ESI MS: m/z = 301.20 [M] (100%).
3.9. Biological Assays
3.9.1. In Vitro Evaluation of Antimicrobial Activity
Microorganisms used in this study were as follows: Gram-positive bacteria: S. aureus NCTC 4163, S. aureus ATCC 25923, S. aureus ATCC 6538, S. aureus ATCC 29213, S. epidermidis ATCC 12228, S. epidermidis ATCC 35984, Enterococcus hirae ATCC 10541, Enterococcus faecalis ATCC 29212, Bacillus subtilis ATCC 6633, Bacillus cereus ATCC 11778, Micrococcus luteus ATCC 9341, M. luteus ATCC 10240; Gram-negative rods: E. coli ATCC 10538, E. coli ATCC 25922, E. coli NCTC 8196, P. vulgaris NCTC 4635, P. aeruginosa ATCC 15442, P. aeruginosa NCTC 6749, P. aeruginosa ATCC 27863, B. bronchiseptica ATCC 4617 and yeasts: C. albicans ATCC 10231, C. albicans ATCC 90028, C. parapsilosis ATCC 22019. All of listed microorganisms used were obtained from the collection of the Department of Pharmaceutical Microbiology, Medical University of Warsaw, Poland.
3.9.2. Media, Growth Conditions and Antimicrobial Activity Assays
Antibacterial activity was examined by the disc-diffusion method under standard conditions using Mueller-Hinton II agar medium (Becton Dickinson, Franklin Lakes, NJ, USA) according to CLSI (previously NCCLS) guidelines [41]. Antifungal activities were assessed using Mueller-Hinton agar 2% glucose and 0.5 mg/ml Methylene Blue Dye Medium [41]. Sterile filter paper discs (9 mm diameter, Whatman No 3 chromatography paper) were dripped with tested compound solutions (in DMSO) to load 400 mg of a given compound per disc. Dry discs were placed on the surface of appropriate agar medium. The results (diameter of the growth inhibition zone) were read after 18 h of incubation at 35 °C.
3.9.3. Cytotoxicity and Antiviral Assays
CD4+ human T-cells containing an integrated HTLV-1 genome (MT-4) were purchased from American Type Culture Collection (ATCC). Laboratory strain IIIB of Human Immunodeficiency Virus type-1 (HIV-1) was obtained from the supernatant of the persistently infected H9/IIIB cells (NIH 1983). Compounds’ activity against HIV-1 was based on inhibition of virus-induced cytopathogenicity in MT-4 cell acutely infected with a multiplicity of infection (MOI) of 0.01, as described in Drzewiecka et al. [45]. After a 4-day incubation at 37 °C, cell viability was determined by the MTT method [46]. Efavirenz was used as reference inhibitor.
4. Conclusions
We successfully synthesized five groups of compounds: thiosemicarbazides 1–7, 1,2,4-triazoles 1a–7a, 1,3,4-thiadiazoles 1b–7b, 1,3,4-oxadiazoles 1f–7f and thiourea derivatives 1e–7e to check their microbiological activity, cytotoxicity and anti HIV activity. Introduction of the 1,2,4-triazole, thiadiazole and oxazole ring to a 2-pyridyl moiety by a methylene linker decreased the antimicrobial activity and cytotoxicity. A second substituent like a phenyl ring connected with halogen atoms (chlorine, bromine or fluorine) had no influence on the biological activity. It could be summarized that all obtained derivatives possess moderate activity and their structure-activity relationships need to be evaluated.
Acknowledgments
This work was supported by the Medical University of Warsaw and carried out with the use of CePT infrastructure financed by the European Union—the European Regional Development Fund within the Operational Programme Innovative Economy for 2007–2013.
Supplementary Materials
Supplementary Materials are available online.
Author Contributions
Daniel Szulczyk performed the syntheses of triazoles, thiadiazoles and oxadiazoles, Piotr Tomaszewski collected biological data and wrote part of the paper, Michał Jóźwiak did the spectral data analyses, Anna E. Kozioł and Tadeusz Lis collected and analyzed X-ray diffraction data, David Collu and Filippo Iuliano performed cytotoxicity and antiviral activity assay, Marta Struga was the principal investigator of the project and provided the research funding. All authors approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available.
