Abstract
We synthesized oligomeric anthocyanins from grape skin-derived monomeric anthocyanins such as anthocyanidin and proanthocyanidin by a fermentation technique using Aspergillus niger, crude enzymes and glucosidase. The biosyntheses of the oligomeric anthocyanins carried out by the conventional method using Aspergillus niger and crude enzymes were confirmed by ESI-MS. The molecular weight of the synthesized anthocyanin oligomers was determined using MALDI-MS. The yield of anthocyanin oligomers using crude enzymes was higher than that of the synthesis using Aspergillus fermentation. Several studies have been demonstrated that oligomeric anthocyanins have higher antioxidant activity than monomeric anthocyanins. Fermentation-based synthesis of oligomeric anthocyanins is an alternative way of producing useful anthocyanins that could support the food industry.
Keywords: oligomeric anthocyanin, fermentation, crude enzyme, Aspergillus niger, glucosidase
1. Introduction
Anthocyanins are naturally occurring water-soluble plant pigments belonging to the group of phytochemicals known as flavonoids [1]. Anthocyanins are present in many plants which display colorful flowers, and different kinds of fruits and vegetables [2,3,4]. The quality and nutritional value of fruits and their products is commonly associated with the color that is derived from anthocyanins [5,6]. Anthocyanins are very useful for the food industry, due to their good water solubility and safety. They have been recognized internationally for their applications, including the replacement of synthetic colorants [7,8]. Anthocyanins have antioxidant activity which contributes to many biological activities such as anticancer, cardiovascular protection, ocular protection and protection against some other chronic diseases [9,10,11,12]. Several studies have been demonstrated that the oligomeric derivatives of anthocyanin have higher activity than the monomeric versions. For example, the anthocyanin oligomers derived from bilberry fruit such as small anthocyanidin glycoside polymers, particularly in the form of dimers, trimers, tetramers and pentamers have higher antioxidant activity than the monomers. These compounds are highly hydro- and liposoluble in nature and are not known to accumulate in the human body [13].
The biosynthesis of oligomeric anthocyanins is the best alternative to overcome the problem of deficiency. At present, studies on the synthesis of anthocyanin oligomers are scarce, and only one related paper is available [13]. Aspergillus species such as Aspergillus niger, A. sojae and A. oryzae have long been used for the production of traditional fermented foods such as doenjang, cheonggukjang, soy sauce and sake in Asian countries [14,15]. Fungi are rich sources of citric acid [16], C8 volatiles [14] and many enzymes such as xylanase, cellulose [17], amyloglucosidase and exopolygalacturonase [18]. However, in the industrial applications of these fungi overcoming their contamination is a big challenge. The present study focuses on the synthesis of oligomeric anthocyanins by fermentation of monomeric anthocyanins with Aspergillus niger, as well as with crude enzymes derived from the fungus.
2. Results and Discussion
The oligomeric anthocyanins were successfully synthesized by fermentation using Aspergillus niger (Figure 1) as well as crude enzyme (Figure 2) as confirmed by ESI-MS (Figure 3 and Figure 4). The oligomeric anthocyanins showed higher peak values and higher molecular weight than the monomeric anthocyanins. The higher peak value might be attributed to the presence of higher amount of oligomeric anthocyanins under similar experimental conditions [19]. It was confirmed that the yield of oligomeric anthocyanins derived from the fermentation by crude enzyme was better than that derived from the fermentation with Aspergillus niger (Table 1).
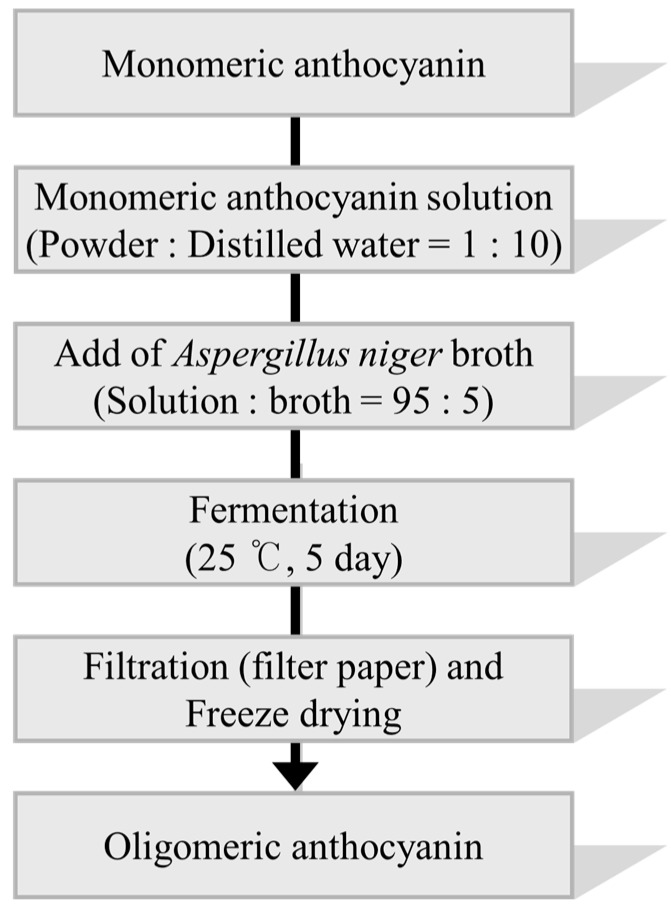
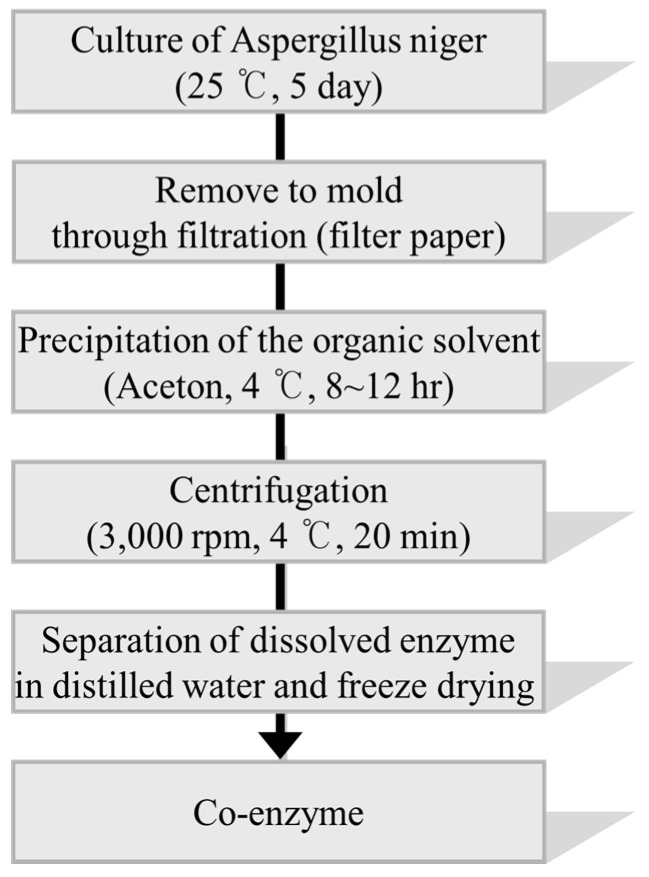
Figure 1.
