Abstract
Opportunistic pathogens (OPs) in drinking water, like Legionella spp., mycobacteria, Pseudomonas aeruginosa and free-living amobae (FLA) are a risk to human health, due to their post-treatment growth in water systems. To assess and manage these risks, it is necessary to understand their variations and environmental conditions for the water routinely used. We sampled premise tap (Ncold=26, Nhot=26) and shower (Nshower=26) waters in a bathroom and compared water temperatures to levels of OPs via qPCR, and identified Legionella spp. by 16S rRNA gene sequencing. The overall occurrence and cell-equivalent quantities (CE L−1) of Mycobacterium spp. were highest (100%, 1.4×105), followed by Vermamoeba vermiformis (91%, 493), Legionella spp. (59%, 146), P. aeruginosa (14%, 10), and Acanthamoeba spp. (5%, 6). There were significant variations of OP’s occurrence and quantities, and water temperatures were associated with their variations, especially for Mycobacterium spp., Legionella spp. and V. vermiformis. The peaks observed for Legionella, mainly consisted of L. pneumophila sg1 or L. anisa, occurred in the temperature ranged from 19 to 49°C, while Mycobacterium spp. and V. vermiformis not only co-occurred with Legionella spp. but also trended to increase with increasing temperatures. There were higher densities of Mycobacterium in 1st than 2nd draw water samples, indicating their release from faucet/shower head biofilm. Legionella spp. were mostly at detectable levels and mainly consisted of L. pneumophila, L. anisa, L. donaldsonii, L. tunisiensis and an unknown drinking water isolate based on sequence analysis. Results from this study suggested potential health risks caused by opportunistic pathogens when exposed to warm shower water with low chlorine residue and the use of Mycobacterium spp. as an indicator of premise pipe biofilm and the control management of those potential pathogens.
Keywords: Legionella, opportunistic pathogen, tapwater, shower water, qPCR
Introduction
Exposure to opportunistic pathogens (OPs) within aerosols generated from use of taps and showers within healthcare facilities is an increasingly recognized drinking water risk (Beer et al. 2015). Legionella pneumophila, non-tuberculous mycobacteria (NTM), Pseudomonas aeruginosa and Acanthamoeba spp. may cause opportunistic infections, such as legionellosis by L. pneumophila, pulmonary infections by various Mycobacterium spp., skin infection from Pseudomonas aeruginosa and Acanthamoeba keratitis in susceptible humans (Ashbolt 2015). L. pneumophila is the leading water-associated pathogen causing severe pneumonia and death in immuno-compromised individuals (Neil &Berkelman 2008). Pulmonary disease and other health risks are associated with inhalation of both viable and non-viable bacteria or their components (Falkinham III 2003, Thorn 2001). There are increasing numbers of humans who are at risk from developing life-threatening opportunistic infections, such as women elders, AIDS patients, individuals undergoing therapy for solid organ transplantation, cancer chemotherapy etc. where immune-suppressants may be administered. Hence, to better protect such immune-compromised people there is a need to identify anthropogenic reservoirs of OPs (Exner et al. 2005, Falkinham III 2003). Considering OPs are often amoeba-resisting microorganisms (ARMs) and their growth in amoebae may be a critical feature of OP life cycles, the presence of opportunistic pathogenic free-living amoebae (FLA), and bacteria in building plumbing systems is an important but poorly studied public health issue. Particularly lacking are studies over periods of months to years. OPs have been detected in tap and shower water, including NTM, L. pneumophila and P. aeruginosa generated within shower heads and hot-water faucets (Aumeran et al. 2007, Falkinham III 2003, Falkinham et al. 2015, Rohr et al. 1998, Vianelli et al. 2006, Zacheus &Martikainen 1994, Zichichi et al. 2000). Some OP exposures were associated with waterborne infections (Bartley et al. 2015, Bédard et al. 2016, Demirjian et al. 2015, Exner et al. 2005), Legionnaires’ disease (Alary &Joly 1991) and other pulmonary and wound infections (Falkinham III et al. 2008, Nishiuchi et al. 2007). Several studies have traced both L. pneumophilia and M. avium, leading water-associated NTM infections in hospitalized patients, to microbes in their home showers, implying home exposures were the source of their infections (Falkinham III et al. 2008, Nishiuchi et al. 2007, Pedro-Botet et al. 2002). Those findings indicated that tap and shower water (specifically, pipe biofilms) are potential sources of daily exposure to OPs.
However, year around sampling of OPs to ascertain trends or possible hot-spots of contamination within household plumbing are rarely reported (Chen et al. 2005, Cooper et al. 2008), yet more information is available from healthcare settings (Chen et al. 2005, Stout 2007). In the present study, we examined the annual occurrences and quantities of OPs with emphasis on Legionella spp. and analyzed the relationships between the OPs and some environmental parameters for tap water routinely used and within a recently built tap/shower unit to simulate a house bathroom, in which water temperature appears to play a major selective role.
Materials and methods
Sample collection and processing
Water samples were collected from an engineered bathroom of a research building in Cincinnati, OH. The bathroom was constructed with a cold and hot faucet/tap and a shower water mixer connected to a showerhead in January 2011. Both taps and shower were fed with the same building tap water. Total 13 sample dates across 2012 (Mar 28-Apr. 24 and Oct. 24 – Dec. 27) and 2013 (Jan. 30 – Apr. 23, Jun. 5 – Jul. 25 and Dec. 18) were used. For each sample date (usually on Wednesday and occasionally on Tuesday or Thursday), two 1-L tap and shower water samples were taken into sterile flasks at early morning (around 7:00). For taps, the first sample (1st draw) was taken immediately after turning tap on, while the second sample (2nd draw) was done after 3 min of flow. For shower water, which flows through showerhead, both samples were collected after 3 min of flow. Thus, the samples included 1st draw cold/hot, 2nd draw cold/hot tap and shower water. A number of non-bath cold/hot tap water samples, which were taken after 3 min of flow from taps of a laboratory in the same building and within the circulation system as the bath unit, were also included as reference for phylogenetic analysis. All samples were measured for total chlorine, free chlorine and temperature. Each water sample was filtered onto a sterile 0.4-μm pore-size polycarbonate membrane, then placed into a bead-beating tube with lysing matrix A (MP Biomedicals, Santa Ana, CA) and stored at −80°C, until use.
DNA extraction
DNA was extracted as described previously for filter-concentrated drinking water samples (Lu et al. 2016). Briefly, the 1.5 mL micro-tubes containing a filter and 300 μL 1 × T&C (cell and tissue) solution was disrupted and lysed using a Mini-Beadbeater-16 (BioSpec Products, Inc., Bartlesville, OK) for 2 × 30 s. The mixture was then centrifuged at 10,000 g for 8 min, supernatant transferred to a sterile tube and DNA extracted and purified using the MasterPure Complete DNA Purification Kit™ (Epicentre Biotechnologies, Madison, WI) per manufacture’s instruction. DNA concentrations were estimated with a Nanodrop ND-1000 Spectrophotometer (NanoDrop Technologies, Inc., Wilmington, Delaware). DNA extracts were stored at −80°C until qPCR and PCR were performed in the same period with the same prepared reagents, standards, supplies and equipment.