References
- 1.Shelke S.H., Mhaske P.C., Kasam S.K., Babade V.D. Synthesis and pharmacological evaluation of a novel series of 2-((2-Aryl thiazol-4-yl)methyl)-5-(alkyl/alkylnitrile thio)-1,3,4-oxadiazole derivatives as possible antifungal agents. J. Heterocycl. Chem. 2014;51:1893–1897. doi: 10.1002/jhet.1910. [DOI] [Google Scholar]
- 2.Wang B.L., Zhang Y., Liu X.H., Zhang L.Y., Zhan Y.Z., Zhang X., Wang L.Z., Li Y.H., Li Z.M. Synthesis and biological activity of novel dimethylpyrazole and piperazine-containing (bis)1,2,4-triazole derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2017;192:34–41. doi: 10.1080/10426507.2016.1223077. [DOI] [Google Scholar]
- 3.Ayati A., Emami S., Asadipour A., Shafiee A., Foroumadi A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015;15:699–718. doi: 10.1016/j.ejmech.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Kamboj V.K., Verma P.K., Dhanda A., Ranjan S. 1,2,4-triazole derivatives as potential scaffold for anticonvulsant activity. Cent. Nerv. Syst. Agents Med. Chem. 2015;15:17–22. doi: 10.2174/1871524915666150209100533. [DOI] [PubMed] [Google Scholar]
- 5.Guan L.P., Quan Z.S. 3,4-DHQLO and triazole and its related analogues with anticonvulsant effects. Mini Rev. Med. Chem. 2016;16:323–342. doi: 10.2174/1389557515666150101100909. [DOI] [PubMed] [Google Scholar]
- 6.Plech T., Kaproń B., Luszczki J.J., Paneth A., Siwek A., Kołaczkowski M., Żołnierek M., Nowak G. Studies on the anticonvulsant activity of 4-alkyl-1,2,4-triazole-3-thiones and their effect on GABAergic system. Eur. J. Med. Chem. 2014;30:690–699. doi: 10.1016/j.ejmech.2014.09.034. [DOI] [PubMed] [Google Scholar]
- 7.Plech T., Luszczki J.J., Wujec M., Flieger J., Pizoń M. Synthesis, characterization and preliminary anticonvulsant evaluation of some 4-alkyl-1,2,4-triazoles. Eur. J. Med. Chem. 2013;60:208–215. doi: 10.1016/j.ejmech.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Li Z., Cao Y., Zhan P., Pannecouque C., Balzarini J., De Clercq E., Liu X. Synthesis and anti-HIV evaluation of novel 1,2,4-triazole derivatives as potential non-nucleoside HIV-1 reverse transcriptase inhibitors. Lett. Drug Des. Discov. 2016;10:27–34. [Google Scholar]
- 9.Aneja R., Rashad A.A., Li H., Kalyana Sundaram R.V., Duffy C., Bailey L.D., Chaiken I. Peptide triazole inactivators of HIV-1 utilize a conserved two-cavity binding site at the junction of the inner and outer domains of Env gp120. J. Med. Chem. 2015;14:3843–3858. doi: 10.1021/acs.jmedchem.5b00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapleo C.B., Myers M., Myers P.L., Saville J.F., Smith A.C.B., Stillings M.R., Tulloch I.F., Walter D.S., Welbourn A.P. Substituted 1,3,4-thiadiazoles with anticonvulsant activity. 1. Hydrazines. J. Med. Chem. 1986;29:2273–2280. doi: 10.1021/jm00161a024. [DOI] [PubMed] [Google Scholar]
- 11.Chapleo C.B., Myers P.L., Smith A.C., Stillings M.R., Tulloch I.F., Walter D.S. Substituted 1,3,4-thiadiazoles with anticonvulsant activity. 4. Amidines. J. Med. Chem. 1988;31:7–11. doi: 10.1021/jm00396a004. [DOI] [PubMed] [Google Scholar]
- 12.Turner S., Myers M., Gadie B., Nelson A.J., Pape R., Saville J.F., Doxey J.C., Berridge T.L. Antihypertensive thiadiazoles. 1. Synthesis of some 2-aryl-5-hydrazino-1,3,4-thiadiazoles with vasodilator activity. J. Med. Chem. 1988;31:902–906. doi: 10.1021/jm00400a003. [DOI] [PubMed] [Google Scholar]