The flow chart of oligomeric anthocyanin synthesis using Aspergillus niger.
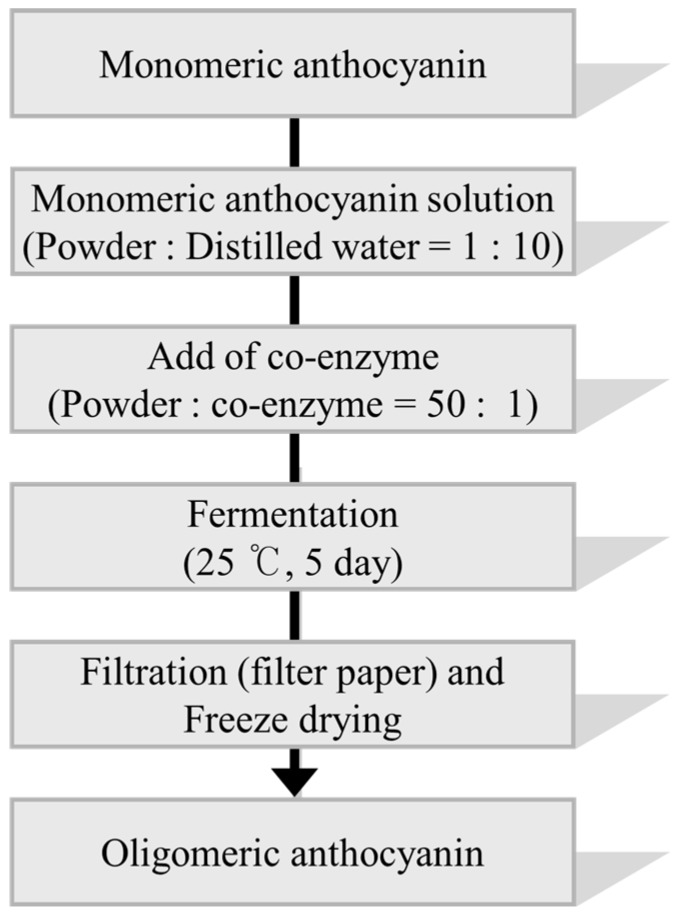
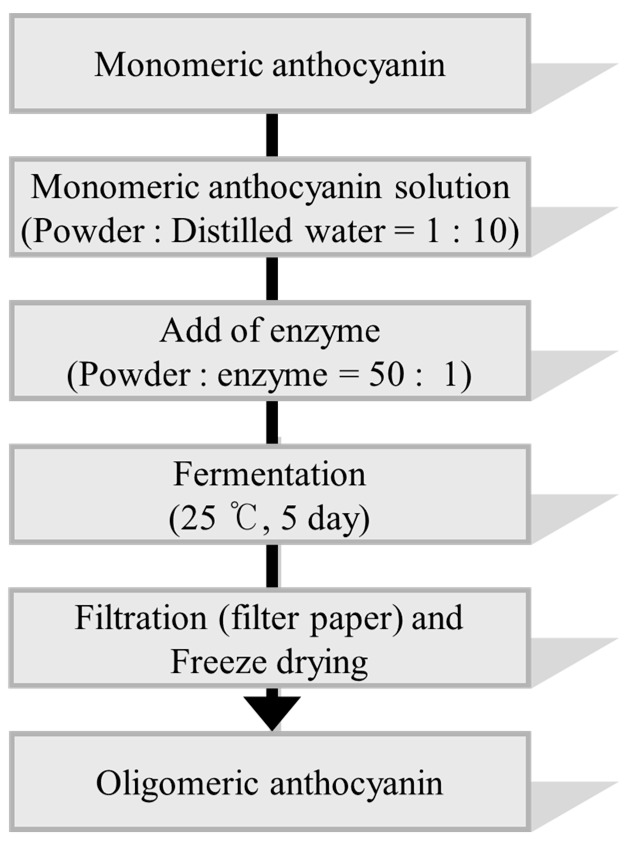
Figure 2.
The flow chart for the synthesis of oligomeric anthocyanins using crude enzyme from Aspergillus niger.
