ABSTRACT
Objective:
The aim of the study is to test the hypothesis of a positive relationship between initial dose of pancreatic enzyme replacement therapy (PERT) in infants with cystic fibrosis (CF) and optimal weight gain over the first 2 years of life.
Methods:
Using the CF Foundation Patient Registry, we identified 502 children born in 2010 and used multivariable models to compare as our primary analysis their 2-year changes in weight-for-age z score (WAZ) and as our secondary analysis weight-for-length percentile (W/L%) by initial PERT dose. We focused on initial dose without reference to subsequent changes in treatment to avoid confounding by indication (severity).
Results:
Initial PERT dose demonstrated a linear relationship to change in WAZ and W/L% at age 2 years. An initial dose of >1500 lipase units/kg/largest meal resulted in a higher likelihood of attaining WAZ at 2 years at or above the birth WAZ (adjusted odds ratio [aOR] 1.87, 95% confidence interval [CI] 1.22–2.86) and at the top quartile for improvement over 2 years in WAZ (aOR 1.90, 95% CI 1.19–3.05). There was no correlation between initial PERT dose and weight at initial PERT encounter (P = 0.35). Findings were similar for W/L% and when the cohort was restricted to infants who began PERT in the first 3 months of life.
Conclusions:
Infants receiving higher initial PERT dose demonstrate better weight-related outcomes, as reflected by attainment of favorable changes in WAZ and W/L%, at age 2 years.
Keywords: comparative effectiveness, infant and childhood nutrition, neonatal nutrition, nutrition, pancreatic insufficiency, weight-for-age z score, weight-for-length percentile
What Is Known
Optimal pancreatic enzyme replacement therapy dosing is essential to minimize malabsorption and maximize growth in children with cystic fibrosis.
Clinical data are still lacking on the best dose of pancreatic enzyme replacement therapy to achieve recommended weight goals.
What Is New
We found a linear relationship between initial dose and attainment of favorable changes in weight-for-age z score and weight-for-length percentile at age 2 years.
Specifically, an initial pancreatic enzyme replacement therapy dose ≥ 1500 lipase units/kg/largest meal resulted in an increased likelihood of attaining favorable weight-related outcomes.
Pancreatic exocrine insufficiency is present in 85% to 90% of cystic fibrosis (CF) patients and is the primary challenge to achieving adequate nutrition in CF (1–3). Multiple studies report that early growth of children with CF is a significant predictor of lung function, morbidity, and survival (4–8). A high-fat, high-protein diet along with pancreatic enzyme replacement therapy (PERT) is recommended to meet energy demands and achieve optimal weight gain, growth, and development (1,9).
The determination of optimal PERT dosing is essential to minimize malabsorption and help attain the goal of maximizing growth of children with CF. Clinical data are, however, still lacking on the optimal dose of PERT to achieve recommended weight goals (8,10,11). The current recommendations for PERT dosing were made in the mid-1990s in response to the recognition that excessively high doses were associated with the development of fibrosing colonopathy (12,13). Current guidelines for PERT dosing recommend 2000 to 5000 lipase units (LU) per 120 mL feed in infants <12 months of age and 1000 to 2500 LU/kg/meal with a maximum daily dose of 10,000 LU/kg in children 12 months to 4 years (10,14,15). An analysis of 2010 CF Foundation (CFF) Patient Registry data revealed that infant PERT dosing varied widely in clinical practice, and that many infants receive PERT doses either lower than or in excess of the recommendation (10).
The CFF Patient Registry includes data that can provide insight into the real-world relationship between clinical practice and health outcomes (16,17). We present the results of an analysis of this registry investigating the association between the initial reported PERT dose in infants and weight-related outcomes at 2 years. We hypothesized that infants who were started on higher doses of PERT would be more likely to achieve satisfactory weight status by age 2 years, as defined primarily as achievement of weight-for-age z score (WAZ) at or above the WAZ at birth (6).
METHODS
Patient Population
Using the CFF Patient Registry (3), we identified individuals diagnosed with CF who were born in 2010, and were prescribed PERT starting in 2010. Each participating CF Program that contributes data to the CFF registry obtains local institutional review board approval and written informed consent and assent, as appropriate, from participants and/or their legal guardians (3). Anonymized data were obtained from the CFF following a review of the protocol by the CFF Patient Registry Committee. We excluded infants with a reported dose >4000 LU/kg/largest meal and those with an absolute WAZ >3 as outliers. Patients with a documented weight and length in at least 2 of the 3 years between 2010 and 2012, separated by at least 12 months between the first and the last encounters, were included in the analysis.
