Abstract
Continuous chemical investigation of the gorgonian coral Pinnigorgia sp. resulted in the isolation of two new sterols, 5α,6α-epoxy-(22E,24R)-3β,11-dihydroxy-9,11-secoergosta-7-en-9-one (1) and (22R)-acetoxy-(24ξ)-ergosta-5-en-3β,25-diol (2). The structures of sterols 1 and 2 were elucidated using spectroscopic methods. Sterol 1 displayed inhibitory effects on the generation of superoxide anions and the release of elastase by human neutrophils with IC50 values of 8.65 and 5.86 μM, respectively. The structure of a known metabolite, pubinernoid A (3), is revised as (+)-loliolide (4).
Keywords: gorgonian, Pinnigorgia, anti-inflammatory, superoxide anion, elastase, pubinernoid A, loliolide
1. Introduction
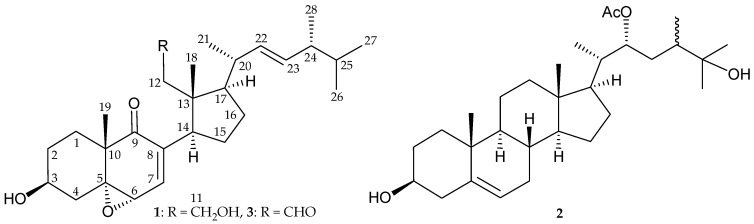
Gorgonian corals belonging to the genus Pinnigorgia have proven to be a rich source of sterols with unusual structural features [1,2,3,4,5,6]. In continuation of our effort to discover new natural products of this organism, its ethyl acetate extract exhibited anti-inflammatory activities by inhibiting the expression of superoxide anions and elastase by human neutrophils with IC50 values of 1.89 and 1.57 μg/mL, respectively. Two new sterols, 5α,6α-epoxy-(22E,24R)-3β,11-dihydroxy-9,11-secoergosta-7-en-9-one (1) and (22R)-acetoxy-(24ξ)-ergosta-5-en-3β,25-diol (2) (Figure 1 and Supplementary Figures S1–S14), were isolated. The structures of these two sterols were established by spectroscopic analyses and sterol 1 was found to display anti-inflammatory activity.
Figure 1.
Chemical structures of 5α,6α-epoxy-(22E,24R)-3β,11-dihydroxy-9,11-secoergosta-7-en-9- one (1), (22R)-acetoxy-(24ξ)-ergosta-5-en-3β,25-diol (2), and 3-O-deacetylluffasterol B (3) [7].
2. Results and Discussion
The new sterol, 5α,6α-epoxy-(22E,24R)-3β,11-dihydroxy-9,11-secoergosta-7-en-9-one (1), was isolated as colorless oil. The high-resolution electrospray ionization mass spectrum (HRESIMS) showed a signal at m/z 467.31308 (calcd. for C28H44O4 + Na, 467.31373), and therefore the molecular formula of 1 was determined to be C28H44O4 (7° of unsaturation degrees). The 13C and distortionless enhancement polarization transfer (DEPT) spectrum of 1 showed that this compound has 28 carbons including six methyls, seven sp3 methylenes, seven sp3 methines, an sp3 oxygenated tertiary carbon, two sp3 quaternary carbons, three sp2 methines, an sp2 tertiary carbon and a ketonic carbonyl (Table 1). The IR spectrum of 1 revealed the presence of hydroxy (νmax 3398 cm−1) and α,β-unsaturated ketonic carbonyl (νmax 1682 cm−1) groups. The latter structural feature was confirmed by the presence of signals at δC 201.9 (C-9), 141.4 (C-8) and 139.3 (CH-7) in the 13C-NMR spectrum. A disubstituted olefin was identified from the signals of carbons at δC 134.4 (CH-22) and 133.0 (CH-23) and was confirmed by two olefin proton signals at δH 5.21 (1H, dd, J = 15.2, 6.4 Hz, H-23) and 5.24 (1H, dd, J = 15.2, 6.8 Hz, H-22) (Table 1). Four doublets at δH 1.03 (3H, J = 6.8 Hz), 0.91 (3H, J = 6.8 Hz), 0.83 (3H, J = 7.2 Hz) and 0.82 (3H, J = 6.8 Hz) were due to the Me-21, Me-28, Me-26 and Me-27 groups, respectively. Two sharp singlets for H3-18 and H3-19 appeared at δH 0.68 and 1.25, respectively. A trisubstituted epoxide was elucidated from the signals of an oxygenated tertiary carbon at δC 63.2 (C-5) and an oxymethine at δC 53.5 (CH-6); and further confirmed by the proton signal of a methine doublet at δH 3.39 (1H, d, J = 4.8 Hz, H-6). On the basis of the unsaturation data overall, 1 was concluded to be a secosterol molecule possessing four rings.