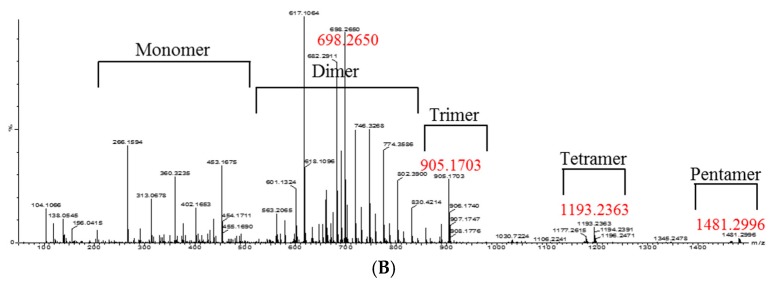
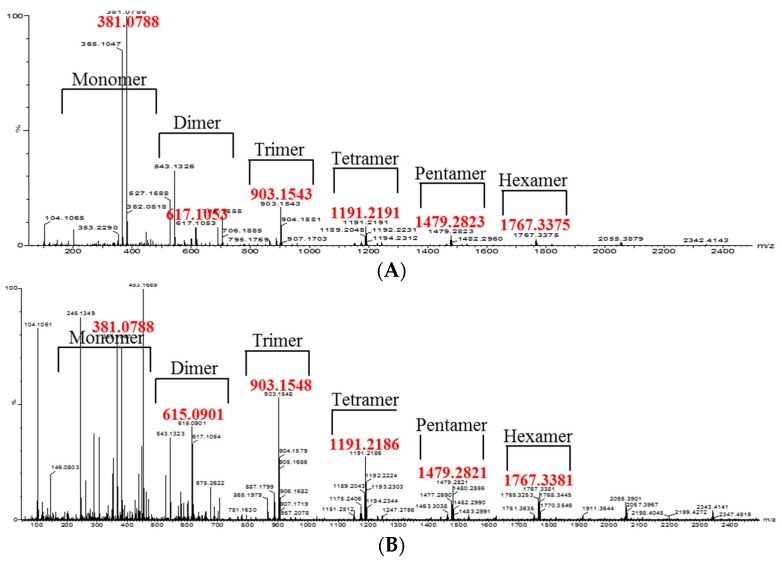
Figure 3.
The ESI-MS observation of synthesis of oligomeric anthocyanins from monomeric anthocyanin using Aspergillus niger. (A) Before biosynthesis; (B) after biosynthesis.
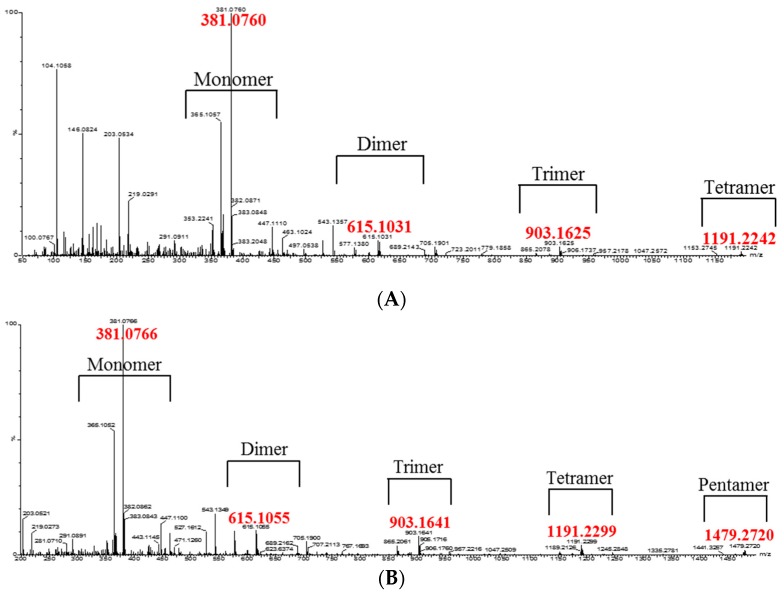
Figure 4.
The synthesis of oligomeric anthocyanins from another monomeric anthocyanin (proanthocyanidin) using crude enzyme derived from Aspergillus niger. (A) Before biosynthesis; (B) after biosynthesis.
Table 1.
The yield of oligomeric anthocyanins derived by fermentation as well as crude enzyme.
| Fermentation | Monomeric Anthocyanin (g) | Oligomeric Anthocyanin (g) | Yield (%) |
|---|---|---|---|
| Aspergillus niger | 10.1254 | 7.9835 | 78.27 ± 1.99 a |
| 5.0354 | 4.0235 | ||
| 1.0024 | 0.7624 | ||
| Crude enzyme | 10.0038 | 8.7685 | 87.93 ± 0.36 b |
| 2.1539 | 1.9025 | ||
| 1.0657 | 0.9357 | ||
| Glucosidase | 10.0387 | 8.5168 | 85.11 ± 0.45 b |
| 5.1348 | 4.3578 | ||
| 1.0222 | 0.8753 |
Different letters indicate significant different (p < 0.05) among samples as determined by the t-test. Values are the mean ± SD of triplicate determinations.
We have previously reported the synthesis and characterization of anthocyanin oligomers produced by A. niger fermentation using anthocyanin monomers as substrate [13]. The molecular weight of the anthocyanin oligomers was determined using Matrix Assisted Laser Desorption/Ionization Mass Spectrometry (MALDI-MS) [20]. In this study, the biosynthesis of oligomeric anthocyanins was detected using the relative absorbance values of compounds estimated by ESI-MS. ESI-MS is an important technique to detect femtomole quantities of sample, including non-volatile and thermally labile biomolecules that are difficult to analysis by other conventional techniques [21]. Liu et al. [22] detected the monomers, dimers, tetramers and hexamers of purified oligomeric proanthocyanins using ESI-MS. Therefore, the present study also used ESI-MS to analyze the various structures of the oligomeric proanthocyanins.
The monomeric anthocyanins such as anthocyanidin and proanthocyanidin give peaks at 288 m/z (Figure 3A) and 381 m/z (Figure 4A), respectively. The oligomeric anthocyanins synthesized from anthocyanidin monomers showed the peak values of m/z 905, 1193 (Figure 3B). Similarly, the oligomeric anthocyanins synthesized from the other monomer (proanthocyanidin) showed the following highest peak values: m/z 903, 1191, 1479 (Figure 4B). Therefore, the differentiation of peak values between before and after fermentation confirmed the synthesis of oligomeric anthocyanins using crude enzyme as well as fermentation with Aspergillus niger. The amount of oligomeric anthocyanin synthesized from fermented crude enzyme was higher than that synthesized from fermentation with Aspergillus niger.