Study Design
This was a retrospective cohort analysis, which included a nested case-control component, of the relationship between initial reported PERT dose and subsequent weight-related outcomes in infants in the CFF Patient Registry. PERT dose is reported at each encounter in the Registry as LU per weight in kilograms at the patient's largest meal. Length and weight at each encounter are entered into the registry, and the registry software calculates percentiles based on patient's birth date. Using age in months at each encounter, we calculated WAZ using the Centers for Disease Control and Prevention 2000 growth charts (ages 0 to <20 years) (http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm). The WAZ and weight-for-length percentile (W/L%) from the patient's initial encounter and from the 21st, 22nd, or 23rd month were compared for analysis.
The change in WAZ was predetermined as the primary outcome of interest, and the change in W/L% as the secondary outcome. For analyses of dichotomous outcomes, we defined a priori as a successful outcome, the eventual attainment of a WAZ score in 2012 at or above the initial 2010 WAZ score (adapted from the analysis performed by Lai et al (6))—approximately half the subjects achieved this outcome. We then evaluated PERT dose associated with the likelihood of attaining an increase in WAZ at the top 25th percentile. Similarly, we evaluated the relationship between enzyme dose and the likelihood of attaining the 50th percentile or the 75th percentile for increase in W/L%.
We focused on the starting PERT dose to avoid confounding by indication (severity bias)—subsequent increases in PERT dosing would be made in response to poor weight gain and would therefore obscure the therapeutic relationship between PERT dose and weight gain (18). To determine whether our findings were sensitive to decisions regarding the timing of enzyme initiation that may have been biased by severity at presentation, a subgroup analysis was performed that was limited to patients who started PERT within the first 3 months of life (months 0, 1, and 2), designated as the early dosing cohort.
Statistical Methods
Initial descriptive analyses evaluated the marginal association between demographic, clinical characteristics as well as nutritional therapies, and weight-related outcomes. Group characteristics were compared using Wilcoxon rank-sum test and Fisher's exact test for continuous and categorical variables, respectively. The Pearson correlation was used to measure the association between initial PERT dose and weight at initial PERT encounter. The Spearman correlation was used to measure the association between initial PERT dose and change in W/L%.
Simple and multiple linear regressions were used to quantify the relationship between initial PERT dose in 2010 and the change in WAZ or in W/L%. The covariates used in the multiple linear models (determined a priori or on the basis of the initial results shown in Table 1) included weight at initial PERT dose encounter, history of prematurity, diagnosis by newborn screening, diagnosis of meconium ileus, history of Pseudomonas aeruginosa on any respiratory culture, proton pump inhibitor use, reported history of distal intestinal obstruction syndrome (DIOS), and cystic fibrosis transmembrane conductance regulator (CFTR) genotype, categorized as severe (Class I–III), mild (Class IV–V), and unknown (if the genotype was not completely identified or if the CFTR genotype was not categorized by the CFF Patient Registry). Plots of the mean change in WAZ or W/L% versus PERT dose over 250 LU/kg/meal intervals were used to identify dose windows for additional analyses, specifically the cutpoint of initial PERT doses of <1500 versus ≥1500 LU/kg/largest meal. Odds ratios (ORs) of achieving a successful outcome between patients with initial PERT doses of <1500 and ≥1500 LU/kg/largest meal were calculated using logistic regression, adjusting for the same variables as those in the multiple linear regression. All analyses were performed with SAS 9.4 (SAS Institute Inc, Cary, NC).
TABLE 1.