Quantitative PCR (qPCR) for screening, quantification and PCR cloning
The PCR and qPCR assays used and reactions performed for detection of microbial pathogens are described in detail previously (Lu et al. 2016). In brief, the qPCR assay was performed with a 7900 HT Fast Real-Time Sequence Detector (Applied Biosystems) with reaction mixtures (20 μL) contained 10 μL 2× qPCR Master Mix (Applied Biosystems), 0.2 μM primers, 0.08 μM probe (final concentration) and 2 μL of template DNA. Initial DNA treatment consisted of 50°C for 2 min with UNG (Uracil-N-Glycosylase) to prevent carryover contamination, then 95°C for 10 min for DNA denaturing. The following quantification cycling protocol was used: 40 cycles at 95°C for 15 s and at 60°C or at the Tm (°C) specified by the assay developers referred by Lu et al. (2016) for 30 s with an extension at 72°C for 30 s and a final hold at 72°C for 5 min, with qPCR reactions for each DNA sample undertaken in duplicate. The standard curves of targets were constructed with the surrogates; Bacteroides thetaiotaomicron, Campylobacter jejuni, Legionella pneumophila, Salmonella enterica, Pseudomonas aeruginosa, Mycobacterium avium, Vermamoeba vermiformis, Acanthamoeba polyphaga trophozoites, Cryptosporidium parvum oocysts and Giardia duodenalis cysts. Target cells in the extracts are reported as numbers of spiked cell or cyst equivalents (CE). Each DNA extract was assayed for potential qPCR inhibitors with 10-fold dilution vs. neat DNA and with the addition of the TaqMan Exogenous Internal Positive Control Reagents (a VIC-labeled probe) manufactured by ABI™. Furthermore, each qPCR run included a DNA standard curve in the first row of the 96-well plate, and a no-template control for each row of each 96-well plate assayed. For qPCR positive Legionella samples, PCR products from five reactions were pooled and cloned into pCR4.1 TOPO (Invitrogen). Individual clones (total 5 plates or 12 colonies per sample selected) were sequenced by using BigDye Terminator chemistry and an Applied Biosystems PRISM 3730XL as described by Lu et al.(Lu et al. 2008). Almost all of the clone sequences were proved true positive. Raw sequences were processed using Sequencher 4.9 software (Gene Codes, Ann Arbor, MI) for editing, comparing and alignment, using protocols, homology searches, chimera check and phylogenetic analysis as previously described (Lu et al. 2008). Representative Legionella 16S rRNA gene sequences from clone libraries were deposited in GenBank with accession numbers (KX238910-KX238948).
Data analysis
For data management and calculations, Microsoft Excel 2003 and SAS Systems version 9.2 (SAS, Cary, NC) were used. For each pathogen in each sampling category, the occurrence was expressed as frequency of detection (FOD) and calculated as the ratio of the number positive to the total sample number, while the mean was calculated as a geometric mean using log (base 10) transformed data from different sample dates for each site. Correlations used to determine the relationship among OPs and between the densities of OPs and other parameters (temperatures and chlorine) were analyzed using generalized linear models (GLM).
Results
The qPCR targets: Salmonella, Campylobacter jejuni, Escherichia coli O157, Giardia duodenalis and Cryptosporidium spp., and human faecal indicator Bacteroides were not detected, while potential opportunistic pathogens, such as P. aeruginosa, L. pneumophila, Mycobacterium spp., and possible host amoeba Acanthamoeba spp. and V. vermiformis were consistently detected (Table S1). The overall occurrence and cell-equivalent quantities (CE L−1) of Mycobacterium spp. were highest (100%, 1.4×105), followed by Vermamoeba vermiformis (91%, 493), Legionella spp. (59%, 146), P. aeruginosa (14%, 10), and Acanthamoeba spp. (5%, 6).
OPs in the bath tap water
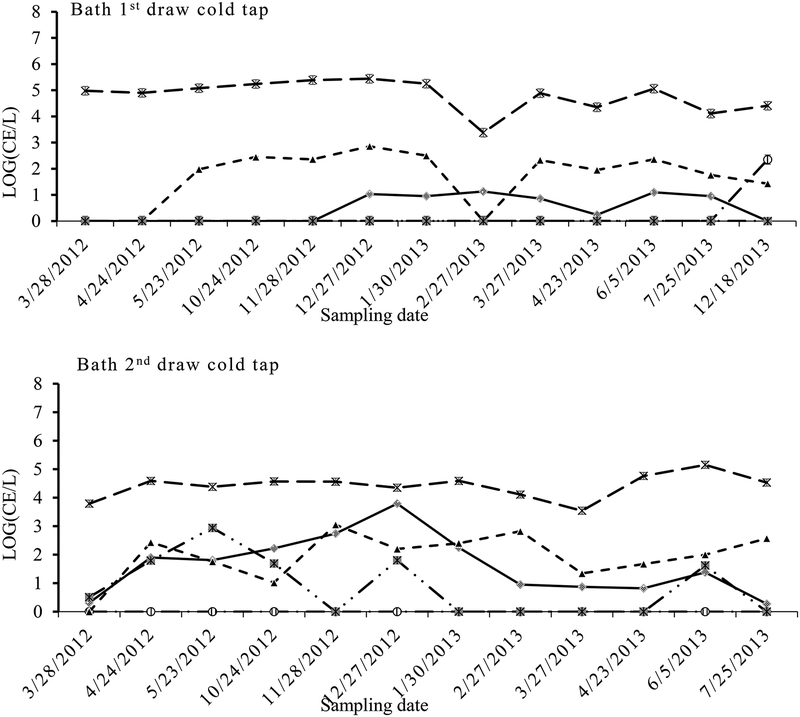
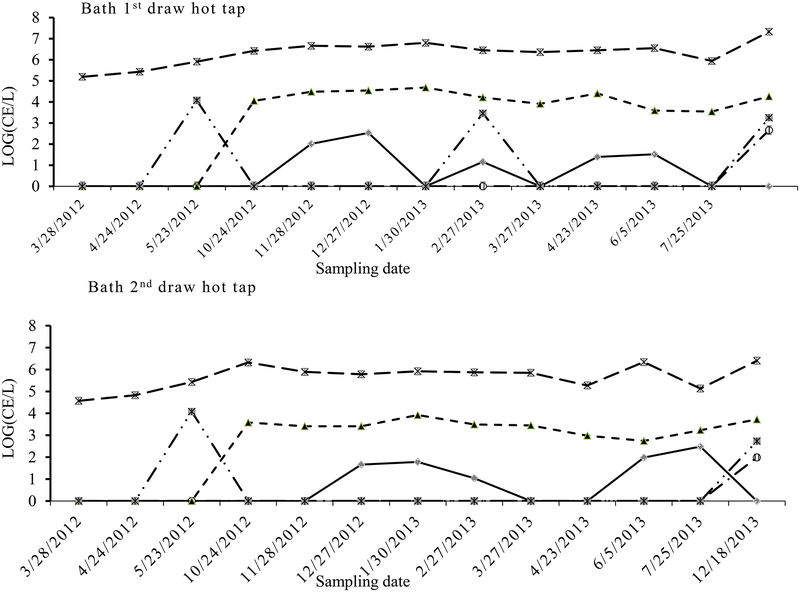
Fluctuations in OPs detections in the tap water were mainly evident with Legionella, Mycobacterium and V. vermiformis, and the densities of Mycobacterium spp. and V. vermiformis showed less variation annually compared to Legionella in the samples from both the tap and shower water (Fig. 1, 2 and 3). There appeared to be a consistent peak in Legionella spp. in the cold tap (19–36°C), hot tap (21–49°C) and shower water (32–44°C) in December 27. Their densities showed significant differences between sample types (cold vs. hot and 1st draw vs. 2nd draw). Comparing the densities (geometric mean for each category) between the cold tap and hot tap waters, total Legionella were higher in the cold tap water (11 CE L−1) than in the hot tap water (2 CE L−1) (PT-test=0.002), while Mycobacterium were higher in the hot (9.7×104 CE L−1) than in the cold tapwater (3.8×104 CE L−1) (PT-test=0.011). Also, Legionella were significantly more numerous in the 2nd draw cold tap water (42 CE L−1) than in the 1st draw cold tap water (3 CE L−1) samples (PT-test=0.004) (Table 1). Conversely, Mycobacterium were significantly higher in the 1st draw cold tap water (6.4×104 CE L−1) than in the 2nd draw cold tap water (2.3×104 CE L−1) samples (PT-test =0.032) (Table 1). For the hot water samples Legionella were at low levels (4~5 CE L−1) close to the detection limit, while Mycobacterium and V. vermiformis were significantly higher in the 1st draw (2.1×106 and 1.6×103 CE L−1, respectively) than in 2nd draw (4.6×105 and 4.1×102 CE L−1, respectively) samples (both PT-test <0.001) (Table 1). The significant correlations of quantities between 1st and 2nd draw samples (R2 = 0.87 and 0.98, respectively) indicated that levels of both Mycobacterium and V. vermiformis were source-related. The highest frequency of detection (FOD) and mean maximum densities of Legionella occurred in the 2nd draw cold tap water, while those of Mycobacterium presented in the 1st draw hot tap water,
Fig. 1.