Table 1.
1H- and 13C-NMR data, 1H-1H COSY, and HMBC correlations for secosterol 1 and the 1H- and 13C-NMR data for 3-O-deacetylluffasterol B (3).
| Position | 1 | 3 | ||||
|---|---|---|---|---|---|---|
| δH (J in Hz) a | δCb | 1H-1H | HMBC | δH (J in Hz)c | δCc | |
| 1a/b | 2.09 m; 1.72 m | 27.8, CH2 | H2-2 | C-5 | 27.8, CH2 | |
| 2a/b | 2.09 m; 1.65 m | 30.5, CH2 | H2-1, H-3 | C-10 | 2.09 m; 1.68 m | 30.5, CH2 |
| 3 | 3.98 m | 68.3, CH | H2-2, H2-4 | n. o. d | 3.98 m | 68.3, CH |
| 4α | 1.57 m | 37.4, CH2 | H-3, H-4β | C-2, -3, -5 | 1.56 m | 37.5, CH2 |
| β | 2.18 dd (12.8, 11.6) | H-3, H-4α | C-3 | 2.18 m | ||
| 5 | 63.2, C | 63.5, C | ||||
| 6 | 3.39 d (4.8) | 53.5, CH | H-7 | C-7, -8 | 3.40 d (4.6) | 53.5, CH |
| 7 | 6.81 d (4.8) | 139.3, CH | H-6 | C-5, -6, -9, -14 | 6.84 dd (4.6, 1.0) | 139.7, CH |
| 8 | 141.4, C | 140.5, C | ||||
| 9 | 201.9, C | 200.6, C | ||||
| 10 | 45.6, C | 45.4, C | ||||
| 11a | 3.81 ddd (10.4, 10.4, 6.0) | 59.1, CH2 | H-11b, H2-12 | n. o. | 9.88 dd (3.8, 1.7) | 203.4, CH |
| b | 3.68 ddd (10.4, 8.8, 6.0) | H-11a, H2-12 | n. o. | |||
| 12a | 1.61 m | 40.4, CH2 | H2-11, H-12b | n. o. | 2.27 dd (15.9, 3.8) | 50.8, CH2 |
| b | 1.12 m | H2-11, H-12a | C-11, -13, -17 | 2.00 dd (15.9, 1.7) | ||
| 13 | 46.1, C | 46.3, C | ||||
| 14 | 3.37 dd (10.8, 8.0) | 43.8, CH | H2-15 | n. o. | 3.51 dd (10.3, 9.2) | 45.0, CH |
| 15a/b | 1.69–1.56 m | 26.9, CH2 | H-14, H2-16 | C-13, -14 | 1.78 m; 1.71 m | 26.7, CH2 |
| 16a/b | 1.69 m; 1.44 m | 25.4, CH2 | H2-15, H-17 | n. o. | 25.8, CH2 | |
| 17 | 1.74 m | 49.6, CH | H2-16, H-20 | n. o. | 51.9, CH | |
| 18 | 0.68 s | 17.8, CH3 | C-12, -13, -14, -17 | 0.76 s | 17.1, CH3 | |
| 19 | 1.25 s | 21.4, CH3 | C-1, -5, -9, -10 | 1.21 s | 20.0, CH3 | |
| 20 | 2.15 m | 38.8, CH | H-17, H3-21, H-22 | n. o. | 2.18 m | 43.0, CH |
| 21 | 1.03 d (6.8) | 21.4, CH3 | H-20 | C-17, -20, -22 | 1.00 d (6.8) | 19.7, CH3 |
| 22 | 5.24 dd (15.2, 6.8) | 134.4, CH | H-20, H-23 | C-20, -24 | 5.20 dd (17.6, 7.4) | 133.4, CH |
| 23 | 5.21 dd (15.2, 6.4) | 133.0, CH | H-22, H-24 | C-20, -24 | 5.24 dd (17.6, 7.4) | 134.0, CH |
| 24 | 1.86 m | 43.0, CH | H-23, H-25, H3-28 | C-22, -23, -25 | 1.87 m | 38.8, CH |
| 25 | 1.47 m | 33.1, CH | H-24, H3-26, H3-27 | C-23, -24, -28 | 1.47 m | 33.2, CH |
| 26 | 0.83 d (7.2) | 20.0, CH3 | H-25 | C-24, -25, -27 | 0.82 d (6.8) | 21.9, CH3 |
| 27 | 0.82 d (6.8) | 19.7, CH3 | H-25 | C-24, -25, -26 | 0.83 d (6.8) | 21.1, CH3 |
| 28 | 0.91 d (6.8) | 17.5, CH3 | H-24 | C-23, -24, -25 | 0.91 d (7.0) | 17.8, CH3 |
a Spectra recorded at 400 MHz in CDCl3. b Spectra recorded at 100 MHz in CDCl3. c Selected 1H-NMR and 13C-NMR data were reported by Rueda et al. (see ref. [7]). These data were recorded at 400 MHz for 1H and 100 MHz for 13C in CDCl3. d n. o. = not observed.