Baseline characteristics for weight-for-age z score categories
| All patients | ΔWAZ < median (2012WAZ < 2010WAZ) | ΔWAZ ≥ median (2012WAZ ≥ 2010WAZ) | |
| Characteristic | (N = 502) | (n = 246) | (n = 256) |
| Gender, % | |||
| Male | 52.2 | 50.0 | 54.3 |
| Race, % | |||
| White | 91.8 | 91.5 | 92.2 |
| Black | 6.8 | 6.1 | 7.4 |
| Ethnicity, % | |||
| Hispanic | 11.2 | 12.6 | 9.8 |
| Insurance, % | |||
| Private | 41.8 | 39.4 | 44.1 |
| Medicaid | 42.8 | 40.2 | 45.3 |
| Diagnosis suggested by: % | |||
| Acute respiratory symptoms | 3.2 | 4.5 | 2 |
| Failure to thrive/malnutrition | 6.0 | 5.7 | 6.3 |
| Family history | 11.4 | 12.6 | 10.2 |
| Genotype | 21.1 | 19.5 | 22.7 |
| Meconium ileus | 15.7 | 13.8 | 17.6 |
| Prenatal diagnosis (CVS, AC) | 6.6 | 4.9 | 8.2 |
| Newborn screening | 80.5 | 80.9 | 80.1 |
| Steatorrhea | 5.8 | 5.3 | 6.3 |
| CFTR genotype, % | |||
| Severe (Class I–III) | 79.3 | 78.5 | 80.1 |
| Mild (Class IV–V) | 2.8 | 2.4 | 3.1 |
| Unknown | 17.9 | 19.1 | 16.8 |
| Any form of supplemental nutrition, % | 83.9 | 89.4 | 78.5* |
| Oral supplemental feeding | 82.9 | 88.6 | 77.3* |
| Nasogastric supplemental feeding | 3.8 | 3.7 | 3.9 |
| Gastrostomy tube feeding | 12.7 | 13.8 | 11.7 |
| H2 blocker use, % | 51.8 | 48.4 | 55.1 |
| Proton pump inhibitor use, % | 55.0 | 52.8 | 57.0 |
| Exclusive breast milk feeding reported at any visit, % | 28.7 | 31.7 | 25.8 |
| Breast milk and formula reported at any visit, % | 32.5 | 35.4 | 29.7 |
| Exclusive formula feeding reported at any visit, % | 71.5 | 67.9 | 75 |
| Normal flora on sputum culture, % | 77.5 | 76.4 | 78.5 |
| Pseudomonas aeruginosa present on respiratory culture, % | 50.4 | 48.4 | 52.3 |
| DIOS, % | 8 | 7.3 | 8.6 |
| Gastroesophageal reflux disease, % | 40.4 | 37.8 | 43 |
| Cystic fibrosis liver disease, % | 4.4 | 2.8 | 5.9 |
| Age at initial PERT encounter (mo), mean (range) | 1.4 (0, 2) | 1.2 (0, 2) | 1.6 (0, 2) |
| Prematurity, % | 11.8 | 4.1 | 19.1* |
| Birth weight (kg), median (IQR) | 3.2 (2.7, 3.5) | 3.3 (3.0, 3.6) | 2.9 (2.6, 3.3)* |
| Weight at initial PERT encounter (kg), median (IQR) | 4.6 (3.7, 5.7) | 4.8 (3.9, 5.7) | 4.1 (3.5, 5.4)* |
| Weight-for-age percentile at initial PERT encounter, median (IQR) | 18.4 (5.9, 40.1) | 30.4 (13.6, 56.3) | 8.7 (2.5, 26.3)* |
| W/L% at initial PERT encounter, median (IQR) | 35.1 (12.0, 60.0) | 42 (17.5, 67.4) | 27.3 (9.1, 54.6)* |
| WAZ at initial PERT encounter, median (IQR) | −0.3 (−1.1, 0.5) | 0.2 (−0.6, 0.9) | −0.8 (−1.5, −0.2)* |
| Median initial PERT dose, LU/kg/largest meal, median (IQR) | 932 (682, 1389) | 1200 (790, 1200)* | |
AC = amniocentesis; CFTR = cystic fibrosis transmembrane conductance regulator; CVS = chorionic villus sampling; DIOS = distal intestinal obstruction syndrome; IQR = interquartile range; LU = lipase units; PERT = pancreatic enzyme replacement therapy; W/L% = weight-for-length percentile; WAZ = weight-for-age z score.
Insurance, DIOS, Pseudomonas infection, H2 blocker, proton pump inhibitor, and supplemental feeding are recorded for the year.
*P ≤ 0.05, ΔWAZ ≥ median versus ΔWAZ < median.
RESULTS
Study Patients
Of 910 patients in the CFF Patient Registry born in 2010, 710 were documented to have been started on PERT and a total of 502 met all inclusion criteria (Supplemental Digital Content Figure 1, Supplemental Digital Content).