Quantity (CE L−1) variations of Legionella spp. ( ), Mycobacterium spp. (
), Mycobacterium spp. (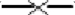 ), Acanthamoeba (
), Acanthamoeba (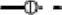 ), V. vermiformis (
), V. vermiformis ( ) and P. aeruginosa (
) and P. aeruginosa ( ) along sampling dates in the bath 1st draw cold tap water and bath 2nd draw cold tap water
) along sampling dates in the bath 1st draw cold tap water and bath 2nd draw cold tap water
Fig. 2.
Quantity (CE L−1) variations of Legionella spp. (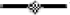 ), Mycobacterium spp. (
), Mycobacterium spp. (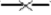 ), Acanthamoeba (
), Acanthamoeba ( ), V. vermiformis (
), V. vermiformis ( ) and P. aeruginosa (
) and P. aeruginosa ( ) along sampling dates in the bath 1st draw hot tap water and bath 2nd draw hot tap water
) along sampling dates in the bath 1st draw hot tap water and bath 2nd draw hot tap water
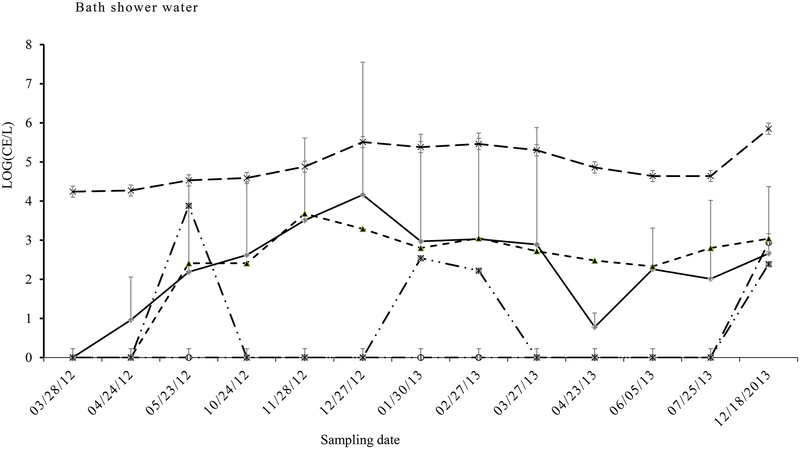
Fig. 3.
Quantity (CE L−1) variations of Legionella spp. ( ), Mycobacterium spp. (
), Mycobacterium spp. ( ), Acanthamoeba (
), Acanthamoeba ( ), V. vermiformis (
), V. vermiformis ( ) and P. aeruginosa (
) and P. aeruginosa ( ) along sampling dates in the bath shower water
) along sampling dates in the bath shower water
Table 1.
Opportunistic microbial contamination based on frequency of detection (FOD: %), geometric mean concentration (MeC: cell equivalent or CE L−1) and maximum concentration (MaC: CE L−1) analyzed using qPCR from bathroom water (n=13) from 2012 (Mar 28-Apr. 24 and Oct. 24 – Dec. 27) to 2013 (Jan. 30 – Apr. 23, Jun. 5 – Jul. 25 and Dec. 18)
| Site | 1st draw cold tap | 2nd draw cold tap | 1st draw hot tap | 2nddraw hot tap | shower |
|---|---|---|---|---|---|
| Legionella FOD (%) | 37 | 95 | 46 | 38 | 77 |
| Legionella MeC (StDev) (CE/L) | 3(3) | 42(10) | 4(8) | 4(9) | 204 (14) |
| Legionella MaC (CE/L) | 13 | 6262 | 34 | 30 | 1642 |
| Acanthamoeba FOD (%) | 4 | 4 | 4 | 4 | 8 |
| Acanthamoeba MeC (StDev) (CE/L) | 1(4) | 1(2) | 1(5) | 1(3) | 1(6) |
| Acanthamoeba MaC (CE/L) | 225 | 21 | 46 | 10 | 150 |
| Vermamoeba vermiformis FOD (%) | 87 | 95 | 87 | 87 | 100 |
| Vermamoeba vermiformis MeC (StDev) (CE/L) | 49(10) | 90(6) | 1601 (72) | 407 (32) | 242 (13) |
| Vermamoeba vermiformis MaC (CE/L) | 732 | 1135 | 4872 | 836 | 648 |
| Mycobacterium FOD (%) | 100 | 100 | 100 | 100 | 100 |
| Mycobacterium MeC (StDev) (CE/L) | 6.4×104(3) | 2.3×104 (2) | 2.1×106(3) | 4.6×105 (3) | 8.6×lO4 (3) |
| Mycobacterium MaC (CE/L) | 2.8×105 | 1.4×105 | 2.2×106 | 2.6×105 | 7.2×104 |
| Pseudomonas aeruginosa FOD (%) | 0 | 37 | 12 | 8 | 12 |
| Pseudomonas aeruginosa MeC (StDev) (CE/L) | 0 | 8(10) | 6(38) | 3(20) | 7(23) |
| Pseudomonas aeruginosa MaC (CE/L) | 0 | 878 | 1176 | 878 | 1021 |
| # of instances Rank 1 FOD | 1 | 2 | |||
| # of instances Rank 1 MeC | 1 | 1 | 2 | ||
| # of instances Rank 1 MaC | 1 | 1 | 2 | 1 |
OPs in the bath shower water
The mean densities of Legionella in the shower water (204 CE L−1) were higher than those in tap water, but those of Mycobacterium (8.6×104 CE L−1) and V. vermiformis (242 CE L−1) in the shower water were lower than those in the hot tap water samples (Table 1), indicating Legionella increased in the shower water and Mycobacterium and V. vermiformis were probably released/sourced to a greater extent from hot tap water than the shower water. Similarly to the 2nd draw cold tap water samples, the highest density of Legionella spp. (1.5×103 CE L−1) (Fig. 3), which mainly consisted of L. pneumophila sg1 (Fig. 4), also occurred in Dec. 27 (31°C), indicating high Legionella densities were attributed to the mixing of the cold tap water with the hot tap water, with the former likely being the main source of these legionellae. Interestingly, variations in Legionella were highly correlated with V. vermiformis (R2=0.79) and Mycobacterium (R2=0.57). Specific qPCR for L. pneumophila (Lp) and serum group 1 (sg1) showed that mean density of Lp or sg1 was 95±17 CE L−1. There were significant close correlation between the densities of Legionella and Lp or sg1 (R2 (Leg-Lp or sg1) =0.93~0.97). Sequence data showed that the presence of Lp or sg1 corresponded to the dominant qPCR signals, especially during the peak Legionella period (Fig. 4).