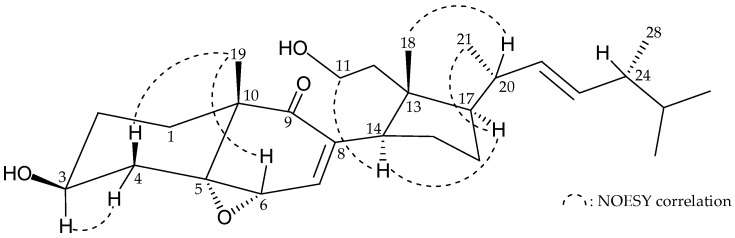
From the 1H-NMR coupling information and 1H–1H correlation spectroscopy (COSY) of 1 (Table 1), the following correlations were revealed: H2-1/H2-2/H-3/H2-4, H-6/H-7, H2-11/H2-12, H-14/H2-15/H2-16/H-17/H-20/H-22/H-23/H-24/H-25/H3-26, H-20/H3-21, H-24/H3-28 and H-25/H3-27. These data, together with the key heteronuclear multiple bond coherence (HMBC) correlations between H2-1, H2-4, H3-19/C-5; H-6/C-8; H-7, H3-19/C-9; H2-2, H3-19/C-10; and H2-12, H2-15, H3-18/ C-13 (Table 1), all the information allowed determination of the carbon skeleton of 1. The stereochemistry of 1 was elucidated by analysis of the results of a nuclear Overhauser effect spectroscopy (NOESY) experiment. Assuming the β-orientation of H3-18 and H3-19, H-14 was found to exhibit correlations with H-11a (δH 3.81) and H-17, but not with H3-18, indicating that this proton was of an α-orientation at C-14. In addition, the main NOESY correlation for 1 were interactions between H-3/H-4α, H-4β/H3-19, H-6/H3-19, H-17/H3-21 and H3-18/H-20; thus, the 3-hydroxy and 5,6-epoxy groups in 1 should be positioned on the β- and α-face, respectively (Figure 2).
Figure 2.
Selected NOESY correlations observed for 1.
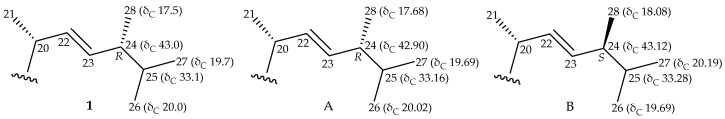
A large coupling constant observed between H-22 and H-23 (J = 15.2 Hz) supported a trans relationship between H-22 and H-23. The configuration of C-24 was suggested to be R on the basis of the 13C-NMR chemical shift of C-28 (δC 17.5). It was reported that the 13C-NMR value of C-28 resonates at δC 17.68 ppm in the 24R epimer of a known sterol, (22E,24R)-24-methylcholesta-5,22-dien-3β-ol, with the same chain, and the 24S epimer, (22E,24S)-24-methylcholesta-5,22-dien-3β-ol, has a relative 0.4 ppm downfield chemical shift (Figure 3) [8].
Figure 3.
The 13C-NMR chemical shifts of the side-chain of secosterol 1, (22E,24R)-24-methyl-cholesta-5,22-dien-3β-ol (A) and (22E,24S)-24-methylcholesta-5,22-dien-3β-ol (B) [8].