The enzyme for the synthesis of oligomeric anthocyanin (Figure 5 and Figure 8) was electrophoresed by SDS-PAGE (Figure 6 and Figure 7) and the tryptic peptides obtained from each gel slice (Figure 7) were analyzed by LC-MS/MS run on a Q-STAR Pulsar ESI-hybrid Q-TOF instrument (Table 2). According our literature search, some of the carbohydrate hydrolases mentioned in Table 2 were found to produce condensation reactions [23]. After the synthesis (Figure 8), the content of anthocyanin was determined based on the presence of glucosidase in the product (Figure 9). The results demonstrate the similarity of pattern between Figure 3 and Figure 4. Figure 9 indicates a difference in molecular weight of m/z 288 for each oligomeric anthocyanin peak, which corresponds to the molecular weight of cyanidin. Consequently, the structure of anthocyanins was presumed to be as shown in Figure 10.
Figure 5.
The flowchart of method to obtain the crude enzyme from Aspergillus niger.
Figure 8.
The flow chart for the synthesis of oligomeric anthocyanins using glucosidase from Aspergillus niger.
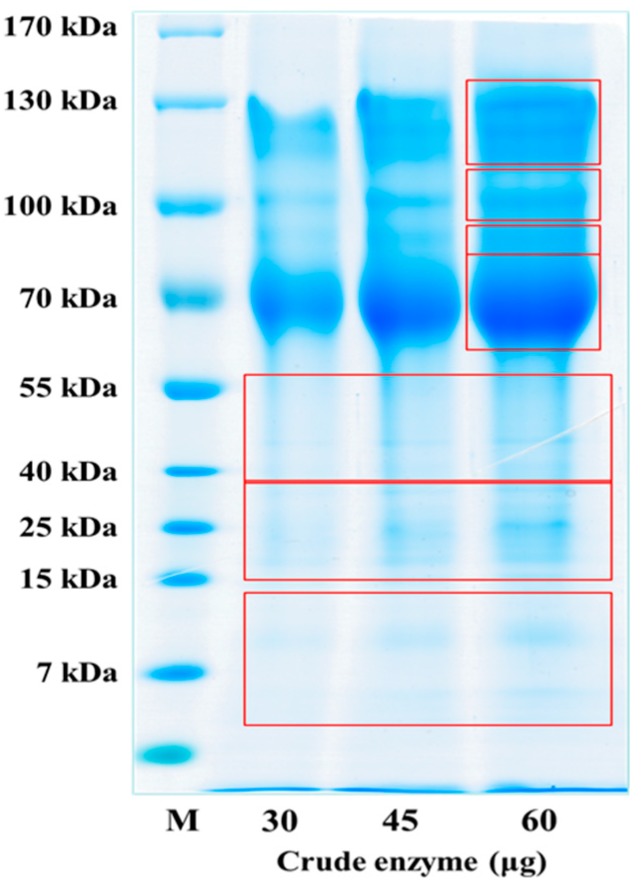
Figure 6.
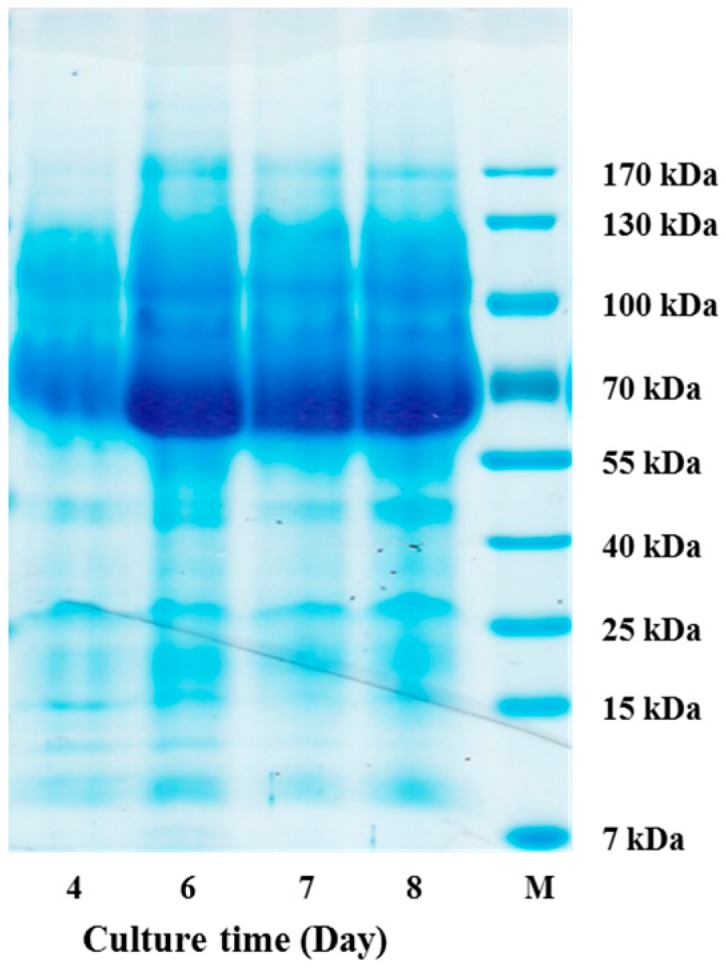
Confirmation of the crude enzyme using SDS-PAGE for each culture day.
Figure 7.
Separation of co-enzyme using SDS-PAGE.
Table 2.