Fig. 4.
Quantity (CE L−1) variations of Legionella spp. ( ), rtxA for L. pneumophila (
), rtxA for L. pneumophila ( ) and P65/66 (
) and P65/66 ( ) for L. pneumophila sg1 along sampling dates in the bath cold tap water, hot tap water and shower water
) for L. pneumophila sg1 along sampling dates in the bath cold tap water, hot tap water and shower water
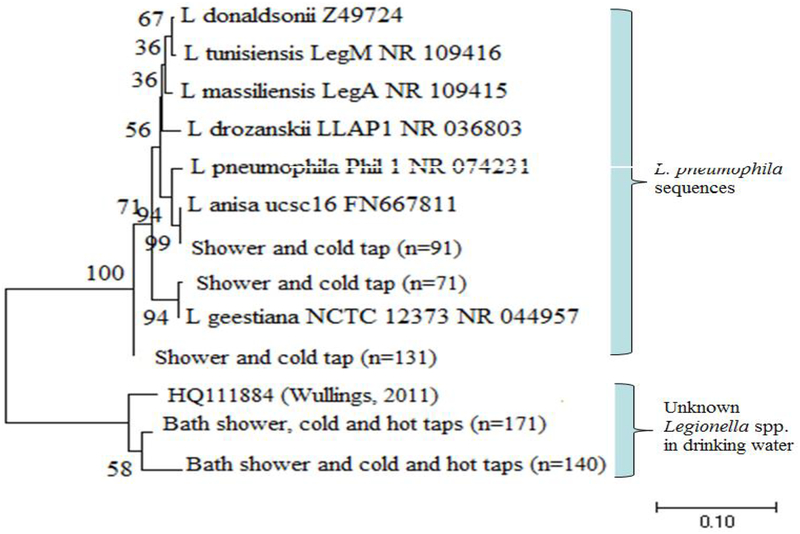
Phylogenetic tree of identified Legionella spp.
Two distinct phylogenetic clades of Legionella OTU were categorized from 16S rRNA gene sequences (Fig. 5). The first clade on the top of the tree was 99% identical to clinically-relevant strains L. anisa, L. pneumophila, L. geestiana, L. massiliansis, L. drozanskii, L. tunisiensis or L. donaldsonii. The sequences mainly derived from the samples of shower, 2nd draw cold tap and some non-bath hot/cold tap water. The other clade, being mostly from non-bath cold (major) and hot (a few) tap water, was 99% identical to those previously described from drinking water distribution systems (Lu et al. 2016) or drinking water biofilms (Keinänen-Toivola et al. 2006) and low temperature drinking water (Wullings &van der Kooij 2006), respectively.
Fig. 5.
Unrooted neighbor-joining tree of 16S rRNA gene amplified for Legionella spp. sequences obtained from clone libraries of the building taps and bath water. Sequences were aligned, and a bootstrap consensus tree was created with MEGA6 (1% divergence). Bootstrap values greater than 50 (1000 replicate) are shown at the nodes
Impact of water temperature on OP presence
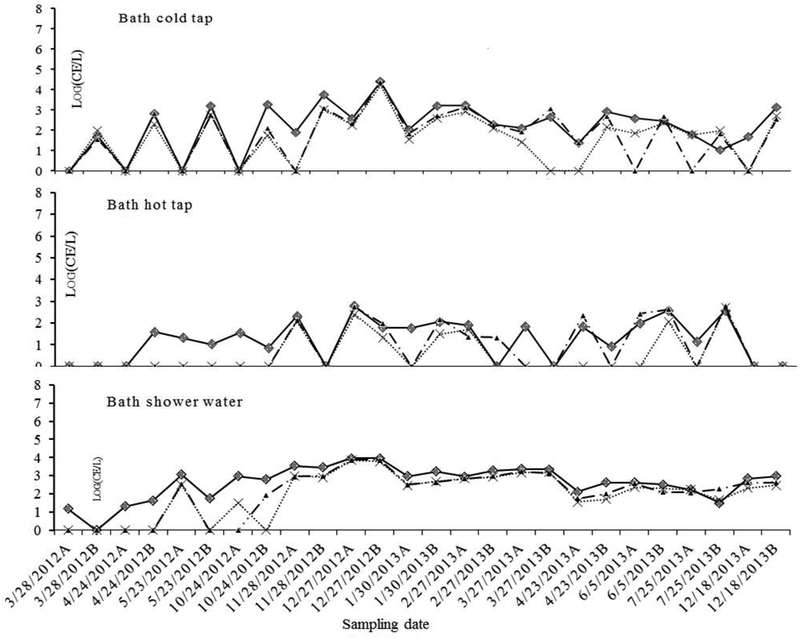
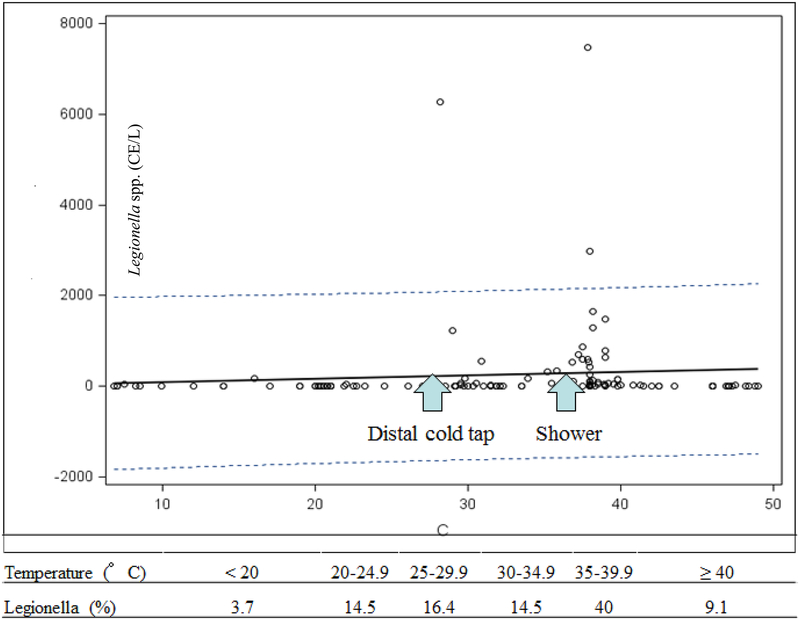
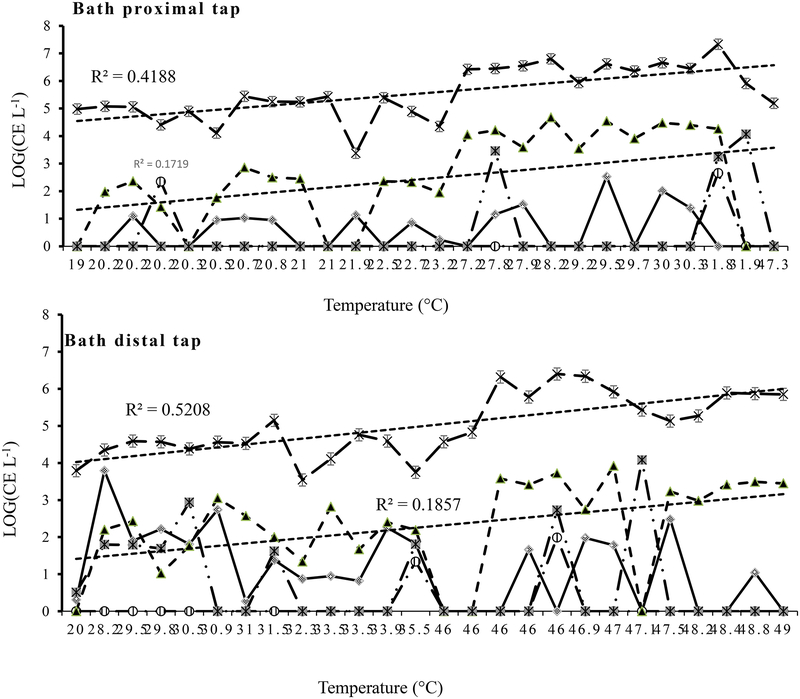
Overall, water temperatures were positively associated with the densities of Legionella (R2=0.33, P<0.001), V. vermiformis (R2=0.31, P<0.001) and Mycobacterium (R2=0.38, P<0.001). The linear regression (including the samples of non-bath water) of Legionella and temperatures showed a very low slope and three points, which occurred in the same period, beyond upper 95% confident interval (corresponding to the peaks in the 2nd draw cold tap and shower water, respectively) (Fig. 6). Most of the Legionella positive samples (71%) were detected between 25–40°C (Fig. 6). However, there were different temperature trends in the density distributions of the three OP targets in 1st draw versus 2nd draw bath tap water (Fig. 7). Generally, both Mycobacterium and V. vermiformis densities increased with temperature from 19 to 50°C, but the former had a higher correlation than the latter (R2=0.42 and 0.17 in the 1st draw tap water and 0.52 and 0.19 in the 2nd draw tap water, Fig. 7). However, in the 1st (21–47°C) and 2nd draw hot tap water (45–49°C) samples, the densities of Legionella were at low levels (<34 CE L−1), while V. vermiformis and Mycobacterium maintained much higher densities than Legionella. Mycobacterium spp. were higher in the 1st than 2nd draw tap water and in the hot than cold tap water, respectively. Only in the 2nd draw cold tap (20–36°C) and shower water (32–42°C) were there high densities of Legionella (Table 1).
Fig. 6.
Correlation of Legionella spp. with temperatures
Fig. 7.
Correlations between temperatures and the quantity (CE L−1) of Legionella spp. ( ), Mycobacterium spp. (
), Mycobacterium spp. ( ), Acanthamoeba (
), Acanthamoeba ( ), V. vermiformis (
), V. vermiformis ( ) and P. aeruginosa (
) and P. aeruginosa ( ) in the bath water
) in the bath water
Discussion
Variations, factors and potential risk