It was found that the NMR data of 1 were similar to those of a known 9,11-secosterol derivative, 3-O-deacetylluffasterol B (3) (Figure 1), isolated from the sponge Spongia agaricina [7], except that the signals corresponding to the 11-hydroxy group in 1 were replaced by signals for an aldehyde group in 3 [7] (Table 1). Furthermore, by comparison of the NMR data of 1 with those of 3, we found that the 13C NMR chemical shifts of methines C-20 and C-24 for 3 (δC 43.0 and 38.8, respectively) should be interchangeable by comparison with those of 1 (δC 38.8 and 43.0, respectively) (Table 1), which was further confirmed by 2D NMR experiments. In a previous study, the structure of 1 as presented in this paper had been reported [9]. However, by comparison of the NMR data of 1 with those of reported data, we found that the NMR data (1H and 13C) for this compound differ significantly from those of 1 that reported herein (Table 1), because the structure of 1 has been established by extensive spectroscopic analysis, particularly with 2D NMR experiments. The authors suggested that the compound which was reported to possess the same structure as that of 1 in Reference [9] should be re-examined.
(22R)-Acetoxy-(24ξ)-ergosta-5-en-3β,25-diol (2) was isolated as a colorless needles and its molecular formula was established as C30H50O4 (6° of unsaturation) by HRESIMS at m/z 497.36015 (calcd. for C30H50O4 + Na, 497.36193). The IR spectrum of 2 indicated the presence of hydroxy (νmax 3414 cm−1) and ester carbonyl (νmax 1730 cm−1) groups. The whole series of spectroscopic data obtained from 1D and 2D NMR experiments (Table 2) clearly indicated that sterol 2 had the same core rings A−D and side chain as those of known sterols, 22(R),28-oxido-24ξ-methylcholest-5-en-3β,25,28-triol (lobophytosterol) and (22R)-5β,6β-epoxy-24ξ-methylcholestan-3β,22(R),25-triol diacetate, respectively [10]. The 1H-1H COSY and HMBC correlations observed fully supported the locations of the functional groups, and, hence, (22R)-acetoxy-(24ξ)-ergosta-5-ene-3β,25-diol (2) was assigned as structure 2, with the same relative configurations as 22(R),28-oxido-24ξ-methylcholest-5-en-3β,25,28-triol in the core rings A–D. The configuration of the C-22 stereogenic center was assigned as R on the basis of the NMR chemical shifts of C-22 oxymethine (δH 5.02, 1H, ddd, J = 10.8, 2.8, 2.4 Hz, H-22; δC 78.3, CH-22). It was reported that the 1H- and 13C=NMR values of C-22 oxymethine (δH 4.99, 1H, dt, J = 10.2, 2.5 Hz, H-22; δC 78.3, CH-22) in a 22R epimer of a known sterol, 5β,6β-epoxy-24ξ-methylcholestan-3β,22(R),25-triol diacetate [10], was found to possess the same side chain as that of 2. The proton coupling constants and NMR chemical shift data also further supported these findings, though the configuration of C-24 was not determined at this stage.
Table 2.
1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data and 1H-1H COSY and HMBC correlations for sterol 2.
| Position | δH (J in Hz) | δC, Multiple | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1a/b | 1.84 m; 1.06 m | 37.2, CH2 | H2-2 | C-2, -5 |
| 2a/b | 1.84 m; 1.51 m | 31.6, CH2 | H2-1, H-3 | n. o. a |
| 3 | 3.52 m | 71.8, CH | H2-2, H2-4 | n. o. |
| 4a/b | 2.30 m; 2.24 m | 42.3, CH2 | H-3 | C-2, -3, -5, -6, -10 |
| 5 | 140.7, C | |||
| 6 | 5.35 d (5.2) | 121.6, CH | H2-7 | C-4, -7, -8, -10 |
| 7a/b | 1.97 m; 1.53 m | 31.8, CH2 | H-6, H-8 | C-6, -14 |
| 8 | 1.46 m | 31.9, CH | H2-7, H-9, H-14 | C-14 |
| 9 | 0.92 m | 50.1, CH | H-8, H2-11 | C-7, -8 |
| 10 | 36.5, C | |||
| 11a/b | 1.50–1.40 m | 21.1, CH2 | H-9, H2-12 | C-9 |
| 12a/b | 1.97 m; 1.17 m | 39.7, CH2 | H2-11 | n. o. |
| 13 | 42.7, C | |||
| 14 | 0.98 m | 56.3, CH | H-8, H2-15 | n. o. |
| 15a/b | 1.56 m; 1.07 m | 24.3, CH2 | H-14, H2-16 | C-14 |
| 16a/b | 1.81 m; 1.53 m | 27.2, CH2 | H2-15, H-17 | n. o. |
| 17 | 1.15 m | 53.1, CH | H2-16, H-20 | C-12, -20 |
| 18 | 0.67 s | 11.9, CH3 | C-12, -13, -14, -17 | |
| 19 | 1.00 s | 19.4, CH3 | C-1, -5, -9 | |
| 20 | 1.75 m | 39.8, CH | H-17, H3-21, H-22 | n. o. |
| 21 | 0.93 d (7.2) | 13.0, CH3 | H-20 | C-17, -20, -22 |
| 22 | 5.02 ddd (10.8, 2.8, 2.4) | 78.3, CH | H-20, H2-23 | n. o. |
| 23a/b | 1.84 m; 1.15 m | 29.3, CH2 | H-22, H-24 | C-20, -22 |
| 24 | 1.43 m | 43.1, CH | H2-23, H3-28 | n. o. |
| 25 | 73.6, C | |||
| 26 | 1.13 s | 25.0, CH3 | C-24, -25, -27 | |
| 27 | 1.20 s | 28.4, CH3 | C-24, -25, -26 | |
| 28 | 0.91 d (7.2) | 16.9, CH3 | H-24 | C-23, -24, -25 |
| 22-OAc | 171.0, C | |||
| 2.04, s | 21.6, CH3 | Acetate carbonyl |
a n. o. = not observed.