Weight-for-Age z Score Analysis
The analysis of the changes in WAZ showed that, at their last measurement to their second birthday, 246 patients (49%) did not achieve and 256 (51%) were at or above their initial WAZ measured in 2010. Thus, a change of ≥0 in WAZ for the 502 patients was approximately the 50th percentile and an appropriate cutpoint for our analysis. An increase in WAZ of ≥0.8 was achieved by the top quartile of subjects. Population characteristics are shown in Table 1. Patients achieving their initial 2010 WAZ 2 years later in 2012 were more likely to be premature and to have a lower birth weight, and a lower weight, weight-for-age, W/L%, and WAZ at their initial clinic encounter. They were also less likely to have received nutritional supplements during the follow-up period. There was no significant correlation between initial PERT dose and weight at initial PERT encounter (P = 0.3458, by Pearson correlation). The differences in baseline characteristics were similar when the WAZ 75th percentile cutpoint was used (Supplemental Digital Content Table 1, Supplemental Digital Content). The median initial reported PERT dose in those who achieved their initial 2010 WAZ score in 2012 was significantly larger (1200 LU/kg/largest meal, interquartile range [IQR] 790, 1667) compared with those who did not achieve it (932 LU/kg/largest meal, IQR 682, 1389) (P < 0.001) (Supplemental Digital Content Figure 2, Supplemental Digital Content). Figure 1 shows the relationship between initial PERT dose (grouped into 250 LU/kg/largest meal intervals) and mean change in WAZ score. Patients with an initial PERT dose <1500 LU/kg/largest meal (from here on designated as being in the low-dose group) had a negative mean change in WAZ score, whereas those with an initial PERT dose ≥1500 LU/kg/largest meal (the high-dose group) averaged a positive change. Simple and multiple linear regressions both showed that the change in WAZ was positively associated with a higher initial PERT dose (P = 0.001 and P = 0.005, respectively).
FIGURE 1.
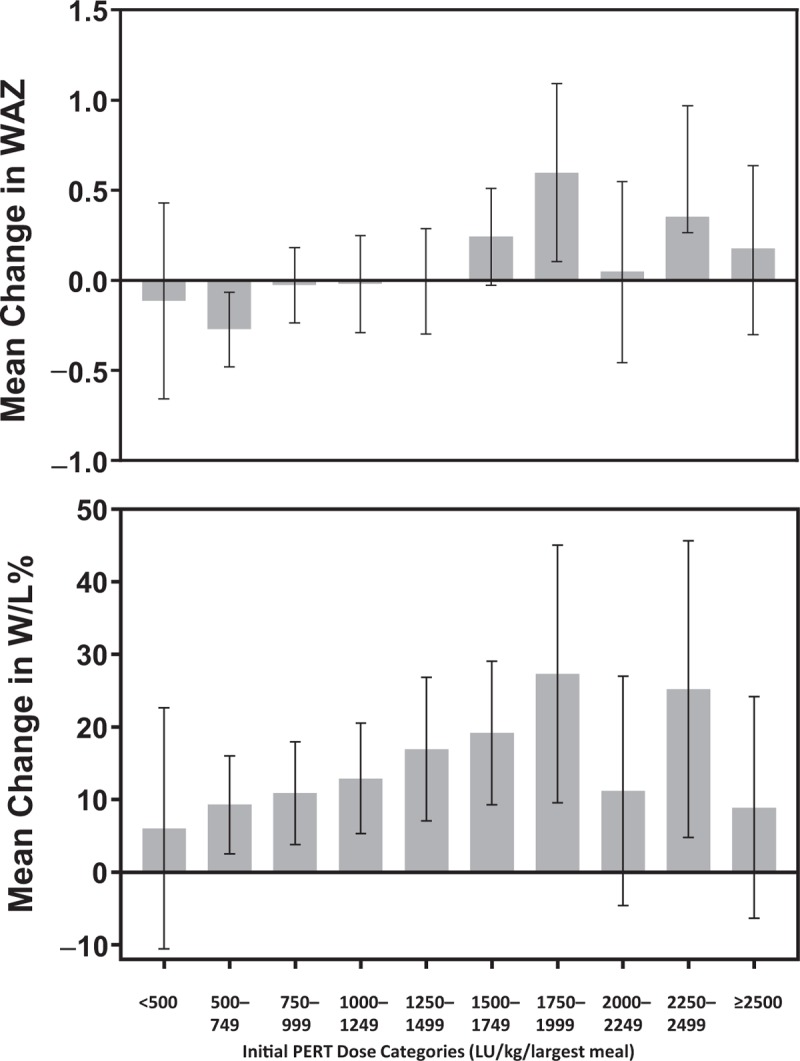
Relationship of initial PERT dose to change in WAZ from birth to age 2 (top panel) and W/L% at age 2 (lower panel). IQR = interquartile range; LU = lipase units; PERT = pancreatic enzyme replacement therapy; WAZ = weight-for-age z score; W/L% = weight-for-length percentile. N = 502. Error bars are 95% confidence intervals.