Legionella spp. appear ubiquitous in drinking water and building plumbing systems, albeit at generally very low concentrations, where they survive and some strains at least replicate inside several species of amoeba that colonize biofilms (Brooks et al. 2004, Dupuy et al. 2016, Fields et al. 2002). However, unlike Legionella in cooling tower biofilms, where Legionella densities reached up to 105 cell L−1 at near optimal temperatures of ~35°C (Ikedo &Yabuuchi 1986, Yamamoto et al. 1992), they are normally in low densities to non-detectable in drinking water (< 103 CFU or cell L−1). For example, the mean Legionella spp. were 187±458 in two chlorinated drinking water systems in southwest Virginia (Wang et al. 2012) and 85 ±154 CE/L in a recently investigated chlorinated metropolitan distribution water system (Lu et al. 2016), and 290±190 /L in a unchlorinated drinking water supply in the Netherlands (Wullings et al. 2011). According to previous documents, the normal quantity of L. pneumophila in water samples should not exceed 103 CFU L−1 (Guillemet et al. 2010, WHO 2007). Hence, our results with qPCR values < 103 CE L−1 for Legionella spp. seem within normal levels for sampled drinking water. A recent report for hospital tap systems, where legionnaires’ disease outbreaks occurred and L. pneumophila was detected > 103 CE L−1 (Bédard et al. 2016), implicated that high densities of L. pneumophila (> 103 CE L−1) could be associated with health risk. Furthermore, our building legionellae clustered within two phylogenetic groups previously reported in drinking water distribution systems/chlorinated biofilm or an engineering biofilm (Keinänen-Toivola et al. 2006, Lu et al. 2016, Wullings &van der Kooij 2006).
What is of potential concern, however, was the peak period (e.g. December 27) when higher concentrations of L. pneumophila sg1 were detected. The peak of OPs might have caused by stagnant pipe water, because of less water use during Christmas holiday season, and appropriate water temperatures. L. pneumophila sg1 along with Mycobacterium and V. vermiformis in the cold water and shower mixer of the unit (2nd draw cold tap water and shower water) reached peak, which potentially increased risk to water users, especially during morning showering events. This Legionella risk appeared to be reduced, if water temperatures are low (<20°C) (Ashbolt 2015, Buse et al. 2012), and high enough (>42°C) (Ohno et al. 2003), although they would still survive under high temperatures up to 70°C (Allegra et al. 2008, Bédard, 2016 #135, Allegra, 2011 #138). The significant low densities of Legionella (4 CE L−1) detected in the hot tap (46–49°C) in this study indicated Legionella spp. were suppressed. However, Mycobacterium and V. vermiformis demonstrated to be resistant to high temperature in this study (46–49°C) and previous studies (Schulze-Röbbecke &Buchholtz 1992). According to Ohno et al.(2003), L. pneumophila becomes non-cultivable at 42°C along with significantly reduced metabolic activity at >45°C, while for Mycobacterium spp., 10 isolates from water supply systems examined by Schulze-Röbbecke and Buchholtz (1992) showed cultivable at high temperatures ranged from 50–70°C. It has been hypothesized that the rise in pulmonary infections by NTM over recent decades is linked to increased use of showers (O’Brien et al. 2000), possibly because various Mycobacterium spp. can cause pulmonary disease both in healthy people and those predisposed to pulmonary infection, and they are highly resistant to various treatments. In many hospitals, Mycobacterium (mostly NTM) infections now outnumber M. tuberculosis detections reported by clinical laboratories (Heifets 2004).