The sterol analogues isolated from Pinnigorgia sp. were found to display interesting anti-inflammatory activities [1,2,3,5,6]. Based on these findings, the anti-inflammatory testing of sterols 1 and 2 were assayed and 1 showed inhibitory effects on the generation of superoxide anions and the release of elastase, respectively, by human neutrophils (Table 3).
Table 3.
Inhibitory effects of sterols 1 and 2 on superoxide anion generation and elastase release by human neutrophils in response to fMet-Leu-Phe/Cytochalastin B.
| Compound | Superoxide Anions | Elastase Release |
|---|---|---|
| IC50 (μM) a | IC50 (μM) | |
| 1 | 8.65 ± 0.19 | 5.86 ± 0.95 |
| 2 | > 10 | > 10 |
| LY294002 b | 1.06 ± 0.06 | 3.85 ± 1.25 |
a Concentration necessary for 50% inhibition (IC50); results are presented as mean ± S.E.M. (n = 3). b LY294002 (2-morpholin-4-yl-8-phenylchromen-4-one) was used as a reference compound.
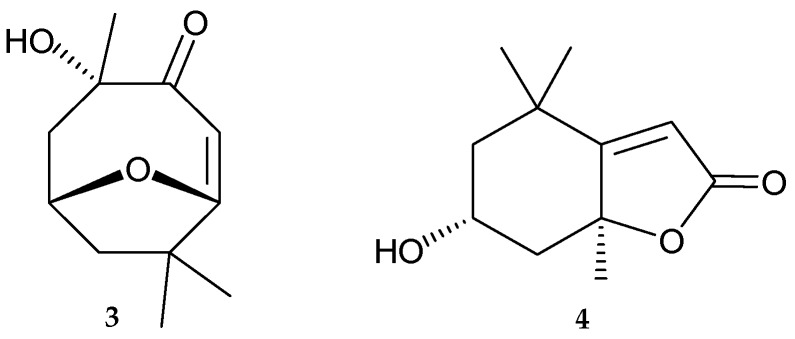
In a previous study, we reported the isolation and structure elucidation of a natural product, pubinernoid A (3), from Pinnigorgia sp. [11] and this compound which has been previously isolated from a traditional Chinese medicinal plant Schisandra pubescens var. pubinervis [12]. Based on the detailed spectroscopic analysis and by comparing the 1H- and 13C-NMR chemical shifts in 3 with those of known carotenoid metabolites, (±)-loliolide, [13,14,15,16,17,18,19], the structure of pubinernoid A (3) should be revised as (+)-loliolide as presented in 4 (Figure 4). Because (±)-loliolide were synthesized by chemical methods [18] and the structure of (–)-loliolide was established by X-ray diffraction analysis [19], the structure of pubinernoid A (3) should be revised as (+)-loliolide (4).
Figure 4.
Chemical structures of pubinernoid A (3) and (+)-loliolide (4).