As shown in Table 2, 62.9% of patients with initial PERT dose ≥1500 LU/kg/largest meal were at or above their initial WAZ at their last measurement in 2012, compared with 46.4% of those with a lower initial PERT dose. The adjusted odds ratio (aOR) of finishing in 2012 at or above their initial WAZ was 1.87 in the high-dose group compared with the low-dose group (95% confidence interval [CI] 1.22–2.86, P = 0.004). Similarly, 33.6% of patients in the high-dose group and 21.0% of patients in the low-dose group were in the top quartile for change in WAZ in 2012 (aOR 1.90, CI 1.19–3.05, P = 0.007). Similar findings were obtained when comparing infants who had been started on ≥1750 LU/kg/largest meal with those started on <1750 LU/kg/largest meal, with aOR of 1.86 for achieving WAZ at or above initial WAZ from the logistic model (CI 1.11–3.14, P = 0.019), and for achieving a change in WAZ in the top quartile (aOR 1.66, CI 0.95–2.88, P = 0.073).
TABLE 2.
Change in weight-for-age z score from 2010 to 2012 by initial pancreatic enzyme replacement therapy dose category
| Initial PERT dose category (2010) | |||||||
| Change in WAZ 2012–2010, n (%) | <1500 LU/kg/largest meal | ≥1500 LU/kg/largest meal | P | <1750 LU/kg/largest meal | ≥1750 LU/kg/largest meal | P | Total |
| (n = 362) | (n = 140) | (n = 421 | (n = 81) | (N = 502) | |||
| <0 | 194 (53.6) | 52 (37.1) | 218 (51.8) | 28 (34.6) | 246 (49.0) | ||
| ≥0 (median) | 168 (46.4) | 88 (62.9) | <0.001 | 203 (48.2) | 53 (65.4) | 0.005 | 256 (51.0) |
| <0.8 | 286 (79.0) | 93 (66.4) | 326 (77.4) | 53 (65.4) | 379 (75.5) | ||
| ≥0.8 (top quartile) | 76 (21.0) | 47 (33.6) | 0.003 | 95 (22.6) | 28 (34.6) | 0.021 | 123 (24.5) |
LU = lipase units; PERT = pancreatic enzyme replacement therapy; WAZ = weight-for-age z score.
When the WAZ analysis was restricted to the early dosing cohort of 348 patients who received their initial PERT dose in the first 3 months of life, similar results were obtained (Supplemental Digital Content Figure 3, Supplemental Digital Content).
Weight-for-Length Percentile Analysis
Analysis of the relationship between change in W/L% and PERT dosing was done as a secondary analysis and is described in detail in the Supplemental Digital Content Text and Supplemental Digital Content Table 2 (Supplemental Digital Content). To summarize, we found a significant association of median initial PERT dose with changes in W/L quartile (P = 0.019) at follow-up in 2012 (Supplemental Digital Content Figure 2, Supplemental Digital Content), and a significant linear relationship between starting PERT dose up to 2000 LU/kg/largest meal and mean attainment in W/L % (P = 0.005 for the multiple regression model) (Fig. 1). The relationship of initial PERT dose with W/L% lost significance when doses ≥2000 LU/kg/largest meal were included in the multivariable model (P = 0.08), suggesting a threshold effect. We also found that infants started on an initial PERT dose ≥1500 LU/kg/largest meal had a greater likelihood of achieving a change in the top quartile of W/L% compared with the patients whose dose was <1500 LU/kg/largest meal (aOR 1.81, CI 1.14–2.87, P = 0.013). The relationship of PERT dosing ≥1750 LU/kg/largest meal to improvement in W/L% followed similar trends but did not reach statistical significance (Supplemental Digital Content Table 3, Supplemental Digital Content). As in the WAZ analysis, the W/L% analysis resulted in similar findings when restricted to patients who were started on PERT before the third month of life (Supplemental Digital Content Figure 4, Supplemental Digital Content).