As showed in the results, the generally detrimental impact of higher water temperatures in giving higher densities of major OPs were significant, but the effect seemed to differ by potential OPs, the sample type and the range of temperatures. Mycobacterium (presumably including various NTM) increased with temperatures in a linear trend (R2: 0.41~0.52, P<0.0001) within the range (7–49°C) surveyed for bath and non-bath water. There might have been higher mycobacteria densities in locally sloughed biofilms (more from 1st draw tap water than from 2nd draw tap water) and these same trends might have been across different water sample types (non-bath cold/hot, and bath 1st and 2nd draw tap water). Legionella acted differently with more variations than other OPs. It seemed that most sampled water (non-bath cold: 7–28°C, 1st draw and 2nd draw hot tap water: 46–49°C) were not favorable to Legionella development, but the water temperatures (37–39°C) possibly favored both Legionella and Mycobacterium compared to the water temperatures (7–29°C). Our findings are in agreement with previous studies, for example, Legionella is able to survive or/and grow at temperature ranging from 20 to 50°C in distribution systems (Stout et al. 1985, Wadowsky et al. 1985). Wadowsky et al. (1985) also reported that naturally occurring L. pneumophila multiplied at a temperature between 25 and 37°C (Wadowsky et al. 1985). Rodgers (1994) noted that L. pneumophila accounted for a low proportion of biofilm microbiota at 20°C, while it was most abundant at 40°C in biofilms, where it accounted for up to 50% of the total bacterial biomass (Rogers et al. 1994). As for the major source of actual OP colonization, Legionella and Mycobacterium could be different. Considering the higher densities of Mycobacterium in 1st than 2nd draw samples, they were probably from the biofilm at the tap faucet or shower head and hot water released more organisms as their quantities were 1st > 2nd draw tap and shower. These findings were in agreement with biofilms from various faucets and shower heads, which showed Mycobacterium spp. to be the major members (Feazel et al. 2009). By contrast, the major factor contributing to Legionella contamination could be from cold water or pipes and increased due to elevated temperatures in the re-circulating hot tap water and the warm basement (2nd draw cold tap water). Therefore, higher concentrations in the 2nd draw cold vs. hot tap water samples were possibly due to appropriate temperatures (19–36°C) for Legionella replication. These findings were different from those reported from hospital building tap water, where Legionella in tap and shower water generally were thought to derive from hot water or sediment of a hot water tank (Allegra et al. 2011, Bartley et al. 2015, Demirjian et al. 2015, Stout et al. 1985, Wadowsky et al. 1982).
Chlorine disinfectant was used in the drinking water distributed to the building and kept at the level regulated by US EPA (~ 1 mg/L, Table S2) at the non-bath and bathroom outlets in our study. However, all Legionella detections occurred at the free chlorine concentrations < 0.4 mg L−1, mostly at <0.1 mg L−1 (Fig. S1). The peak of Legionella co-occurred with no detectable free/total chlorine, and there was a negative correlation between Legionella densities and free chlorine after excluding the peak values, indicating that maintaining a chlorine residual should also be a factor controlling Legionella. Considering chlorine concentrations measured were lower in 1st than 2nd draw water sampling sites (Table S2), chlorine residual alone could not be the causal factor in the Legionella densities being less in 1st than 2nd draw water. Regarding the potential effects of amoeba hosts on Legionella and Mycobacterium, it was frequently observed that V. vermiformis correlated with Legionella and Mycobacterium spp. presence, especially with the latter. Previously it has been demonstrated that the growth of pathogenic Legionella is positively supported by free-living amoebae, biofilm and algae (Greub &Raoult 2004, Tison et al. 1980, Wadowsky et al. 1988). In a distribution system, positive correlations between V. vermiformis and total bacteria, mycobacteria and V. vermiformis, and Legionella and V. vermiformis have been reported (Delafont et al. 2014, Wang et al. 2012). As of P. aeruginosa, low level of occurrence and densities compared to the major three OP groups were also observed previously in distribution system (Lu et al. 2016) and storage tank sediments (Lu et al. 2015). It was reported that P. aeruginosa were more prevalent in drains (51%) than manual faucet water (14%), in the 1st draw than the 2nd draw (Charron 2014), and in outlet point than plumbing system (Christina 2015).
Indicators of problematic biofilm growth: Mycobacterium spp.
A previous study on drinking water distribution system showed that total Legionella could be used to indicate saprozoic (environmental) opportunistic bacterial growth for piped microbial water quality (Lu et al. 2016). In the current study, mycobacteria occurred more frequently and at higher density in the 1st than in the 2nd draw samples, possibly because they were released from biofilms within faucets or shower heads. Mycobacteria, including NTM, readily form persistent biofilms due to their generally waxy cell walls and slow growth rates, favorable attributes to resist shear forces generated during shower operation (Wadowsky et al. 1988, Wadowsky et al. 1982) and as evident in numerous tap and shower water and biofilm samples (Covert et al. 1999, du Moulin et al. 1988, Kusnetsov et al. 2003, O’Brien et al. 2000, Rusin et al. 1997, van der Wielen &van der Kooij 2013, Wang et al. 2012) using culture-based methods. For example, the relative abundance of mycobacteria accounted for 38%, 32% and 12% of total bacterial community in drinking water tubing biofilm, shower head biofilm and tap water, respectively (Feazel et al. 2009, Lu et al. 2014). However, sampling of biofilm in distribution systems or on premise is challenging in most cases. Yet, so-called first flush water samples (1st draw samples in this study) yield one estimate for biofilm-derived mycobacteria, which may provide an indication of biofilms of potential concern in premises. In addition to the common presence of various mycobacteria in drinking water biofilms, biofilms are subject to detachment due to abrasion effects from sediments (Brading et al. 2003), and the rate of erosion and sloughing of biofilm increases with increased biofilm thickness and fluid shear at the biofilm-bulk liquid interface (Characklis &Marshall 1990). Furthermore, Mycobacterium spp. presence also correlates with V. vermiformis (Liu et al. 2012), presumably present with general increases in bacterial biomass in biofilms. We also report that increased mycobacteria correlated with Legionella and V. vermiformis. These characteristics might make total Mycobacterium and Legionella ssp. suitable indicators for problematic biofilm and water quality, respectively, noting that both genera include non-pathogenic and environmental members.
Supplementary Material
Acknowledgements & Disclaimer
This research was supported by U-SSW2.2B for pathogen detection and Research Effort of SSWR 6.01C for Distribution System Indicators and Plumbing Water. The views expressed in this article are those of the author(s) and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Footnotes
Conflict of interest
No conflict of interest exists.