Identification of proteins recovered from the secretory proteins from Aspergillus niger.
| Gene I.D. | Protein Name | Probability | Molecular Weight |
|---|---|---|---|
| gi|224027 | Glucoamylase G1 | 627 | 65,448 |
| gi|134081727 | Unnamed protein product [Aspergillus niger] | 274 | 75,190 |
| gi|765328 | Acid phosphatase, orthophosphoric monoester phosphohydrolase, APase (EC 3.1.3.2) [Aspergillus ficuum, NRRL 3135, Peptide, 583 aa] | 265 | 64,211 |
| gi|257187 | α-Glucosidase P2 subunit, ANP P2 subunit {EC 3.2.1.20} [Aspergillus niger, Peptide, 719 aa] | 181 | 79,656 |
| gi|2344 | Preproglucoamylase G2 [Aspergillus niger] | 531 | 56,695 |
| gi|145242978 | Hypothetical protein ANI_1_1546094 [Aspergillus niger CBS 513.88] | 351 | 59,208 |
| gi|145231236 | Phospholipase C PLC-C [Aspergillus niger CBS 513.88] | 410 | 49,652 |
| gi|145235505 | Serine carboxypeptidase [Aspergillus niger CBS 513.88] | 297 | 62,560 |
| gi|145252338 | Phosphatidylglycerol specific phospholipase [Aspergillus niger CBS 513.88] | 261 | 53,895 |
| gi|4185610 | Phytase [Aspergillus niger] | 218 | 50,997 |
| gi|145241119 | 3-Phytase B [Aspergillus niger CBS 513.88] | 256 | 52,453 |
| gi|145241490 | 1,3-β-Glucanosyltransferase gel3 [Aspergillus niger CBS 513.88] | 161 | 56,721 |
| gi|83655609 | Acid phosphatase [Aspergillus niger] | 142 | 52,725 |
| gi|145242970 | Hypothetical protein ANI_1_1540094 [Aspergillus niger CBS 513.88] | 128 | 45,753 |
| gi|145256696 | Protein ecm33 [Aspergillus niger CBS 513.88] | 125 | 41,026 |
| gi|317026828 | Serine-type carboxypeptidase F [Aspergillus niger CBS 513.88] | 118 | 57,756 |
| gi|145248273 | Polyamine oxidase [Aspergillus niger CBS 513.88] | 110 | 58,728 |
| gi|145248205 | Aspartic-type endopeptidase opsB [Aspergillus niger CBS 513.88] | 104 | 50,958 |
| gi|145234270 | Glutaminase GtaA [Aspergillus niger CBS 513.88] | 99 | 75,470 |
| gi|350633205 | Hypothetical protein ASPNIDRAFT_55058 [Aspergillus niger ATCC 1015] | 87 | 22,487 |
| gi|350631594 | Hypothetical protein ASPNIDRAFT_53033 [Aspergillus niger ATCC 1015] | 63 | 57,162 |
| gi|145235707 | FAD binding domain protein [Aspergillus niger CBS 513.88] | 59 | 61,292 |
| gi|145233743 | α-Galactosidase B [Aspergillus niger CBS 513.88] | 392 | 48,796 |
| gi|317031802 | Histidine acid phosphatase [Aspergillus niger CBS 513.88] | 153 | 53,047 |
| gi|317025164 | Aspartic endopeptidase (AP1) [Aspergillus niger CBS 513.88] | 483 | 46,701 |
| gi|145242664 | Sulphydryl oxidase [Aspergillus niger CBS 513.88] | 264 | 43,471 |
| gi|74626383 | RecName: Probable α-galactosidase B; AltName: Melibiase B; Flags: Precursor | 175 | 48,753 |
| gi|134083538 | Unnamed protein product [Aspergillus niger] | 173 | 45,226 |
| gi|400801 | RecName: Pectin lyase A; Short=PLA; AltName: Full-Pectinlyase II; Short=PLII; Flags: Precursor | 135 | 39,830 |
| gi|145235303 | Hypothetical protein ANI_1_496034 [Aspergillus niger CBS 513.88] | 103 | 52,301 |
| gi|134055991 | Unnamed protein product [Aspergillus niger] | 85 | 41,620 |
| gi|134076313 | Unnamed protein product [Aspergillus niger] | 85 | 45,581 |
| gi|145251519 | Phosphoglycerate mutase family protein [Aspergillus niger CBS 513.88] | 79 | 19,282 |
| gi|350633205 | Hypothetical protein ASPNIDRAFT_55058 [Aspergillus niger ATCC 1015] | 73 | 22,487 |
| gi|145232359 | Endopolygalacturonase C [Aspergillus niger CBS 513.88] | 241 | 37,796 |
| gi|145235523 | Glucan endo-1,3-β-glucosidase eglC [Aspergillus niger CBS 513.88] | 129 | 46,778 |
| gi|145230419 | Glycosidase crf1 [Aspergillus niger CBS 513.88] | 107 | 39,862 |
| gi|129935 | RecName: Full=Endopolygalacturonase II; Short=EPG-II; AltName: Full=Pectinase 2; AltName: Full=PolygalacturonaseII; Short=PG-II; AltName: Full=Polygalacturonase X2; Flags: Precursor | 89 | 37,489 |
| gi|133176 | RecName: Full=Ribonuclease M; Short=RNase M | 89 | 26,590 |
| gi|134055750 | Unnamed protein product [Aspergillus niger] | 84 | 27,072 |
| gi|145229151 | Endo-1,3(4)-β-glucanase [Aspergillus niger CBS 513.88] | 83 | 46,311 |
| gi|134075575 | Hypothetical protein An07g00170 [Aspergillus niger] | 69 | 90,993 |
| gi|134083538 | Unnamed protein product [Aspergillus niger] | 67 | 45,226 |
| gi|145252266 | GPI anchored cell wall protein [Aspergillus niger CBS 513.88] | 64 | 19,022 |
| gi|83638302 | Xylanase [Aspergillus phoenicis] | 117 | 10,944 |
| gi|350633205 | Hypothetical protein ASPNIDRAFT_55058 [Aspergillus niger ATCC 1015] | 92 | 22,487 |
Figure 9.
The synthesis of oligomeric anthocyanin from monomeric anthocyanin using glucosidase from Aspergillus niger. (A) Before biosynthesis; (B) after biosynthesis.
Figure 10.