DISCUSSION
These results suggest a significant association between the PERT starting dose prescribed to infants with CF and achievement of weight gain in the first 2 years of life. We looked at this relationship in different ways and found concordance among them. First, median initial PERT dose correlated with categories of anthropometric improvement using change in either WAZ or W/L% as our outcome. Second, a positive linear relationship was found between the initial PERT dose and the change in WAZ and W/L% in these infants, modeled so as to adjust for potential confounders. Third, an initial PERT dose ≥1500 LU/kg/largest meal was associated with a higher likelihood of achieving a change in WAZ above the median and above the 75th percentile by age 2 years; similar findings were seen in association with the changes in W/L%. The findings were similar when the study cohort was limited to the infants who were started on PERT before 3 months of age.
While these findings do not substitute for clinical trial data, they do reflect clinical experience in the real-life practice setting and contribute insights regarding the relationship of initial PERT dosing with weight-related outcomes in young children with CF. Current recommendations to limit PERT dosing in patients with CF to <10,000 LU/kg/day are based upon expert-consensus guidelines that were made in the absence of strong empirical data (15,19) and were influenced by concerns regarding the association of high PERT dosing with the development of fibrosing colonopathy (12,13). These recommendations have been carried forward (8,14,20) without any additional evidence regarding the optimal PERT dosage for minimizing malabsorption and maximizing growth in patients of any age (11). Given that the average infant feeds at least 6 times a day, our findings suggest that infants who are more likely to achieve preferred nutritional outcomes are also more likely to be receiving PERT doses above the recommended 10,000 LU/kg/day (10).
Determining optimal PERT dosing is essential for minimizing malabsorption to help attain the goal of maximizing growth of children with CF. Multiple studies and analyses show a correlation between nutritional status and pulmonary outcomes in CF (1), and this relationship may be especially salient in infancy. Konstan et al (5) analyzed data on 931 children from the Epidemiologic Study of CF and found that those with weight-for-age less than fifth percentile at age 3 had an average forced expiratory volume in 1 second of 86 ± 20% predicted at age 6 compared with 102 ± 18% predicted in those with weight-for-age >75th percentile at age 3. Lai et al (6) studied 63 children aged 2 through 6 years who were enrolled in the Wisconsin CF Neonatal Screening Project, and found that recovery of birth weight z score within 2 years of CF diagnosis was associated with fewer cough symptoms, higher lung function, and better chest radiograph scores at age 6. Most recently, Yen et al (21) studied 3142 children in the CFF Registry born between 1989 and 1992 and found that a higher weight-for-age percentile at 4 years was associated with greater height, better lung function, fewer complications of CF, and improved survival through 18 years.
Previously published studies evaluating PERT dosing used older preparations, were generally performed on small numbers of patients, and had important methodological weaknesses (22,23). For example, a CFF Registry study of 1215 patients >4 years from 33 US CF centers that was published in 2005 found no association between PERT dose and growth or gastrointestinal symptoms (24). The findings of that analysis were, however, likely confounded by indication bias, an important pitfall when observational databases and registries are used to evaluate the association between therapies and outcomes. Clinicians often prescribe higher doses or additional therapies to patients whose condition is more severe, so naïve analyses of observational data may find the apparent effectiveness of evidence-based therapies to be dampened, lost, or even apparently reversed (25). Multivariable modeling may adjust for indication bias if all indications for treatment are measured and recorded, but residual confounding will occur when modeling is unable to adjust for undocumented influences on prescribing (16,25). Novel techniques have been proposed to eliminate confounding by undocumented indication, but indication bias remains an important challenge to comparative effectiveness research (26). Haupt et al (27) used CFF Patient Registry data to investigate the association between PERT dosing and body mass index (BMI) percentile in patients 2 to 20 years, using CF center practice as the unit of analysis to avoid indication bias. They found a higher adjusted median PERT dose prescribed at centers in the highest quartile for BMI compared with that prescribed at centers in the lowest BMI quartile (1755 LU/kg/meal, 95% CI 1722–1788 vs 1628 LU/kg/meal, 95% CI 1595–1660, P < 0.001).
Although the present analysis aligns with previous publications suggesting that lower PERT dosing may be associated with worse weight-related outcomes, certain limitations must be considered. Pitfalls of secondary analyses of observational data are well described and include selection bias, information bias, and confounding (16). Selection and information biases are likely to be minimal in the CFF Patient Registry (3). The potential impact of confounding by indication, or indication bias, as described above, must be acknowledged (16,25). This was minimized by our study design, which focused on the PERT initial dose prescribed at early encounters with infants whose primary risk factors, such as genotype, mode of diagnosis, and current weight, are all recorded and therefore easily adjusted with multivariable modeling techniques. Furthermore, to determine whether our findings were sensitive to unmeasured factors that may have developed before treatment initiation (18), we duplicated our analysis using a population restricted to those who were started on PERT in the first 3 months of life, and found no substantive difference. Another potential confounding factor is the possibility that the prescription of higher enzyme doses may be correlated with a more aggressive overall approach to nutritional management. The analysis was adjusted for the prescribed use of nutritional supplements in the dataset, but other potential factors are not recorded in the CFF Registry. Finally, as data on fecal elastase in the CFF Patient Registry is incomplete, it is possible that some patients receiving PERT were not pancreatic insufficient. Our model, however, included CFTR mutation class, which is a strong predictor of pancreatic function (28).