- 13.Turner S., Myers M., Gadie B., Hale S.A., Horsley A., Nelson A.J., Pape R., Saville J.F., Doxey J.C., Berridge T.L. Antihypertensive thiadiazoles. 2. Vasodilator activity of some 2-aryl-5-guanidino-1,3,4-thiadiazoles. J. Med. Chem. 1988;31:906–913. doi: 10.1021/jm00400a004. [DOI] [PubMed] [Google Scholar]
- 14.Mazzone G., Pignatello R., Mazzone S., Panico A., Penisi G., Castana R., Mazzone P. Synthesis and local anesthetic activity of alkylaminoacyl derivatives of 2-amino-1,3,4-thiadiazole. Farmaco. 1993;48:1207–1224. doi: 10.1002/chin.199412195. [DOI] [PubMed] [Google Scholar]
- 15.Chou J.Y., Lai S.Y., Pan S.L., Jow G.M., Chern J.W., Guh J.H. Investigation of anticancer mechanism of thiadiazole-based compound in human non-small cell lung cancer A549 cells. Biochem. Pharmacol. 2003;66:115–124. doi: 10.1016/S0006-2952(03)00254-5. [DOI] [PubMed] [Google Scholar]
- 16.Xu F., Jia Y., Wen Q., Wang X., Zhang L., Zhang Y., Yang K., Xu W. Synthesis and biological evaluation of N-(4-hydroxy-3-mercaptonaphthalen-1-yl)amides as inhibitors of angiogenesis and tumor growth. Eur. J. Med. Chem. 2013;64:377–388. doi: 10.1016/j.ejmech.2013.03.043. [DOI] [PubMed] [Google Scholar]
- 17.Hajimahdi Z., Zarghi A., Zabihollahi R., Aghasadeghi M.R. Synthesis, biological evaluation, and molecular modeling studies of new 1,3,4-oxadiazole- and 1,3,4-thiadiazole-substituted 4-oxo-4H-pyrido[1,2-a]pyrimidines as anti-HIV-1 agents. Med. Chem. Res. 2013;22:2467–2475. doi: 10.1007/s00044-012-0241-5. [DOI] [Google Scholar]
- 18.Song Y., Connor D.T., Sercel A.D., Sorenson R.J., Doubleday R., Unangst P.C., Roth B.D., Beylin V.G., Gilbertsen R.B., Chan K., et al. Synthesis, structure-activity relationships, and in vivo evaluations of substituted di-tert-butylphenols as a novel class of potent, selective, and orally active cyclooxygenase-2 inhibitors. 2. 1,3,4- and 1,2,4-thiadiazole series. J. Med. Chem. 1999;42:1161–1169. doi: 10.1021/jm980570y. [DOI] [PubMed] [Google Scholar]
- 19.Labanauskas L., Kalcas V., Udrenaite E., Gaidelis P., Brukstus A., Dauksas V. Synthesis of 3-(3,4-dimethoxyphenyl)-1H-1,2,4-triazole-5-thiol and 2-amino-5-(3,4-dimethoxyphenyl)-1,3,4-thiadiazole derivatives exhibiting anti-inflammatory activity. Pharmazie. 2001;56:617–619. [PubMed] [Google Scholar]
- 20.Hanna M.A., Girges M.M., Rasala D., Gawinecki R. Synthesis and pharmacological evaluation of some novel 5-(pyrazol-3-yl)thiadiazole and oxadiazole derivatives as potential hypoglycemic agents. Arzneimittelforschung. 1995;45:1074–1078. doi: 10.1002/chin.199607149. [DOI] [PubMed] [Google Scholar]
- 21.Khan M.U., Akhtar T., Al-Masoudi N.A., Stoeckli-Evans H., Hameed S. Synthesis, crystal structure and anti-HIV activity of 2-adamantyl/adamantylmethyl-5-aryl-1,3,4-oxadiazoles. Med. Chem. 2012;8:1190–1197. doi: 10.2174/1573406411208061190. [DOI] [PubMed] [Google Scholar]
- 22.El-Sayed W.A., El-Essawy F.A., Ali O.M., Nasr B.S., Abdalla M.M., Abdel-Rahman A.A. Anti-HIV activity of new substituted 1,3,4-oxadiazole derivatives and their acyclic nucleoside analogues. Z. Naturforsch. C. 2009;64:773–778. doi: 10.1515/znc-2009-11-1203. [DOI] [PubMed] [Google Scholar]