The structure of oligomeric anthocyanin.
Comparing the yield of the oligomeric anthocyanins, it was seen that the highest yield was obtained using crude enzyme (Table 1), but crude enzymes are difficult to obtain. Therefore, it was thought that it might be more economical to use a commercially available glucosidase. In addition, as seen in Figure 4B and Figure 9B, the oligomeric anthocyanins synthesized with glucosidase showed higher oligomer content than oligomeric anthocyanin synthesized with crude enzyme.
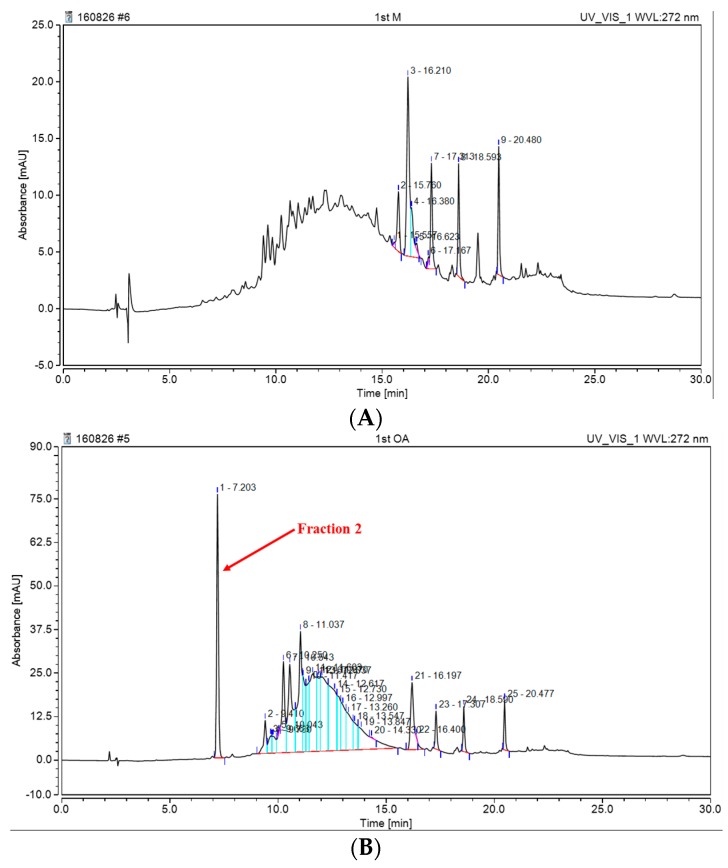
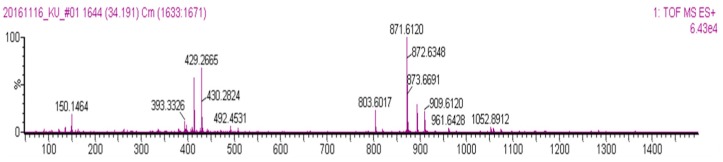
The comparison of monomeric and oligomeric anthocyanins using HPLC with UV detection confirmed that the fractions have different patterns (Figure 11). Different patterns were fractionated and analyzed by LC/MS. In the second fraction, a single substance showing a molecular weight of m/z 429, 871 was identified at 7 min (Figure 12). It was assumed that the compound with molecular weight m/z 871 was the dimer and that with m/z 429 was the monomer. This assumption was correlated to the results obtained by ESI-MS for the m/z 871 peak (Figure 13A). Based on the results of Figure 13, the m/z 429 compound consisted of anthocyanidin (m/z 310) and glucose. The m/z 871 peak is the dimeric form of the m/z 429 species. An NMR study is needed for better understanding of these molecular structures.
Figure 11.
HPLC patterns of monomeric and oligomeric anthocyanins. (A) Before biosynthesis; (B) after biosynthesis using glucosidase.
Figure 12.
LC/MS analysis of two fractions of the oligomeric anthocyanin.
Figure 13.
ESI-MS analysis of 871 m/z material. (A) m/z 871 peak of the LC/MS analysis results; (B) m/z 927 peak of the ESI-MS analysis results.
In summary, the biosynthesis of oligomeric anthocyanins using fermentation is an alternative approach to overcome the problem of their natural scarcity to avoid the overexploitation of natural resources.
3. Materials and Methods
3.1. Materials
5-Dimethyl-1-pyrroline-N-oxide (DMPO), FeSO4, and H2O2 were purchased from Sigma Chemical Co. (St. Louis, MO, USA). KH2PO4, KCl and NaCl were purchased from Junsei (Tokyo, Japan). Saccharose, dextrose, urea, MgSO4, MnSO4, and ZnSO4 were purchased from Deajung (Siheung, Korea). Peptone G was purchased from Acumedia (Lansing, MI, USA). The grape skin- derived anthocyanins were purchased from Kitolife Co. Ltd. (Pyeongtaek, Korea).
3.2. Culture Medium of Aspergillus niger
The Aspergillus niger culture was maintained at 25 °C in a shaking incubator. The culture media contained saccharose (60 g/L), peptone G (10 g/L), dextrose (5 g/L), urea (0.5 g/L), MgSO4 (0.25 g/L), KH2PO4 (0.04 g/L), KCl (0.075 g/L), NaCl (0.075 g/L), MnSO4 (0.01 g/L) and ZnSO4 (0.005 g/L).