In conclusion, higher initial PERT dosing (≥1500 LU/kg/largest meal) in infants with CF appears to be associated with an increased likelihood of favorable weight-related outcomes at 2 years, as measured by the change in WAZ or W/L%. This dosing regimen is associated with a total daily dose of PERT that is likely to exceed the current guideline recommendations. Although our findings cannot be used in isolation to prove causation or to identify or recommend a specific initial PERT dose for patients with CF, they suggest that current recommendations for initial PERT dosing should be re-evaluated and tested in a prospective clinical trial to evaluate both efficacy and safety.
Supplementary Material
Acknowledgments
The authors would like to thank the Cystic Fibrosis Foundation for the use of Cystic Fibrosis Foundation Patient Registry data to conduct this study. In addition, the authors would like to thank the individuals with cystic fibrosis, care providers, and clinic coordinators at cystic fibrosis centers throughout the United States for their contributions to the Cystic Fibrosis Foundation Patient Registry. Medical writing and editorial support was provided by Larry Deblinger and Robin Smith, PhD, of The Curry Rockefeller Group, LLC, Tarrytown, NY, and Michael J. Theisen, PhD, and Richard M. Edwards, PhD, of Complete Publications Solutions, LLC, North Wales, PA, and was funded by AbbVie Inc.
Footnotes
M.S.S. has no financial relationship with AbbVie Inc and received no funding for his work on this manuscript. He receives research support from the NIH, CF Foundation, Anthera, and Novartis; and has had consulting relationships with AbbVie, Vertex, Novartis, Gilead, Genentech, and Celtaxsys. S.M. is a consultant to AbbVie Inc. She is also a technical advisor to MVW Nutritionals, makers of cystic fibrosis-specific vitamin products. M.K. and R.K. are employed by AbbVie Inc, and may own stock. S.L. was an employee of AbbVie Inc, during the analysis. Her current affiliation is Astellas Inc, Northbrook, IL. B.W.S. was an employee of AbbVie Inc, during the analysis. His current affiliation is Rhythm Pharmaceuticals, Boston, MA. M.H. was an employee of AbbVie Inc, during the analysis. His current affiliation is ARIEL Precision Medicine, Pittsburgh, PA. Funding for this study was provided by AbbVie Inc.
The authors report no conflicts of report.
REFERENCES
- 1.Culhane S, George C, Pearo B, et al. Malnutrition in cystic fibrosis: a review. Nutr Clin Pract 2013; 28:676–683. [DOI] [PubMed] [Google Scholar]
- 2.Fieker A, Philpott J, Armand M. Enzyme replacement therapy for pancreatic insufficiency: present and future. Clin Exp Gastroenterol 2011; 4:55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knapp EA, Fink AK, Goss CH, et al. The Cystic Fibrosis Foundation Patient Registry. Design and methods of a National Observational Disease Registry. Ann Am Thorac Soc 2016; 13:1173–1179. [DOI] [PubMed] [Google Scholar]
- 4.Konstan MW, Butler SM, Schidlow DV, et al. Patterns of medical practice in cystic fibrosis: part II. Use of therapies. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Pediatr Pulmonol 1999; 28:248–254. [DOI] [PubMed] [Google Scholar]
- 5.Konstan MW, Butler SM, Wohl ME, et al. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr 2003; 142:624–630. [DOI] [PubMed] [Google Scholar]
- 6.Lai HJ, Shoff SM. Farrell PM Recovery of birth weight z score within 2 years of diagnosis is positively associated with pulmonary status at 6 years of age in children with cystic fibrosis. Pediatrics 2009; 123:714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McPhail GL, Acton JD, Fenchel MC, et al. Improvements in lung function outcomes in children with cystic fibrosis are associated with better nutrition, fewer chronic Pseudomonas aeruginosa infections, and dornase alfa use. J Pediatr 2008; 153:752–757. [DOI] [PubMed] [Google Scholar]