References
- Alary M, Joly J (1991): Risk factors for contamination of domestic hot water systems by legionellae. Appl Environ Microbiol 57, 2360–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra S, Berger F, Berthelot P, Grattard F, Pozzetto B, Riffard S (2008): Use of flow cytometry to monitor Legionella viability. Appl Environ Microbiol 74, 7813–7816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra S, Grattard F, Girardot F, Riffard S, Pozzetto B, Berthelot P (2011): Longitudinal evaluation of the efficacy of heat treatment procedures against Legionella spp. in hospital water systems by using a flow cytometric assay. Appl Environ Microbiol 77, 1268–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbolt NJ (2015): Environmental (saprozoic) pathogens of engineered water systems: understanding their ecology for risk assessment and management. Pathogens 4, 390–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumeran C, Paillard C, Robin F, Kanold J, Baud O, Bonnet R, Souweine B, Traore O (2007): Pseudomonas aeruginosa and Pseudomonas putida outbreak associated with contaminated water outlets in an oncohaematology paediatric unit. J Hosp Infect 65, 47–53 [DOI] [PubMed] [Google Scholar]
- Bartley PB, Zakour NB, Stanton-Cook M, Muguli R, Prado L, Garnys V, Taylor K, Barnett TC, Pinna G, Robson J (2015): Hospital-wide eradication of a nosocomial Legionella pneumophila serogroup 1 outbreak. Clin Infct Dis, civ870 [DOI] [PubMed] [Google Scholar]
- Bédard E, Boppe I, Kouamé S, Martin P, Pinsonneault L, Valiquette L, Racine J, Prévost M (2016): Combination of Heat Shock and Enhanced Thermal Regime to Control the Growth of a Persistent Legionella pneumophila Strain. Pathogens 5, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer KD, Gargano JW, Roberts VA, Hill VR, Garrison LE, Kutty PK, Hilborn ED, Wade TJ, Fullerton KE, Yoder JS (2015): Surveillance for wate rborne disease outbreaks associated with drinking water—United States, 2011–2012. MMWR Morb Mortal Wkly Rep 64, 842–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading MG, Jass J, Lappin-Scott HM (2003): Dynamics of Bacterial Biofilm. Microb Biof 5, 46 [Google Scholar]
- Brooks T, Osicki RA, Springthorpe VS, Sattar SA, Filion L, Abrial D, Riffard S (2004): Detection and identification of Legionella species from groundwaters. J Toxicol Environ Health Part A 67, 1845–1859 [DOI] [PubMed] [Google Scholar]
- Buse HY, Schoen ME, Ashbolt NJ (2012): Legionellae in engineered systems and use of quantitative microbial risk assessment to predict exposure. Water Res 46, 921–933 [DOI] [PubMed] [Google Scholar]
- Characklis WG, Marshall KC (1990): Biofilms.
- Chen Y-s, Liu Y-c, Lee SS-j, Tsai H-c, Wann S-r, Kao C-h, Chang C-l, Huang W-k, Huang T-s, Chao H-L (2005): Abbreviated duration of superheat-and-flush and disinfection of taps for Legionella disinfection: lessons learned from failure. Am J Infet Control 33, 606–610 [DOI] [PubMed] [Google Scholar]
- Cooper I, White J, Mahenthiralingam E, Hanlon G (2008): Long-term persistence of a single Legionella pneumophila strain possessing the mip gene in a municipal shower despite repeated cycles of chlorination. J Hosp Infect 70, 154–159 [DOI] [PubMed] [Google Scholar]
- Covert TC, Rodgers MR, Reyes AL, Stelma GN (1999): Occurrence of nontuberculous mycobacteria in environmental samples. Appl Environ Microbiol 65, 2492–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delafont V, Mougari F, Cambau E, Joyeux M, Bouchon D, Héchard Y, Moulin L (2014): First evidence of amoebae–mycobacteria association in drinking water network. Environ. Sci. Technol 48, 11872–11882 [DOI] [PubMed] [Google Scholar]
- Demirjian A, Lucas CE, Garrison LE, Kozak-Muiznieks NA, Brown EW, Wortham JM, Beaudoin A, Casey ML, Marriott C, Ludwig AM (2015): The Importance of Clinical Surveillance in Detecting Legionnaires’ Disease Outbreaks: A Large Outbreak in a Hospital With a Legionella Disinfection System—Pennsylvania, 2011–2012. Clin Infct Dis 60, 1596–1602 [DOI] [PubMed] [Google Scholar]
- du Moulin GC, Stottmeier KD, Pelletier PA, Tsang AY, Hedley-Whyte J (1988): Concentration of Mycobacterium avium by hospital hot water systems. Jama 260, 1599–1601 [DOI] [PubMed] [Google Scholar]
- Dupuy M, Binet M, Bouteleux C, Herbelin P, Soreau S, Héchard Y (2016): Permissiveness of freshly isolated environmental strains of amoebae for growth of Legionella pneumophila. FEMS Microbiol Let 363, fnw022 [DOI] [PubMed] [Google Scholar]
- Exner M, Kramer A, Lajoie L, Gebel J, Engelhart S, Hartemann P (2005): Prevention and control of health care–associated waterborne infections in health care facilities. Am J Infet Control 33, S26–S40 [DOI] [PubMed] [Google Scholar]
- Falkinham J III, Iseman M, Haas P, Soolingen D (2008): Mycobacterium avium in a shower linked to pulmonary disease. Journal of water and health 6, 209–213 [DOI] [PubMed] [Google Scholar]
- Falkinham JO III (2003): Mycobacterial aerosols and respiratory disease. Emerging Infect. Dis 9, 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham JO, Pruden A, Edwards M (2015): Opportunistic premise plumbing pathogens: Increasingly important pathogens in drinking water. Pathogens 4, 373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feazel LM, Baumgartner LK, Peterson KL, Frank DN, Harris JK, Pace NR (2009): Opportunistic pathogens enriched in showerhead biofilms. Proc Natl Acad Sci 106, 16393–16399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields BS, Benson RF, Besser RE (2002): Legionella and Legionnaires’ disease: 25 years of investigation. Clin Microbiol Rev 15, 506–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greub G, Raoult D (2004): Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev 17, 413–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemet T, Levesque B, Gauvin D, Brousseau N, Giroux JP, Cantin P (2010): Assessment of real‐time PCR for quantification of Legionella spp. in spa water. Letters in applied microbiology 51, 639–644 [DOI] [PubMed] [Google Scholar]
- Heifets L (2004): Mycobacterial infections caused by nontuberculous mycobacteria, Seminars in respiratory and critical care medicine, pp. 283–295 [DOI] [PubMed] [Google Scholar]
- Ikedo M, Yabuuchi E (1986): Ecological studies of Legionella species. Microbiol Immunol 30, 413–423 [DOI] [PubMed] [Google Scholar]
- Keinänen-Toivola MM, Revetta RP, Santo Domingo JW (2006): Identification of active bacterial communities in a model drinking water biofilm system using 16S rRNA-based clone libraries. FEMS Microbiol Let 257, 182–188 [DOI] [PubMed] [Google Scholar]
- Kusnetsov J, Torvinen E, Perola O, Nousiainen T, KATILA ML (2003): Colonization of hospital water systems by legionellae, mycobacteria and other heterotrophic bacteria potentially hazardous to risk group patients. Apmis 111, 546–556 [DOI] [PubMed] [Google Scholar]