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured on a Jasco P-1010 digital polarimeter (Japan Spectroscopic Corporation, Tokyo, Japan). Infrared spectra were recorded on a Jasco FT/IR-4100 spectrometer (Japan Spectroscopic Corporation); peaks are reported in cm–1. The NMR spectra were recorded on a 400 MHz Varian Mercury Plus NMR spectrometer (Varian Inc., Palo Alto, CA, USA), using the residual CHCl3 signal (δH 7.26 ppm) as an internal standard for 1H-NMR and CDCl3 (δC 77.1 ppm) for 13C-NMR; coupling constants (J) are given in Hz. ESIMS and HRESIMS were recorded using a Bruker 7 Tesla solariX FTMS system (Bruker, Bremen, Germany). Column chromatography was performed on silica gel (230–400 mesh, Merck, Darmstadt, Germany). TLC was carried out on precoated Kieselgel 60 F254 (0.25 mm, Merck); spots were visualized by spraying with 10% H2SO4 solution followed by heating. Normal-phase HPLC (NP-HPLC) was performed using a system comprised of a Hitachi L-7110 pump (Hitachi Ltd., Tokyo, Japan) and a Rheodyne 7725 injection port (Rheodyne LLC, Rohnert Park, CA, USA). A semi-preparative normal-phase column (Supelco Ascentis Si Cat #:581515-U, 25 cm × 21.2 mm, 5 μm, Sigma-Aldrich, St. Louis, MO, USA) was used for NP-HPLC. Reversed-phase HPLC (RP-HPLC) was performed using a system comprised of a Hitachi L-2130 pump (Hitachi Ltd., Tokyo, Japan), a Hitachi L-2455 photodiode array detector (Hitachi Ltd., Tokyo, Japan) and a Rheodyne 7725 injection port (Rheodyne LLC., Rohnert Park, CA, USA). A reverse phase column (Luna 5 μm C18(2) 100 Å, AXIA Packed, 25 cm × 21.2 mm, Phenomenex Inc., Torrance, CA, USA) was used for RP-HPLC.
3.2. Animal Material
Specimens of the gorgonian corals Pinnigorgia sp. were collected by hand using scuba off the coast of Green Island, Taiwan in August 2012 and stored in a freezer until extraction. A voucher specimen (NMMBA-TW-GC-2012-130) was deposited in the National Museum of Marine Biology & Aquarium, Taiwan. This organism was identified by comparison with previous descriptions [20].
3.3. Extraction and Separation
Sliced bodies of Pinnigorgia sp. (wet weight 1.98 kg; dry weight 0.86 kg) were extracted with ethyl acetate (EtOAc) at room temperature. The EtOAc extract (84.9 g) was partitioned between methanol (MeOH) and n-hexane. The MeOH layer (12.6 g) was separated on Sephadex LH-20 and eluted using a mixture of dichloromethane (DCM) and MeOH (1:1) to yield 7 subfractions A–G. Fraction F was separated by silica gel column chromatography and eluted using n-hexa ne/acetone (stepwise, 1:1–pure acetone) to afford 8 subfractions F1–F8. Fraction F2 was purified by silica gel column chromatography and eluted using n-hexane/acetone (stepwise, 9:1–pure acetone) to yield 13 subfractions F2A–F2M. Fraction F2H was purified by NP-HPLC using a mixture of n-hexane/EtOAc (1:1) to afford 14 subfractions F2H1–F2F14. Fraction F2H12 was re-purified by RP-HPLC using a mixture of MeOH/H2O (90:10, 4.0 mL/min flow rate) to yield 1 (2.8 mg). Fraction F2D was purified by NP-HPLC using a mixture of n-hexane/EtOAc (3:1) to afford 17 subfractions F2D1–F2D117. Fraction F2D12 was re-purified by RP-HPLC using MeOH (1.5 mL/min flow rate) to yield 2 (2.6 mg).
5α,6α-Epoxy-(22E,24R)-3β,11-dihydroxy-9,11-secoergosta-7-en-9-one (1): colorless oil; [α] −35 (c 0.9, CHCl3); IR (neat) νmax 3398, 1682 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data (see Table 1); ESIMS m/z 467 [M + Na]+; HRESIMS m/z 467.31308 (calcd. for C28H44O4 + Na, 467.31373).
(22R)-Acetoxy-(24ξ)-ergosta-5-en-3β,25-diol (2): colorless needles; mp. 130−132 °C; [α] −111 (c 0.7, CHCl3); IR (neat) νmax 3414, 1730 cm−1; 1H (400 MHz, CDCl3) and 13C (100 MHz, CDCl3) NMR data (see Table 2); ESIMS m/z 497 [M + Na]+; HRESIMS m/z 497.36015 (calcd. for C30H50O4 + Na, 497.36193).