- 8.Stallings VA, Stark LJ, Robinson KA, et al. Clinical Practice Guidelines on Growth and Nutrition Subcommittee; Ad Hoc Working Group. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc 2008; 108:832–839. [DOI] [PubMed] [Google Scholar]
- 9.Kalnins D, Wilschanski M. Maintenance of nutritional status in patients with cystic fibrosis: new and emerging therapies. Drug Des Devel Ther 2012; 6:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borowitz D, Gelfond D, Maguiness K, et al. Maximal daily dose of pancreatic enzyme replacement therapy in infants with cystic fibrosis: a reconsideration. J Cyst Fibros 2013; 12:784–785. [DOI] [PubMed] [Google Scholar]
- 11.Somaraju UR, Solis-Moya A. Pancreatic enzyme replacement therapy for people with cystic fibrosis. Cochrane Database Syst Rev 2014; 10:CD008227. [DOI] [PubMed] [Google Scholar]
- 12.Borowitz D, Grand R, Durie P. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. J Pediatr 1995; 127:681–684. [DOI] [PubMed] [Google Scholar]
- 13.FitzSimmons SC, Burkhart GA, Borowitz D, et al. High-dose pancreatic-enzyme supplements and fibrosing colonopathy in children with cystic fibrosis. N Engl J Med 1997; 336:1283–1289. [DOI] [PubMed] [Google Scholar]
- 14.Borowitz D, Robinson KA, Rosenfeld M, et al. Cystic fibrosis foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr 2009; 155 (6 suppl 1):S73–S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinaasappel M, Stern M, Littlewood J, et al. Nutrition in patients with cystic fibrosis: a European Consensus. J Cyst Fibros 2002; 1:51–75. [DOI] [PubMed] [Google Scholar]
- 16.Schechter MS. Patient registry analyses: seize the data, but caveat lector. J Pediatr 2008; 153:733–735. [DOI] [PubMed] [Google Scholar]
- 17.Shah BR, Drozda J, Peterson ED. Leveraging observational registries to inform comparative effectiveness research. Am Heart J 2010; 160:8–15. [DOI] [PubMed] [Google Scholar]
- 18.Psaty BM, Siscovick DS. Minimizing bias due to confounding by indication in comparative effectiveness research: the importance of restriction. JAMA 2010; 304:897–898. [DOI] [PubMed] [Google Scholar]
- 19.Ramsey BW, Farrell PM, Pencharz P. Nutritional assessment and management in cystic fibrosis: a consensus report. The Consensus Committee. Am J Clin Nutr 1992; 55:108–116. [DOI] [PubMed] [Google Scholar]
- 20.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr 2002; 35:246–259. [DOI] [PubMed] [Google Scholar]
- 21.Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr 2013; 162:530.e1–535.e1. [DOI] [PubMed] [Google Scholar]
- 22.Beker LT, Fink RJ, Shamsa FH, et al. Comparison of weight-based dosages of enteric-coated microtablet enzyme preparations in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr 1994; 19:191–197. [DOI] [PubMed] [Google Scholar]
- 23.Brady MS, Rickard K, Yu PL, et al. Effectiveness and safety of small vs. large doses of enteric coated pancreatic enzymes in reducing steatorrhea in children with cystic fibrosis: a prospective randomized study. Pediatr Pulmonol 1991; 10:79–85. [DOI] [PubMed] [Google Scholar]
- 24.Baker SS, Borowitz D, Duffy L, et al. Pancreatic enzyme therapy and clinical outcomes in patients with cystic fibrosis. J Pediatr 2005; 146:189–193. [DOI] [PubMed] [Google Scholar]
- 25.Rothman KJ, Wentworth CE., 3rd Mortality of cystic fibrosis patients treated with tobramycin solution for inhalation. Epidemiology 2003; 14:55–59. [DOI] [PubMed] [Google Scholar]
- 26.Bosco JL, Silliman RA, Thwin SS, et al. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol 2010; 63:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haupt ME, Kwasny MJ, Schechter MS, et al. Pancreatic enzyme replacement therapy dosing and nutritional outcomes in children with cystic fibrosis. J Pediatr 2014; 164:1110.e1–1115.e1. [DOI] [PubMed] [Google Scholar]
- 28.Kristidis P, Bozon D, Corey M, et al. Genetic determination of exocrine pancreatic function in cystic fibrosis. Am J Hum Genet 1992; 50:1178–1184. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