- Liu R, Yu Z, Guo H, Liu M, Zhang H, Yang M (2012): Pyrosequencing analysis of eukaryotic and bacterial communities in faucet biofilms. Sci Total Environ 435, 124–131 [DOI] [PubMed] [Google Scholar]
- Lu J, Santo Domingo JW, Lamendella R, Edge T, Hill S (2008): Phylogenetic diversity and molecular detection of bacteria in gull feces. Appl Environ Microbiol 74, 3969–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Buse H, Gomez‐Alvarez V, Struewing I, Santo Domingo J, Ashbolt NJ (2014): Impact of drinking water conditions and copper materials on downstream biofilm microbial communities and Legionella pneumophila colonization. J Appl Microbiol 117, 905–918 [DOI] [PubMed] [Google Scholar]
- Lu J, Struewing I, Yelton S, Ashbolt N (2015): Molecular survey of occurrence and quantity of Legionella spp., Mycobacterium spp., Pseudomonas aeruginosa and amoeba hosts in municipal drinking water storage tank sediments. J Appl Microbiol 119, 278–288 [DOI] [PubMed] [Google Scholar]
- Lu J, Struewing I, Vereen E, Kirby A, Levy K, Moe C, Ashbolt N (2016): Molecular Detection of Legionella spp. and their associations with Mycobacterium spp., Pseudomonas aeruginosa and amoeba hosts in a drinking water distribution system. J Appl Microbiol 120, 509–521 [DOI] [PubMed] [Google Scholar]
- Neil K, Berkelman R (2008): Increasing incidence of legionellosis in the United States, 1990–2005: changing epidemiologic trends. Clin Infct Dis 47, 591–599 [DOI] [PubMed] [Google Scholar]
- Nishiuchi Y, Maekura R, Kitada S, Tamaru A, Taguri T, Kira Y, Hiraga T, Hirotani A, Yoshimura K, Miki M (2007): The recovery of Mycobacterium avium-intracellulare complex (MAC) from the residential bathrooms of patients with pulmonary MAC. Clinical Infectious Diseases 45, 347–351 [DOI] [PubMed] [Google Scholar]
- O’Brien DP, Currie BJ, Krause VL (2000): Nontuberculous mycobacterial disease in northern Australia: a case series and review of the literature. Clin Infct Dis 31, 958–967 [DOI] [PubMed] [Google Scholar]
- Ohno A, Kato N, Yamada K, Yamaguchi K (2003): Factors influencing survival of Legionella pneumophila serotype 1 in hot spring water and tap water. Appl Environ Microbiol 69, 2540–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedro-Botet M, Stout J, Yu V (2002): Legionnaires’ disease contracted from patient homes: the coming of the third plague? Eur J Clin Microbiol Infect Dis 21, 699–705 [DOI] [PubMed] [Google Scholar]
- Rogers J, Dowsett A, Dennis P, Lee J, Keevil C (1994): Influence of temperature and plumbing material selection on biofilm formation and growth of Legionella pneumophila in a model potable water system containing complex microbial flora. Appl Environ Microbiol 60, 1585–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr U, Weber S, Michel R, Selenka F, Wilhelm M (1998): Comparison of free-living amoebae in hot water systems of hospitals with isolates from moist sanitary areas by identifying genera and determining temperature tolerance. Appl Environ Microbiol 64, 1822–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusin PA, Rose JB, Haas CN, Gerba CP (1997): Risk assessment of opportunistic bacterial pathogens in drinking water, Reviews of environmental contamination and toxicology. Springer, pp. 57–83 [DOI] [PubMed] [Google Scholar]
- Schulze-Röbbecke R, Buchholtz K (1992): Heat susceptibility of aquatic mycobacteria. Appl Environ Microbiol 58, 1869–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout J, Yu V, Best M (1985): Ecology of Legionella pneumophila within water distribution systems. Appl. Environ. Microbiol 49, 221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout J (2007): Preventing legionellosis. ASHRAE journal 49, 58–63 [Google Scholar]
- Thorn J (2001): The inflammatory response in humans after inhalation of bacterial endotoxin: a review. Inflammation Research 50, 254–261 [DOI] [PubMed] [Google Scholar]
- Tison D, Pope D, Cherry W, Fliermans C (1980): Growth of Legionella pneumophila in association with blue-green algae (cyanobacteria). Appl. Environ. Microbiol 39, 456–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wielen PW, van der Kooij D (2013): Nontuberculous mycobacteria, fungi, and opportunistic pathogens in unchlorinated drinking water in The Netherlands. Appl Environ Microbiol 79, 825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianelli N, Giannini MB, Quarti C, Sabattini MB, Fiacchini M, de Vivo A, Graldi P, Galli S, Nanetti A, Baccarani M (2006): Resolution of a Pseudomonas aeruginosa outbreak in a hematology unit with the use of disposable sterile water filters. Haematologica 91, 983–985 [PubMed] [Google Scholar]
- Wadowsky R, Yee R, Mezmar L, Wing E, Dowling J (1982): Hot water systems as sources of Legionella pneumophila in hospital and nonhospital plumbing fixtures. Appl Environ Microbiol 43, 1104–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadowsky R, Butler L, Cook M, Verma S, Paul M, Fields B, Keleti G, Sykora J, Yee R (1988): Growth-supporting activity for Legionella pneumophila in tap water cultures and implication of hartmannellid amoebae as growth factors. Applied and Environmental Microbiology 54, 2677–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadowsky RM, Wolford R, McNamara A, Yee RB (1985): Effect of temperature, pH, and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water. Appl Environ Microbiol 49, 1197–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Edwards M, Falkinham JO 3rd, Pruden A (2012): Molecular survey of the occurrence of Legionella spp., Mycobacterium spp., Pseudomonas aeruginosa, and amoeba hosts in two chloraminated drinking water distribution systems. Appl. Environ. Microbiol 78, 6285–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2007): Legionella and the Prevention of Legionellosis. World Health Organization, WHO Press, Geneva [Google Scholar]
- Wullings BA, van der Kooij D (2006): Occurrence and genetic diversity of uncultured Legionella spp. in drinking water treated at temperatures below 15 C. Appl Environ Microbiol 72, 157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullings BA, Bakker G, van der Kooij D (2011): Concentration and diversity of uncultured Legionella spp. in two unchlorinated drinking water supplies with different concentrations of natural organic matter. Appl. Environ. Microbiol 77, 634–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Sugiura M, Kusunoki S, Ezaki T, Ikedo M, Yabuuchi E (1992): Factors stimulating propagation of legionellae in cooling tower water. Appl Environ Microbiol 58, 1394–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacheus OM, Martikainen PJ (1994): Occurrence of legionellae in hot water distribution systems of Finnish apartment buildings. Can J Microbiol 40, 993–999 [DOI] [PubMed] [Google Scholar]
- Zichichi L, Asta G, Noto G (2000): Pseudomonas aeruginosa folliculitis after shower/bath exposure. Int J Dermatol 39, 270–273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.