3.4. Generation of Superoxide Anions and Release of Elastase by Human Neutrophils
Human neutrophils were obtained by means of dextran sedimentation and Ficoll centrifugation. Measurements of superoxide anion generation and elastase release were carried out according to previously described procedures [21,22]. Briefly, superoxide anion production was assayed by monitoring the superoxide dismutase-inhabitable reduction of ferricytochrome c. Elastase release experiments were performed using MeO-Suc-Ala-Ala-Pro-Valp-nitroanilide as the elastase substrate.
4. Conclusions
Our further studies on Pinnigorgia sp. for the extraction of natural substances have led to the isolation of two new marine sterols, 5α,6α-epoxy-(22E,24R)-3β,11-dihydroxy-9,11-secoergosta-7-en-9-one (1) and (22R)-acetoxy-(24ξ)-ergosta-5-en-3β,25-diol (2), and 1 showed potentially anti- inflammatory activity. These results suggested that continuing investigation of new secondary metabolites together with the potentially useful bioactive substances from Pinnigorgia sp. are worthwhile for future drug development.
Acknowledgments
This research was supported by grants from the National Museum of Marine Biology & Aquarium; the National Dong Hwa University; the National Sun Yat-sen University; and the National Research Program for Biopharmaceuticals, Ministry of Science and Technology (Grant No. MOST 105-2325-B- 291-001, 105-2811-B-291-003, 104-2325-B-291-001, 103-2325-B-291-001, and 104-2320-B-291-001-MY3), awarded to P.-J. S.
Supplementary Materials
Supplementary materials Figure S1–S14 are available online.
Author Contributions
Ping-Jyun Sung designed the whole experiment and contributed to manuscript preparation. Yu-Chia Chang researched data. Tsong-Long Hwang and Chih-Hua Chao analyzed the data and performed data acquisition.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of the compounds 1 and 2 are not available from the authors.
References and Notes
- 1.Chang Y.-C., Kuo L.-M., Su J.-H., Hwang T.-L., Kuo Y.-H., Lin C.-S., Wu Y.-C., Sheu J.-H., Sung P.-J. Pinnigorgiols A–C, 9,11-secosterols with a rare ring arrangement from a gorgonian coral Pinnigorgia sp. Tetrahedron. 2016;72:999–1004. doi: 10.1016/j.tet.2015.12.072. [DOI] [Google Scholar]
- 2.Chang Y.-C., Kuo L.-M., Hwang T.-L., Yeh J., Wen Z.-H., Fang L.-S., Wu Y.-C., Lin C.-S., Sheu J.-H., Sung P.-J. Pinnisterols A–C, new 9,11-secosterols from a gorgonian Pinnigorgia sp. Mar. Drugs. 2016;14:12. doi: 10.3390/md14010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su Y.-D., Cheng C.-H., Wen Z.-H., Wu Y.-C., Sung P.-J. New anti-inflammatory sterols from a gorgonian Pinnigorgia sp. Bioorg. Med. Chem. Lett. 2016;26:3060–3063. doi: 10.1016/j.bmcl.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Chang Y.-C., Chen N.-F., Hwang T.-L., Tseng C.-C., Wu T.-Y., Peng B.-R., Wen Z.-H., Fang L.-S., Wu Y.-C., Sheu J.-H., Sung P.-J. New marine sterols from an algal-bearing gorgonian coral Pinnigorgia sp. Steroids. 2016;115:123–129. doi: 10.1016/j.steroids.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Chang Y.-C., Hwang T.-L., Sheu J.-H., Wu Y.-C., Sung P.-J. New anti-inflammatory 9,11-secosterols with a rare tricyclo[5,2,1,1]decane ring from a Formosan gorgonian Pinnigorgia sp. Mar. Drugs. 2016;14:218. doi: 10.3390/md14120218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Y.-C., Hwang T.-L., Kuo L.-M., Sung P.-J. Pinnisterols D−J, new 11-acetoxy-9,11-secosterols with a 1,4-quinone moiety from Formosan gorgonian coral Pinnigorgia sp. (Gorgoniidae) Mar. Drugs. 2017;15:11. doi: 10.3390/md15010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rueda A., Zubía E., Ortega M.J., Carballo J.L., Salvá J. New metabolites from the sponge Spongia agaricina. J. Nat. Prod. 1998;61:258–261. doi: 10.1021/np970390b. [DOI] [PubMed] [Google Scholar]