3.3. Synthesis of Oligomeric Anthocyanin by Fermentation Using Aspergillus niger
Synthesis of oligomeric anthocyanins using Aspergillus niger was described by Lee et al. [13]. In this study, monomeric anthocyanins such as anthocyanindins and proanthocyanidins were used to synthesize oligomeric anthocyanins. The monomeric anthocyanin powders were fermented with Aspergillus niger at 25 °C in a shaking incubator for 5 days. The fermented cultures were centrifuged at 3000 rpm, 4 °C, for 20 min. The supernatants were filtered with Whatman No. 41 filter paper and the filtrate was freeze-dried by a freeze drier system (SFDSM06, Samwon, Busan, Korea) in order to obtain the synthesized oligomeric anthocyanins. The concentrations of fermented oligomeric anthocyanin produced by fermentation were estimated using Electrospray Ionization-Mass Spectrometry (ESI-MS) at the Korea Basic Science Institute (KBSI, Ochang, Korea). The molecular mass values of the compounds were analyzed by a Synapt G2 HDMS quadrupole time-of-flight (TOF) mass spectrometer equipped with an electrospray ion source (Waters, Milford, MA, UK) in positive ion mode at a spray voltage of 2.5 kV. MS spectra were obtained with the capillary heated to 150 °C. The instrument was calibrated using NaF solution. The sample was dissolved in 100% MeOH and introduced by direct infusion at a flow rate of 20 μL/min into the ion source operating in positive mode. All spectra were acquired at a range of 50 to 2500 m/z. Leucine enkephalin was used as the lock mass for the exact mass measurement correction.
3.4. Separation of Fermented Crude Enzyme from the Culture of Aspergillus niger
The fungal strain Aspergillus niger was cultured in 100 mL saccharose medium or potato dextrose agar (PDA) medium and incubated at 25 °C, for 7 days in an shaking incubator. The Aspergillus niger culture medium was centrifuged at 3000 rpm, 4 °C, for 20 min. The supernatant was precipitated with an equal volume of acetone at 4 °C, overnight (10–12 h) and this mixture was then centrifuged at 3000 rpm, 4 °C for 20 min. After removal of the supernatant, the pellet was dissolved using 5 mL of distilled water and further centrifuged at 13,000 rpm, 4 °C, for 5 min. The supernatant (crude enzyme) was freeze-dried in order to synthesize the oligomeric anthocyanin.
3.5. Synthesis of Oligomeric Anthocyanin Using Crude Enzyme
The anthocyanin powder was fermented with crude enzyme at 25 °C in a shaking incubator for 7 days. The fermented stuff was centrifuged at 4 °C, 3000 rpm for 20 min. The supernatant was filtered with Whatman No. 41 filter paper and the filtrate was freeze-dried in a SFDSM06 freeze drier system in order to obtain the synthesized oligomeric anthocyanins. The concentration of oligomeric anthocyanin was examined by ESI-MS at KBSI.
3.6. Analysis of Crude Enzyme from the Culture of Aspergillus niger
SDS-PAGE gel slicing was used for LC-MS/MS analysis of the secretory proteins from Aspergillus niger. The soluble proteins in urea lysis buffer containing 8 M urea and 4% CHAPS after the acetone precipitation of the secretory fraction from Aspergillus niger was subjected to 6–15% SDS-PAGE and stained with colloidal Coomassie solution. The lanes, which are cut as nine slices, were excised from the gel in three of the protein lanes for the mass spectrometry experiments by destaining and in-gel digestion followed by peptide extraction. The tryptic peptides obtained from each gel slice were analyzed by LC-MS/MS running on the Q-STAR Pulsar ESI-hybrid Q-TOF instrument.
3.7. Synthesis of Oligomeric Anthocyanin Using Various Enzymes
The anthocyanin powder was fermented with various enzymes at 25 °C in a shaking incubator for 7 days. The fermented product was centrifuged at 4 °C, 3000 rpm for 20 min. The supernatant was filtered with Whatman No. 41 filter paper and the filtrate was freeze-dried by a freeze dry system (SFDSM06) in order to obtain the synthesized oligomeric anthocyanins The concentration of oligomeric anthocyanin was examined by ESI-MS at KBSI.
3.8. Isolation of Oligomeric Anthocyanin and Analysis
The monomeric and oligomeric anthocyanins were further isolated using reversed-phase HPLC (RP-HPLC) on a C18 column (4.0 × 250 mm) with a linear gradient of MeOH (0–60%) at a flow rate of 1.0 mL/min. The eluted peaks were detected at 272 nm. The collected samples were pooled and concentrated using a rotary evaporator, then lyophilized for 3 days. The lyophilized sample was further analyzed by LC/MS followed by ESI-MS at KBSI.
3.9. Statistical Analysis
The statistical analyses was carried out by the paired t-test (p < 0.05) and comparisons made between monomeric anthocyanins and oligomeric anthocyanins. The data are presented as mean ± SD. All analyses were performed using the SPSS software (SPSS Institute, Chicago, IL, USA).
4. Conclusions
Our results indicate that the synthesis of oligomeric anthocyanins using glucosidase from A. niger is better than that possible with fermentation of A. niger. Synthesis of oligomeric anthocyanins was confirmed by ESI-MS and HPLC analysis. The present study successfully overcome the problem of fungal contamination during synthesis of oligomeric anthocyanins. Further studies are however required to assess the biological activities of the produced oligomeric anthocyanins.
Acknowledgments
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bio industry Technology Development program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (114068-3).
Author Contributions
The experimental part was carried out by Jin-Woo Hwang, Yon-Suk Kim and Jae Woong Lee; The results analyzed and interpreted by Eun-Kyung Kim, Sang-Ho Moon and Byong-Tae Jeon; The article was written by Jin-Woo Hwang and Sithranga Boopathy Natarajan and the work was designed by Pyo-Jam Park.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available.