- 23.El-Emam A.A., Al-Deeb O.A., Al-Omar M., Lehmann J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg. Med. Chem. 2004;12:5107–5113. doi: 10.1016/j.bmc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 24.Ravichandran V., Shalini S., Sundram K., Sokkalingam A.D. QSAR study of substituted 1,3,4-oxadiazole naphthyridines as HIV-1 integrase inhibitors. Eur. J. Med. Chem. 2010;45:2791–2797. doi: 10.1016/j.ejmech.2010.02.062. [DOI] [PubMed] [Google Scholar]
- 25.Dimova V., Perisic-Janjic N. Solvatochromism studies on UV spectra of 4,5-disubstituted-1,2,4-triazoline-3-thiones. Maced. J. Chem. Chem. Eng. 2009;28:79–89. [Google Scholar]
- 26.Lindsay D.S., Rippey N.S., Cole R.A., Parsons L.C., Dubey J.P., Tidwell R.R., Blagburn B.L. Examination of the activities of 43 chemotherapeutic agents against Neospora caninum tachyzoites in cultured cells. Am. J. Vet. Res. 1994;55:976–981. [PubMed] [Google Scholar]
- 27.Salgin-Gökşen U., Gökhan-Kelekçi N., Göktaş O., Köysal Y., Kiliç E., Işik S., Aktay G., Ozalp M. 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: Synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg. Med. Chem. 2007;15:5738–5751. doi: 10.1016/j.bmc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Tehranchian S., Akbarzadeh T., Fazeli M.R., Jamalifar H., Shafiee A. Synthesis and antibacterial activity of 1-[1,2,4-triazol-3-yl] and 1-[1,3,4-thiadiazol-2-yl]-3-methylthio-6,7-dihydrobenzo[c]thiophen-4(5H)ones. Bioorg. Med. Chem. Lett. 2005;15:1023–1025. doi: 10.1016/j.bmcl.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 29.Turan-Zitouni G., Kaplancikli Z.A., Yildiz M.T., Chevallet P., Kaya D. Synthesis and antimicrobial activity of 4-phenyl/cyclohexyl-5-(1-phenoxyethyl)-3-[N-(2-thiazolyl)acetamido]thio-4H-1,2,4-triazole derivatives. Eur. J. Med. Chem. 2005;40:607–613. doi: 10.1016/j.ejmech.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Kitani H., Kuroda T., Moriguchi A., Ao H., Hirayama F., Ikeda Y., Kawakita T. Synthesis and structural optimization of 7-(3,3-disubstituted-1-pyrrolidinyl)-1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acids as antibacterial agents. Bioorg. Med. Chem. Lett. 1997;7:515–520. doi: 10.1016/S0960-894X(97)00054-1. [DOI] [Google Scholar]
- 31.Tang T., Chen K.X., Jiang H.L., Ji R.Y. QSAR/QSTR of fluoroquinolones: An example of simultaneous analysis of multiple biological activities using neural network method. Eur. J. Med. Chem. 1988;33:647–658. doi: 10.1016/S0223-5234(98)80023-8. [DOI] [Google Scholar]
- 32.Bielenica A., Stefanska J., Stępień K., Napiórkowska A., Augustynowicz-Kopeć E., Sanna G., Madeddu S., Boi S., Giliberti G., Wrzosek M., et al. Synthesis, cytotoxicity and antimicrobial activity of thiourea derivatives incorporating 3-(trifluoromethyl)phenyl moiety. Eur. J. Med. Chem. 2015;101:111–125. doi: 10.1016/j.ejmech.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 33.Bielenica A., Kedzierska E., Fidecka S., Maluszynska H., Miroslaw B., Koziol A.E., Stefanska J., Madeddu S., Giliberti G., Sanna G. Synthesis, Antimicrobial and Pharmacological Evaluation of Thiourea-derivatives of 4H-1,2,4-triazole. Lett. Drug Des. Discov. 2015;12:263–276. doi: 10.2174/1570180811666141001010044. [DOI] [Google Scholar]