- 8.Wright J.L.C., McInnes A.G., Shimizu S., Smith D.G., Walter J.A., Idler D., Khalil W. Identification of C-24 alkyl epimers of marine sterols by 13C nuclear magnetic resonances spectroscopy. Can. J. Chem. 1978;56:1898–1903. [Google Scholar]
- 9.Aiello A., Fattorusso E., Menna M., Carnuccio R., Iuvone T. New cytotoxic steroids from the marine sponge Dysidea fragilis coming from the lagoon of Venice. Steroids. 1995;60:666–673. doi: 10.1016/0039-128X(95)00055-U. The compound that was reported to possess the same structure as that of 1 was listed as compound 7 in this article. [DOI] [PubMed] [Google Scholar]
- 10.Carmely S., Kashman Y. Isolation and structure elucidation of lobophytosterol, depresosterol and three other closely related sterols. Tetrahedron. 1981;37:2397–2403. doi: 10.1016/S0040-4020(01)88896-7. [DOI] [Google Scholar]
- 11.Chang H.-H., Chang Y.-C., Chen W.-F., Hwang T.-L., Fang L.-S., Wen Z.-H., Chen Y.-H., Wu Y.-C., Sung P.-J. Pubinernoid A and apo-9′-fucoxanthinone, secondary metabolites from a gorgonian coral Pinnigorgia sp. Nat. Prod. Commun. 2016;11:707–708. [PubMed] [Google Scholar]
- 12.Huang S.-X., Yang J., Xiao W.-L., Zhu Y.-L., Li R.-T., Li L.-M., Pu J.-X., Li X., Li S.-H., Sun H.-D. Three novel terpenoids from Schisandra pubescens var. pubinervis. Helv. Chim. Acta. 2006;89:1169–1175. doi: 10.1002/hlca.200690114. [DOI] [Google Scholar]
- 13.Hodges R., Porte A.L. The structure of loliolide, a terpene from Lolium perenne. Tetrahedron. 1964;20:1463–1467. doi: 10.1016/S0040-4020(01)99140-9. [DOI] [Google Scholar]
- 14.Isoe S., Hyeon S.B., Katsumura S., Sakan T. Photo-oxygenation of carotenoids. II. The absolute configuration of loliolide and dihydroactinidiolide. Tetrahedron Lett. 1972;13:2517–2520. doi: 10.1016/S0040-4039(01)84863-2. [DOI] [Google Scholar]
- 15.Pettit G.R., Herald C.L., Ode R.H., Brown P., Gust D.J., Michel C. The isolation of loliolide from an Indian Ocean opisthobranch mollusc. J. Nat. Prod. 1980;43:752–755. doi: 10.1021/np50012a009. [DOI] [PubMed] [Google Scholar]
- 16.Ravi B.N., Murphy P.T., Lidgard R.O., Warren R.G., Wells R.J. C18 terpenoid metabolites of the brown alga Cystophora moniliformis. Aust. J. Chem. 1982;35:171–182. doi: 10.1071/CH9820171. [DOI] [Google Scholar]
- 17.Valdes L.J., III Loliolide from Salvia divinorum. J. Nat. Prod. 1986;49:171. doi: 10.1021/np50043a031. [DOI] [PubMed] [Google Scholar]
- 18.Mori K., Khlebnikov V. Synthesis of (+)-dihydroactinidiolide, (+)- and (–)-actinidiolide, (+)- and (–)-loliolide as well as (+)- and (–)-epiloliolide. Liebigs Ann. Chem. 1993:77–82. doi: 10.1002/jlac.199319930113. [DOI] [Google Scholar]
- 19.Sung P.-J., Chen B.-Y., Chen Y.-H., Chiang M.Y., Lin M.-R. Loliolide: Occurrence of a carotenoid metabolite in the octocoral Briareum excavatum (Briareidae) Biochem. Syst. Ecol. 2010;38:116–118. doi: 10.1016/j.bse.2009.12.028. [DOI] [Google Scholar]
- 20.Fabricius K., Alderslade P. Soft Corals and Sea Fans—A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea. 1st ed. Australian Institute of Marine Science; Queensland, Australia: 2001. pp. 218–219. [Google Scholar]
- 21.Yang S.-C., Chung P.-J., Ho C.-M., Kuo C.-Y., Hung M.-F., Huang Y.-T., Chang W.-Y., Chang Y.-W., Chan K.-H., Hwang T.-L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013;190:6511–6519. doi: 10.4049/jimmunol.1202215. [DOI] [PubMed] [Google Scholar]
- 22.Yu H.-P., Hsieh P.-W., Chang Y.-J., Chung P.-J., Kuo L.-M., Hwang T.-L. 2-(2-Fluorobenzamido) benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011;50:1737–1748. doi: 10.1016/j.freeradbiomed.2011.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.