References
- 1.Peterson J., Dwyer J. Flavonoids: Dietary occurrence and biochemical activity. Nutr. Res. 1998;18:1995–2018. doi: 10.1016/S0271-5317(98)00169-9. [DOI] [Google Scholar]
- 2.Delgado-Vargas F., Jiménez A.R., Paredes-López O. Natural pigments: Carotenoids, anthocyanins, and betalains—Characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000;40:173–289. doi: 10.1080/10408690091189257. [DOI] [PubMed] [Google Scholar]
- 3.Glover B.J., Martin C. Anthocyanins. Curr. Biol. 2012;22:147–150. doi: 10.1016/j.cub.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Scotter M.J. Methods for the determination of European Union-permitted added natural colours in foods: A review. Food Addit. Contam. Part A. 2011;28:527–596. doi: 10.1080/19440049.2011.555844. [DOI] [PubMed] [Google Scholar]
- 5.Kovačević D.B., Putnik P., Dragović-Uzelac V., Vahčić N., Babojelić M.S., Levaj B. Influences of organically and conventionally grown strawberry cultivars on anthocyanins content and color in purees and low-sugar jams. Food Chem. 2015;181:94–100. doi: 10.1016/j.foodchem.2015.02.063. [DOI] [PubMed] [Google Scholar]
- 6.Kovačević D.B., Putnik P., Dragović-Uzelac V., Pedisić S., Jambrak A.R., Herceg Z. Effects of cold atmospheric gas phase plasma on anthocyanins and color in pomegranate juice. Food Chem. 2016;190:317–323. doi: 10.1016/j.foodchem.2015.05.099. [DOI] [PubMed] [Google Scholar]
- 7.Chou P.H., Matsui S., Misaki K., Matsuda T. Isolation and identification of xenobiotic aryl hydrocarbon receptor ligands in dyeing wastewater. Environ. Sci. Technol. 2007;41:652–657. doi: 10.1021/es061500g. [DOI] [PubMed] [Google Scholar]
- 8.Bridle P., Timberlake C. Timberlake, Anthocyanins as natural food coloursselected aspects. Food Chem. 1997;58:103–109. doi: 10.1016/S0308-8146(96)00222-1. [DOI] [Google Scholar]
- 9.Kong J.M., Chia L.S., Goh N.K., Chia T.F., Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–933. doi: 10.1016/S0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- 10.Lila M.A. Anthocyanins and human health: An in vitro investigative approach. Biomed. Res. Int. 2004;2004:306–313. doi: 10.1155/S111072430440401X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen P.N., Chu S.C., Chiou H.L., Kuo W.H., Chiang C.L., Hsieh Y.S. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 2006;235:248–259. doi: 10.1016/j.canlet.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Hou D.X. Potential mechanisms of cancer chemoprevention by anthocyanins. Curr. Mol. Med. 2003;3:149–159. doi: 10.2174/1566524033361555. [DOI] [PubMed] [Google Scholar]
- 13.Lee J.H., Lee H.K., Kim C.Y., Hong Y.J., Choe C.M., You T.W., Seong G.J. Purified high-dose anthocyanoside oligomer administration improves nocturnal vision and clinical symptoms in myopia subjects. Br. J. Nutr. 2005;93:895–899. doi: 10.1079/BJN20051438. [DOI] [PubMed] [Google Scholar]
- 14.Günes O., Yüceer Y.K. Biosynthesis of eight-carbon volatiles from tomato and pepper pomaces by fungi: Trichoderma atroviride and Aspergillus sojae. J. Biosci. Bioeng. 2017 doi: 10.1016/j.jbiosc.2016.11.013. in press. [DOI] [PubMed] [Google Scholar]
- 15.Lee S.M., Lee S.R., Singh D., Oh J.Y., Jeon E.J., Ryu H.S., Lee D.W., Kim B.S., Lee C.H. Comparative evaluation of microbial diversity and metabolite profiles in doenjang, a fermented soybean paste, during the two different industrial manufacturing processes. Food Chem. 2017;221:1578–1586. doi: 10.1016/j.foodchem.2016.10.135. [DOI] [PubMed] [Google Scholar]
- 16.Wang B., Chen J., Li H., Sun F., Li Y., Shi G. Pellet-dispersion strategy to simplify the seed cultivation of Aspergillus niger and optimize citric acid production. Bioprocess. Biosyst. Eng. 2017;40:45–53. doi: 10.1007/s00449-016-1673-y. [DOI] [PubMed] [Google Scholar]
- 17.Paulina L., José Manuel S., Armando V., José Manuel D., Isabel B. Ultrasounds pretreatment of olive pomace to improve xylanase and cellulase production by solid-state fermentation. Bioresour. Technol. 2016;214:737–746. doi: 10.1016/j.biortech.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Colla E., Santos L.O., Deamici K., Magagnin G., Vendruscolo M., Costa J.A.V. Simultaneous production of Amyloglucosidase and Exo-Polygalacturonase by Aspergillus niger in a rotating Drum reactor. Appl. Biochem. Biotechnol. 2017;181:627–637. doi: 10.1007/s12010-016-2237-y. [DOI] [PubMed] [Google Scholar]
- 19.Steaphane V., Emmanuelle M., Vea Ronique C., George S., Yoji H. Mass spectrometric evidence for the existence of oligomeric anthocyanins in grape skins. J. Agric. Food Chem. 2004;52:7144–7151. doi: 10.1021/jf048939h. [DOI] [PubMed] [Google Scholar]
- 20.Hwang J.W., Kim E.K., Lee S.J., Kim Y.S., Moon S.H., Jeon B.T., Sung S.H., Kim E.T., Park P.J. Antioxidant activity and protective effect of anthocyanin oligomers on H2O2 triggered G2/M arrest in retinal cells. J. Agric. Food Chem. 2012;60:4282–4288. doi: 10.1021/jf205321j. [DOI] [PubMed] [Google Scholar]
- 21.Ho C.S., Lam C.W.K., Chan M.H.M., Cheung R.C.K., Law L.K., Lit L.C.W., Ng K.F., Suen M.W.M., Tai H.L. Electrospray Ionisation Mass Spectrometry: Principles and Clinical Applications. Clin. Biochem. Rev. 2003;24:3–12. [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y., Feng S., Song L., He G., Chen M., Huang D. Secondary Metabolites in Durian Seeds: Oligomeric Proanthocyanidins. Molecules. 2013;18:14172–14185. doi: 10.3390/molecules181114172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seiya C. A historical perspective for the Catalytic reaction mechanism of Glycosidase; So as to bring about breakthrough in Confusing Situation. Biosci. Biotechnol. Biochem. 2012;76:215–231. doi: 10.1271/bbb.110713. [DOI] [PubMed] [Google Scholar]