- 34.Stefanska J., Nowicka G., Struga M., Szulczyk D., Koziol A.E., Augustynowicz-Kopec E., Napiorkowska A., Bielenica A., Filipowski W., Filipowska A., et al. Antimicrobial and anti-biofilm activity of thiourea derivatives incorporating a 2-aminothiazole scaffold. Chem. Pharm. Bull. 2015;63:225–236. doi: 10.1248/cpb.c14-00837. [DOI] [PubMed] [Google Scholar]
- 35.Uto Y., Ueno Y., Kiyotsuka Y., Miyazawa Y., Kurata H., Ogata T., Yamada M., Deguchi T., Konishi M., Takagi T., et al. Synthesis and evaluation of novel stearoyl-CoA desaturase 1 inhibitors: 1′-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl]pyridazin-3-yl}-3,4-dihydrospiro[chromene-2,4′-piperidine] analogs. Eur. J. Med. Chem. 2010;45:4788–4796. doi: 10.1016/j.ejmech.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 36.Yale H.L., Losee K., Martins J., Holsing M., Perry F.M., Bernstein J. Chemotherapy of experimental tuberculosis. VIII. The synthesis of acid hydrazides, Their derivatives and related compounds. J. Am. Chem. Soc. 1953;75:1933–1934. doi: 10.1021/ja01104a046. [DOI] [Google Scholar]
- 37.Dobosz M., Struga M., Chodkowska A., Jagiełło-Wójtowicz E., Stepniak J., Koziol A.E. Synthesis and some pharmacological properties of 3-(4-phenyl-5-oxo-1,2,4-triazolin-1-ylmethyl)-1,2,4-triazolin-5-thione derivatives. Acta Pol. Pharm. 2002;59:281–290. [PubMed] [Google Scholar]
- 38.Plech T., Kaproń B., Paneth A., Wujec M., Czarnomysy R., Bielawska A., Bielawski K., Trotsko N., Kuśmierz E., Paneth P. Search for human DNA topoisomerase II poisons in the group of 2,5-disubstituted-1,3,4-thiadiazoles. J. Enzym. Inhib. Med. Chem. 2015;30:1021–1026. doi: 10.3109/14756366.2014.995179. [DOI] [PubMed] [Google Scholar]
- 39.Isobe T., Ishikawa T. 2-Chloro-1,3-dimethylimidazolium chloride. 2. Its application to the construction of heterocycles through dehydration reactions. J. Org. Chem. 1999;64:6989–6992. doi: 10.1021/jo9909756. [DOI] [Google Scholar]
- 40.Gavrilyuk J.I., Lough A.J., Batey R.A. Parallel Solution Phase Synthesis of a Library of Amino acid derived 2-Arylamino-[1,3,4]-oxadiazoles. Tetrahedron Lett. 2008;49:4746–4749. doi: 10.1016/j.tetlet.2008.05.110. [DOI] [Google Scholar]
- 41.National Committee for Clinical Laboratory Standards . Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts, 2004. National Committee for Clinical Laboratory Standards; Wayne, PA, USA: 2009. Approved Guideline. NCCLS document M44-A. [Google Scholar]
- 42.CrysAlis PRO. Agilent Technologies Ltd.; Yarnton, UK: 2014. [Google Scholar]
- 43.Sheldrick G.M. A short history of SHELX. Acta Crystallogr. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 44.Sobi M., El-sayed R., Abdullah M. The Effect of Non Ionic Surfactants Containing Triazole, Thiadiazole and Oxadiazole as Inhibitors of the Corrosion of Carbon Steel in 1M Hydrochloric Acid. J. Surfactants Deterg. 2013;16:937–946. doi: 10.1007/s11743-013-1468-y. [DOI] [Google Scholar]
- 45.Drzewiecka A., Koziol A.E., Borowski P., Sanna G., Giliberti G., La Colla P., Zawadowski T., Struga M. Structural and antivirial studies of dipetalactone and its methyl derivative. J. Mol. Struct. 2013;150:1054–1055. doi: 10.1016/j.molstruc.2013.09.020. [DOI] [Google Scholar]
- 46.Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., de Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.